Abstract
Here we present an intriguing new species of the Empididae genus Rhamphomyia (Pararhamphomyia) from the Kashmir Himalayas. The new species, Rhamphomyia (Pararhamphomyia) aquila, has a distinctive appearance due to its highly deformed male hind legs with extremely shortened hind tibia, a feature very peculiar in the genus. The new species is described along with elements of mating behaviour, the mechanism of male hind leg articulation, and its possible role during mating. Also, scanning electron microscopy analysis is used to elucidate the general morphology and sensilla in both sexes of the new species. Details of the male and female internal reproductive system are described and illustrated. A preliminary checklist of 55 species of the Empididae of India is provided.
http://www.zoobank.org/urn:lsid:zoobank.org:pub:A28B850F-6A2D-4FB2-9AC3-E89015C1C5A7
http://www.zoobank.org/urn:lsid:zoobank.org:act:C0249B1D-D0C8-4998-96A2-C25D3DB89810
1. Introduction
The genus Rhamphomyia Meigen, 1822 (Diptera: Empididae) is widespread across multiple zoogeographical regions (Saigusa Citation2012), and currently about 610 species are known worldwide (Rhodén & Wahlberg Citation2020). These are important pollinators in high mountains and in boreal environments (Barták & Kubík Citation2009); they are also important biocontrol agents and important bioindicators (Grootaert et al. Citation2000). So far most of the species have been reported from the Holarctic Region, with some unpublished estimates suggesting the richest diversity to be present in the Nearctic (Barták & Kubík Citation2009; Sinclair et al. Citation2019). Globally it is estimated that there may be as many as 1500 species of Rhamphomyia (Sinclair et al. Citation2019). The genus consists of several subgenera which helps provides a better overview of its diversity (Rhodén & Wahlberg Citation2020). The first attempt at subgeneric grouping was carried out by Frey (Citation1909, Citation1922), and recent papers by Barták (Citation1981, Citation1982), Chvála and Wagner (Citation1989) and Saigusa (Citation2012) remain very relevant in this regard. Currently, the genus consists of about 18 subgenera (Yang et al. Citation2007; Barták & Kubík Citation2009; Saigusa Citation2012), almost exclusively relevant for the Palaearctic fauna; some of those that were revised recently are stable, while others remain problematic and include the presence of extralimital species (Rhodén & Wahlberg Citation2020; Barták et al. Citation2021). The use of plesiomorphic characters for subgeneric classification, the probable polyphyletic nature of the genus and its high diversity, among not only the congeners but also the allied genus Empis L., has made the current subgeneric classification unreliable (Saigusa Citation2012; Chvála & Pont Citation2015). A global comprehensive review to delimit, establish and redefine subgeneric classification and overall diversity of the genus has yet to be performed.
The subgenus Pararhamphomyia Frey, Citation1922 includes about 120 species, mostly known from the eastern parts of the Palaearctic Region (Barták & Kubík Citation2009), but surely diverse in the Nearctic Region as well. These species are characterised by the prosternum and mostly propleura without setae, biserial acrostichals, incomplete anal vein, long setose labella and usually a distinct basal costal seta. The separation of Rhamphomyia s. str. and Pararhamphomyia remains sometimes rather difficult but can be resolved using the decision table provided by Barták (Citation1982).
We provide a description of a new species belonging to Rhamphomyia (Pararhamphomyia) from the Kashmir Himalayas (India). The species placement within the subgenus Pararhamphomyia follows Collin (Citation1961) and Barták and Sinclair (Citation2003). Within the subgenus Pararhamphomyia, the placement of the new species and its association with known species groups remains unclear. With the presence of joint distortion in the male hind leg, the new species can be placed in the basalis species group, but the male terminalia, especially the shape of the cercus and the absence of a subepandrial lobe, do not match those of the basalis group (see also Chillcott Citation1959). The male terminalia, particularly the shape of the cercus, is more similar to that of the members of the pusilla group – from which, however, there are also marked differences (see Remarks). The new species is the fourth Rhamphomyia species to be discovered from India (Brunetti Citation1913, Citation1917, Citation1920; Barták et al. Citation2021).
In addition to description of the male and female specimens elucidated with scanning electron microscopy (SEM) analysis, a discussion on the mating behaviour is provided including a possible role for the modified male hind leg. We also include details of the male and female internal reproductive system and compare them with those of the other recently discovered Rhamphomyia species from India. The comparison suggests the possible use of the male internal reproductive tract as a species-specific character (see Discussion).
2. Materials and methods
2.1. Materials, light and scanning electron microscopy
Specimens were collected by net sweeping and hand picking from the fruit orchards of the Central Institute of Temperate Horticulture (CITH) during the years 2015–2021. CITH is situated at 33.59°N, 74.50°E, at an altitude of 1640 m a.s.l. (). The field photographs were obtained using a Canon 80D DSLR fitted with a 100 mm macro lens. Taxonomic analyses were conducted using an Olympus SZX16 stereo zoom microscope. For digital images, ProgRes0 Capture Pro v. 2.8.0. evolution digital camera was used on the same microscope with Combine ZP-Montage software. Later, images were cleaned up with Adobe Photoshop CS6. Genitalia together with 2–3 pregenital segments were removed and macerated in potassium hydroxide solution (10%) in small vials, for 1–2 h, treated with 8% acetic acid and dissected in glycerine. For bright-field microscopy, prepared slides were placed on a stage prepared from cardboard and provided with an LED lamp (3 W) to produce the transmission light beam. For dark-field microscopy a dark-field illumination filter was used to illuminate unstained specimens. Microphotographs were captured with a digital mirrorless camera (Nikon Z50) with attached infinity-corrected microscope objectives (Lomo 3.7× and Nikon CFI Plan Achromat 10X). The details and description of the type locality and the illustration procedure are similar to those of Barták et al. (Citation2021).
Figure 1. (a, b) Type locality of the new species, Central Institute of Temperate Horticulture, Srinagar, Jammu and Kashmir, India.

The morphological terms used here follow Sinclair and Cumming (Citation2006). All the specimens are deposited in the Central Institute of Temperate Horticulture Insect Collection, Srinagar (CITH). Two paratypes will be deposited in the Natural History Museum, London, UK (NHMUK), four at the Czech University of Life Sciences Prague (CULSP) and two at the University of Silesia, Katowice (DZUS). For studies on leg articulation, the dissection procedure described by Földvári et al. (Citation2019) was followed. Ethanol-stored specimens were bleached and rehydrated in 35% H2O2 for 24 h and then transferred to anhydrous glycerol. Specimens were dissected in glycerol with fine forceps, insect pins and razor blades on concave slides. The straightening and bending movements of the leg were observed to understand interaction between the proximal tibial flexor tendon and the ventral femoral wall. For this the bleached legs were moved with insect pins; these pins were also used to dislodge the locking sclerites, similarly to Földvári et al. (Citation2019). The term “sclerite” was used for less flexible areas of the exoskeleton that are connected to each other by more flexible conjunctiva, as detailed in Klass and Matushkina (Citation2012) and Ronquist and Nordlander (Citation1989). All the procedural videos were taken with the use of a camera attached to the microscope and are provided as supplementary files. Specimens for SEM analyses were preserved in 70% ethanol for several days, processed as described by Barták et al. (Citation2021) and imaged with the Hitachi SU8010 field emission scanning electron microscope (FESEM; Hitachi High-Technologies Corporation, Tokyo, Japan), Institute of Biology, Biotechnology and Environmental Protection, University of Silesia, in Katowice.
2.2. Institutional abbreviations
| CAU | = | China Agricultural University, Beijing, China |
| CITH | = | Central Institute of Temperate Horticulture, Srinagar, India |
| CULSP | = | Czech University of Life Sciences, Prague, Czech Republic |
| DZUS | = | University of Silesia, Katowice, Poland |
| MZLS | = | Musée cantonal de zoologie, Lausanne, Switzerland |
| NHMUK | = | Natural History Museum, London, UK |
| PAN | = | Polish Academy of Sciences, Warsaw, Poland |
| ZFMK | = | Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany |
| ZIN | = | Zoological Institute of the Russian Academy of Sciences, St. Petersburg, Russia |
| ZSI | = | Zoological Survey of India, Kolkata, India |
3. Results
3.1. Taxonomy
Rhamphomyia (Pararhamphomyia) aquila sp. nov.
()
Figure 2. Rhamphomyia aquila sp. nov., male. (a) Head, frontal view; (b) habitus, lateral view; (c) habitus, dorsal view.
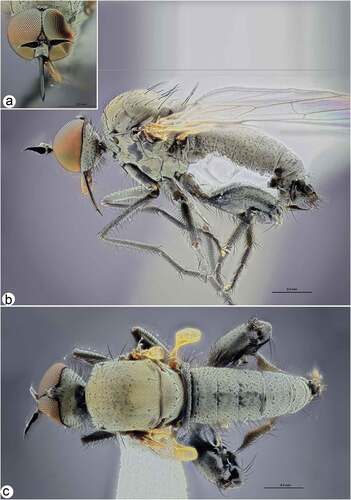
Figure 3. Rhamphomyia aquila sp. nov., male. (a) Mouth parts; (b) antennae; (c–d) legs, male hind leg with deformed femorotibial joint, enlarged view; (e) fore and mid legs; (f) male fore wing with labelled discal cell (dm), cubitus (CuA1) and medial veins (M2); (g–h) male genitalia, cercus (cerc), ejaculatory apodeme (ej apod), epandrium (epand), hypandrium (hypd), phallus (ph).
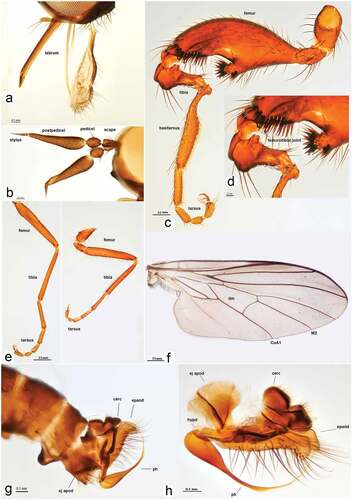
Figure 4. Rhamphomyia aquila sp. nov., female. (a) Head, frontal view; (b) habitus, lateral view; (c) habitus, dorsal view.
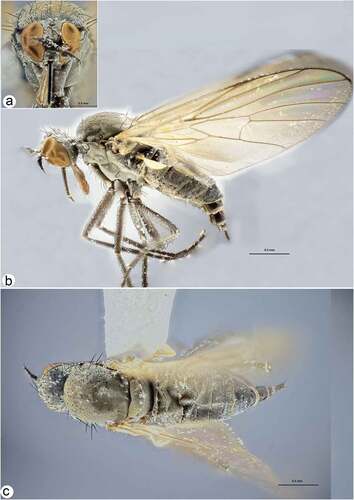
Figure 5. Male and female internal reproductive system of Rhamphomyia aquila sp. nov. (a–d) female internal reproductive tract: (a–b) complete internal reproductive system; (c) right ovary removed and spermatheca extended; (d) separated spermatheca, enlarged view. (e–f) male internal reproductive tract.
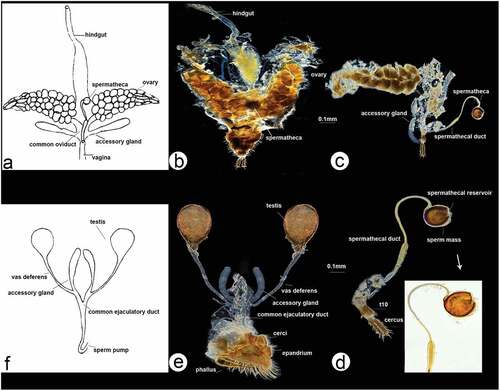
Figure 6. Scanning electron microscopy showing general morphology of (a) female and (b) male of Rhamphomyia aquila sp. nov. body, lateral view; (c) female head, full face view; (d) male head, full face view; (e) female terminalia; (f) male terminalia.
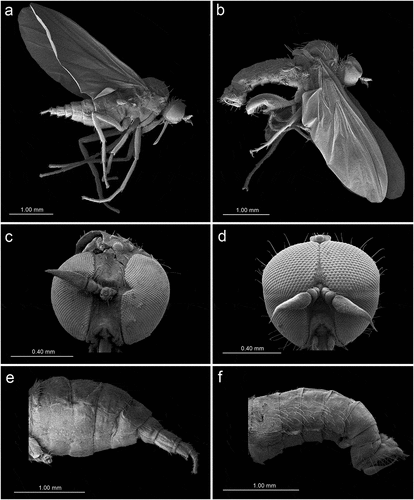
Figure 7. Scanning electron microscopy of Rhamphomyia aquila sp. nov. female head and antennal sensilla. (a) Dichoptic eyes; (b–c) mouth parts with palpi bearing 3–4 long setae (white arrow); (d–e) labium and long stylet labrum with epipharyngeal blades; (f) ventral side of postpedicel with sensilla (white arrows); (g–i) basiconic sensilla (most probably type I) (white star); (j–l) sensilla basiconica with granulated and porous surface.
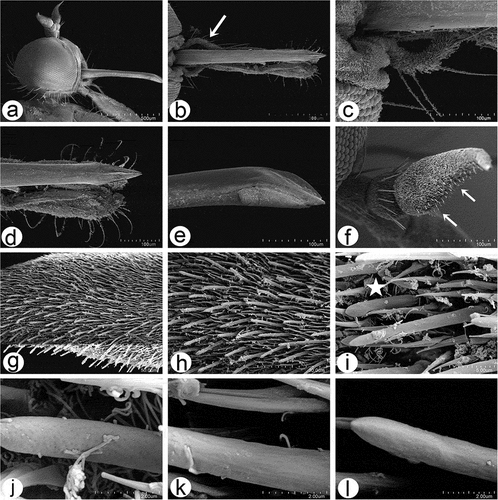
Figure 8. Scanning electron microscopy of Rhamphomyia aquila sp. nov. male head and antennal sensilla. (a–b) Holoptic eyes, with enlarged facets along dorsal half; (c–d) labellum with distinct pseudotracheae; (e) sponge-like general surface of pseudotracheae; (f) labrum with piercing, serrate apical part (white arrows); (g) expanded and convex ventral basal of postpedicel with basiconic sensilla (arrows); (h–k) regularly arranged basiconic sensilla with swollen bases and small pores of sensilla ampullacea (white arrows); (l–o) stylus, mechanoreceptor porous with dense covering of microtrichia.
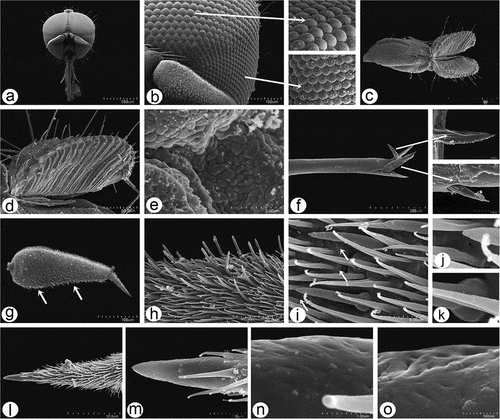
Figure 9. Scanning electron microscopy of Rhamphomyia aquila sp. nov. male antennal sensilla. (a) Basal portion of postpedicel with group of sensilla coeloconica (dotted box); (b) regularly placed sensilla coeloconica (arrows); (c–e) sensilla coeloconica located in depressions (white star); (f–h) peg-shaped sensilla coeloconica surrounded by ring-like cuticular elevation (white dotted arrows).
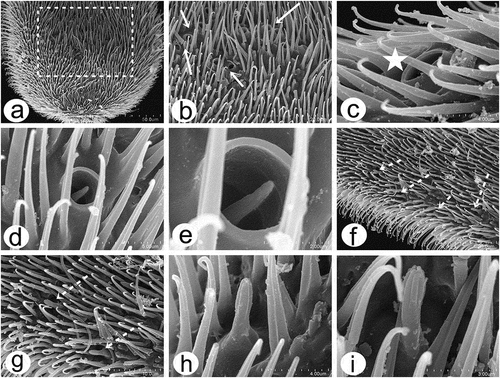
Figure 10. Scanning electron microscopy of Rhamphomyia aquila sp. nov. male detailing wing articulation. (a–c) Microtrichia wing membrane with wax layer; (d–f) long, ribbed chaetic sensilla present along wing edges.
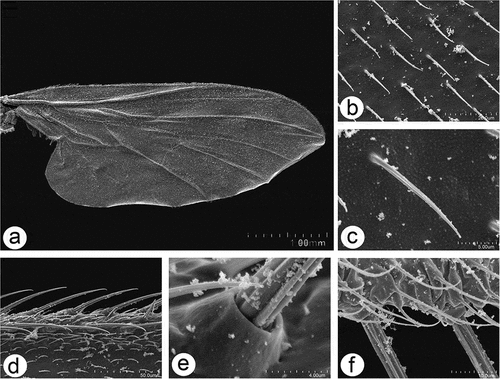
Figure 11. Scanning electron microscopy of Rhamphomyia aquila sp. nov. halter. (a–b) Ventral and dorsal side of the halter with robust scabelum, pedicellus and oval capitellum; (c) sensilla on dorsal scabellum and dorsal pedicellus; (d) scabellus protuberant basal plate sensilla; (e) oriented flanking sensilla on the dorsal pedicel stem; (g–k) single trichoid and campaniform sensillum on capitellum (black arrows : solid, dotted), in addition to the general dense microtrichia.
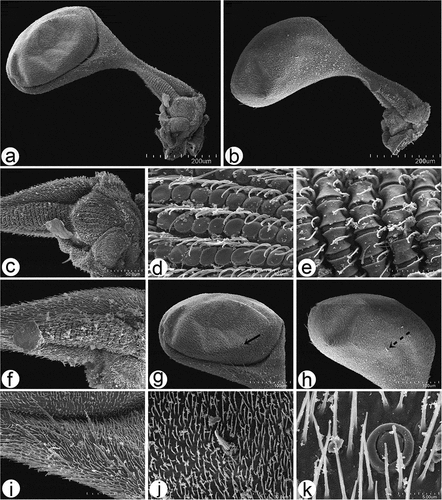
Figure 12. Scanning electron microscopy of Rhamphomyia aquila sp. nov. legs. (a–b) Swollen male hind femur and extremely reduced hind tibia; (c–g) long and stout trichoid and chaetic sensilla on apical third of femur, posteroventrals; (h–j) single campaniform sensillum on the basal inner side of femora (arrow) with small pore near the centre (star); (k–l) elongate basitarsus with large grooved chaetic sensilla; (m–n) trichoid and chaetic sensilla on third tarsomere; (o–q) curved claws and pulvilli (r) fine structure of tenent setae and terminal plates.
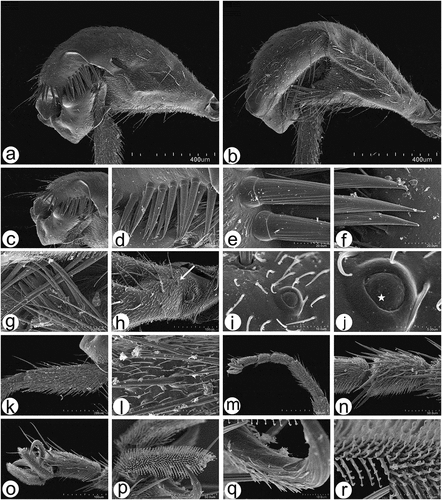
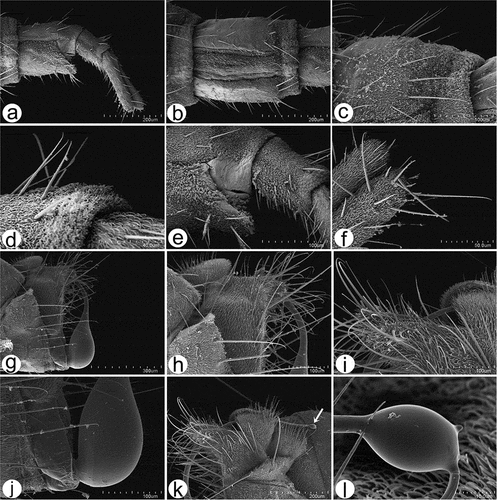
Figure 13. Scanning electron microscopy of Rhamphomyia aquila sp. nov. male and female genitalia. (a) Terminal female abdomen tergites; (b) in ventral view appear partially retracted into proximal segment; (c–e) seventh and eight segments of abdomen with longer chaetic sensilla dorsally; (f) thick, long chaetic sensilla of cerci; (g) smooth basal part of phallus; (h–i) long and rigid chaetic sensilla on genitalia; (j–l) phallus broad, covered by waxy secretions, and bulb-shaped apical portion (arrow).
Diagnosis
Black species of Rhamphomyia (Pararhamphomyia): legs and body setae black, black palpus, acrostichals biserial, dorsocentrals irregularly biserial and much longer than acrostichals; halter yellow, anal lobe well developed with rectangular axillary angle, anal vein incomplete. Male with deformed hind femorotibial joint having tuft of anteroventral bristles; distinctly shortened hind tibia; epandrium subtriangular, truncate, cercus with broad base, tapered at mid-length to truncate apical portion, phallus lustrous with apex having shallow S-shaped curvature.
Material examined
Holotype. ♂, INDIA: Kashmir: Srinagar: Central Institute of Temperate Horticulture, 34.0094°N 74.7984°E, 1640 m.a.s.l., 1 May 2017 (CITH). Paratypes. Same locality as holotype, except: 12 May 2015 (5♂, 8♀, CITH), 28 April 2016 (2♂, 2♀, CITH), 1 May 2017 (13♂, 20♀, CITH), 6 May 2017 (2♂, 2♀, CITH), 11 June 2018 (8♂, 19, CITH), 7 May 2019 (10♂, 13♀, CITH), 15 April 2020 (11♂, 9♀, CITH), 21 May 2020 (3♂, 6♀, CITH), 3 April 2021 (9♂, 2♀, CITH), 8 May 2021 (15♂, 15♀, CITH), Shahid Ali Akbar leg.
Distribution
India (Jammu and Kashmir).
Date of occurrence
April to June.
Description
Male (). Head with pale greyish pruinescence on face, frons, postgena and occiput; eyes holoptic, meeting along median dorsal line, facets in dorsal half of eye considerably enlarged (); frons represented by small triangular space below ocellar tubercle and larger sub-triangular space above antennae, brown, without setulae; face parallel sided, slightly converging dorsally near antennae and divergent towards mouthparts; approx. 0.11–0.14 mm wide, 0.15–0.18 mm long, without setae; microtrichose with pale greyish pruinescence, labrum microtrichose. Gena narrow and microtrichose. Palpus black and short, with several long setae (up to 0.22 mm). Labrum black, shiny, almost as long as head height; labellum with rather long setae (). Ocellar triangle dark brown pruinescent, ocellar setae black, fine, approximately 0.11–0.15 mm long, accompanied by several smaller setulae. Occiput with row of dark, fine postocular setae on upper half of occiput similar in length to ocellars; remaining occipital setae shorter; postocular row incomplete, irregular on lower half. Antenna black, both basal segments short, setose; length of antennal segments (scape:pedicel:postpedicel:stylus) = 0.05–0.07 mm:0.04–0.05 mm:0.22–0.25 mm:0.05–0.06 mm; postpedicel conical, 2.3–2.5× longer than wide ().Thorax pale greyish pruinescent. Thoracic setae dark, posterior dorsocentrals, postalars and scutellars black; acrostichals and dorsocentrals also dark black (). Chaetotaxy: antepronotum with numerous fine setae; postpronotum with 1 long seta and additional 3–4 short dark setae; proepisternum with 2–3 stout, dark setae; upper proepisternum in front of spiracle with 2 setae; prosternum and propleura bare; acrostichals biserial and few in number (6–8 in one row), about 0.10–0.14 mm long; dorsocentrals irregularly biserial, slightly longer than acrostichals, ending in 2–3 long, black prescutellar pairs; 1 presutural intra-alar (intrahumeral), 1 presutural supra-alar (posthumeral) and additional 1–2 dorsocentral setae; 2–3 notopleural setae and 1–2 rather long but fine setae on anterior part of notopleuron; 1–2 supra-alars and 1 small prealar; two pairs of black scutellars.
Legs
Black to dark brown, microtrichose, with black setae (–c, 3c–e). Fore femur with sparse rows of very short anteroventrals and somewhat longer posteroventrals (about half as long as femur depth), dorsal setae shorter. Fore tibia with very short setae, posteroventrals fine and about as long as tibia depth. Mid femur with anteroventral row of setae slightly shorter than femur depth and similar row of posteroventrals, shorter on proximal part, on distal part with several slightly stronger setae, dorsal setae short. Mid tibia with short and fine dorsal setae, only 1–2 antero- or posterodorsals slightly longer than tibia depth, ventral setae stronger, almost spine-like, arranged in two rows, subequal in length to tibia depth. Hind femur with row of strong preapical setae; strongly modified ( b–c, 3c–d): swollen, with long ventral protuberance on basal third to half and strong ventral incision on apical third, on apical third with long ventral setae and comb of posteroventrals, dorsally with short setae. Hind tibia extremely shortened, roundly expanded at base, with dorsal knob, with several strong and long setae. Hind basitarsus extremely elongate, strongly constricted on basal third, much longer than tibia, covered with dense short slightly widened setae on both sides. Tarsi of both fore and mid legs narrow with short setae, with apical circlets of setae and long claws. Posteroapical comb of hind tibia with 1 long seta.
Wing
Clear, veins brownish-black, pterostigma brown, anal vein incomplete; anal lobe well developed with rectangular axillary angle. Halter yellow with brown stem, calypter yellow with whitish fringes ().
Abdomen. Abdomen silvery pruinescent; with short black setae, finer and longer on proximal tergites, stouter and shorter towards terminalia (). Lateral marginal setae on tergites 2–4 longer, dorsal setae much shorter, sternites with uniform long, light brown setae. Sternite 7 produced ventrally. Sternite 8 larger than sternite 7, closely appressed laterally with tergite 8; with long setae posteriorly. Tergite 8 half length of sternite 8 with short marginal setae. Terminalia dark grey, barely longer than abdominal width. Epandrium subtriangular, truncate; apex of epandrium down-curved with tuft of long setae; inner apex thinly sclerotised with short setae. Cercus with broad base, strongly attenuated at mid-length; dorsal margin of cercus bearing many short, fine, erect dark setae. Hypandrium ending in middle of swollen phallus base; phallus lustrous, reddish brown, gently arched between epandrial lamellae; apex with shallow S-shaped curvature. Ejaculatory apodeme fan-shaped with lateral wings shorter than vertical wing ().
Length
Body 4.0–5.0 mm, wing 3.5–4.2 mm.
Female (). Similar to male, but body setae much shorter and lighter; legs simple and unremarkable, without deformed hind femorotibial joint. Eyes dichoptic with all facets almost equal in size (). Frons approximately 0.14–0.18 mm, broad with 4–5 black, rather long setae on sides. Labrum black, labella with rather long setae. Thoracic chaetotaxy: acrostichals and dorsocentrals black, dorsocentrals distinctly longer. Both fore and mid femora with short setae, longest (distal) posteroventrals shorter than femur depth. Both fore and mid tibiae very short, without prominent setae. Hind femur short, anteroventral setae and prominent posteroventrals, shorter than femur depth. Antero- and posterodorsal setae of hind tibia as long as tibia diameter (). Length: body 3–3.3 mm; wing 3.1–3.4 mm.
Female and male internal genital tract (). Internal reproductive tract of female comprises paired ovaries, lateral oviducts, common oviduct, long tubular vagina, spermatheca, spermathecal duct and two accessory glands (). Vagina appears as elongate muscular tube lined internally with thin cuticle; anteriorly fused with common oviduct. Spermathecal duct and ducts of accessory glands open anterolaterally and posterolaterally, respectively, into vagina. Ovaries broad leaf-like with four follicular cell layers, with youngest oocytes near apical germarium and most mature ones near basal region. Eggs empty into lateral oviducts, unite into common oviduct. Spermatheca with broad basal stalk, extending into elongate duct and terminating as round reservoir for sperm mass collection and storage (). Accessory gland appears as small bisected delicate sacs, with thicker basal sac compared with thinner apical sac.
Internal reproductive tract of male comprises paired testes, vas deferens, pair of accessory glands and common ejaculatory duct (). Each testis bulb-shaped, comprising testicular follicles enclosed in common outer sheath. Vas deferens appears as long tube-like structure, without enlarged and differentiated region distinguished as seminal vesicle. Tubular accessory gland ducts along with vas deferens open into anterior portion of ejaculatory duct. Ejaculatory duct short without distinct folds.
Etymology
The species epithet is Latin for eagle and appropriately symbolises the aerial grabbling skills and holding dominance of the male specimens of the new species.
3.2. Notes on SEM morphology of Rhamphomyia aquila sp. nov. ()
The male is generally similar to the female, except for the normal sexual dimorphism. Variations in general morphology, eyes and terminalia can be observed (see ).
3.2.1. Head, antennae and mouthparts
In females, the ommatidia are tightly and regularly deployed (20–25 μm), uniform without dimorphism between the upper and the lower halves (). The palpi are long, cylindrical, rarely strongly compressed laterally (), bearing 3–4 long setae, in addition to fine microtrichia throughout (). Labium with dense cover of microtrichia and several thick, long chaetic sensilla along the dorsal side of the labellum (). Labrum is long. The stylet is covered by a wax layer, with pointed epipharyngeal blades (). The antennae are rather short. Scape, pedicel, postpedicel and stylus are covered by numerous fine sclerotic microtrichia and several types of sensilla especially on the ventral side of the postpedicel (). Most of the basiconic sensilla (most probably type I) are located along the ventral side of the postpedicel, placed regularly, lengthwise (). They are 5–9 μm long, sometimes covered by a wax layer. Some sensilla are characterised by a multiporous surface (), whereas others have rather grooved surfaces ().
In males, the ommatidia of the compound eye exhibits distinct dimorphism with a larger upper half and smaller lower half, tightly and regular deployed (). The distinct pseudotracheae channels and ground surface of the labellum provide capillary action (), whereas the proximal part of the labellum is characterised by a very granular surface (). The labrum has an array of rasps and teeth on the apical part of the epipharyngeal blades, used for piercing (). The antennae are similar to those of the female, and most of the sensilla are located on the ventral side of the postpedicel, a larger segment having an expanded and convex ventral basal part (). The postpedicel is densely covered by prominent microtrichia and numerous, regularly arranged basiconic sensilla (). The basiconic sensilla are located in depressions and characterised by a wide and rounded basal part, and they are mostly without a porous surface (). On the cuticle of the postpedicel some small pores of sensilla ampullacea are also apparent (). The stylus has a dense covering of microtrichia with the most apical part bare (). The sensillum surface of the apical part of the stylus seems to be smooth but at higher magnification it is clear that the cuticle is covered by numerous longitudinal pores (). The basal portion of the male postpedicel latero-ventrally consists of numerous regularly placed sensilla coeloconica, located in deep depressions and surrounded by a large ring-like cuticular collar (). The sensilla are visibly tapered with a wider basal part and a more pointed distal part (). On the other hand, the lateral side of the postpedicel bears more sensilla coeloconica characterised as a raised peg with terminal bulb-like tip and 6–7 well-visible projections ().
3.2.2. Wings
Wings in males and females are similar, with a distinct wax layer and a microtrichose wing membrane, regularly deployed, fine, slightly pointed and slightly ribbed. The wing edges and wing articulation bear chaetic sensilla, long and ribbed with basal groove raised (). The halter is covered by numerous microtrichia with robust scabellum, with well-visible sensilla plates, pedicellus and oval capitellum (). Sensilla on the dorsal scabellum and dorsal pedicellus distinct (), scabellus with densely arranged spherical and protuberant basal plate sensilla (), and similar linearly oriented flanking sensilla on the dorsal pedicel stem (); flanking sensilla are also well marked on the ventral pedicellum (). The capitellum is characterised by a single trichoid and campaniform sensillum along the ventral side and single prominent trichoid sensillum along the dorsal in addition to the general dense microtrichia ().
3.2.3. Legs
Legs in males and females are densely covered by numerous short, fine, pointed microtrichia and mechanoreceptors with longer trichoid sensilla mainly on the coxae. In males, the hind femur is strongly modified and swollen and the hind tibia is extremely reduced (). The hind femur is characterised also by a prominent ventral protuberance on the basal third with a ventral incision on the apical third having long and stout trichoid and chaetic sensilla in the form of a comb, posteroventrals pointed and grooved (). On the basal inner side of the femora, a single campaniform sensillum of about 5.5–6.5 μm in diameter can be found (). The sensillum is characterised by a protuberant inner part with a small but visible pore (). The hind basitarsus is extremely elongate, having larger numbers of grooved chaetic sensilla ().The tarsomeres have large chaetic sensilla, while distal parts bear more trichoid sensilla (). The claws are long, curved, with proximal halves covered by pointed microtrichia and distal halves curved and ribbed, ventral side of the pulvilli with regularly arranged tenent setae with flat capitate terminal plates ().
3.2.4. Male and female terminalia
Female terminal segments with microtrichia, ventral sides and the cerci characterised by longer chaetic sensilla (), most of the terminal tergites appear to have fewer microtrichia as they are partially retracted into the proximal segment (). The seventh and eight segments of the abdomen have a fine appearance with longer chaetic sensilla mostly apparent along the dorsal portion (); chaetic sensilla thickest on tips of cerci (). The terminal part of the male abdomen is covered by dense, very long, rigid and pointed chaetic sensilla (). The cercus and epandrium have numerous microtrichia, and trichoid and long chaetic sensilla (). The basal part of the phallus is smooth, broad, enlarged, and covered by waxy secretions, and the apex is swollen, bulb-shaped ().
3.3. Remarks
Among the known Indian species belonging to the genus Rhamphomyia, the new species appears distinct with its highly modified male hind legs. In addition to the distinct morphological differences between the new species and another recently discovered Indian species (Rhamphomyia bhagati Barták, Akbar, Kanturski, Wachkoo & Maqbool, Citation2021), there is also a difference in the male internal reproductive tract. The testis is more or less rounded with a basal neck leading into the vas deferens in R. bhagati, compared with an oval testis leading into the vas deferens without having a distinct neck in the new species. Although the male hind leg does appear to be modified very similarly to the R. basalis group, the male terminalia, especially the cercus shape and absence of a subepandrial lobe, do not match the basalis group. The cercus is more similar to those of the pusilla group (sensu Saigusa; see also Sinclair et al. (Citation2019)). Moreover, the females of the new species are without pennate setae on the legs and without infuscated wings, both characteristic features of the basalis group (Sinclair & Saigusa Citation2018). From the members of the pusilla group, the new species differs in not having darkened halteres, midlegs without modified tibiae and tarsomere and phallus not long and very fine. Rhamphomyia merzi Bartak, 2000 from Kazakhstan, a species similar to the basalis group on the basis of male terminalia, also has the hind femora deformed, but asymmetrical; only the right femur is strongly swollen while the left hind femur is only slightly swollen (Barták Citation2000), and also not to the extent reported here.
Male hind leg articulation mechanism and involved components (). We present here perhaps the first finding of extremely modified male hind legs in Empidinae; the only remotely similar finding comes from Daugeron et al. (Citation2010) regarding the occurrence of highly modified hind legs in Empis subgenus Enoplempis (see Sinclair et al. Citation2013). Modified hind femora occur in males of some species, e.g. R. merzi. The modified basal part of the tibia is a common feature among the basalis group and many others. A highly thickened or elongated basitarsus of the hind leg is also often reported in the genus. However, a distinctly modified and shortened hind tibia, as reported here, has never been reported previously. The hind basitarsus which is greatly elongated in fact takes over a function of the normal tibia, which is of peculiar occurrence.
Figure 14. Male hind leg articulation mechanism and involved components. (a–d) Locked femorotibial joint and locking apparatus: (a) darkfield micro image of locked femorotibial joint with labelled tibial flexor sclerite (TFS), genuflexor apodeme (GFS) and Heitler’s lump (HL); (c) bright-field micro image of same structure; (b) bright-field micro image of locking apparatus, lateral view with labelled tibial extensor muscles and Heitler’s lump, and flexor sclerite separated from Heitler’s lump; (d) bright-field micro image of same structure. (e–f) locking apparatus in ventro- and dorso-lateral views; (g–j) femorotibial joint in flexed position (g, i) with tibial flexor sclerite (TFS) and Heitler’s lump in physical contact, and in straight position (h, j) with tibial flexor sclerite (TFS) and Heitler’s lump separated.
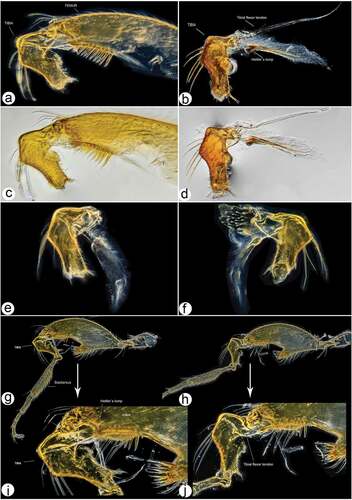
The enlarged tibial flexor apodeme, presence of robust teeth on the ventral surface, and thickened femur having an enlarged tibial flexor muscle mass, indicates its usage in grasping/holding; this is confirmed by our behavioural observations in the field. The dark-field microphotographs of the femorotibial joint in the male hind leg also reveal a unique locking mechanism. It allows the leg to remain in a flexed position for an extended period of time; this is perhaps an efficient energy-saving feature useful during mating and for prolonged holding of the female. The occurrence of similar structural features and their diverse implicational usage in insect legs are detailed by Földvári et al. (Citation2019). Of the three stated universal locking mechanisms in insect legs, the flexor sclerite lump-engaging mechanism (Type 1) explains best the femorotibial joint articulation of the new species. The locking apparatus consists of an enlarged tibial flexor muscle, relatively reduced tibial extensor muscles, tibial flexor sclerite (TFS), genuflexor apodeme (GFS) and Heitler’s lump (HL) (). The lock is present between the tibial flexor sclerite and Heitler’s lump. In the locked state (), the convex transparent ventral surface of the tibial flexor sclerite remains in physical contact with Heitler’s lump (); thereafter the leg can only be opened after forcing the tibial flexor sclerite over and in front of Heitler’s lump (). Our laboratory observations and the field observations lead us to postulate that the apparent male hind leg deformation is a secondary sexual character employed to exhibit territoriality and for attracting females. We have also observed the presence of a single campaniform sensillum on the proximal part of the hind leg femur, the presence of which is also supportive of enhanced muscle synergies in substrate grip, similar to that discussed in other studies (Zill et al. Citation2017).
3.4. Courtship rituals ()
The courtship behaviour of the new species illustrated here in brief is somewhat similar to that discussed for a recently described sympatric species in Barták et al. (Citation2021). However, capturing photographic evidence of dance fly behaviour is challenging, as these are fast-moving insects and difficult to focus on, and the interpretation of intriguing aerial images is at times quite difficult. As in the new species of Barták et al. (Citation2021), and some of the other previously reported species (Downes Citation1970), males raid and prey on small insects and form aerial swarms to display their catch to nearby females in “aerial holding flights”. Such mating swarms often use visual environmental markers and always appear at those certain stationary positions. Once the female accepts the gift and commits to mating, the tangled couple moves at a slower pace and drops down towards the nearby vegetation, where they remain until copulation is completed.
Figure 15. Courtship rituals of Rhamphomyia aquila sp. nov. (a–h) male aerial flight postures displaying territoriality; (i–k) copula with male hind leg usage to detain wilting female; (l) male with prey; (m) male and female in courtship.
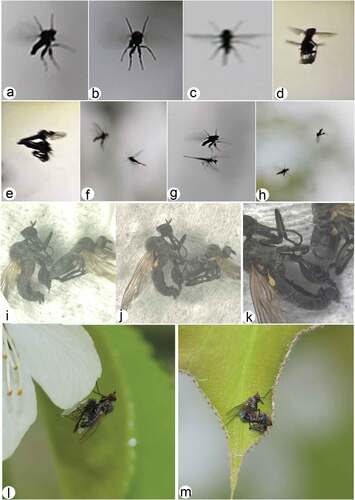
Descriptive analysis of observed courtship characters (such as lek height, copulation duration, etc.) mainly depend on the environment and are more or less the same as stated in Barták et al. (Citation2021). Some significant differences do occur among the two species, however. The new species exhibits prey polymorphism, preying on a number of insect species such as psyllids, small flies, etc. Prey polymorphism was not reported in R. bhagati, which preyed only on a specific chironomid species. In both species chironomids are mostly preferred, but the new species preys on relatively larger chironomid species compared with R. bhagati which preyed on a smaller and different chironomid species. The new species is more frequent in occurrence compared with the other species, exhibits strong territoriality and often displays offensive aerial postures (). The modified hind legs are displayed and used as specialised structures for holding females in male bias mating swarms (). At places with similar lekking sites, the males of the two species are observed to encounter each other. The males of the new species chase off males of the other species during such encounters and retain the area (). The distinct trait morphology of the new species is certainly associated with mating success and prolonged copula duration (). We are currently quantifying more precisely the role of legs as specialised structures in aerial display signalling, and their selective advantage. The deformed legs have given a selective advantage to the species and have favoured their predominance in the region.
4. Discussion
Males have a much larger potential benefit from engaging in copulation (Parker Citation1979). Selection pressure leads males towards mating, while females shy away. This pressure on occasion leads to the development of male-specific morphological characters for coercing unwilling females to mate (Bergsten Citation2005). We observed that the deformed male hind legs are used to take hold of the female abdomen for a proper genital union and to detain an initially wilting female until the ejaculate is transferred (). In future research we will generate predictions based on these functions and test whether these predictions are consistent with observations. Among possible male phenotypes the degree of leg deformation should covary with the duration of copulation and vice versa. Our discovery adds to the list of already apparently deceptive traits in empidid flies and suggests them as a potential model to evaluate inconclusive empirical evidence about sexual selection and secondary sexual traits as drivers for speciation.
The external as well as the internal genital tract in Diptera is generally poorly known, with only a few detailed comparative studies available (Sinclair et al. Citation2007; Puniamoorthy et al. Citation2010). The same is true in the family Empididae, where apart from Smith (Citation1969) nothing much can be cited. In addition to the external male and female genital tract we herewith have detailed the internal genital system, which perhaps represents the first such detail for the genus as well. Previously works of Loew (Citation1841), Dufour (Citation1851), Keuchenius (Citation1913) and Smith (Citation1969) are rather brief without labels or proper descriptions. The spermatheca is here illustrated in greater detail for the family Empididae for the first time, although it was previously reported and illustrated for other insect orders and various families within the order Diptera (Pascini & Martins Citation2017). Whether the structure in this species aids post-copulatory sperm competition remains a fascinating topic to be explored. Not only do the details of the internal reproductive tract have taxonomic significance, but its functional advantages vary among species. It is postulated that the length of the sperm duct between the sperm pump and opening roughly corresponds to the length of the male phallus (Barták, unpublished data). By clasping copulating pairs quickly by hand, and subsequently dissecting them, it can be observed that the whole length of the phallus, even if it is very long (as in Rhamphomyia (Holoclera) nigripennis group), is inserted into the female.
5. Checklist of Indian Empididae
An updated checklist of the family Empididae (Diptera) of India is presented. It includes 55 species belonging to 13 genera. The checklist is primarily based on the available literature with all the names of described species being presented in accordance with the most recent classification. Notes about type localities, depositories, and relevant references to each species record are given. The list provides a synthesis of the regional taxonomical work carried out to date and will serve as a baseline for future studies. Within the Indian Empididae, the genus Chelifera is the most species rich, represented by 10 species, followed by Clinocera (nine), Empis (eight), Hemerodromia (six) and Rhamphomyia (four). The genera Heleodromia, Hilara, and Roederiodes are each represented by three species, while Trichoclinocera and Chelipoda are each represented by two species and the genera Trichopeza and Wiedemannia are each represented by a single species. For the genus Rhamphomyia we had previously stated the presence of four species – R. bhagati Barták, Akbar, Kanturski, Wachkoo and Maqbool, Citation2021, R. griseonigra Brunetti, Citation1913, R. himalayana Brunetti, Citation1913 and R. unifasciata Brunetti, Citation1913 – from the region (Barták et al. Citation2021). However, R. unifasciata is now treated as Ocydromia unifasciata (Brunetti, Citation1913), a member of Hybotidae, and is therefore excluded from the list. As such, the genus in India is represented by three previously described species only. The new species described here represents the fourth species belonging to the genus Rhamphomyia to be reported from India. The classification of Empidoidea suggested by Wahlberg and Johanson (Citation2018) is followed here. According to their study, the family Empididae globally comprises 3200 known species, and more than 80 genera, in three subfamilies (Brachystomatinae, Clinocerinae and Empidinae).
Family Empididae Latreille, 1804
Subfamily Brachystomatinae Melander, 1908
Tribe Trichopezini Vaillant, 1981
Genus Heleodromia Haliday, 1833
Heleodromia baculifera Tokarczyk and Kovalev, Citation1986
Heleodromia baculifera Tokarczyk and Kovalev, Citation1986: 56. Holotype ♂, Lahaul, Pancha [Panch] Nala Valley Himachal Pradesh, India [PAN].
Distribution
India (Himachal Pradesh) (Tokarczyk & Kovalev Citation1986: 58; Yang et al. Citation2007: 451).
Heleodromia obscura (Brunetti, Citation1913)
Clinocera obscura Brunetti, Citation1913: 34. Syntypes 2 ♂♂, Shimla [Simla], Himachal Pradesh, India [ZSI].
Distribution
India (Himachal Pradesh) (Brunetti Citation1913: 34, Citation1917: 80, Citation1920: 366; Yang et al. Citation2007: 452).
Heleodromia rami Wagner and Leese, 2004
Heleodromia rami Wagner and Leese in Wagner et al. Citation2004: 15. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 15; Yang et al. Citation2007: 452).
Genus Trichopeza Róndani, 1856
Trichopeza fusca Brunetti, Citation1913
Trichopeza fusca Brunetti, Citation1913: 31. Holotype ♀, Kurseong, Darjeeling [Darjiling], West Bengal, India [ZSI].
Distribution
India (West Bengal) (Brunetti Citation1913: 32, Citation1920: 373; Yang et al. Citation2007: 457).
Subfamily Clinocerinae Collin, 1928
Genus Clinocera Meigen, 1803
Clinocera cuspidata Wagner and Leese, 2004
Clinocera cuspidata Wagner and Leese in Wagner et al. Citation2004: 16. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 16; Yang et al. Citation2007: 55).
Clinocera longicercus Wagner and Leese, 2004
Clinocera longicercus Wagner and Leese in Wagner et al. Citation2004: 16. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 16; Yang et al. Citation2007: 56).
Clinocera lunata Wagner and Leese, 2004
Clinocera lunata Wagner and Leese in Wagner et al. Citation2004: 18. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 18; Yang et al. Citation2007: 56).
Clinocera lunatoides Wagner and Leese, 2004
Clinocera lunatoides Wagner and Leese in Wagner et al. Citation2004: 18. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 18; Yang et al. Citation2007: 56).
Clinocera marginesetosa Wagner and Leese, 2004
Clinocera marginesetosa Wagner and Leese in Wagner et al. Citation2004: 16. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 18; Yang et al. Citation2007: 56).
Clinocera minutissima (Vaillant, Citation1960)
Atalanta minutissima Vaillant, Citation1960: 172. Lectotype ♂, Khorogon Gunt River, Tajikistan [MZLS].
Distribution
India (Himachal Pradesh), Tajikistan (Vaillant Citation1960: 172; Wagner et al. Citation2004: 17; Yang et al. Citation2007: 56; Sinclair & Shamshev Citation2020: 267).
Clinocera setosa Wagner and Leese, 2004
Clinocera setose Wagner and Leese in Wagner et al. Citation2004: 18. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 18; Yang et al. Citation2007: 57).
Clinocera sinclairi Wagner and Leese, 2004
Clinocera sinclairi Wagner and Leese in Wagner et al. Citation2004: 19. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 19; Yang et al. Citation2007: 57).
Clinocera stackelbergi (Vaillant, Citation1960)
Atalanta stackelbergi Vaillant, Citation1960: 174. Lectotype ♂, Khorogon Gunt River, Tajikistan [ZIN].
Distribution. India (Himachal Pradesh), Tajikistan (Vaillant Citation1960: 174; Wagner et al. Citation2004: 18; Yang et al. Citation2007: 58; Sinclair & Shamshev Citation2020: 268).
Genus Dolichocephala Macquart, 1823
Dolichocephala flamingo Smith, Citation1965
Dolichocephala flamingo Smith, Citation1965: 98. Holotype ♂, Tumlingtar, Arun Valley, Nepal [NHMUK].
Dolichocephala tibetensis Liu, Tang and Yang, Citation2014: 1023. Holotype ♂, Bomi, Galonglashan, Tibet [CAU].
Distribution
China, India (Himachal Pradesh), Nepal (Smith Citation1965: 99; Wagner et al. Citation2004: 22; Yang et al. Citation2007: 60; Liu et al. Citation2014: 1024; Sinclair Citation2017: 291).
Dolichocephala panesari Wagner and Leese, 2004
Dolichocephala panesari Wagner and Leese inWagner et al. Citation2004: 20. Holotype ♂, Nishala [Nishalla] Nalla, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 20; Yang et al. Citation2007: 62).
Dolichocephala rotundinota Wagner and Leese, 2004
Dolichocephala rotundinota Wagner and Leese in Wagner et al. Citation2004: 20. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 20; Yang et al. Citation2007: 62).
Dolichocephala septemnotata Brunetti, Citation1913
Dolichocephala septemnotata Brunetti, Citation1913: 35. Holotype ♂, Shimla [Simla], Himachal Pradesh, India [ZSI].
Distribution
India (Himachal Pradesh) (Brunetti Citation1913: 36, Citation1917: 81, Citation1920: 371; Yang et al. Citation2007: 62).
Genus Roederiodes Coquillett, 1901
Roederiodes bilobatus Wagner and Leese, 2004
Roederiodes bilobatus Wagner and Leese in Wagner et al. Citation2004: 22. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 22; Yang et al. Citation2007: 69).
Roederiodes naggarense Wagner and Leese, 2004
Roederiodes naggarense Wagner and Leese in Wagner et al. Citation2004: 22. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 22; Yang et al. Citation2007: 70).
Roederiodes schwoerbeli Wagner and Leese, 2004
Roederiodes schwoerbeli Wagner and Leese in Wagner et al. Citation2004: 22. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 22; Yang et al. Citation2007: 70).
Genus Trichoclinocera Collin, 1941
Trichoclinocera fluviatilis (Brunetti, Citation1913)
Clinocera fluviatilis Brunetti, Citation1913: 34. Holotype ♂, Bhowali, Uttarakhand, India [ZSI].
Distribution
India (Himachal Pradesh, Uttarakhand) (Brunetti Citation1913: 35, Citation1920: 366; Sinclair Citation1994: 1016; Wagner et al. Citation2004: 25; Sinclair & Saigusa Citation2005: 199; Yang et al. Citation2007: 71).
Trichoclinocera serrata Wagner and Leese, 2004
Trichoclinocera serrata Wagner and Leese in Wagner et al. Citation2004: 25. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 25; Yang et al. Citation2007: 72).
Genus Wiedemannia Zetterstedt, 1838
Wiedemannia glaucescens (Brunetti, Citation1917)
Clinocera glaucescens Brunetti, Citation1917: 80. Syntypes 2 ♀♀, Phagu, Himachal Pradesh, India [ZSI].
Acanthoclinocera saigusai Smith, Citation1965: 102. Holotype ♂, Dobhan, Taplejung, Nepal [NHMUK].
Wiedemannia tibetensis Wang, Wang and Yang, Citation2015: 45. Holotype ♂, Nyingchi, Sejilashan, Tibet [CAU].
Distribution
China, India (Himachal Pradesh), Nepal (Brunetti Citation1917: 81; Smith Citation1965: 103; Wagner et al. Citation2004: 17; Yang et al. Citation2007: 77; Wang et al. Citation2015: 46; Sinclair Citation2017: 291).
Subfamily Empidinae Latreille, 1809
Tribe Chelipodini Hendel, 1936
Genus Chelipoda Macquart, 1823
Chelipoda flavida Brunetti, Citation1913
Chelipoda flavida Brunetti, Citation1913: 32. Syntypes ♂, ♀, Chota Nagpur, Jharkhand; Darjeeling [Darjiling], Paresnath, West Bengal, India; Dawna Hills, Myanmar [ZSI].
Distribution
India (Jharkhand, West Bengal), Myanmar, Thailand (Brunetti Citation1913: 33, Citation1920: 369; Plant Citation2009: 257; Yang et al. Citation2007: 252).
Chelipoda indica (Brunetti, Citation1913)
Litanomyia indica Brunetti, Citation1913: 36. Holotype ♀, Darjeeling [Darjiling], West Bengal, India [ZSI].
Distribution
India (West Bengal) (Brunetti Citation1913: 36, Citation1920: 370; Yang et al. Citation2007: 253).
Tribe Empidini Collin Citation1961
Genus Empis Linnaeus, 1758
Empis amplitarsis Brunetti, Citation1920
Empis amplitarsis Brunetti, Citation1920: 349. Holotype ♂, Belgaum, Karnataka, India [NHMUK].
Distribution
India (Karnataka) (Brunetti Citation1920: 350; Yang et al. Citation2007: 130).
Empis centralis Brunetti, Citation1913
Empis centralis Brunetti, Citation1913: 26. Holotype ♀, Mundali, Uttar Pradesh, India [ZSI].
Distribution. India (Uttar Pradesh) (Brunetti Citation1913: 27, Citation1920: 354; Yang et al. Citation2007: 132).
Empis elegans Brunetti, Citation1913
Empis elegans Brunetti, Citation1913: 26. Syntypes 2 ♀♀, Mundali, Uttar Pradesh, India [ZSI].
Distribution
India (Uttar Pradesh) (Brunetti Citation1913: 26, Citation1920: 357; Yang et al. Citation2007: 133).
Empis griseonigra Brunetti, Citation1913
Empis griseonigra Brunetti, Citation1913: 25. Holotype ♂, Mundali, Uttar Pradesh, India [ZSI].
Distribution
India (Uttar Pradesh) (Brunetti Citation1913: 26, Citation1920: 353; Yang et al. Citation2007: 134).
Empis inconspicua Brunetti, Citation1913
Empis inconspicua Brunetti, Citation1913: 28. Syntypes 2 ♂♂, Lucknow, Uttar Pradesh, India [ZSI].
Distribution
India (Uttar Pradesh) (Brunetti Citation1913: 28, Citation1920: 357; Yang et al. Citation2007: 135).
Empis marginata Brunetti, Citation1917
Pachymeria marginata Brunetti, Citation1917: 79. Holotype ♀, Between Kufri and Phagu, Himachal Pradesh, India [ZSI].
Distribution
India (Himachal Pradesh) (Brunetti Citation1917: 80, Citation1920: 356; Yang et al. Citation2007: 136).
Empis rostrata Brunetti, Citation1913
Empis rostrata Brunetti, Citation1913: 25. Holotype ♀, Theog, Shimla [Simla], Himachal Pradesh, India [ZSI].
Distribution
India (Himachal Pradesh) (Brunetti Citation1913: 25, Citation1917: 79, Citation1920: 356; Yang et al. Citation2007: 140).
Empis subcilipes Brunetti, Citation1913
Empis subcilipes Brunetti, Citation1913: 24. Holotype ♀, Mundali, Uttar Pradesh, India [ZSI].
Distribution
India (Uttar Pradesh) (Brunetti Citation1913: 25, Citation1920: 355; Yang et al. Citation2007: 141).
Genus Rhamphomyia Meigen, 1822
Rhamphomyia bhagati Barták, Akbar, Kanturski, Wachkoo and Maqbool, Citation2021
Rhamphomyia (Pararhamphomyia) bhagati Barták, Akbar, Kanturski, Wachkoo and Maqbool, Citation2021: 70. Holotype ♂, Central Institute of Temperate Horticulture, Srinagar, Jammu and Kashmir [CITH].
Distribution
India (Jammu and Kashmir) (Barták et al. Citation2021: 72).
Rhamphomyia griseonigra Brunetti, Citation1913
Rhamphomyia griseonigra Brunetti, Citation1913: 29. Holotype ♂, Mundali, Uttar Pradesh, India [ZSI].
Distribution
India (Uttar Pradesh) (Brunetti Citation1913: 30, Citation1920: 353; Yang et al. Citation2007: 183).
Rhamphomyia himalayana Brunetti, Citation1913
Rhamphomyia himalayana Brunetti, Citation1913: 28. Holotype ♀, Matiana, Himachal Pradesh, India [ZSI].
Distribution
India (Himachal Pradesh) (Brunetti Citation1913: 29, Citation1917: 80, Citation1920: 346; Yang et al. Citation2007: 183).
Tribe Hemerodromiini Schiner, 1862
Genus Chelifera Macquart, 1823
Chelifera accomodata Wagner and Leese, 2004
Chelifera accomodata Wagner and Leese in Wagner et al. Citation2004: 10. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 10; Yang et al. Citation2007: 259).
Chelifera brevidigitata Wagner and Leese, 2004
Chelifera brevidigitata Wagner and Leese in Wagner et al. Citation2004: 10. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 10; Yang et al. Citation2007: 259).
Chelifera curvata Wagner and Leese, 2004
Chelifera curvata Wagner and Leese in Wagner et al. Citation2004: 7. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 7; Yang et al. Citation2007: 260).
Chelifera digitata Wagner and Leese, 2004
Chelifera digitata Wagner and Leese in Wagner et al. Citation2004: 10. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 10; Yang et al. Citation2007: 260).
Chelifera haeselbarthae Wagner and Leese, 2004
Chelifera haeselbarthae Wagner and Leese in Wagner et al. Citation2004: 11. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 10; Yang et al. Citation2007: 261).
Chelifera insueta Wagner and Leese, 2004
Chelifera insueta Wagner and Leese in Wagner et al. Citation2004: 5. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 5; Yang et al. Citation2007: 261).
Chelifera multiseta Wagner and Leese, 2004
Chelifera multiseta Wagner and Leese in Wagner et al. Citation2004: 6. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 6; Yang et al. Citation2007: 261).
Chelifera multisetoides Wagner and Leese, 2004
Chelifera multisetoides Wagner and Leese in Wagner et al. Citation2004: 7. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 7; Yang et al. Citation2007: 261).
Chelifera rhombicercus Wagner and Leese, 2004
Chelifera rhombicercus Wagner and Leese in Wagner et al. Citation2004: 7. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 7; Yang et al. Citation2007: 263).
Chelifera stauderae Wagner and Leese, 2004
Chelifera stauderae Wagner and Leese in Wagner et al. Citation2004: 8. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 8; Yang et al. Citation2007: 263).
Genus Hemerodromia Meigen, 1822
Hemerodromia acutata Grootaert, Yang and Saigusa, Citation2000
Hemerodromia acutata Grootaert, Yang and Saigusa, Citation2000: 73. Holotype ♂, Menglun, Xishuangbanna, China [Holotype, ♂ CAU].
Distribution
China, India (Himachal Pradesh), Thailand (Grootaert et al. Citation2000: 75; Wagner et al. Citation2004: 11; Plant Citation2015: 7; Yang et al. Citation2007: 267).
Hemerodromia chitaoides Wagner and Leese, 2004
Hemerodromia chitaoides Wagner and Leese in Wagner et al. Citation2004: 13. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 13; Yang et al. Citation2007: 268).
Hemerodromia dorsalis (Brunetti, Citation1913)
Chelipoda dorsalis Brunetti, Citation1913: 33. Syntypes ♂, ♀, Barog [Barogh], Himachal Pradesh, India [ZSI].
Distribution
India (Himachal Pradesh) (Brunetti Citation1913: 34, Citation1917: 81, Citation1920: 367; Yang et al. Citation2007: 269).
Hemerodromia elongatiodes Wagner and Leese, 2004
Hemerodromia elongatiodes Wagner and Leese in Wagner et al. Citation2004: 13. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 13; Yang et al. Citation2007: 269).
Hemerodromia rhomboides Wagner and Leese, 2004
Hemerodromia rhomboides Wagner and Leese in Wagner et al. Citation2004: 13. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 13; Yang et al. Citation2007: 273).
Hemerodromia subspinosa Yang, Zhang and Zhang, 2007
Hemerodromia spinosa Wagner and Leese, 2004: 13. Holotype ♂, Kullu Valley, Himachal Pradesh, India [ZFMK].
Distribution
India (Himachal Pradesh) (Wagner et al. Citation2004: 13; Yang et al. Citation2007: 274).
Tribe Hilarini Collin Citation1961
Genus Hilara Meigen, 1822
Hilara bares Walker, Citation1849
Hilara bares Walker, Citation1849: 491. Holotype ♂, India [East India] [NHMUK].
Distribution
India (Walker Citation1849: 491; Brunetti Citation1920: 359; Yang et al. Citation2007: 211).
Hilara compacta Brunetti, Citation1913
Hilara compacta Brunetti, Citation1913: 30. Syntypes, 3 ♂♂, Shimla [Simla], Himachal Pradesh, India [ZSI].
Distribution
India (Himachal Pradesh) (Brunetti Citation1913: 31, Citation1917: 80, Citation1920: 359; Yang et al. Citation2007: 214).
Hilara rufithorax (Brunetti, Citation1913)
Empimorpha rufithorax Brunetti, Citation1913: 30. Holotype ♂, Darjeeling [Darjiling], West Bengal, India [ZSI].
Distribution
India (West Bengal) (Brunetti Citation1913: 30, Citation1920: 360; Yang et al. Citation2007: 232).
Supplemental Material
Download MP4 Video (23.3 MB)Acknowledgements
The first author thanks the Department of Science and Technology (DST), Govt. of India, New Delhi, for research encouragement and financial assistance rendered via the N-PDF Fellowship programme, File No: PDF/2015/000866. The authors also thank Darryl Gwynne (University of Toronto, Mississauga, Canada) and Luc Bussière (Biological and Environmental Sciences, University of Stirling, United Kingdom) for their valuable suggestions. We are highly thankful to Adrian Plant (Mahasarakham University, Thailand) and Paul Beuk (Natuurhistorisch Museum Maastricht) for their help with the literature. We also thank the reviewers for immensely improving the manuscript. Sincere thanks are due to the director of CITH for making facilities available.
Disclosure statement
No potential conflict of interest was reported by the authos.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2022.2139864
Additional information
Funding
References
- Barták M. 1981. A revision of the Rhamphomyia albosegmentata-group (Diptera, Empididae), with descriptions of new species. Acta Universitatis Carolinae – Biologica 1979:361–407.
- Barták M. 1982. The Czechoslovak species of Rhamphomyia (Diptera, Empididae), with description of a new species from Central Europe. Acta Universitatis Carolinae – Biologica 1980:381–461.
- Barták M. 2000. Rhamphomyia species (Diptera: Empididae) of Middle Asia. Acta Universitatis Carolinae, Biologica 44:111–131.
- Barták M, Akbar SA, Kanturski M, Wachkoo AA, Maqbool A. 2021. SEM morphology and courtship rituals of a new species of Rhamphomyia (Diptera: Empididae: Empidinae) from the Kashmir Himalayas (India). Bonn Zoological Bulletin 70(1):67–84. DOI: 10.20363/BZB-2021.70.1.067.
- Barták M, Kubík Š. 2009. Two new East Palaearctic Rhamphomyia (Pararhamphomyia) (Diptera: Empididae). Entomological News 120(1):76–86. DOI: 10.3157/021.120.0114.
- Barták M, Sinclair BJ. 2003. Case 3269. Rhamphomyia (Rhamphomyia) Meigen, 1822 and Rhamphomyia (Pararhamphomyia) Frey, 1922 (Insecta: Diptera): Proposed conservation of usage of the subgeneric names by designation of Empis sulcata Meigen, 1804 as the type species of Rhamphomyia. Bulletin of Zoological Nomenclature 60(3):203–205.
- Bergsten J. 2005. Taxonomy, phylogeny, and secondary sexual character evolution of diving beetles, focusing on the genus Acilius. Umeå: Department of Ecology and Environmental Science, Umeå University. pp. 36.
- Brunetti E. 1913. New Indian Empidae. Records of Indian Museum 9:11–45.
- Brunetti E. 1917. Diptera of the Simla district. Records of Indian Museum 13:59–101.
- Brunetti E. 1920. Diptera Brachycera. The Fauna of British India, including Ceylon and Burma.Vol. 1. London: Taylor and Francis.
- Chillcott JG. 1959. Studies on the genus Rhamphomyia Meigen: A revision of the Nearctic species of the basalis group of the subgenus Pararhamphomyia Frey (Diptera: Empididae). The Canadian Entomologist 91(5):257–275. DOI: 10.4039/Ent91257-5.
- Chvála M, Pont AC. 2015. Revision of the European Empis (s. str.) alpicola group of species (Diptera: Empididae), with a new synonymy of Rhamphomyia subgenus Aclonempis Collin with the subgenus Empis Linnaeus s. str. Studia Dipterologica 21(1):53–68.
- Chvála M, Wagner R. 1989. Empididae. In: Soós Á, Papp L, editors. Catalogue of Palaearctic Diptera, Therevidae – Empididae. Vol. 6. Amsterdam & Budapest: Elsevier Science Publishers & Akadémiai Kiadó. pp. 228–336.
- Collin JE. 1961. Empididae. In: ‘British Flies. Vol. VI. Cambridge University Press: Cambridge, UK., pp. 782.
- Daugeron C, Plant A, Winkler I, Stark A, Baylac M. 2010. Extreme male leg polymorphic asymmetry in a new empidine dance fly (Diptera: Empididae). Biology Letters 7:11–14. DOI: 10.1098/rsbl.2010.0726.
- Downes JA. 1970. The feeding and mating behaviour of the specialized Empidinae (Diptera); observations on four species of Rhamphomyia in the high Arctic and a general discussion. The Canadian Entomologist 102:769–791. DOI: 10.4039/Ent102769-7.
- Dufour L. 1851. Recherches anatomiques et physiologiques sur les Diptères. Mémoires présentés par divers savants à l’Académie des Sciences de l’Institut de France et imprimés par son ordre: Sciences mathématiques et physiques. Paris 11: Academie Des Sciences. pp. 171–360.
- Földvári M, Mikó I, Ulmer JM, Dos Santos Rolo T, Csősz S, Pomiankowski A, Baumbach T, van de Kamp T. 2019. Jumping and Grasping: Universal Locking Mechanisms in Insect Legs. Insect Systematics and Diversity 3(6):1–16. DOI: 10.1093/isd/ixz018.
- Frey R. 1909. Mitteilungenu¨ber Finnala¨ndische Dipteren. Aca Societatis pro Fauna et Flora Fennica 31:1–23.
- Frey R. 1922. Vorarbeitenzueiner Monogrphie der Gattung Rhamphomyia Meig. (Dipt., Empididae). Notulae Entomologicae2 110:33–45, 65–77.
- Grootaert P, Yang D, Saigusa T. 2000. Empididae (Diptera: Empidoidea) from Xishuangbanna, Yunnan (I): Hemerodromiinae. Bulletin de L’Institute Royal Des Sciences Naturelles de Belgique 70:71–80.
- Keuchenius PE. 1913. The structure of the internal genitalia of some male Diptera. Zeitschriftfür Wissenschaftliche Zoologie 105:501–536.
- Klass KD, Matushkina NA. 2012. The exoskeleton of the female genitalic region in Petrobiellustakunagae (Insecta: Archaeognatha): Insect wide terminology, homologies, and functional interpretations. Arthropod Structure & Development 41:575–591. DOI: 10.1016/j.asd.2012.06.003.
- Liu X, Tang C, Yang D. 2014. Dolichocephala (Diptera: Empididae) newly found in Tibet with description of a new species. Florida Entomologist 97(3):1021–1025. DOI: 10.1653/024.097.0303.
- Loew H. 1841. Horae Anatomicae, Beiträge zur Genaueren Antomischen Kenntniß der Evertebraten. Abteilung I. Entomotomien. Heine: Posen.
- Parker GA. 1979. Sexual selection and sexual conflict. In: Blum MS, N.a B, editors. Sexual selection and sexual conflict. New York: Academic Press. pp. 123–166.
- Pascini TV, Martins GF. 2017. The insect spermatheca: An overview. Zoology (Jena) 121:56–71. DOI: 10.1016/j.zool.2016.12.001.
- Plant AR. 2009. Diversity of Chelipoda Macquart, 1823 (Diptera: Empididae:Hemerodromiinae) in northern Thailand with discussion of a biodiversity ‘hot-spot’ at Doi Inthanon. Raffles Bulletin of Zoology 57:255–277. DOI: 10.5281/zenodo.5342006.
- Plant AR. 2015. Diversity of Hemerodromia Meigen, 1822 (Diptera: Empididae) in Thailand, the tip of a tropical iceberg? Zootaxa 4039(1):1–56. DOI: 10.11646/zootaxa.4039.1.1.
- Puniamoorthy N, Kotrba M, Meier R. 2010. Unlocking the “Black box”: Internal female genitalia in Sepsidae (Diptera) evolve fast and are species-specific. BMC Evolutionary Biology 10:275. DOI: 10.1186/1471-2148-10-275.
- Rhodén C, Wahlberg E. 2020. The phylogeny of Empis and Rhamphomyia (Diptera, Empididae) investigated using UCEs including an over 150 years old museum specimen. Evolutionary Systematics 4:21–33. DOI: 10.3897/evolsyst.4.49537.
- Ronquist F, Nordlander G. 1989. Skeletal morphology of an archaic cynipoid, Ibalia rufipes (Hymenoptera: Ibaliidae). Scandinavian Society of Entomology 33:1–60.
- Saigusa T. 2012. A new Asio-Nearctic subgenus of Rhamphomyia (Diptera: Empididae:Empidinae). The Canadian Entomologist 144(2):291–322. DOI: 10.4039/tce.2012.28.
- Sinclair BJ. 1994. Revision of the Nearctic species of Trichoclinocera Collin (Diptera: Empididae; Clinocerinae). The Canadian Entomologist 126:1007–1059. DOI: 10.4039/Ent1261007-4.
- Sinclair BJ. 2017. Two new synonyms from Tibet (Diptera: Empididae: Clinocerinae). Zootaxa 4268(2):291–292. DOI: 10.11646/zootaxa.4268.2.8.
- Sinclair BJ, Borkent A, Wood DM. 2007. The male genital tract and aedeagal components of the Diptera with a discussion of their phylogenetic significance. Zoological Journal of the Linnean Society 150:711–742. DOI: 10.1111/j.1096-3642.2007.00314.x.
- Sinclair BJ, Brooks SE, Cumming JM. 2013. Revision of the Empis subgenus Enoplempis Bigot, east of the Rocky Mountains (Diptera: Empididae). Zootaxa 3736(5):401–456. DOI: 10.11646/zootaxa.3736.5.1.
- Sinclair BJ, Cumming JM. 2006. The morphology, higher-level phylogeny and classification of the Empidoidea (Diptera). Zootaxa 1180:1–172. DOI: 10.11646/zootaxa.1180.1.1.
- Sinclair BJ, Saigusa T. 2005. Revision of the Trichoclinocera dasyscutellum group from East Asia (Diptera:Empididae: Clinocerinae). Bonner zoologische Beiträge 53 (2004)(1–2):193–209.
- Sinclair BJ, Saigusa T. 2018. Revision of Francis Walker’s female types of North American Rhamphomyia Meigen (Diptera, Empididae). Bonn Zoological Bulletin 67(2):129–143. DOI: 10.20363/bzb-2018.67.2.129.
- Sinclair BJ, Shamshev IV GJL. 2020. Revision of the aquatic dance flies (Diptera:Empididae: Clinocerinae) described by F. Vaillant in two 1960 publications. Bonn Zoological Bulletin 69(2):263–274. DOI: 10.20363/BZB-2020.69.2.263.
- Sinclair BJ, Vajda ÉA, Saigusa T, Shamshev IV, Wheeler TA. 2019. Rhamphomyia Meigen of the Canadian Arctic Archipelago, Greenland and Iceland (Diptera:Empididae). Zootaxa 4670(1):001–094. DOI: 10.11646/zootaxa.4670.1.1.
- Smith KGV. 1965. Diptera from Nepal Empididae. Bulletin of the British Museum (Natural History. Entomology 17(2):63–112. DOI: 10.5962/bhl.part.14808.
- Smith KGV. 1969. The Empididae of Southern Africa (Diptera). Annals of the Natal Museum 19:1–342.
- Tokarczyk G, Kovalev VG. 1986. Heleodromia baculifera sp. nov. (Insecta: Diptera:Empididae) from Lahaul Himalaya in India. Zoology (Journal of Pure and Applied Zoology) 1:55–59.
- Vaillant F. 1960. Quelques EmpididaeAtalantinaed’Asie russe [Dipt.]. Bulletin de la Société entomologique de France 65:170–186. DOI: 10.3406/bsef.1960.20543.
- Wagner R, Leese F, Panesar AR. 2004. Aquatic dance flies from a small Himalayan mountain stream (Diptera: Empididae: Hemerodromiinae, Trichopezinae and Clinocerinae). Bonner zoologische Beiträge 52(1–2):3–32.
- Wahlberg E, Johanson KA. 2018. Molecular phylogenetics reveals novel relationships within Empidoidea (Diptera). Systematic Entomology 43(4):619–636. DOI: 10.1111/syen.12297.
- Walker F. 1849. List of the Specimens of Dipterous Insects in the Collection of the British Museum. London: Part III. Printed by order of the Trustees [British Museum]. pp. 485–687.
- Wang N, Wang B, Yang D. 2015. Wiedemannia (Diptera: Empididae) newly found in China with description of a new species from Tibet. Florida Entomologist 98(1):44–46. DOI: 10.1653/024.098.0108.
- Yang D, Zhang K, Yao G, Zhang J. 2007. World catalog of Empididae (Insecta: Diptera). Beijing: China Agricultural University Press. pp. 599.
- Zill SN, Neff D, Chaudhry S, Exter A, Schmitz J, Büschges A. 2017. Effects of force detecting sense organs on muscle synergies are correlated with their response properties. Arthropod Structure & Development 46:564–578. DOI: 10.1016/j.asd.2017.05.004.
