Abstract
Nesting habits of the digger wasp Lindenius albilabris (Fabricius, 1793) at two localities in Poland were studied. The female hunts nymphs or adults of plant bugs Lygus rugulipennis Poppius, 1911 and L. pratensis (Linnaeus, 1758), depositing up to 10 individuals per cell, depending on their size. Nests are built in a wide variety of sandy habitats, on dirt roads, and in wastelands. The nest consists of a 10–12 cm long main burrow and one to three cells. Males are usually found on flowers of wild carrots Achillea millefolium, Daucus carota, Peucedanum oreoselinum, Pimpinella sp. and Tanacetum vulgare. The nest kleptoparasite Metopia argyrocephala was recorded in nests of this species. The mature larva is first described in this study and its morphology is compared with other species of the genus.
Introduction
The genus Lindenius Lepeletier and Brullé, 1835 comprises 64 species, 57 of which occur in the Old World (Pulawski Citation2022). Representatives of this genus are small, stocky and black pigmented with widely spaced hind ocelli, which distinguishes them from the similar genus Crossocerus Lepeletier and Brullé, 1834. Miller and Kurczewski (Citation1975) reviewed the world literature on the ethology of three Palaearctic species – L. albilabris (Fabricius, 1793), L. panzeri (Vander Linden, 1829) and L. pygmaeus (Rossi, 1794) – and added data on the nesting behaviour of L. armaticeps (Fox, 1895), L. buccadentis Mickel, 1916 and L. columbianus errans (Fox, 1895) from the Nearctic region. Bohart and Menke (Citation1976) added at least partial information on the habits of three Nearctic species: L. columbianus (Kohl, 1892), L. tecuya Pate, 1947 and L. tylotis Court and Bohart, 1958.
Central European Lindenius usually nest in flat terrain, on both sandy and harder ground (Blösch Citation2000). The nest entrance is always surrounded by a prominent mound, and is not closed during the provisioning period, so that an approaching female with prey can easily get inside.
The prey is held belly up by the middle or hind legs or is impaled on the sting (Kazenas Citation2001). According to Kazenas (Citation2001), nests can contain up to 24 cells and are usually built from the bottom up along the main channel, which can be 20 cm deep. Females prey on small insects from three orders: Diptera, Hemiptera and Hymenoptera (Lomholdt Citation1984). The egg is laid on one of the first prey individuals introduced into the cell and is placed under the head of the prey on the ventral side. The larva eats all prey within 1–5 days. The cocoon is covered with the remains of the prey. Adult digger wasps visit flowering plants from multiple families, e.g. Apiacae, Lamiaceae, Asteraceae, Euphorbiaceae and many others (Kazenas Citation2001). Kleptoparasites are Miltogramminae (Sarcophagidae), Myrmosa (Tiphidae) and Hedychridium (Chrysididae) (Bohart & Menke Citation1976).
Lindenius albilabris is the most widespread species of Lindenius in the Palaearctic (Kohl Citation1915). Females of this species hunt prey belonging to two orders: Hemiptera – Miridae (Nielsen Citation1900; Bouwman Citation1911; Grönblom Citation1925; Hamm & Richards Citation1926; Minkiewicz Citation1931, Citation1933) and Diptera – Chloropidae (Hamm & Richards Citation1926), Empididae (Bristowe Citation1948), and Anthomyiidae (Hüsing & Jäger Citation1964).
The egg of this species is white and banana-shaped, attached to the prosternum obliquely (Minkiewicz Citation1931) or almost transversely (Adlerz Citation1910). Bonelli (Citation1967) described the development of the larva and the cocoon construction. Interestingly, males of L. albilabris are able to dig a short burrow ending in a single cell in which they spend the night (Minkiewicz Citation1931). According to Bonelli (Citation1967) under laboratory conditions, the larva reached maturity within four days and the cocoon is yellow-brown, covered with sand particles. Grönblom (Citation1925) found smaller, darker cocoons in Finland and noticed that they were covered with prey remains, larvae droppings and gravel particles.
The objective of the study is to provide information on the nesting habits of L. albilabris, such as: (1) nest construction, including number of cells, length and diameter of the main burrow and transport of prey to the nest; (2) larval food, including species composition and the number of individuals per cell; (3) description of the mature larva; and (4) kleptoparasites.
Materials and methods
The research on L. albilabris was carried out in the town of Kowalewo Pomorskie (53°10ʹ05.7″N, 18°52ʹ15.5″E) and the village of Sierakowo (53°10ʹ19.0”N, 18°52ʹ21.7”E) both in Poland, from early June to late September in 2020 and 2021 in insignificantly varying weather conditions of sunny and warm days (with a temperature of at least 18°C) and in comparable timed samples (). The site in Kowalewo Pomorskie was an agricultural wasteland overgrown with segetal and ruderal vegetation, dominated by Tanacetum vulgare L., Taraxacum officinale F.H. Wigg., Geranium pusillum L., Trifolium arvense L., Lactuca serriola L., Cerastium holosteoides Fr. em. Hyl., Berteroa incana (L.) DC., Artemisia vulgaris L., Achillea millefolium L., Daucus carota L. and Potentilla anserina L. This site partly covered the same area that was described in our papers on nesting biology of Dryudella stigma (Olszewski et al. Citation2021a), Oxybelus variegatus (Olszewski et al. Citation2021b) and Alysson spinosus (Olszewski et al. Citation2022). The second site, in Sierakowo, was described in the paper on nesting biology of A. spinosus (Olszewski et al. Citation2022) and consisted of sandy loam, dominated by Helichrysum arenarium (L.), Peucedanum oreoselinum (L.), Sedum spp. and Senecio vernalis Waldst. et Kit. The surroundings of the studied sites included an agricultural landscape dominated by the cultivation of maize, cereals and potatoes. Nesting activity (i.e. nest digging and prey transportation) was analysed based on direct observations and on-site notes. Photographs were taken with a Canon EOS M50 camera; an additional Raynox M-250 macroscopic lens was also used. The foraging range was determined by nest inspection. The structure of the nest was analysed by unearthing it. During the females’ activity (from late June to late September), their nests were dug out and larvae were collected for analysis. Larvae and pupae of kleptoparasitic wasps were grown in Eppendorf tubes. The larvae were fixed in Pampel’s solution.
Table I. Number of 30 minute observation samples per month in 2020 and 2021.
To describe the larval specimens, we transferred some of the larvae into Pampel solution (30 volumes of distilled water, 15 volumes of 96% ethanol, 6 volumes of formaldehyde and 4 volumes of glacial acetic acid) as described by Švácha and Danilevsky (Citation1987). After taking photographs of the intact larvae, we examined their sclerotised parts. For this purpose, we placed the larvae into a 10% solution of hot (60°C) KOH for 12 h to dilute all body parts except the integument. We then coloured the integument in 5% Chlorazol Black E (Sigma Aldrich) for 2 s and then moved it into 96% ethanol. To observe the species-specific characteristics, we placed the integument into glycerol and separately observed the head, mouthparts, spiracles and other parts under a light microscope. We used the same specimens to study small structures such as setae, sensilla or mouthparts. We drew figures of (1) the head, with a focus on the clypeus, labrum, maxillae and labium; (2) mandibles, in anterior view; and (3) spiracles of larvae. Specimens of kleptoparasitic flies and prey are deposited in the first author’s collection and larvae are deposited at the University of Hradec Králové, Czech Republic.
Results
Environmental preferences
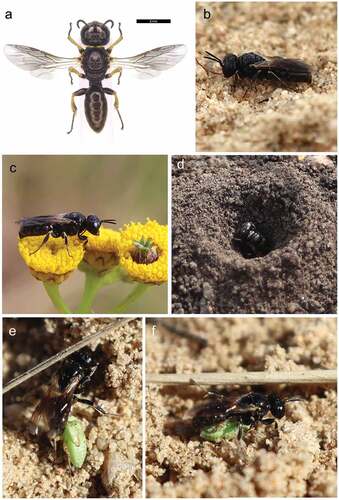
Adult individuals were observed from early June to late September 2020 and 2021, with females () most frequently observed carrying prey to the nest ( or accompanied by a male ()), on plants such as Achillea millefolium, Daucus carota, Peucedanum oreoselinum, Pimpinella sp. and Tanacetum vulgare. A total of 12 nests were found during the research, of which four nests (two in sandy loam and two in agricultural wasteland) were inspected. The area with nests in the wasteland area was strongly hardened, surrounded by rich segetal and ruderal vegetation. In Sierakowo, nests were built in bare ground surrounded by rich thermophilic vegetation. The nests were usually built as single structures, or rarely in pairs in close proximity to each other ()), with the nearest distance between the channel entrances being 10 cm (N = 4). The circular nest entrance, with a diameter of 3–4 mm, was surrounded by a mound (which, depending on the site, may have been blown away by the wind) approximately 25 mm wide × 5 mm high. Provisioning females usually entered directly into the main canal while still in flight (by diving). Females in flight transported their prey impaled on the sting or supported it with their middle legs.
Figure 2. Nest of Lindenius albilabris. (a) Lateral view of nest and brood cells; (b–d) top view of the nest entrance.
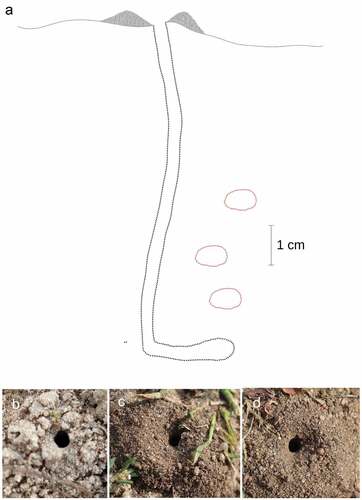
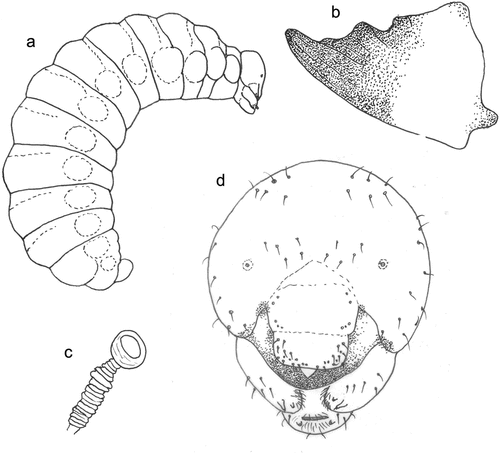
Figure 5. Larva of Lindenius albilabris. (a) Body, lateral view; (b) mandible, frontal view; (c) spiracle; (d) head, frontal view.
The provisioning flights took place with varying intensity, mainly between 9.30a.m. and 4.30p.m., from late June to late September. The highest density of active females was observed in the afternoon from 12.00p.m. to 4.00p.m. The female did not leave the main burrow at night or during unfavourable weather conditions. The kleptoparasitic sarcophagid fly Metopia argyrocephala (Meigen, 1824) was spotted entering the nest during the absence of the female ().
Nest structure
During the excavation of four nests, pupae of M. argyrocephala and two larvae of L. albilabris were excavated (; ). No eggs were recorded and no larvae of L. albilabris were reared. The nest consisted of a nearly vertical main tunnel about 10–12 cm long (3–4 mm in diameter). From half of the tunnel depth to the kink there were one to three cells, then the tunnel curved horizontally (about 2 cm) and ended with another cell (). A single brood cell contained up to 10 dead specimens paralysed or dead plant bugs ().
Table II. Nests of Lindenius albilabris.
Description of larva
Material examined
Two larvae, Sierakowo, 29 August 2021.
Diagnosis
The larva of L. albilabris is similar in most details to those of L. pygmaeus and L. tylotis. The main difference is the size, L. albilabris being larger than the other two. Grandi (Citation1928, Citation1961) described the mature larva of L. pymaeus. According to him, the mandible of that species has three teeth as opposed to the four in L. albilabris and L. tylotis. However, one tooth is lateral and may have not been taken into account by the author. The mandible of L. albilabris, however, is more massive, with a length-to-width ratio of 10:6, and the teeth are more rounded. The shape and measurements of the mandible were not mentioned in the short description of mature larva of L. tylotis by Evans (Citation1959), so we cannot make any comparison. Additionally, the diameter of the basal ring of the antenna is about 1/5 the distance from the closest point of the ring to the centre of the anterior tentorial pit, so it is larger than that of L. pygmaeus. In all other characters, the mature larva of L. albilabris generally agrees with those of L. pygmaeus and L. tylotis.
Description
Body
Body length 7.5 and 8.7 mm (N = 2) (). Body covered with densely distributed short spicules, and with several slender, pale setae, tapering to fine points, arising from small but distinct alveoli; setae not elongate. Several setae on mouthparts, mandibles, area around mandibular condyli and maxillar and labial palpi brownish, remaining body whitish. Other body parts with only several setae, except last three metasomal segments more setose. Body of postdefecating larva fusiform and only very slightly dorsoventrally flattened, robust; body segments similarly wide along whole length. Paired body tubercles present and well developed on all mesosomal and metasomal segments except T10, tubercles similarly large on all segments but most conspicuous on mesothorax and metathorax and on T7–T9. Dorsal tubercles wide, flat and well developed on all three thoracic segments, most conspicuous on T4–T5. In predefecating larva, T3–T6 have the greatest diameter in lateral outline. Abdominal segment 9 more hirsute than previous ones, segment 10 attached to middle of segment 9 in lateral view; anus positioned medially and transverse. Spiracles unpigmented, subequal in diameter; atrium globular, slightly wider than deep (), projecting little above body wall, with rim; atrial opening diameter vs. peritreme width ratio 1:4; atrial inner surface with rows of wrinkles concentric with primary tracheal opening; primary tracheal opening without collar; subatrium short, with about 10 chambers of approximately equal size except one or two next to atrium slightly larger in diameter. Sex characters unknown.
Head
Head moderately small in relation to body size; oriented in normal, hypognathous position relative to thorax. Setae long but sparse on upper part of head capsule; those of maxillary and labial apices large, straight and conspicuous (). Head capsule unpigmented except at points of articulations with mandibles; mandible conspicuously pigmented; labrum and maxillary sclerites faintly pigmented; salivary lips projecting but unpigmented; maxillary and labial palpi all uniformly moderately pigmented. Coronal and postoccipital ridges absent. Tentorium mostly absent because of impending ecdysis. Parietal bands absent. In lateral view, clypeus globularly projecting beyond frons, antenna arising from ill-developed prominence, and labrum extending beyond clypeus. Diameter of basal ring of antenna about 1/5 distance from closest point on ring to centre of anterior tentorial pit; antennal papilla only slightly pigmented, very small and not elongate, bearing two sensilla apically. Frontal area between antennae with two groups of five setae and many spinules. Parietal region with many setae – three setae from pleurostomal ridge to front tentorial pit and multiple sensilla on the sides. Clypeus wide with ill-developed basal and apical margins, with two sensilla basally on sides and three small sensilla more medially on each side. Labrum not emarginated apically in the middle, with a group of nine conspicuous setae and several smaller sensilla on each side subapically; labral sclerite not defined and only poorly pigmented. Epipharynx simple with large spinulae on the whole surface. Mandible moderately robust; darkly pigmented, with apical tooth longest and moderately blunt, conspicuous lateral tooth, and with one subbasal and two subapical tubercles; outer mandibular surface without setae (). Maxillary apex strongly bent mesad in frontal view, maxillary palpus subapical, with many small elongated setae. Cardo distinct, posterior end directed towards posterior tentorial pit; stipes weakly sclerotised; maxillary palpi elongate, more than three times basal diameters, both pigmented. Stipes with five conspicuous setae. Labium not divided into prementum and postmentum; apex moderately narrow in frontal view, with three setae on each side. Spinneret transversal and well visible, with inner surface bearing parallel longitudinal grooves; lip width slightly more than double width of maxillary palpus. Labial palpus elongate, with two sensilla in middle.
Discussion
The biology of Lindenius species is incompletely known; the available information is mainly limited to the food base of larvae, plants as a source of nectar for adults, and environmental preferences for several Palaearctic and New World species (Bohart & Menke Citation1976). Our data on L. albilabris, including the description of the adult larva, nesting habits, time of nest construction, and food of larvae, mostly agree with previously reported observations (Nielsen Citation1900; Adlerz Citation1910; Minkiewicz Citation1931, Citation1933; Bristowe Citation1948; Bonelli Citation1967). The varying number of cells in individual nests may depend on the abundance and size of food or the type of substrate.
In this study, the food base from two orders (Hemipetra and Diptera) for larvae of L. albilabris was not confirmed. Minkiewicz (Citation1931) emphasised the homogeneity of Lygus pratensis (L.) prey carried by the female into the cells. During our research, we have confirmed the homogeneity of prey with respect to the genus Lygus (). During this study, L. rugulipennis is reported for the first time as prey of L. albilabris. It seems that the choice of both L. pratensis and L. rugulipennis as prey results from their common availability near the nesting site. Both species are broadly distributed throughout Poland and Europe, being the most numerous and widespread representatives of the genus (Gorczyca & Wolski Citation2011), with the vast majority being nymphs. Besides the relative availability of these heteropterans, the habitats selected by L. albilabris largely overlap with those occupied by Lygus pratensis and L. rugulipennis: crop lands and grasslands. In such habitats, L. rugulipennis and L. pratensis are among the most numerous true-bug species (Holopainen & Varis Citation1991; Gorczyca & Wolski Citation2011).
Plant bugs of the genus Lygus feed on various cultivated species – trees, vegetables and cereals – and are among the most important pest on a wide range of crops in Europe (Wheeler Citation2000). Thus, L. albilabris feeds the brood in such habitats exclusively with Lygus, and plays a hitherto undiscovered and highly advantageous role as a natural enemy of these pests (Wheeler Citation2001).
The egg of L. albilabris is attached to the prosternum obliquely (Minkiewicz Citation1931) or almost transversely (Adlerz Citation1910). No rearing of larvae of L. albilabris was conducted in this study. Breeding results were presented by Bonelli (Citation1967), who described the cocoon as yellow-brown covered with sand particles. Interestingly, Grönblom (Citation1925) found smaller, darker cocoons in Finland and noticed that they were covered with prey remains, larvae droppings and gravel particles. Lomholdt (Citation1984) reported two ecophenotypes, one of which, as mentioned above, uses Hemiptera as food for larvae, while the other, apparently a more southern type, uses smaller flies. According to Adlerz (Citation1910), both types are represented in Sweden but they are not sympatric. Adlerz (Citation1910) additionally described a population that uses both flies and plant bugs for larval provisioning. The issue of L. albilabris species identity certainly requires examination of the material of both ecophenotypes by molecular tests to clarify their status.
Lindenius pygmaeus also preys on a wide spectrum of prey, which includes insects from two orders: Hymenoptera (Braconidae, Formicidae, Ophioninae, Pteromalidae) and Diptera (Ceratopogonidae and Sciaridae) (Miller & Kurczewski Citation1975). On the other hand, L. panzeri seems to choose only Diptera (Chloropidae, Milichiidae, Simuliidae, Tephritidae) (Miller & Kurczewski Citation1975).
To date, the morphology of the larvae of only two species of Lindenius is known. Grandi (Citation1928, Citation1961) provided a description of L. pygmaeus larvae, while Evans (Citation1959) provided a description of L. tylotis larvae. The larva of L. albilabris is mostly similar to the larvae of the two already known species, but is generally larger. However, the available data show that all known larvae are morphologically very similar, and it is hard to compare anything with the short description of Evans (Citation1959). Features such as eyes naked, mandibles without ventral emargination, and short galeae are known in all three species identified so far and can be used to distinguish Lindenius from related genera.
Conclusions
Reviewing the nesting biology of L. albilabris, we can conclude that nests are usually built as single structures, or rarely in pairs within a short distance of each other. Entrances, surrounded by a mound of earth, were open all the time during the provisioning period. Provisioning females were usually falling directly into the main canal while still in flight. The female collects up to 6–10 nymphs or imagines of L. rugulipennis or L. pratensis per cell, depending on their size. Females transported their prey in flight impaled on the sting or supported with their middle legs. Provisioning flights took place with varying intensity, mainly between 9.30a.m. and 4.30p.m. The nest consisted of an almost vertical main channel and, from mid-depth down, one to three cells. There were on average six paralysed or dead plant bugs in each cell, belonging to two species: L. rugulipennis and L. pratensis. The larva of this species is larger in size compared to other known larvae of this genus. It differs in having more rounded and stronger mandibles. In addition, the diameter of the basal antennal ring is about 1/5 of the distance from the closest point on the ring to the centre of the anterior tentorial pit.
Acknowledgements
We thank Karolina and Łukasz Musiał for the possibility of conducting our research in Kowalewo Pomorskie and Kamila Ludwik for the possibility of conducting our research in Sierakowo. We also thank Wojciech Pulawski (California Academy of Science) for his critical review of the manuscript and Krzysztof Szpila (Nicolaus Copernicus University) for identifying the kleptoparasitic fly.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Adlerz G. 1910. Lefnadsförhållanden och instinkter inom familjerna Pompilidae och Sphegidae. III. Kungliga Svenska Vetenskapsakademiens Handlingar 45(12):1–75.
- Blösch M. 2000. Die Grabwespen Deutschlands. Sphecidae s. str., Crabronidae. Lebensweise, Verhalten, Verbreitung. In: Blank SM, Taeger A, editors. Die Tierwelt Deutschlands. 71. Teil. Keltern, Germany: Goecke & Evers. pp. 480.
- Bohart RM, Menke AS. 1976. Sphecid wasps of the world. A generic revision. Berkeley: University of California Press. pp. ix + 695.
- Bonelli B. 1967. Osservazioni biologiche sugli Imenotteri melliferi e predatori della Val di Fiemme. XXV. Bollettino dell’Istituto di Entomologia della Università di Bologna 28:291–303.
- Bouwman BE. 1911. Crabro’s. Levende Natuur 16:173–177.
- Bristowe WS. 1948. Notes on the habits and prey of twenty species of British hunting wasps. Proceedings of the Linnean Society of London 160:12–37. DOI: 10.1111/j.1095-8312.1948.tb00502.x.
- Evans HE. 1959. Studies on the larvae of digger wasps (Hymenoptera, Sphecidae). Part V: Conclusion. Transactions of the American Entomological Society 85:137–191.
- Gorczyca J, Wolski A. 2011. A catalogue of plant bugs (Heteroptera: Miridae) of Poland. Part II. Subfamilies: Mirinae. Warszawa: Natura optima dux Foundation.
- Grandi G. 1928. Contributi alla conoscenza biologica e morfologica degli Imenotteri melliferi e predatori. VII. Bollettino del Laboratorio di Entomologia del R. Istituto Superiore Agrario di Bologna 1:259–326.
- Grandi G. 1961. Studi di un Entomologo sugli Imenotteri superiori. Bollettino dell’Istituto di Entomologia della Università di Bologna 25:1–659.
- Grönblom T. 1925. Bidrag till kännedom om levnadssättet hos våra rovsteklar (Hymenopt., Sphegidae) I. Notulae Entomologicae 5:1–9.
- Hamm AH, Richards OW. 1926. The biology of the British Crabronidae. The Transactions of the Entomological Society of London 74:297–331. DOI: 10.1111/j.1365-2311.1926.tb02241.x.
- Holopainen JL, Varis AL. 1991. Host plants of the European tarnished plant bug Lygus rugulipennis Poppius (Heteroptera, Miridae). Journal of Applied Entomology 111:484–498. DOI: 10.1111/j.1439-0418.1991.tb00351.x.
- Hüsing JO, Jäger K. 1964. Zur Verbreitung, Biologie und Ökologie der Grabwespen (Hym. Sphec.) in der näheren Umgebund von Haalle/Saale mit speciellen Bemerkungen über Mellinus arvensis L. Hercynia (Neue Folge) 1:186–206.
- Kazenas VL. 2001. Fauna and biology of sphecid wasps (Hymenoptera, Sphecidae) of Kazakhstan and Central Asia. Almaty, Kazakhstan: Kazgos INTI. pp. 333.
- Kohl FF. 1915. Die Crabronen (Hymenopt.) der paläarktischen Region. Monographisch bearbeitet. Annalen des k.k. Naturhistorischen Hofmuseums 29:1–453.
- Lomholdt O. 1984. The Sphecidae (Hymenoptera) of Fennoscandia and Denmark. Fauna Entomologica Scandinavica. 4. 2nd ed. Leiden, Copenhagen: E.J. Brill/Scandinavian Science Press. pp. 452.
- Miller RC, Kurczewski FE. 1975. Comparative behavior of wasps in the genus Lindenius (Hymenoptera: Sphecidae, Crabroninae). Journal of the New York Entomological Society 83:82–120.
- Minkiewicz R. 1931. Nids et proies des Sphégiens de Pologne. Fragments éthologiques (première série). Polskie Pismo Entomologiczne 10:196.
- Minkiewicz R. 1933. Gniazda i zwierzyna łowna Grzebaczowatych – Nids et proies des Sphégiens de Pologne. Deuxième série de fragments éthologiques. Polskie Pismo Entomologiczne 11:98–112.
- Nielsen JC. 1900. Biologiske Studier over Gravehvepse – Recherches biologiques sur les hyménoptères fouisseurs. Videnskabelige Meddelelser fra den Naturhistoriske Forening i Kjøbenhavn 1900:255–280.
- Olszewski P, Bogusch P, Klejdysz T, Szpila K. 2022. Nesting behaviour and description of the larva of Alysson spinosus (Panzer, 1801) (Hymenoptera: Bembicidae). The European Zoological Journal 891:958–966. DOI:10.1080/24750263.2022.2100494.
- Olszewski P, Bogusch P, Mięsikowski M, Baños-Picon L, Puchałka R. 2021a. Behavioural and ecological data on Dryudella stigma (Panzer, 1809) (Hymenoptera, Astatidae) with the first description of the mature larva. Journal of Hymenoptera Research 82:305–316. DOI: 10.3897/jhr.82.63594.
- Olszewski P, Bogusch P, Szpila K. 2021b. Life history of Oxybelus variegatus Wesmael, 1852 (Hymenoptera: Crabronidae) with a description of the mature larva. Insects 12(2):100. DOI: 10.3390/insects12020100.
- Pulawski W. 2022. Catalog of Sphecidae. San Francisco: California Academy of Sciences. Available: http://research.calacademy.org/sites/research.calacademy.org/files/Departments/ent/sphecidae/Genera_and_species_pdf/Lindenius.pdf. Accessed Jan 2022 6.
- Švácha P, Danilevsky ML. 1987. Cerambycoid larvae of Europe and Soviet Union. Part I.- Acta Universitatis Carolinae. Biologica 30:1–176.
- Wheeler Jr. AG. 2001. Biology of the plant bugs (Hemiptera, Miridae), pest, predators, opportunists. Ithaca and London: Cornell University Press. pp. 507.
- Wheeler Jr. AG. 2000. Plant bugs (Miridae) as plant pests. In: Schaefer CW, Panizzi AR, editors. Heteroptera of economic importance. Boca Raton; London; New York; Washington, DC: CRC Press. pp. 37–83, 828.