Abstract
Numerous rainbow sardine specimens, previously identified as Dussumieria elopsoides Bleeker, 1849, due to their elongate body, scales without posterior striae, and numerous gill rakers and branchiostegal rays, were examined and confirmed as representing four valid species: true D. elopsoides (restricted to Indonesia), Dussumieria hasseltii Bleeker, 1851 (previously regarded as a junior synonym of D. elopsoides; Arabian Sea to Philippines and Indonesia), Dussumieria modakandai Singh, Jayakumar, Kumar, Murali, Mishra, Singh and Lal, 2021 (Arabian Sea to Ryukyu Islands), and Dussumieria productissima Chabanaud, 1933 (previously regarded as a junior synonym of D. elopsoides; Gulf of Aden, Red Sea and eastern Mediterranean). Each species is redescribed in detail and a key based on morphological characters provided. The phylogenetic relationships among seven species of Dussumieria (including three of the species examined in the present study) were reconstructed from the cytochrome oxidase I (COI) gene, the genetic distinctiveness of each species being indicated by at least 4.1% mean p-distance divergence from the others.
Introduction
Dussumieria Valenciennes, Citation1847 is a genus of rainbow sardines characterized by 14–18 anal-fin rays, 12–18 branchiostegal rays, the pelvic-fin insertion below the dorsal-fin base, and the isthmus pointed anteriorly (Whitehead Citation1985; Munroe et al. Citation1999; Hata & Motomura Citation2020). The genus was initially reviewed by Whitehead (Citation1963), who considered it to be monotypic, including only Dussumieria acuta Valenciennes, Citation1847. Subsequently, Whitehead (Citation1985) considered the genus to comprise two valid species, D. acuta and Dussumieria elopsoides Bleeker, 1849, the latter being distinguished by an elongate body (body depth <22% of standard length vs. >22% in D. acuta), higher counts of lower gill rakers on the first gill arch (21–32 vs. 19–26) and branchiostegal rays (13–17 vs. 12–15), and posterior striae absent on the body scales (vs. numerous longitudinal striae). Whitehead (Citation1985) also regarded two nominal species (Dussumieria hasseltii Bleeker, Citation1851 and Dussumieria productissima Chabanaud, Citation1933) as junior synonyms of D. elopsoides, the latter being widely distributed in the Indo-West Pacific. However, recent taxonomic studies have revealed or suggested the existence of more than two valid species, including Dussumieria modakandai Singh, Jayakumar, Kumar, Murali, Mishra, Singh and Lal, Citation2021, morphologically resembling D. elopsoides, which was described from recently collected Indian specimens. During a revisionary study of the genus, re-examination of specimens previously identified as D. elopsoides following Whitehead’s (Citation1985) key confirmed the validity of four species: D. elopsoides, D. modakandai, and the two nominal species regarded by Whitehead (Citation1985) as synonyms of D. elopsoides. These four species are redescribed in detail herein. To support the morphological comparisons, partial cytochrome oxidase I (COI) gene sequences from 102 Dussumieria specimens were used to examine their genetic distinctiveness, and reconstruct the phylogenetic relationships of seven available species, including D. hasseltii, D. productissima and D. modakandai.
Material and methods
Counts and measurements follow Hata and Motomura (Citation2017). Standard length is abbreviated as SL. Gill raker numbers are given for the upper and lower limbs, plus total gill rakers, abbreviated as UGR, LGR and TGR, respectively, with preceding numbers indicating the specific gill arch. Distances from the dorsal-fin origin to the pectoral-fin insertion, from the dorsal-fin origin to the pelvic-fin insertion, between the dorsal-fin and anal-fin origins, between the pectoral-fin and pelvic-fin insertions, and from the pelvic-fin insertion to the anal-fin origin are abbreviated as D–P1, D–P2, D–A, P1–P2, and P2–A, respectively. Osteological characters, including vertebral counts, were observed from radiographs of 7, 14, 33 and 16 specimens of D. elopsoides, D. hasseltii, D. modakandai and D. productissima, respectively. Institutional codes follow Sabaj (Citation2020). All specimens listed in this paper, except for type specimens of D. modakandai, were examined in this study; data for the above type specimens are from Singh et al. (Citation2021). Analysis of covariance (ANCOVA) was performed with EZR (Kanda Citation2012).
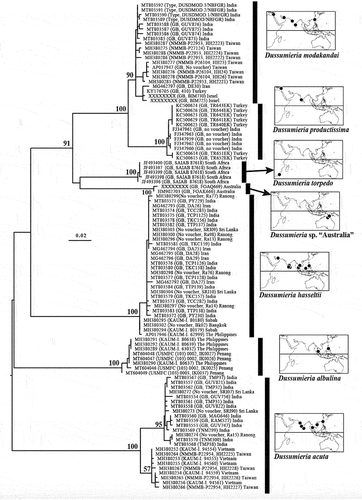
The genetic profiles of 102 specimens, comprising six described and one undescribed species of Dussumieria, were compared using the partial (648 bp) COI gene. This gene was newly sequenced for eight specimens of D. modakandai and 10 of D. hasseltii, and the resulting data were combined with COI sequences published by Lakra et al. (Citation2011), Keskin and Atar (Citation2013), Steinke et al. (Citation2016), Lavoué et al. (Citation2017), Hata et al. (Citation2020) and Singh et al. (Citation2021) (), from which the phylogenetic relationships among the species were inferred. The phylogenetic tree was rooted at its mid-point.
Table I. Molecular marker sampling of 102 specimens of Dussumieria examined in this study. Bold accession numbers indicate sequences determined in the present study. “-” indicates the corresponding sequence has no GenBank number. Abbreviation: COI, cytochrome oxidase I.
DNA was extracted from 95% ethanol-preserved tissue samples. Polymerase chain reaction (PCR) amplification and sequencing of the COI gene followed standard protocols (Ward et al. Citation2005), with annealing at 55°C. Amplification of the partial (about 670 bp) COI gene used the following primers: forward COI_FishF1 (5’-TCA ACC AAC CAC AAA GAC ATT GGC AC-3’) and reverse COI_FishR1 (5’- CCG GTC TGA ACT CAG ATC ACG T-3’). PCR products were purified, and then sequenced in both directions with Sanger Sequencing technology using the same PCR primers. Sequences generated in this study have been deposited in the GenBank database (accession numbers given in ).
Alignments of the COI sequences were determined separately by eye, requiring neither insertions nor deletions. The final alignment combining the gene (for 102 specimens) comprised 648 nucleotide positions. Uncorrected pairwise genetic distances among and within species were calculated with MEGA X (Stecher et al. Citation2020). The maximum likelihood (ML) method of phylogenetic reconstruction was used to infer relationships within and between species. Weighting all positions equally, the ML phylogenetic tree was calculated under the general time-reversible model of nucleotide substitution with rate heterogeneity following a discrete gamma distribution and an estimated proportion of invariable sites, using the software MEGA X (Stecher et al. Citation2020). Bootstrap support (500 replicates) was calculated to assess the robustness of each relationship.
Results
Molecular comparisons
Dussumieria hasseltii, D. productissima and D. modakandai diverged from each other (and from other species of Dussumieria) by at least 4.1% COI-based mean uncorrected genetic distance (min–max = 4.1–12.6%) (). In contrast, each species was genetically uniform, with intra-group differentiation not exceeding 1% (except for D. modakandai [1.1%] and Dussumieria acuta [1.8%]). The ML phylogenetic tree using the COI gene () was fully resolved, with each species forming a well-supported monophyletic group (bootstrap proportion >90%) in agreement with their genetic distinctiveness, thereby confirming their taxonomic status. The genetic results and morphological observations complement each other, supporting the validity of Dussumieria hasseltii, D. productissima and D. modakandai, among other species. COI sequences of two unexamined specimens collected off northern Australia (identified as Dussumieria sp. “Australia” in ) indicate that the species diversity of Dussumieria is still underestimated.
Table II. Cytochrome oxidase I (COI) mean pairwise uncorrected genetic distances (p distances) between species of Dussumieria. All ambiguous positions are removed for each sequence pair (pairwise deletion option) as implemented in Mega X software.
Figure 1. Maximum-likelihood phylogenetic tree of Dussumieria, based on the cytochrome oxidase I (COI) gene (total: 648 base pairs) from 102 specimens, each species forming a monophyletic group. Each specimen is identified by COI sequence GenBank (GB) number followed by Museum Registration Number, and/or sequence origin (GB) and/or specimen code (see text and for details) in parentheses, and geographical origin. Tree rooted at midpoint; branch lengths proportional to number of substitutions; bootstrap proportions (if >50%) indicated at nodes. Abbreviations: GB, GenBank; COI, cytochrome oxidase I.
Morphological comparisons
Welch’s T-test for comparison of meristic characters among the four species showed significant differences (p < 5) in at least four characters between each pair of species (–). In addition, ANCOVA of 22 morphometric characters, shown in –, showed significant differences (p < 5) in at least 10 characters between each species. Detailed comparisons with and identification of each species are included in “Comparisons” under each species account, and an identification key to the five species is provided at the end of the taxonomic account. As a result, the validity of D. elopsoides, D. modakandai and two nominal species (D. hasseltii and D. productissima) previously regarded as junior synonyms of D. elopsoides by Whitehead et al. (1988) is confirmed.
Table III. Meristics of Dussumieria elopsoides.
Table IV. Meristics of Dussumieria hasseltii.
Table V. Meristics of Dussumieria modakandai.
Dussumieria elopsoides Bleeker, 1849
[English common name: slender rainbow sardine]
(; , , , )
Figure 2. (a) non-type specimen of Dussumieria elopsoides (preserved) (KAUM–I. 97199, 107.7 mm SL, Timor, Indonesia); (b) lectotype of D. elopsoides (BMNH 1867.11.28.17, 124.7 mm SL, Java, Indonesia). Abbreviations: KAUM, Kagoshima University Museum; BMNH, British Museum of Natural History; SL, standard length.

Dussumieria elopsoides Bleeker, Citation1849b: 12 [original type locality: several localities in Indonesia including Madura Strait near Kammal and Surabaya, Java Sea near Batavia (currently Jakarta), Samarang (Semarang); type locality: Java, Indonesia, based on lectotype designated by Whitehead et al. (Citation1966)]; Günther Citation1868: 466 (in part: Java, Indonesia); Whitehead et al. Citation1966: 30 (Java, Indonesia; lectotype designation); Hata et al. Citation2022: 21 (in part: Jakarta, Java, Indonesia).
Dussumieria acuta (not of Valenciennes): Whitehead Citation1963: 312 (in part: Java, Indonesia).
Lectotype of Dussumieria elopsoides
BMNH 1867.11.28.17, 124.7 mm SL, Java, Indonesia, P. Bleeker.
Non-type specimens
7 specimens (71.6–119.9 mm SL). INDONESIA: KAUM–I. 97199, 107.7 mm SL, KAUM–I. 97453, 84.9 mm SL, Oeba, Kec. Kota Lama, Kota Kupang, Nusa Tenggara Timur, Timor (obtained at Oeba Fish Market); LBRC-F 5042, 110.7 mm SL, Ambon, Moluccas; USNM 329498, 108.1 mm SL, Gulf of Tomini, Buka Buka Island; ZMA.PISC.115.770, 82.2 mm SL, Saleyer Island, south of Sulawesi; ZMA.PISC.129.136, 119.9 mm SL, Balikpapan, East Kalimantan, Borneo; ZUMT 62628, 105.9 mm SL, Jakarta, Java.
Diagnosis
A species of Dussumieria with the following combination of characters: 1UGR 12–14 (modally 12), 1LGR 22–26 (23), 1TGR 34–39 (37); 2UGR 8–10 (9), 2LGR 20–24 (22), 2TGR 28–33 (31); 3UGR 7–8 (8), 3LGR 14–16 (15), 3TGR 21–24 (23); 4UGR 3–8 (5), 4LGR 9–10 (10), 4TGR 12–17 (15); gill rakers 2–4 (3) on hind face of third gill arch; scale rows in longitudinal series 55–57 (56); branchiostegal rays 13–15 (15); vertebrae 55–58 (55, 56, 58); body elongate, 18.7–21.8% of SL; pre-dorsal-fin length 54.6–55.9% of SL, 44.2–45.4% of total length; head length 25.6–27.1% of SL; D–P1 31.6–33.7% of SL; D–A 29.6–32.4% of SL; P1–P2 31.5–35.7% of SL; P2–A 18.9–21.1% of SL; pelvic fin short, 9.5–10.7% of SL; dorsal-fin base short, 12.6–14.7% of SL; lateral body scales without posterior striae; melanophores scattered on 4th to 7th pectoral-fin rays from the uppermost ray; parasphenoid and vomer without teeth; tooth patches on anterior parts of palatine and pterygoid broad, with numerous rows of conical teeth.
Description
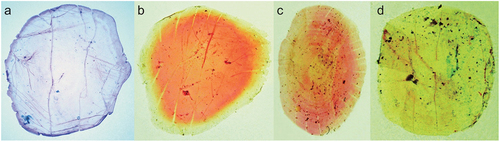
Counts and measurements, expressed as percentages of SL, are given in and . Data for the lectotype are presented first, followed by other specimen data in parentheses (if different). Body cylindrical, deepest at dorsal-fin origin. Head and caudal peduncle laterally compressed. Dorsal profile of body elevated from snout tip to dorsal-fin origin, thereafter decreasing to uppermost point of caudal-fin base. Ventral profile of body lowering from lower-jaw tip to pelvic-fin insertion and subsequently rising to lowermost point of caudal-fin base. Abdomen soft, not covered with keeled scutes. Pelvic scute on pelvic girdle, W-shaped. Mouth terminal, small, posterior tip of maxilla not reaching vertical through anterior margin of orbit. Premaxilla rectangular. Single row of conical teeth on ventral margins of premaxilla and maxilla. The 1st and 2nd supramaxillae elongate. Vomer and parasphenoid without teeth. Tooth patches on anterior parts of palatine and pterygoid broad, with numerous rows of conical teeth. Pterygoids densely covered with small conical teeth ()). Lower jaw with a single row of conical teeth. Basihyal with small dense conical teeth. Orbit, eye and iris round. Eye completely covered by well-developed adipose eyelid. Vertical slit opening on eyelid exposing central part of iris. Interorbital space flat. Nostrils close to each other, located on middle of snout. Posterior margins of opercle and preopercle smooth. Preopercular margin angular. Gill rakers long, slender. Pseudobranchial filaments present. Isthmus pointed anteriorly. Anteriormost point of pectoral-fin insertion slightly posterior to posteriormost point of opercle. Upper, posterior and ventral margins of pectoral fin nearly linear; posterior tip of pectoral fin pointed, not reaching to vertical through dorsal-fin origin. Dorsal-fin origin anterior to pelvic-fin insertion. End of dorsal-fin base posterior to posteriormost point of pelvic-fin insertion. Pelvic-fin insertion directly below origin of 11th (8th to 11th) dorsal-fin ray. Posterior tip of depressed pelvic fin slightly beyond vertical through posterior end of dorsal-fin base. Anal-fin origin posterior to vertical through posteriormost point of dorsal-fin base. Caudal fin forked, both lobes with nearly straight outer profiles, posterior tips pointed. Anus on ventral midline, slightly anterior to anal-fin origin, posterior to midpoint of body. No lateral line. All lateral body scales detached in lectotype (scales cycloid, thin, deciduous, without posterior longitudinal striae; )). Bases of dorsal and anal fins with low scaly sheaths. Predorsal scales arranged in median row. Scales absent on head and fins.
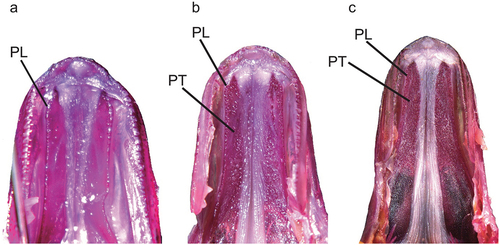
Figure 3. Ventral view of palate: (a) Dussumieria elopsoides (LBRC-F 5042, 110.7 mm SL, Ambon, Indonesia); (b) D. modakandai (KAUM–I. 129055, 145.6 mm SL, off Dong-gang, south-western Taiwan); and (c) D. productissima (NSMT-P 138482, 146.5 mm SL, Shihr, Gulf of Aden, Yemen). PL, palatine; PT, pterygoid; alizarin Red S stain. Abbreviations: LBRC, Technical Implementation Unit for Marine Biota Conservation; KAUM, Kagoshima University Museum; NSMT, National Museum of Nature and Science; SL, standard length.
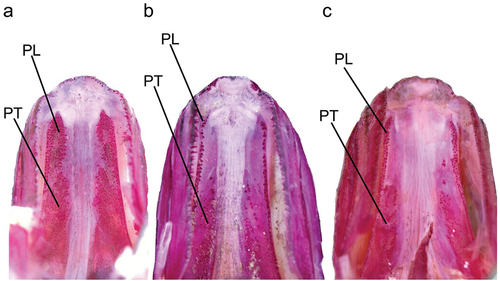
Figure 4. Photographs of stained lateral body scales of (a) Dussumieria elopsoides (KAUM–I. 97199, 107.7 mm SL, Timor, Indonesia; scale just above anal fin), (b) Dussumieria hasseltii (KAUM–I. 24111, 135.0 mm SL, Gulf of Thailand; scale just below dorsal fin), (c) D. modakandai (KAUM–I. 129056, 150.1 mm SL, off Dong-gang, south-western Taiwan; scale just below dorsal-fin base), and (d) D. productissima (BMNH 1962.3.16.211–214, 146.6 mm SL, Shihr, Gulf of Aden, Yemen; scale just below dorsal fin); alizarin Red S stain. Abbreviations: KAUM, Kagoshima University Museum; BMNH, British Museum of Natural History; SL, standard length.
Distribution
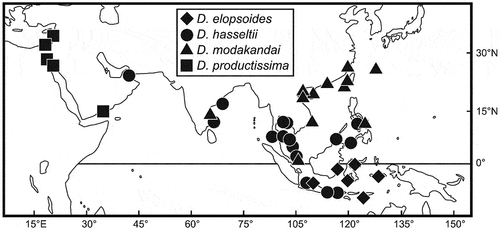
Dussumieria elopsoides occurs only in Indonesian waters ().
Morphological comparisons
Dussumieria acuta Valenciennes, Citation1847, Dussumieria albulina (Fowler, 1934), D. elopsoides, Dussumieria modakandai Singh, Jayakumar, Kumar, Murali, Mishra, Singh and Lal Citation2021 and Dussumieria torpedo Hata, Lavoué and Motomura, Citation2021 have all been regarded as valid species of Dussumieria (Whitehead Citation1985; Munroe et al. Citation1999; Hata et al. Citation2020, Citation2021; Singh et al. Citation2021), D. elopsoides having been considered conspecific with D. hasseltii and D. productissima (under the name D. elopsoides) (e.g. Whitehead Citation1985; Munroe et al. Citation1999). Together with D. modakandai (also redescribed herein), D. elopsoides, D. hasseltii and D. productissima are easily separable from all other valid species as follows: body depth less than 22% of SL (vs. 20% or more in specimens larger than 70 mm SL of the latter); and body scales without numerous posterior longitudinal striae or small pores (vs. numerous posterior longitudinal striae in D. acuta and D. albulina, and small posterior pores in D. torpedo) (Whitehead Citation1985; Munroe et al. Citation1999; Hata et al. Citation2020, Citation2021).
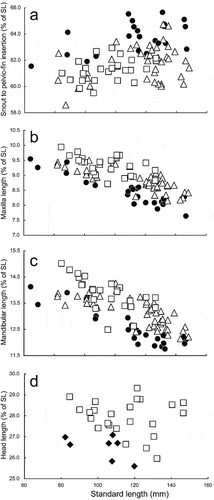
Dussumieria elopsoides most closely resembles D. hasseltii, both species having similar numbers of gill rakers and broad tooth bands on the palatines and pterygoids. However, the former has a shorter pre-dorsal-fin length (54.6–55.9% of SL vs. 58.0–60.6% in D. hasseltii; )), D–P1 (30.7–33.7% of SL vs. 34.6–39.4%; )), P1–P2 (31.5–35.7% of SL vs. 33.3–38.8%; )), and longer D–A (30.0–32.4% of SL vs. 25.8–29.4%; )), P2–A (18.9–21.1% of SL vs. 16.5–19.0%; )), dorsal-fin base (12.6–14.7% of SL vs. 10.2–12.9%; )) and pelvic fin (9.5–10.7% of SL vs. 7.5–8.7%; )), plus fewer longitudinal series of scale rows (55–57 vs. 57–60) and vertebrae (total vertebrae 55–58 vs. 57–60) ( and ). Detailed comparisons of D. elopsoides with D. modakandai and D. productissima are given in “Morphological comparisons” under the latter two species.
Figure 6. Relationships of (a) pre-dorsal-fin length (as % of standard length; SL), (b) distance between dorsal-fin origin and pectoral-fin insertion (D–P1; as % of SL), (c) distance between insertions of pectoral and pelvic fins (P1–P2; as % of SL), (d) distance between origins of dorsal and anal fins (D–A; as % of SL), (e) distance between pelvic-fin insertion and anal-fin origin (P2–A; as % of SL), (f) dorsal-fin base length (as % of SL), and (g) pelvic-fin length (as % of SL) to SL in Dussumieria hasseltii (diamonds), D. elopsoides (circles), D. modakandai (triangles), and D. productissima (squares).

Remarks
Dussumieria elopsoides was described from specimens collected from Madura Strait (Kammal and Surabaya) and the Java Sea [Batavia (currently Jakarta) and Samarang (Semarang)], Java, Indonesia, by Bleeker (Citation1849b). Subsequently, Whitehead et al. (Citation1966) designated BMNH 1867.11.28.17 ()) as the lectotype of the nominal species. Although Whitehead (Citation1985) regarded D. elopsoides as being widely distributed in the Indo-West Pacific, the species as recognized here is limited to Indonesian waters (). There is no genetic data available for D. elopsoides.
Dussumieria hasseltii Bleeker, Citation1851
[New English common name: Hasselt’s rainbow sardine]
(; , , , )
Table VI. Meristics of Dussumieria productissima.
Table VII. Morphometrics of Dussumieria elopsoides.
Table VIII. Morphometrics of Dussumieria hasseltii.
Dussumieria hasseltii Bleeker, Citation1851: 422 [original type locality: Batavia (currently Jakarta), Cheribon (Chirebon), Samarang (Semarang), and Surabaja (Surabaya), Java, Indonesia; type locality: Java, Indonesia, based on the neotype, newly designated herein]; Whitehead et al. Citation1966: 31 (Java, Indonesia).
Dussumieria elopsoides (not of Bleeker): Günther Citation1868: 466 (in part: Java, Indonesia); Whitehead Citation1985: 29 (in part: Arabian Sea to Indonesia); Wongratana 1999: 1793 (in part: Arabian Sea to Indonesia); Kimura Citation2009: 30, unnumbered fig. (Andaman Sea, Thailand); Kimura Citation2011: 38, second fig. from bottom (in part: off Terengganu, Malaysia); Kimura Citation2013: 37, unnumbered fig. (Bang Pakong, Gulf of Thailand, Thailand); Lavoué et al. Citation2017: 46, table 2 (Panay, Philippines); Hata Citation2017: 40, unnumbered fig. (Panay, Philippines); Hata and Motomura Citation2020: 11, fig. 1 (Terengganu, Malaysia); Singh et al. 2022: table 2, fig. 1b (India); Hata et al. Citation2022: 21 (in part: Jolo Island, Philippines).
Dussumieria acuta (not of Valenciennes): Whitehead Citation1963: 312 (in part: Java, Indonesia).
Dussumieria cf. elopsoides: Hata et al. Citation2020: table 2, fig. 9 (in part: Panay, Philippines); Hata et al. Citation2021: table 1, fig. 6 (in part: Panay, Philippines).
Neotype
BMNH 1867.11.28.21, 134.4 mm SL, Java, Indonesia.
Non-type specimens
25 specimens (64.4–166.7 mm SL). PHILIPPINES: KAUM–I. 62999, 135.7 mm SL, off Antique, Panay; ZUMT 41001, 101.1 mm SL, Jolo Island. THAILAND: BMNH 1982.9.6.102, 166.7 mm SL, Phuket; FRLM 48592, 148.0 mm SL, Phuket; KAUM–I. 23352, 140.3 mm SL, KAUM–I. 23353, 150.8 mm SL, mouth of Bang Pakong River, Chachoengsao Province; KAUM–I. 24111, 135.0 mm SL, Gulf of Thailand, Mahachai, Samut Prakan Province; NSMT-P 23077, 101.5 mm SL, NSMT-P 23078, 107.1 mm SL, Songkhla; NSMT-P 138074, 165.1 mm SL, NSMT-P 138075, 165.8 mm SL, west of Phuket. MALAYSIA: KAUM–I. 17130, 146.0 mm SL, off Kuala Terengganu, Terengganu; KAUM–I. 80179, 64.4 mm SL, KAUM–I. 80180, 69.2 mm SL, Marudu Bay, Sabah, Borneo (06°43ʹ14″N, 116°57ʹ05″E), 0–25 m depth; KAUM–I. 105295, 88.1 mm SL, off Bidong Island, Kuala Terengganu, Terengganu (05°37ʹN, 103°03ʹE). INDONESIA: BPBM 29946, 128.4 mm SL, Ampenan Utara, Lombok; URM-P 43940, 151.8 mm SL, URM-P 43943, 152.8 mm SL, Bintan Island, Riau Archipelago; RMNH.PISC.7128, 2 specimens, 131.0–132.0 mm SL, East Indies; ZMA.PISC.115.780, 128.7 mm SL, Banyuwangi, Jawa Timur, Java; ZMA.PISC.129.138, 88.4 mm SL, Jakarta, Java. INDIA: BMNH 1868.10.28.39, 132.7 mm SL, Visakhapatnam; BPBM 41428, 152.0 mm SL, Chennai. UAE: BMNH 1970.10.2.17, 107.5 mm SL, Khor Fakkan, Trucial States.
Diagnosis
A species of Dussumieria with the following combination of characters: 1UGR 12–15 (modally 13), 1LGR 21–25 (23), 1TGR 33–39 (36); 2UGR 8–10 (9), 2LGR 19–23 (21), 2TGR 28–32 (29); 3UGR 7–9 (7), 3LGR 14–16 (15), 3TGR 21–24 (22); 4UGR 4–6 (5), 4LGR 9–11 (10), 4TGR 13–17 (15); gill rakers 3–5 (4) on hind face of third gill arch; scale rows in longitudinal series 57–60 (58); branchiostegal rays 13–17 (16); vertebrae 40–42 (41) + 17–19 (17) = 57–60 (58, 59); body elongate, 16.7–22.0% of SL; pre-dorsal-fin length 58.0–60.6% of SL, 46.8–50.3% of total length; D–P1 34.6–39.4% of SL; D–A 25.8–29.4% of SL; P1–P2 33.3–38.8% of SL; P2–A 16.5–19.0% of SL; pelvic fin short, 7.5–8.7% of SL; dorsal-fin base short, 10.2–12.9% of SL; snout to pelvic-fin insertion 61.5–65.6% of SL; maxilla rather short, 7.6–9.5% of SL; lower jaw rather short, 11.8–14.1% of SL; lateral scales without posterior striae; melanophores scattered on 5th to 9th (rarely 12th) pectoral-fin rays from uppermost ray; parasphenoid and vomer without teeth; tooth patches on anterior parts of palatine and pterygoid broad, with numerous rows of conical teeth in individuals larger than 80 mm SL.
Description
Counts and measurements, expressed as percentages of SL, are given in and . Data for neotype are presented first, followed by other specimen data in parentheses (if different). Body cylindrical, deepest at dorsal-fin origin. Head and caudal peduncle laterally compressed. Dorsal profile of body elevated from snout tip to dorsal-fin origin, thereafter decreasing to uppermost point of caudal-fin base. Ventral profile of body lowering from lower-jaw tip to pelvic-fin insertion, subsequently rising to lowermost point of caudal-fin base. Abdomen soft, not covered with keeled scutes. Pelvic scute joined to pelvic girdle, W-shaped. Mouth terminal, small, posterior tip of maxilla not reaching vertical through anterior margin of orbit. Premaxilla rectangular. Single row of conical teeth on ventral margins of premaxilla and maxilla. The 1st and 2nd supramaxillae elongate. Vomer and parasphenoid without teeth. Tooth patches on anterior parts of palatine and pterygoid broad, with numerous rows of conical teeth (width of tooth patches on palatine and pterygoid varying with body size; see “Morphological comparisons”). Pterygoids densely covered with small conical teeth (). Lower jaw with a single row of conical teeth. Basihyal with small dense conical teeth. Orbit, eye and iris round. Eye completely covered by well-developed adipose eyelid. Vertical slit opening on eyelid exposing central part of iris. Interorbital space flat. Nostrils close to each other, located on middle of snout. Posterior margins of opercle and preopercle smooth. Preopercular margin angular. Gill rakers long, slender. Pseudobranchial filaments present. Isthmus pointed anteriorly. Anteriormost point of pectoral-fin insertion slightly posterior to posteriormost point of opercle. Upper, posterior and ventral margins of pectoral fin nearly linear; posterior tip of pectoral fin pointed, not reaching to vertical through dorsal-fin origin. Dorsal-fin origin anterior to pelvic-fin insertion. Dorsal-fin base end posterior to posteriormost point of pelvic-fin insertion. Pelvic-fin insertion directly below origin of 9th (7th to 11th) dorsal-fin ray. Posterior tip of depressed pelvic fin slightly beyond vertical through posterior end of dorsal-fin base. Anal-fin origin posterior to vertical through posteriormost point of dorsal-fin base. Caudal fin forked, both lobes with nearly straight outer profiles, posterior tips pointed. Anus on ventral midline, slightly anterior to anal-fin origin, posterior to midpoint of body. No lateral line. All lateral body scales detached in neotype (scales cycloid, thin, deciduous, without posterior longitudinal striae; )). Bases of dorsal and anal fins with low scaly sheaths. Predorsal scales arranged in median row. Scales absent on head and fins.
Figure 7. Non-type specimens of Dussumieria hasseltii (fresh condition): (a) KAUM–I. 17130, 146.0 mm SL, Terengganu, Malaysia; (b) KAUM–I. 80179, 64.4 mm SL, Sabah, Malaysia); and (c) neotype of D. hasseltii (BMNH 1867.11.28.21, 134.4 mm SL, Java, Indonesia). Abbreviations: KAUM, Kagoshima University Museum; BMNH, British Museum of Natural History; SL, standard length.

Figure 8. Ventral views of palate of Dussumieria hasseltii: (a) KAUM–I. 80179, 64.4 mm SL, Sabah, Borneo, Malaysia; (b) NSMT-P 23078, 107.1 mm SL, Songkhla, Thailand; and (c) KAUM–I. 23352, 140.3 mm SL, Gulf of Thailand). PL, palatine; PT, pterygoid; alizarin Red S stain. Abbreviations: KAUM, Kagoshima University Museum; NSMT, National Museum of Nature and Science; SL, standard length.
Fresh coloration [based on color photographs of KAUM–I. 17130 ()), 23352, 23353, 62999]
Lateral surface of body uniformly whitish-silver. Dorsum dark blue. Dark blue and silver areas on body separated by light blue and electric green longitudinal bands. Lateral surface of head silver. Region between anterior margin of preopercle and posterior margin of opercle reddish. Melanophores densely scattered on head from tips of both jaws to occiput. Iris silver, pupil black. Dorsal fin whitish-yellow, with black margin anteriorly. Pectoral, pelvic and anal fins semitransparent, white. Caudal fin yellow, with black posterior margin. In juveniles [based on color photographs of KAUM–I. 80179, 64.4 mm SL ()) and KAUM–I. 80180, 69.2 mm SL], body uniformly milky-white with a silver longitudinal band from opercle to caudal-fin base. Lateral surface of head silver. Dorsal edge of body margined with melanophores.
Coloration of preserved specimens
Dorsum to upper part of lateral surface of body brownish to purplish-black, lateral surface thereafter uniformly pale or silver. All fins pale yellow. Posterior margin of caudal fin dusky. Melanophores scattered on 5th to 9th (rarely 12th) pectoral-fin rays from uppermost ray.
Distribution
Dussumieria hasseltii is widely distributed in the Indo-West Pacific, from the Persian Gulf to the Philippines and Indonesia ().
Morphological comparisons
Dussumieria hasseltii and D. elopsoides have broad tooth bands on the palatines and pterygoids (), distinguishing them from all other species of the genus treated herein. Lower gill raker counts on each gill arch also characterize the former two species (–; ). Detailed comparisons between D. hasseltii and D. elopsoides are given in “Morphological comparisons” under the latter species. Dussumieria hasseltii is further characterized by a pre-dorsal-fin length >58% of SL (vs. <58% of SL in D. elopsoides, D. modakandai and D. productissima) ()).
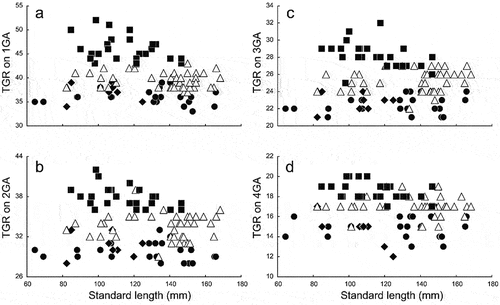
Figure 9. Relationships of total gill-raker numbers (TGR) on (a) first gill arch (1GA), (b) second gill arch (2GA), (c) third gill arch (3GA), and (d) fourth gill arch (4GA) to standard length in Dussumieria elopsoides (closed diamonds), D. hasseltii (closed circles), D. productissima (closed squares), and D. modakandai (open triangles).
The form of the palatine tooth patch in D. hasseltii changes with growth, individuals <80 mm SL having the patch restricted to a single or two rows of conical teeth. Additionally, pterygoid teeth are absent ()). In specimens 80–108 mm SL, the palatine tooth patch became broad, comprising several rows on the anterior part of the palatine and continuing onto the pterygoid ()). Individuals >108 mm had broad tooth patches on the anterior parts of the palatine and pterygoid, comprising numerous rows of conical teeth ()).
Remarks
Dussumieria hasseltii Bleeker, Citation1851 was described on the basis of 20 specimens (80–160 mm total length) collected from Batavia (currently Jakarta), Cheribon (Chirebon), Samarang (Semarang), and Surabaja, Java, Indonesia, the author noting that the species had distinct teeth on the tongue, palatine and pterygoids, and the dorsal-fin origin located on the mid-point of the body. Dussumieria hasseltii defined herein closely matches Bleeker’s (Citation1851) D. hasseltii in having numerous conical teeth on the tongue, palatine and pterygoids, and a pre-dorsal-fin length 46.8–50.3% of total length (cf. 44.2–45.4% in D. elopsoides). Therefore, the species described by Bleeker (Citation1851) is most likely D. hasseltii, as defined here. Because no syntypes of D. hasseltii could be found, Whitehead et al. (Citation1966) selected BMNH 1867.11.28.21 ()), a specimen collected from Java, Indonesia, as the “putative neotype” for D. hasseltii. Although Whitehead et al. (Citation1966) described that specimen in detail, having shown that neotype designation was necessary, a formal designation was not in fact given, therefore failing to meet the full mandatory requirements listed under Article 75.3.1 (ICZN Citation1999). To avoid taxonomic confusion among species of Dussumieria, we designate BMNH 1867.11.28.21 as the neotype of D. hasseltii, its collection locality becoming the type locality of D. hasseltii under Article 76.3 (ICZN Citation1999).
Most specimens reported as D. elopsoides from the Indo-West Pacific region since Whitehead (Citation1985) are probably D. hasseltii (see synonym list).
Dussumieria modakandai Singh, Jayakumar, Kumar, Murali, Mishra, Singh and Lal, Citation2021
[English common name: soft rainbow sardine]
(; , , , )
Table IX. Morphometrics of Dussumieria modakandai.
Table X. Morphometrics of Dussumieria productissima.
Dussumieria acuta (not of Valenciennes): Whitehead Citation1963: 312 (in part: Foochow, Amoy, China and Hong Kong).
Dussumieria elopsoides (not of Bleeker): Günther Citation1868: 466 [in part: Amoy (currently Xiamen), China]; Whitehead Citation1985: 30, unnumbered fig. (in part: southern coast of China); Uyeno and Sato Citation1984: 18, pl. 21-A (Okinawa Prefecture, Japan); Aonuma Citation1993: 203, unnumbered fig. (Okinawa Prefecture, Japan); Wongratana 1999: 1793, unnumbered fig. (in part: southern coast of China); Aonuma Citation2000: 243, unnumbered fig. (Okinawa Prefecture, Japan); Zhang Citation2001: 56, fig. II-15 (Chinese coast from Yangtze Estuary to Hainan Island); Aonuma Citation2002: 243, unnumbered fig. (Okinawa Island, Ryukyu Islands, Japan); Aonuma and Yagishita Citation2013: 297, unnumbered fig. (Okinawa Prefecture, Japan).
Dussumieria cf. elopsoides: Lavoué et al. Citation2017: 46, table 2 (Taiwan); Hata Citation2019: 215, unnumbered figs (Dong-gang, south-western Taiwan); Hata et al. Citation2020: 9, table 1 (in part: Ke-tzu-liao, Kaohsiung, Taiwan); Hata et al. Citation2021: fig. 6 (in part: Taiwan); Hata et al. Citation2021: table 1, fig. 6 (in part: Taiwan).
Dussumieria modakandai: Singh et al. Citation2021: 6, fig. 1a (type locality: Chennai coast, Tamil Nadu State, India); Hata et al. Citation2022: 21 (Ryukyu Islands, Japan; Tamsui and Tainan, Taiwan; Hainan Island, China; Philippines).
Holotype
DUSDMOD/NBFGR 145 mm SL, Chennai coast, Bay of Bengal, India, coll. By T.T. Ajith Kumar and Teena Jayakumar T.K. [counts and measurements cited from original description, Singh et al. (Citation2021)].
Paratypes
DUSDMOD.1/NBFGR; DUSDMOD.2/NBFGR; DUSDMOD. 3/NBFGR, 148–163 mm SL, same collection details as holotype [counts and measurements cited from original description, Singh et al. (Citation2021)].
Non-type specimens
42 specimens (82.1–168.3 mm SL). JAPAN: BSKU 109910, 168.3 mm SL, Okinawa City, Okinawa Island, Ryukyu Islands; URM-P 6390, 147.8 mm SL, URM-P 6625, 163.0 mm SL, Okinawa Island, Ryukyu Islands; ZUMT 11155, 103.0 mm SL, ZUMT 11156, 120.4 mm SL, ZUMT 11157, 120.9 mm SL, Ryukyu Islands. CHINA: BMNH 1936.10.7.21, 117.3 mm SL, Foochow; BMNH 1965.7.5.73, 140.8 mm SL, Aberdeen Fish market, Hong Kong, purse seine; BMNH 1988.8.1.63, 154.7 mm SL, Qinglan Market, Hainan. TAIWAN: KAUM–I. 110283, 153.6 mm SL, KAUM–I. 110284, 149.9 mm SL, KAUM–I. 110285, 154.7 mm SL, KAUM–I. 110286, 159.0 mm SL, KAUM–I. 110287, 141.1 mm SL, KAUM–I. 110288, 145.5 mm SL, KAUM–I. 110290, 166.0 mm SL, KAUM–I. 110291, 154.5 mm SL, KAUM–I. 110292, 148.0 mm SL, KAUM–I. 110293, 164.7 mm SL, KAUM–I. 129055, 145.6 mm SL, KAUM–I. 129056, 150.1 mm SL, NMMB-P17817, 2 specimens, 133.7–140.5 mm SL, NMMB-P27124, 150.0 mm SL, off Dong-gang, Pingtung County (obtained at Dong-gang Fishing Port); ZUMT 14875, 142.5 mm SL, ZUMT 14876, 143.8 mm SL, ZUMT 14877, 146.2 mm SL, Tainan. PHILIPPINES: ZUMT 39500, 108.2 mm SL, ZUMT 39501, 101.3 mm SL, ZUMT 39502, 95.2 mm SL, ZUMT 39503, 101.4 mm SL, Philippines. VIETNAM: KAUM–I. 117471, 105.7 mm SL, off Nha Trang, Khánh Hòa (purchased at Vĩnh Lương Fish Landing Port); NSMT-P 66177, 2 specimens, 82.1–87.1 mm SL, Long Chau; NSMT-P 66193, 110.0 mm SL, NSMT-P 138483, 82.5 mm SL, Hon Dau. INDONESIA: URM-P 43938, 142.4 mm SL, URM-P 43939, 136.5 mm SL, URM-P 43941, 147.0 mm SL, URM-P 43942, 151.3 mm SL, URM-P 43944, 151.2 mm SL, Bintan Island, Riau Archipelago. INDIA: BPBM 41429, 129.8 mm SL, Chennai.
Diagnosis
A species of Dussumieria with the following combination of characters: 1UGR 12–16 (modally 14), 1LGR 24–29 (26), 1TGR 37–43 (38); 2UGR 9–11 (10), 2LGR 20–28 (24), 2TGR 29–39 (35); 3UGR 6–9 (8), 3LGR 16–18 (17), 3TGR 22–27 (24); 4UGR 5–8 (6), 4LGR 10–12 (11), 4GR 15–19 (17); gill rakers 1–5 (4) on hind face of third gill arch; scale rows in longitudinal series 56–60 (58); branchiostegal rays 13–17 (15); vertebrae 39–41 (40) + 15–19 (16) = 55–58 (usually 56 or 57); body elongate, 18.7–21.9% of SL; pre-dorsal-fin length 54.7–57.9% of SL; P1–P2 31.0–37.3% of SL; P2–A 17.9–22.5% of SL; snout to pelvic fin short, 59.6–63.6% of SL; maxilla rather long, 8.2–9.9% of SL; lower jaw rather short, 12.1–14.3% of SL; lateral scales with 10 or fewer posterior longitudinal striae; melanophores scattered on 5th to 9th pectoral-fin rays from uppermost ray; parasphenoid and vomer without teeth; tooth patch on palatine narrow, with one or two rows of conical teeth.
Description
Counts and measurements, expressed as percentages of SL, are given in and . The description is based only on specimens examined in this study (type specimens not included). Body cylindrical, deepest at dorsal-fin origin. Head and caudal peduncle laterally compressed. Dorsal profile of body elevated from snout tip to dorsal-fin origin, thereafter lowering to uppermost point of caudal-fin base. Ventral profile of body lowering from lower-jaw tip to pelvic-fin insertion, subsequently rising to lowermost point of caudal-fin base. Abdomen soft, not covered with keeled scutes. Pelvic scute joined to pelvic girdle W-shaped. Mouth terminal, small, posterior tip of maxilla not reaching vertical through anterior margin of orbit. Premaxilla rectangular. Single row of conical teeth on ventral margins of premaxilla and maxilla. First and second supramaxillae elongate. Vomer and parasphenoid without teeth. One or two rows of conical teeth on palatine. Pterygoids densely covered with small conical teeth ()). Lower jaw with a single row of conical teeth. Basihyal with small dense conical teeth. Orbit, eye and iris round. Eye completely covered by well-developed adipose eyelid. Vertical slit opening on eyelid exposing central part of iris. Interorbital space flat. Nostrils close to each other, located on middle of snout. Posterior margins of opercle and preopercle smooth. Preopercular margin angular. Gill rakers long, slender. Pseudobranchial filaments present. Isthmus pointed anteriorly. Anteriormost point of pectoral-fin insertion slightly posterior to posteriormost point of opercle. Upper, posterior and ventral margins of pectoral fin nearly linear; posterior tip of pectoral fin pointed, not reaching to vertical through dorsal-fin origin. Dorsal-fin origin anterior to pelvic-fin insertion. Dorsal-fin base end posterior to posteriormost point of pelvic-fin insertion. Pelvic-fin insertion directly below origin of 7th to 11th dorsal-fin ray. Posterior tip of depressed pelvic fin slightly beyond vertical through posterior end of dorsal-fin base. Anal-fin origin posterior to vertical through posteriormost point of dorsal-fin base. Caudal fin forked, both lobes with nearly straight outer profiles, posterior tips pointed. Anus on ventral midline, slightly anterior to anal-fin origin, posterior to midpoint of body. No lateral line. Scales on body cycloid, thin, deciduous. Ten or fewer longitudinal striae posteriorly on lateral body scales ()). Bases of dorsal and anal fins with low scaly sheaths. Predorsal scales arranged in median row. Scales absent on head and fins.
Fresh coloration [based on color photographs of KAUM–I. 110283, 110284, 117471 and NMMB-P27124 ()]
Lateral surface of body uniformly whitish-silver. Dorsum dark blue. Dark blue and silver areas separated by light blue and yellowish-green longitudinal bands. Lateral surface of anterior part of head yellowish-silver. Melanophores densely scattered on head from tips of both jaws to occiput. Iris yellowish-silver, pupil black. Preopercle reddish. Dorsal fin whitish-yellow. Pectoral, pelvic and anal fins semitransparent white. Caudal fin yellowish-white, with dusky posterior margin. The fresh coloration of the holotype is shown in the original description by Singh et al. (Citation2021, fig. 1a).
Coloration of preserved specimens
Lateral surface of body uniformly pale or silver. Dorsum to upper lateral body surface dark brown or purple. All fins pale yellow. Caudal fin with black margin. Melanophores scattered on 5th to 9th pectoral-fin rays from uppermost ray.
Distribution
Specimens of D. modakandai are known from the Indo-west Pacific from Chennai, Tamil Nadu State, eastern coast of India to Riau Archipelago and the Ryukyu Islands, Japan (Singh et al. Citation2021; this study). Moreover, molecular evidence indicates that the species is distributed westward to the Mediterranean (Singh et al. Citation2021; this study, ). Because BPBM 41429 (collected from Chennai, eastern coast of India) was caught with D. hasseltii (BPBM 41428, 152.0 mm SL) and D. albulina (BPBM 20500, 149.7 mm SL), the three species probably co-occur at least in the Bay of Bengal. Dussumieria modakandai is an abundant bycatch in midwater trawls targeting shrimps, and is mainly used for nutritional purposes in aquaculture in southern Taiwan (Hata Citation2019 as Dussumieria cf. elopsoides; this study).
Morphological comparisons
Dussumieria modakandai keys to D. elopsoides following Whitehead (Citation1985) and Munroe et al. (Citation1999), having body scales lacking numerous posterior striae, a body depth <22% SL, and branchiostegal rays and 1LGR numbering more than 12 and 23, respectively. However, D. modakandai clearly differs from true D. elopsoides, D. hasseltii and D. productissima in having body scales with 10 or fewer posterior longitudinal striae ()) (vs. no distinct longitudinal striae in the latter three species; )). Moreover, the narrow palatine tooth patch (1 or 2 rows) in D. modakandai separates that species from D. elopsoides and D. hasseltii (both having a broad palatine tooth patch, comprising numerous rows; ). Dussumieria modakandai has a number of gill rakers intermediate between that of D. productissima and those of D. elopsoides and D. hasseltii (see –; ), and further differs from D. hasseltii in having a shorter pre-dorsal-fin length (54.7–57.9% of SL vs. 58.0–60.6% in D. hasseltii; )), D–P1 (33.1–37.5% of SL vs. 34.6–39.4%; )), P1–P2 (31.0–37.3% of SL vs. 33.3–38.8%; )), snout to pelvic-fin insertion (59.6–63.6% of SL vs. 61.5–65.6%; )), maxilla length (8.2–9.9% of SL vs. 7.6–9.5%; )), and lower jaw (12.1–14.3% of SL vs. 11.8–14.4%; )), and longer D–A (28.1–31.9% of SL vs. 25.8–29.4%; )), P2–A (17.9–22.5% of SL vs. 16.5–19.0%; )), and pelvic fin (8.7–9.8% of SL vs. 7.5–8.7%; )). Additionally, D. productissima has fewer vertebrae (total vertebrae 56–58 vs. 57–60 in D. hasseltii; ). Although D. modakandai is closely similar to D. productissima in the form of the palatine/pterygoid tooth patches, the former has fewer total gill rakers on each gill arch ( and ; ) and higher numbers of longitudinal series of scale rows [56–61 (modally 58) vs. 53–56 (55) in D. productissima; ] and total vertebrae [56–58 (usually 56 or 57; modally 57) vs. 55 or 56 (modally 55); ].
Figure 11. Relationships of (a) distance from snout to pelvic-fin insertion (as % of standard length; SL), (b) maxilla length (as % of SL), (c) mandibular length (as % of SL), and (d) head length (as % of SL) to SL in Dussumieria elopsoides (diamonds), D. hasseltii (circles), D. modakandai (triangles), and D. productissima (squares).
Remarks
Dussumieria modakandai, described by Singh et al. (Citation2021), was based on four specimens collected off the Chennai coast, eastern coast of India. Although the authors suggested that the species was also distributed in the eastern Mediterranean (considered as Lessepsian migration), Iranian waters and Taiwan (based on molecular evidence), no examples of the species from these areas have been reported. In the present study, examples of D. modakandai were observed from a wide area in the western Pacific, from the Riau Archipelago, Indonesia, to the Ryukyu Islands and Philippines ().
Dussumieria productissima Chabanaud, Citation1933
(New English common name: javelin rainbow sardine)
(; , –)
Dussumieria productissima Chabanaud, Citation1933: 4, figs 3–6 (original locality: Gulf of Suez, Great Bitter Lake, and Timsah Lake, Egypt; type locality: Gulf of Suez, based on newly designated lectotype).
Dussumieria acuta (not of Valenciennes): Whitehead Citation1963: 312, fig. 5 (in part: Gulf of Aden); Whitehead Citation1965: 234 (in part: Gulf of Aden).
Dussumieria elopsoides (not of Bleeker): Whitehead Citation1985: 29 (in part: Red Sea and eastern Mediterranean); Munroe et al. Citation1999: 1793 (in part: Red Sea and eastern Mediterranean).
Lectotype of Dussumieria productissima
MNHN 1966–0259, 106.0 mm SL, Gulf of Suez, Egypt (29°54ʹ00”N, 32°31ʹ01”E), 16–20 m depth, 16 Jan. 1929, coll. by R.P. Dollfus.
Paralectotypes
7 specimens, 98.6–115.2 mm SL. MNHN 3997, 2 specimens, 110.1–115.2 mm SL, Red Sea, 1928, coll. by R.P. Dollfus; MNHN 1966–0258, 107.3 mm SL, MNHN 1966–0260, 105.1 mm SL, MNHN 1966–0262, 100.1 mm SL, MNHN 1966–0263, 98.6 mm SL, MNHN 1966–0264, 98.0 mm SL, Gulf of Suez, Egypt (29°54ʹ00”N, 32°31ʹ01”E), 16–20 m depth, 16 Jan. 1929, coll. by R.P. Dollfus.
Non-type specimens
17 specimens, 84.7–146.6 mm SL. LEBANON: BMNH 1967.2.1.20–24, 5 specimens, 105.4–132.2 mm SL, Antelias. EGYPT: MNHN 1966–0265, 2 of 4 specimens, 117.5–117.6 mm SL, Gulf of Suez (29°55ʹ12”N, 32°33ʹ00”E); RMNH.PISC. 34258, 4 specimens, 84.7–96.2 mm SL, off Bardawil, 8 fathoms (approx. 14.6 m) depth. YEMEN: BMNH 1962.3.26.211–214, 4 specimens, 121.4–146.6 mm SL, KAUM–I. 146374, 121.3 mm SL, NSMT-P 138482, 146.5 mm SL, Shihr, Gulf of Aden.
Diagnosis
A species of Dussumieria with the following combination of characters: 1UGR 14–17 (modally 15), 1LGR 27–35 (31), 1TGR 43–52 (44); 2UGR 9–12 (11), 2LGR 25–30 (26), 2TGR 36–42 (37); 3UGR 7–10 (9), 3LGR 18–22 (19), 3TGR 25–32 (28); 4UGR 5–7 (6), 4LGR 11–13 (12), 4TGR 17–20 (18); gill rakers 2–5 (4) on hind face of third gill arch; scale rows in longitudinal series 53–56 (55); branchiostegal rays 13–16 (14); vertebrae 38–40 (39) + 16–18 (17) = 55 or 56 (55); body elongate, 17.1–22.0% of SL; pre-dorsal-fin length 55.4–57.8% of SL; P1–P2 short, 30.5–34.0% of SL; P2–A rather long, 18.0–22.9% of SL; snout to pelvic fin short, 59.5–62.9% of SL; maxilla rather long, 8.7–9.9% of SL; lower jaw rather long, 12.5–15.0% of SL; lateral scales without posterior striae; melanophores scattered on 8th to 11th pectoral-fin rays from uppermost ray; parasphenoid and vomer without teeth; tooth patch on palatine narrow, with one or two rows of conical teeth.
Description
Counts and measurements, expressed as percentages of SL, are given in and . Data for the lectotype are presented first, followed by other specimen data in parentheses (if different). Body cylindrical, deepest at dorsal-fin origin. Head and caudal peduncle laterally compressed. Dorsal profile of body elevated from snout tip to dorsal-fin origin thereafter lowering to uppermost point of caudal-fin base. Ventral profile of body lowering from lower-jaw tip to pelvic-fin insertion, subsequently rising to lowermost point of caudal-fin base. Abdomen soft, not covered with keeled scutes. Pelvic scute joined to pelvic girdle, W-shaped. Mouth terminal, small, posterior tip of maxilla not reaching vertical through anterior margin of orbit. Premaxilla rectangular. Single row of conical teeth on ventral margins of premaxilla and maxilla. The 1st and 2nd supramaxillae elongate. Vomer and parasphenoid without teeth. One or two rows of conical teeth on palatine. Pterygoids densely covered with small conical teeth ()). Lower jaw with a single row of conical teeth. Basihyal with small dense conical teeth. Orbit, eye and iris round. Eye completely covered by well-developed adipose eyelid. Vertical slit opening on eyelid exposing central part of iris. Interorbital space flat. Nostrils close to each other, located on middle of snout. Posterior margins of opercle and preopercle smooth. Preopercular margin angular. Gill rakers long, slender. Pseudobranchial filaments present. Isthmus pointed anteriorly. Anteriormost point of pectoral-fin insertion slightly posterior to posteriormost point of opercle. Upper, posterior and ventral margins of pectoral fin nearly linear; posterior tip of pectoral fin pointed, not reaching to vertical through dorsal-fin origin. Dorsal-fin origin anterior to pelvic-fin insertion. Dorsal-fin base end posterior to posteriormost point of pelvic-fin insertion. Pelvic-fin insertion directly below origin of 9th (6th to 10th) dorsal-fin ray. Posterior tip of depressed pelvic fin slightly beyond vertical through posterior end of dorsal-fin base. Anal-fin origin posterior to vertical through posteriormost point of dorsal-fin base. Caudal fin forked, both lobes with nearly straight outer profiles, posterior tips pointed. Anus on ventral midline, slightly anterior to anal-fin origin, posterior to midpoint of body. No lateral line. All lateral body scales detached in lectotype (scales cycloid, thin, deciduous, without posterior longitudinal striae; )). Bases of dorsal and anal fins with low scaly sheaths. Predorsal scales arranged in median row. Scales absent on head and fins.
Coloration of preserved specimens
Lateral surface of body uniformly pale or silver. Dorsum to upper lateral body surface dark brown. Numerous melanophores densely scattered on upper part of body. All fins pale yellow. Melanophores scattered along fin rays of dorsal and caudal fins. Caudal fin margin blackish. Melanophores scattered on 8th to 11th pectoral-fin rays from uppermost ray.
Distribution
Dussumieria productissima is distributed in the Gulf of Aden (Yemen), Red Sea (Egypt) and eastern Mediterranean (). The distribution of the species in the Mediterranean is considered to represent Lessepsian migration. In addition, molecular evidence indicates that the species is also distributed along the western coast of India (), where it co-occurs with D. modakandai.
Morphological comparisons
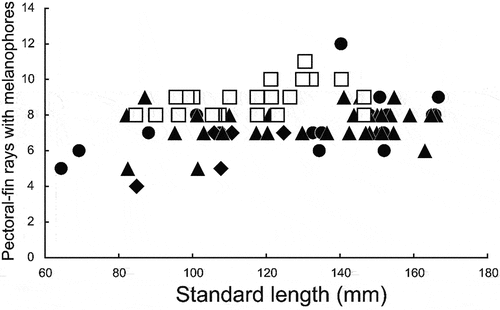
Dussumieria productissima resembles D. elopsoides, D. hasseltii and D. modakandai, all species having a slender body (<22% of SL), lacking numerous longitudinal striae on the lateral body scales, and lacking parasphenoid teeth. However, D. productissima has the highest gill raker numbers in the genus, separating it from the aforementioned three species (Hata et al. Citation2020, Citation2021; this study; –; ), as well as more pectoral-fin rays bearing melanophores [melanophores scattered on the 8th to 11th pectoral-fin rays from the uppermost ray in D. productissima vs. 4th to 7th in D. elopsoides, and 5th to 9th (rarely 12th in D. hasseltii) in D. hasseltii and D. modakandai (–; ). Additionally, the palatine tooth patch in D. productissima (narrow with one or two rows of conical teeth; )) differs from that in D. elopsoides and D. hasseltii (broad with numerous rows of conical teeth; ). Dussumieria productissima differs from D. hasseltii in having a shorter pre-dorsal-fin length (55.4–57.8% of SL vs. 58.0–60.6% in D. hasseltii; )), distance from snout to pelvic-fin insertion (59.5–62.9% of SL vs. 61.5–65.6%; )), D–P1 (31.7–35.3% of SL vs. 34.6–39.4%; )), P1–P2 (30.5–34.0% of SL vs. 33.3–38.8%; )), maxilla (8.7–9.9% of SL vs. 7.6–9.5%; )), and lower jaw (12.5–15.0% of SL vs. 11.8–14.4%; )), and longer D–A (28.9–31.0% of SL vs. 25.8–29.4%; )), P2–A (18.0–22.9% of SL vs. 16.5–19.0%; )), and pelvic fin (8.7–10.4% of SL vs. 7.5–8.7%; )), in addition to fewer longitudinal series of scale rows (53–56 vs. 57–60; ) and vertebrae (total vertebrae 55 or 56 vs. 57–60; ). Dussumieria productissima further differs from D. elopsoides in having greater head (26.0–29.3% of SL vs. 25.6–27.1%; )) and pre-dorsal-fin lengths (55.4–57.8% of SL vs. 54.6–55.9%; )). Detailed comparisons with D. productissima and D. modakandai are given under Morphological comparisons for the latter.
Table XI. Frequency distribution of scale rows in longitudinal series in Dussumieria elopsoides, D. hasseltii, D. modakandai, and D. productissima, based on specimens examined in this study.
Table XII. Frequency distribution of total vertebrae in Dussumieria elopsoides, D. hasseltii, D. modakandai, and D. productissima, based on specimens examined in this study.
Remarks
Dussumieria productissima, described by Chabanaud (Citation1933), was based on 16 specimens collected from the Gulf of Suez and Suez Canal. Although Fricke et al. (Citation2021) listed MNHN 1966–0258 to 0264 (one specimen in each lot), MNHN 3997 (two specimens) and some MOM specimens as the syntypes of the nominal species, those deposited in MOM were not found (Fricke et al. Citation2021). Moreover, MNHN 1966–0261 has been identified as Sardinella gibbosa (Bleeker, Citation1849a), a species of Clupeidae (Stern et al. Citation2015; this study). Because Chabanaud (Citation1933) gave the number of branchiostegal rays of D. productissima as 14–16 (6 branchiostegal rays in Sardinella species; Whitehead Citation1985; Munroe et al. Citation1999), MNHN 1966–0261 is not considered to have been originally included among the type specimens of D. productissima. Consequently, only eight of the 16 syntypes are confirmed here as extant. For nomenclatural stability, a syntype (MNHN 1966–0259, 106.0 mm SL; ), from the Gulf of Suez, Egypt, is designated herein as the lectotype of D. productissima, the remaining syntypes becoming paralectotypes, and the Gulf of Suez, Egypt, becoming the type locality of the nominal species.
Figure 12. Lectotype of Dussumieria productissima, MNHN 1966–0259, 106.0 mm SL, Gulf of Suez, Egypt. Abbreviations: MNHN, Muséum National d'Histoire naturelle; SL, standard length.

Figure 13. Relationships of number of pectoral-fin rays bearing melanophores to standard length in Dussumieria elopsoides (diamonds), D. hasseltii (circles), D. modakandai (triangles), and D. productissima (squares).
Although Whitehead (Citation1985), who reviewed the genus, regarded this nominal species as a junior synonym of D. elopsoides, the validity of both nominal species was confirmed in the present study. Moreover, all nominal species included in the genus by Whitehead (Citation1985), except for Clupea flosmaris Richardson, Citation1846, a name suppressed due to ambiguity in its original description by Opinion 901 (ICZN Citation1970), are regarded herein as valid species (Hata et al. Citation2020, Citation2021; Singh et al. Citation2021; this study).
Whitehead’s (Citation1985) D. elopsoides was diagnosed by the lateral scales lacking numerous posterior longitudinal striae, a slender body (depth <22% of SL), 1LGR 21 or more, and branchiostegal rays 13 or more.
1a. Tooth band on anterior part of palatine broad, comprising numerous rows of conical teeth; total gill rakers (1TGR) ≤ 392
1b. Tooth band on anterior part of palatine narrow, comprising one or two rows of conical teeth; total gill rakers (1TGR) ≥37 . 3
2a. Pre-dorsal-fin length <56% of SL; pelvic fin >9.5% of SL; total vertebrae 55–58 D. elopsoides (Indonesia)
2b. Pre-dorsal-fin length >58% of SL; pelvic fin <8.7% of SL; total vertebrae 57–60 . D. hasseltii (Persian Gulf to Thailand and Indonesia)
3a. Total gill rakers (1TGR) ≥43); total vertebrae 55 or 56 (modally 55); no distinct longitudinal striae posteriorly on lateral body scales D. productissima (western coast of India, Red Sea, and eastern Mediterranean)
3b. Total gill rakers (1TGR) ≤ 43; total vertebrae usually 56 or 57 (rarely 55 or 58); lateral body scales with (10 or fewer) distinct posterior longitudinal striae . D. modakandai (Red Sea to Riau Archipelago and Ryukyu Islands)
Key to species identified as Dussumieria elopsoides by Whitehead (Citation1985)
Comparative material examined
Dussumieria albulina, BPBM 20500, 149.7 mm SL, off Chennai, India, collected with BPBM 41428 (D. hasseltii), and BPBM 41429 (D. modakandai).
Acknowledgements
We thank O. Crimmen, J. Maclaine, and N. Martin (BMNH), A. Suzumoto and L. O’Hara (BPBM), H. Endo (BSKU), S. Kimura (FRLM), T. Peristiwady (LBRC), R. Causse, P. Pruvost, Z. Gabsi, and J. Pfilger (MNHN), H.-C. Ho (NMMB), K. Matsuura, G. Shinohara, and M. Nakae (NSMT), K. Miyamoto (OCF), E. Dondorp (RMNH), T. Yoshino (URM), J. Williams, K. Murphy, S. Raredon, and D. Pitassy (USNM), and M. Aizawa, K. Sakamoto, R. Ueshima, and K. Koeda (ZUMT) for opportunities to examine specimens of Dussumieria. We also thank Y. Haraguchi and other volunteers and students of KAUM and NSMT for their curatorial assistance, and G. Hardy (Ngunguru, New Zealand) for reading the manuscript and providing help with English. Malaysian specimens were collected during the JSPS Asian Core Program, “Establishment of Research and Education Network on Coastal Marine Science in Southeast Asia”, and the JSPS Core-to-Core Program: B Asia-Africa Science Platforms, supported by the Ministry of Higher Education (Government of Malaysia), University Putra Malaysia, and University Malaysia Terengganu. Finally, we thank the two anonymous reviewers for their insightful comments which helped to improve this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aonuma Y. 1993. Clupeidae, herrings. In: Nakabo T, editor. Fishes of Japan with pictorial keys to the species. Tokyo: Tokai University Press. pp. 203–207, 1257–1258.
- Aonuma Y. 2000. Clupeidae, herrings. In: Nakabo T, editor. Fishes of Japan with pictorial keys to the species. 2nd ed. Tokyo: Tokai University Press. pp. 243–247, 1464.
- Aonuma Y. 2002. Clupeidae, herrings. In: Nakabo T, editor. Fishes of Japan with pictorial keys to the species. English ed. Tokyo: Tokai University Press. pp. 243–247, 1463.
- Aonuma Y, Yagishita N. 2013. Clupeidae, herrings. In: Nakabo T, editor. Fishes of Japan with pictorial keys to the species. 3rd ed. Hadano: Tokai University Press. pp. 297–301, 1811–1812.
- Bleeker P. 1849a. A contribution to the knowledge of the ichthyological fauna of Celebes. Journal of the Indian Archipelago and Eastern Asia 3 (1):65–74.
- Bleeker P. 1849b. Bijdrage tot de kennis der ichthyologische fauna van het eiland Madura, met beschrijving van eenige nieuwe soorten. Verhandelingen van het Bataviaasch Genootschap van Kunsten en Wetenschappen 22 (8):1–16.
- Bleeker P. 1851. Bijdrage tot de kennis der ichthyologische fauna van Riouw. Natuurkundig Tijdschrift voor Nederlandsch Indië 2 (3):469–497.
- Chabanaud P. 1933. Sur divers poissons de la mer Rouge et du canal de Suez. Description de deux espèces nouvelles. Bulletin de l’Institut Océanographique 627:1–12.
- Fricke R, Eschmeyer WN, Vander Laan R, editors. 2021. Eschmeyer’s catalog of fishes: genera, species, references. Available: http://researcharchive.calacademy.org/research/ichthyoogy/catalog/fishcatmain.asp. Accessed Dec 2021 12.
- Günther A. 1868. Catalogue of the fishes in the British Museum. Catalogue of the Physostomi, containing the families Heteropygii, Cyprinidae, Gonorhynchidae, Hyodontidae, Osteoglossidae, Clupeidae, Chirocentridae, Alepocephaluidae, Notopteridae, Halosaulidae, in the collection of the British Museum. Vol. 7. London: Taylor and Francis. pp. xx + 512.
- Hata H. 2017. Dussumieria elopsoides Bleeker 1849. In: Motomura H, Alama UB, Muto N, Babaran RP, Ishikawa S, editors. Commercial and bycatch market fishes of Panay Island, Republic of the Philippines. Kagoshima: The Kagoshima University Museum; Iloilo: University of the Philippines Visayas; Kyoto: Research Institute for Humanity and Nature. p. 40.
- Hata H. 2019. Clupeidae. In: Koeda K, Ho H-C, editors. Fishes of southern Taiwan. Pingtung: National Museum of Marine Biology & Aquarium. pp. 212–224.
- Hata H, Koeda K, Aizawa M, Sakamoto K, Ueshima R. 2022. A list of Clupeiformes (Actinopterygii: Teleostei) specimens deposited in the Department of Zoology, The University Museum, The University of Tokyo. The University Museum, The University of Tokyo. Bulletin 128:17–58.
- Hata H, Lavoué S, Motomura H. 2020. Redescriptions of Dussumieria acuta Valenciennes 1847 and Dussumieria albulina (Fowler 1934), two valid species of rainbow sardines (Clupeiformes: Dussumieriidae). Ichthyological Research 68:1–13. DOI: 10.1007/s10228-020-00778-y.
- Hata H, Lavoué S, Motomura H. 2021. Dussumieria torpedo (Teleostei: Clupeiformes: Dussumieriidae), a new rainbow sardine from the east African coast. Ichthyology and Herpetology 109 (4):991–997. DOI: 10.1643/i2020159.
- Hata H, Motomura H. 2017. A new species of anchovy, Encrasicholina auster (Clupeiformes: Engraulidae), from Fiji, southwestern Pacific Ocean. New Zealand Journal of Zoology 44:122–128. DOI: 10.1080/03014223.2016.1268177.
- Hata H, Motomura H. 2020. Assessment of standard Japanese name for the family Dussumieriidae (Clupeiformes). Ichthy, Natural History of Fishes of Japan 1:11–14.
- ICZN (The International Commission on Zoological Nomenclature). 1970. Opinion 901. Richardson fish names: Suppressed under the plenary powers. Bulletin of Zoological Nomenclature 26:217–220.
- ICZN (The International Commission on Zoological Nomenclature). 1999. International code of zoological nomenclature. 4th ed. Adopted by the General Assembly of the International Union of Biological Sciences. London: International Trust for Zoological Nomenclature. pp. xxix + 306.
- Kanda Y. 2012. Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant 48:452–458. DOI: 10.1038/bmt.2012.244.
- Keskin E, Atar HH. 2013. DNA barcoding commercially important fish species of Turkey. Molecular Ecology Resources 13:788–797. DOI: 10.1111/1755-0998.12120.
- Kimura S. 2009. Dussumieria elopsoides Bleeker, 1849. In: Kimura S, Satapoomin U, Matsuura K, editors. Fishes of Andaman Sea, west coast of southern Thailand. Tokyo: National Museum of Nature and Science. pp. 30.
- Kimura S. 2011. Dussumieria elopsoides Bleeker, 1849. In: Matsunuma M, Motomura H, Matsuura K, Shazili NAM, Ambak MA, editors. Fishes of Terengganu – East coast of Malay Peninsula, Malaysia. Tokyo: National Museum of Nature and Science; Terengganu: Universiti Malaysia Terengganu; Kagoshima Kagoshima University Museum. p. 38.
- Kimura S. 2013. Dussumieria elopsoides Bleeker, 1849. In: Yoshida T, Motomura H, Musikasinthorn P, Matsuura K, editors. Fishes of northern Gulf of Thailand. Tsukuba: National Museum of Nature and Science; Kyoto: Research Institute for Humanity and Nature; Kagoshima: Kagoshima University Museum. p. 37.
- Lakra WS, Verma MS, Goswami M, Lal KK, Mohindra V, Punia P, Gopalakrishnan A, Singh KV, Ward RD, Hebert P. 2011. DNA barcoding Indian marine fishes. Molecular Ecology Resources 11:60–71. DOI: 10.1111/j.1755-0998.2010.02894.x.
- Lavoué S, Bertrand JAM, Chen W-J, Ho H-C, Motomura H, Sado T, Miya M. 2017. Phylogenetic position of the rainbow sardine Dussumieria (Dussumieriidae) and its bearing on the early evolution of the Clupeoidei. Gene 623:41–47. DOI: 10.1016/j.gene.2017.04.032.
- Munroe TA, Wongratana T, Nizinski MS. 1999. Clupeidae Herrings (also, sardines, shad, sprats, pilchard, and menhadens). In: Carpenter KE, Niem VH, editors. FAO species identification guide for fishery purposes. The living marine resources of the western central Pacific, vol 3. Batoid fishes, chimaeras and bony fishes part 1 (Elopidae to Linophrynidae). Rome: FAO. pp. 1775–1821.
- Richardson J. 1846. Report on the ichthyology of seas of China and Japan. Report of the British Association for the Advancement of Science 15th Meeting 1845:187–320.
- Sabaj MH. 2020. Codes for natural history collections in Ichthyology and Herpetology. Copeia 108:593–669. DOI: 10.1643/ASIHCODONS2020.
- Singh M, Jayakumar TTK, Kumar TTA, Murali S, Mishra A, Singh A, Lal KK. 2021. Integrative taxonomy based discovery of Dussumieria modakandai sp. nov. from India. Journal of Fish Biology 100 (1):268–278. DOI: 10.1111/jfb.14943.
- Stecher G, Tamura K, Kumar S. 2020. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Molecular Biology and Evolution 37:1237–1239. DOI: 10.1093/molbev/msz312.
- Steinke D, Connell AD, Hebert PD. 2016. Linking adults and immatures of South African marine fishes. Genome 59:959–967. DOI: 10.1139/gen-2015-0212.
- Stern N, Rinkevich B, Goren M. 2015. First record of the Goldstripe sardinella – Sardinella gibbosa (Bleeker, 1849) in the Mediterranean Sea and confirmation for its presence in the Red Sea. BioInvasions Records 4:47–51. DOI: 10.3391/bir.2015.4.1.08.
- Uyeno T, Sato T. 1984. Dussumieria elopsoides Bleeker. In: Masuda H, Amaoka K, Araga C, Uyeno T, Yoshino T, editors. The fishes of the Japanese Archipelago. Tokyo: Tokai University Press. pp. 18, pl. 21A., pl. 21A.
- Valenciennes A. 1847. Chapitre II. Des Sardinelles. In: Cuvier G, Valenciennes A, editors. Histoire naturelle des poissons. Vol. 20. Paris: P Bertrand. pp. 260–276.
- Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PDN. 2005. DNA barcoding Australia’s fish species. Philosophical Transactions of the Royal Society B Biological Sciences 360:1847–1857. DOI: 10.1098/rstb.2005.1716.
- Whitehead PJP. 1963. A revision of the recent round herrings (Pisces: Dussumieriidae). Bulletin of the British Museum (Natural History) Zoology 10 (6):308–380. DOI: 10.5962/bhl.part.20529.
- Whitehead PJP. 1965. A review of the elopoid and clupeoid fishes of the Red Sea and adjacent regions. Bulletin of the British Museum (Natural. History) 12:227–281.
- Whitehead PJP. 1985. FAO species catalogue. Vol 7. Clupeoid fishes of the world (suborder Clupeoidei). An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, anchovies and wolf-herrings. Part 1 – Chirocentridae, Clupeidae and Pristigasteridae. FAO Fisheries Synopsis, No 125 7:1–303.
- Whitehead PJP, Boeseman M, Wheeler AC. 1966. The types of Bleeker’s Indo-Pacific elopoid and clupeoid fishes. Zoologische Verhandelingen 84:1–159.
- Zhang S. 2001. Fauna Sinica Osteichthyes. Acipenseriformes, Elopiformes, Clupeiformes, Gonorhynchiformes. Beijing: Science Press. pp. 209.