Abstract
Here, we present a revision of the poorly studied aphid genus, Sinolachnus from the tribe Tuberolachnini of the subfamily Lachninae (Hemiptera: Aphididae) modified to include eight species. Apterous and alate viviparous females of the type species S. niitakayamensis are redescribed together with alate viviparous females of Sinolachnus elaeagnensis. Alate viviparous females of a new species Sinolachnus takahashii sp. nov. and apterous and alate viviparous females of Sinolachnus yushanensis sp. nov. associated with Elaeagnus oldhamii, from Taiwan are described. Sinolachnus is, for the first time, recorded from Japan and the alate viviparous female of Sinolachnus nipponicus sp. nov. is described. Additionally, we recognize two other species of the Lachninae as members of this genus: Sinolachnus plurisensoriatus (Zhang, Citation1988) comb. nov. (= Cinara plurisensoriata Zhang, Citation1988) from China, and Sinolachnus rubi (Ghosh & Raychaudhuri, 1972) comb. nov. (= Maculolachnus rubi Ghosh & Raychaudhuri, 1972) from India. We have designated the lectotype for Sinolachnus niitakayamensis. For the first time, we have provided scanning electron microscopy analyses to show the morphology and sensilla of this genus. Following comparative analyses with other Tuberolachnini and Lachninae genera, we propose to transfer the genus Sinolachnus from the tribe Tuberolachnini to the Tramini.
http://urn:lsid:zoobank.org:pub:ED1A226C-6DEA-4F17-9669-1D2E33E86566
1. Introduction
Owing to their complicated morphology, polymorphism and life cycles, aphids are fascinating insects. They play an important role in ecosystems and as plant pests and viruses vectors, making them one of the most economically significant groups. Members of the subfamily Lachninae are one of the most interesting groups, not only within Aphididae but among insects generally. Members of the Lachninae can feed on both green and woody parts of both conifers and broadleaved plants (Chen et al. Citation2016). From the beginning of the 21st century, Lachninae aphids have received attention from many researchers in terms of their phylogeny and evolution (Normark Citation2000; Jousselin et al. Citation2013; Chen et al. Citation2016; Théry et al. Citation2018) as well as their morphology, biodiversity, systematics and taxonomy (Kanturski & Wieczorek Citation2014; Kanturski et al. Citation2016, Citation2017a, Citation2017b). The Asiatic representatives of Lachninae have received the least attention in recent years in studies involving scanning electron microscopy and molecular research (Chen et al. Citation2017; Kanturski et al. Citation2017c, Citation2018a, Citation2017d, Citation2017e, Citation2018b; Chakrabarti et al. Citation2020). One of the most important and interesting Lachninae groups are the poorly known members of the tribe Tuberolachnini, which, besides the almost cosmopolitan Tuberolachnus salignus Gmelin, are characterized to be Asiatic Lachninaes. Recognition of the Tubelolachnini as a tribe has been validated by molecular studies (Normark Citation2000; Chen et al. Citation2016) and it comprises four genera: Tuberolachnus Mordvilko, Nippolachnus Matsumura, Pyrolachnus Basu & Hille Ris Lambers and Sinolachnus Hille Ris Lambers of which Sinolachnus is the least known. Representatives of Sinolachnus are known from their association with Elaeagnus species (maybe also with other Elaeagnaceae) where they feed on branches and are constantly visited by ants. Sinolachnus spp. most probably prefer higher mountain areas of South-East Asia. The first species of the genus Sinolachnus was described from Taiwan by Takahashi (Citation1925) as Lachnus niitakayamensis; Takahashi noted that the only known alate viviparous female was characterized by antennae with numerous sensilla on each segment, previously known only in the males of aphids (Takahashi Citation1925). Two years later, Takahashi (Citation1927) described the apterous viviparous females and in the next years (Takahashi Citation1931) transferred L. niitakayamensis to the genus Lachniella Del Guercio. Hille Ris Lambers (Citation1956) erected Sinolachnus, a new genus for L. niitakayamensis mostly due to the “sensoriation of the antennae of alatae, which is unique in aphids”. Additionally, Hille Ris Lambers highlighted the “first tarsal joints with several short sensillae and some longer hairs”. The genus remained monotypic until the description of the second species, known from a single alate viviparous female captured in a Malaise trap: S. taiwanus (Tao, Citation1989). The least known species of this genus, S. elaeagnensis, has been described by Chakrabarti and Das (Citation2015) from Bhutan (Remaudière & Remaudière Citation1997; Favret Citation2022). Sinolachnus still remains the most poorly known genus not only in Tuberolachnini but also within Lachninae as a whole. Representatives of this genus so far never have been investigated in terms of morphology or relationships with other genera beyond the rather arbitrary decision of Chen et al. (Citation2016) to put Sinolachnus within Tuberolachnini genera. In the course of several years of research by the first author on Asiatic Lachninae, all known available material of Sinolachnus has been gathered together with the material of species described in other genera (e.g. Cinara Curtis and Maculolachnus Gaumont). We hereby present the results of the revision of the poorly known genus Sinolachnus to include eight species. We report Sinolachnus from Japan for the first time with a description of a new species, and describe a new species from Taiwan. We propose to transfer two species already described in other Lachninae genera: Cinara plurisensoriata Zhang, Citation1988 and Maculolachnus rubi Ghosh & Raychaudhuri, Citation1972 to Sinolachnus. We provide detailed scanning electron microscopy analyses of apterous and alate viviparous females as well as of alatoid nymphs to show the morphology and sensilla of Sinolachnus for the first time. Moreover, after comparison with other Lachninae genera, we propose to transfer Sinolachnus to the tribe Tramini.
This paper is a part of the project “Morphology, diversity and phylogeny of Asiatic Tuberolachnini aphids”.
2. Materials and methods
2.1. Study material and light microscopy
The slide-mounted material, obtained from different collections, was fixed in Canada balsam. Freshly collected samples were preserved in 70% ethanol for several days. Alcohol-fixed samples were transferred to 10% KOH and boiled. After maceration and removing the embryos, the samples were transferred to chloral phenol for one hour and finally to the Chloral hydrate. Samples were then transferred to a Faure-Berlese solution and dried in an incubator for about one week in 40°C.
All existing and available slides of representative Sinolachnus have been examined for this study. The specimens were examined using a light microscope Leica DM 3000 LED and photographed by Leica MC 190 HD camera. The measurements were done according to Ilharco and van Harten (Citation1987); Blackman and Eastop (Citation2006). Measurements are given in millimeters. Material examined of each species is given in the review of species. Actual host plant names are given according to The Plant List (Citation2013). Final figure processing was done using Photoscape 3.7 (photoscape.org) and Irfanview 64 (irfanview.com).
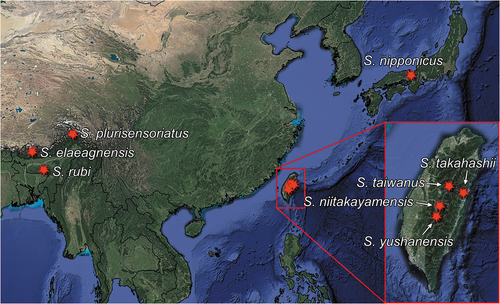
Locality data were obtained from specimens studied in the museum collections. All localities were georeferenced using Google Earth 7.3.2.5776 (Google Inc Citation2022; http://www.google.com/earth/index.html) (coordinates were collected in decimal degrees, datum: WGS84). The map was prepared in QGIS 3.26.0 (QGIS Development Team Citation2022; http://www.qgis.org) using the WGS84 datum and EPSG: 4326.
The following abbreviations are used:
| ANT | = | antennae or their lengths |
| ANT I–VI | = | antennal segments I, II, III, IV, V, VI or their lengths (ratios between antennal segments are simply given as e.g. “VI: III”) |
| BASE | = | basal part of last antennal segment or its length |
| BD III | = | basal articular diameter of ANT III |
| BL | = | body length |
| FT I | = | first segment of fore tarsus |
| HW | = | greatest head width across compound eyes |
| HT I | = | first segment of hind tarsus |
| HT Ib | = | basal length of hind first segment of hind tarsus |
| HT Id | = | dorsal length of hind first segment of hind tarsus |
| HT Iv | = | ventral length of hind first segment of hind tarsus |
| HT II | = | second segment of hind tarsus or its length |
| LS ANT III | = | length of longest setae of ANT III |
| MT I | = | first segment of middle tarsus |
| PT | = | processus terminalis of last antennal segment or its length |
| SIPH | = | siphunculi sclerite width |
| URS | = | ultimate segments of rostrum (IV + V) or their length |
| III FEMORA | = | hind femora length |
| III TIBIAE | = | hind tibiae length |
Depositories of the material examined (including type material):
| NHMUK | = | Natural History Museum, London, United Kingdom |
| DZUS | = | Hemiptera Collection of the Department of Zoology, University of Silesia in Katowice, Poland |
| MNHN | = | Muséum national d’Histoire naturelle, Paris, France |
| NTU | = | National Taiwan University, Taipei, Taiwan |
| NZMC | = | National Zoological Museum of China Institute of Zoology, Chinese Academy of Sciences, Beijing, P.R. China |
| TARIIC | = | Taiwan Agricultural Research Institute Insect Collection, Taichung, Taiwan |
| VCK | = | Vidyasagar College, Kolkata, India. |
2.2. Scanning electron microscopy
Specimens for SEM analyses were preserved in 70% ethanol for several days. From ethanol, the specimens were transferred into 6% phosphotungstic acid (PTA) solution in 70% ethanol for 24 hours. Dehydration was accomplished through an ethanol series of 80%, 90%, 96% and two changes of absolute ethanol for 10 minutes each. Absolute ethanol dehydrated specimens were treated with chloroform for 24 h. Dehydrated and cleaned samples were dried using the Leica EM CPD 300 auto-critical point dryer (Leica Microsystems, Vienna, Austria). Dry samples were mounted on aluminum stubs with double-sided adhesive carbon tape and sputter-coated with 30 nm gold layer in a Quorum 150 T ES Plus sputter coater (Quorum Technologies Ltd, Laughton, East Sussex, UK). The specimens were imaged by the Hitachi SU8010 field emission scanning electron microscope FESEM (Hitachi High-Technologies Corporation, Tokyo, Japan) at 7 and 10 kV accelerating voltage with a secondary electron detector (ESD). Final figure processing was done using Photoscape 3.7 (photoscape.org) and Irfanview 64 (irfanview.com).
3. Results
3.1. Notes on Sinolachnus SEM morphology based on S. yushanensis
3.1.1. General morphological characters
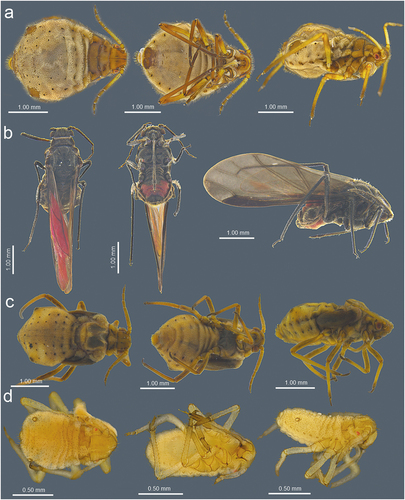
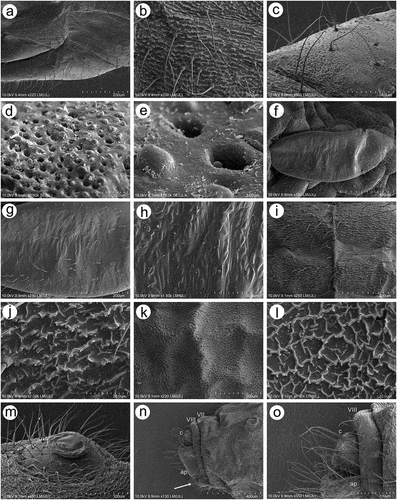
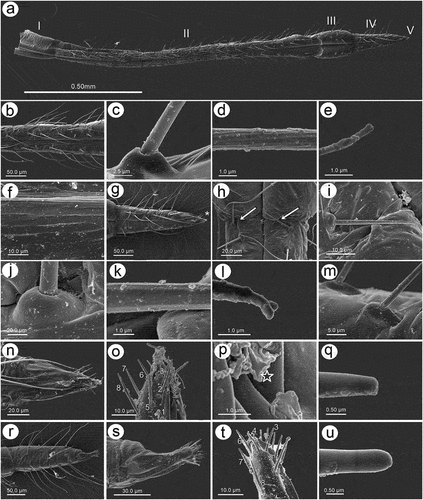
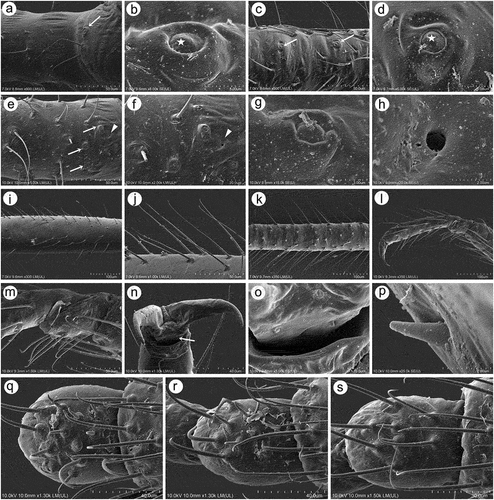
Apterous viviparous females of S. yushanensis are pear-shaped and very densely covered by numerous long, fine and pointed setae (trichoid sensilla). The head is separated from thorax of which each segment (pronotum, mesonotum and metanotum) is also separated from each other and from ABD I. Abdominal segments I, ABD VII and VIII are well separated from one another ()). Siphunculi are quite low and situated on very protuberant and hairy sclerites. Additionally, the surface of SIPH sclerite is wrinkled or ruffled ()). The perianal structures of the end of the abdomen are well adapted to ant attendance with short or even hidden cauda, the almost vertical anal plate with characteristically protruded hairs forming a “basket-like” structure (). Between anal and genital plates, three rudimentary gonapophyes are visible ()). The cuticular surface varies by bodily region. All body parts and appendages are densely covered by trichoid sensilla (setae), which are characterized by very protuberant sockets. Trichoid sensilla on the body are long, sometimes hairlike, with very fine and pointed apices (). The cuticle on the head and pronotum cuticle is smooth with moderate corrugation ()). The surface of mesonotum is visibly covered by numerous minute scales or denticles that are similar to triangles with pointed apices (). The metanotum and the remaining abdominal tergites (especially ABD I–VI) are characterized by cuticle with very clear polygonal sculpturing in the form of pentagons or hexagons (). The edges between each polygon are rather irregular and often, additional inner rigidity is visible within each polygon ()). The polygonation is not only restricted to the dorsal part of the body, but also lateral sides in the area of spiracles are polygonal and richly sculptured ()). As mentioned previously, the trichoid sensilla are long and fine, their surface is longitudinally ribbed and the sockets are quite narrow and protuberant (). Alate viviparous females are characterized by rather well developed and sclerotized thorax. Both alate viviparous females, as well as alatoid nymphs, are densely covered by numerous setae (trichoid sensilla) and their cuticular surface characters are the same as in apterous viviparous females (). In alate viviparous females, the eyes are larger with more ommatidia () and three ocelli are visible (). Triommatidia are distinct and placed on protuberant tubercle ()). Similarly as in the apterous viviparous female, SIPH sclerite surface in alate females is wrinkled or ruffled and the apex of the SIPH terminates in a rather flat flange and semicircular operculum ()). The perianal structures are very hairy and similar to those of apterous females ()). The dorsal abdomen of the alate female of S. yushanensis is covered by some amount of wax in the form of granular and oblong secretions (). Those secretions were visible despite of chloroform treating. Alate viviparous females are characterized by numerous surfaces covered by polygonal (most probably strengthening) sculpture which is visible even on the distal half of the antennal pedicel (). Most of the body surface is also covered by a thin but visible wax layer that moreover has not been removed during the treatment with a chloroform bath. This layer can be noted on the head ()) and mostly on the abdomen (). In some places when the secretions have been removed, the cuticle presents a serrated sculpture (). Alatoid nymphs present a similar head morphology to the alate female ()). Only the eyes are characterized by smaller number of ommatidia which are absent from the dorso-proximal part (). Moreover, the cuticle of the dorso-proximal part of the eyes bears a few small but well-visible openings that may be openings to sensory structures (). Cuticle of the alatoid nymph is rather similar as in the apterous and alate viviparous females with some distinct exceptions in the area and directly on the wing buds. The wing buds are in general smooth or slightly wrinkled, covered by numerous trichoid sensilla (). The cuticle of the proximal part of especially fore wings buds are characteristically porous or even spongy (). The thoracic cuticle is strongly wrinkled with wide denticles forming rather transverse lines rather than polygonal cells () which are clearly visible on the abdomen (). SIPH sclerites are of the same characters as in apterous and alate females but lying on smaller and lower sclerites ()), and the perianal structures (especially the shape of cauda and anal plate) are almost the same as in the apterous female ().
Figure 1. Scanning electron microscopy (SEM) of apterous viviparous female of S. yushanensis general morphology: (a) habitus, (b) head and pronotum, (c) thorax, Pr-pronotum, Ms-mesonotum, Mt-metanotum.
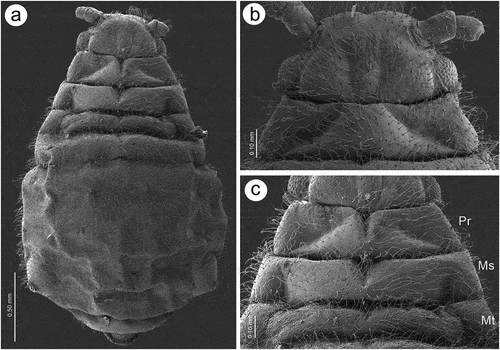
Figure 2. SEM of apterous viviparous female of S. yushanensis general morphology: (a) siphunculus densely covered by setae, (b) lateral side of the end of abdomen showing perianal structures with well-visible anal plate (ap), genital plate (gp) and poorly visible cauda (arrowhead), (c) back side of the end of abdomen with visible perianal structures and spiracles, VII-abdominal tergite VII, VIII-abdominal tergite VIII, c-cauda, ap-anal plate, arrow-rudimentary gonaphyses, gp-genital plate, (d) chaetotaxy of cauda and anal plate, (e) cauda and anal plate from the dorsal view, (f) ultrastructure of rudimentary gonapophyses.
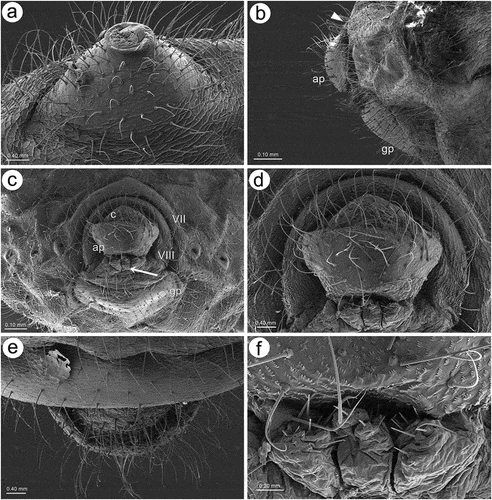
Figure 3. SEM of apterous viviparous female of S. yushanensis dorsal chaetotaxy and cuticle characters: (a) head chaetotaxy, (b) structure of the basal part of head trichoid sensilla and their sockets, (c) pronotum smooth cuticle surface, (d) surface of cuticle of the rest of thorax with numerous denticles, (e) ultrastructure of the cuticle surface and denticles, (f) dorsal abdominal chaetotaxy, (g, h) dorsal abdominal cuticle sculpture, (i) ultrastructure of the cuticle sculpture, (j) structure of the lateral abdomen cuticle and spiracles openings, (k) ultrastructure of the basal part of abdominal trichoid sensillum with the socket, (l) ultrastructure of the socket opening.
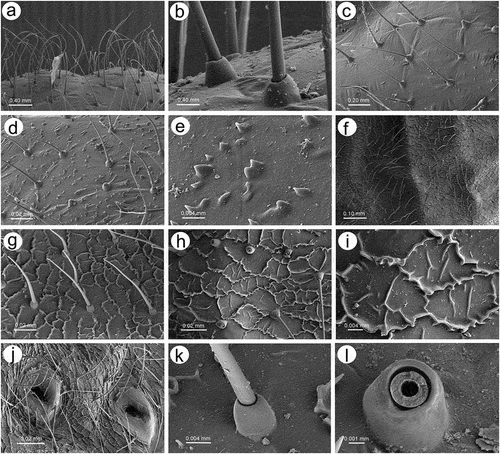
Figure 4. SEM of alate viviparous female and alatoid nymph of S. yushanensis: (a) dorsal side of alate viviparous female, (b) lateral side of alate viviparous female, (c) dorsal side of last instar alatoid nymph, (d) lateral side of last instar alatoid nymph.
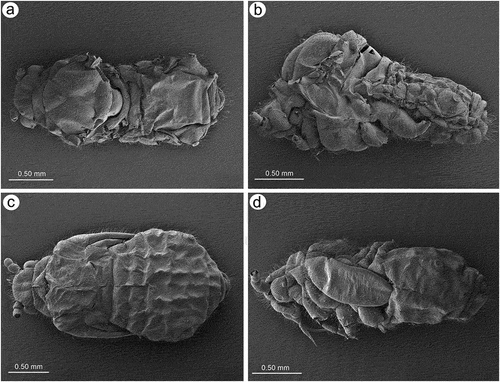
Figure 5. SEM of general morphological characters of alate viviparous female S. yushanensis: (a) head and pronotum, (b) dorsal side of head with large compound eye, lateral ocellus (star) and triommatidium (asterisk), (c) lateral side of head with large compound eye, lateral ocellus (star) and triommatidium (asterisk), (d) ultrastructure of triommatidium, (e) siphunculus on large sclerotic cone, (f) suphuncular opening with operculum, (g) lateral side of the end of abdomen showing siphunculus (s), abdominal segment VIII (VIII), short cauda (c), anal plate (ap) and large genital plate (gp), (h) waxy secretion on the body, (i) ultrastructure of the waxy secretion.
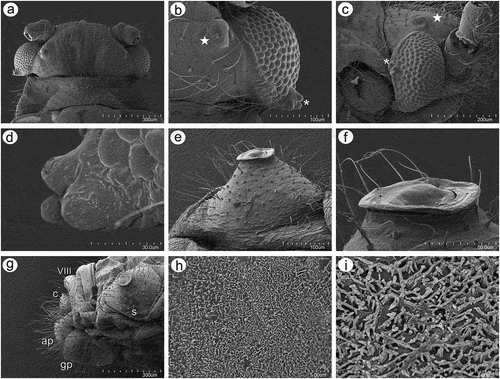
Figure 6. SEM of body surfaces of alate viviparous female of S. yushanensis: (a) pedicel with polygonal strengthenings, (b) ultrastructure of the strengthenings on the pedicel, (c) head cuticle with trichoid sensilla, (d) surface of abdominal dorsum, (e) ultrastructure of the dorsal cuticle, (f) structure of trichoid sensillum on the abdomen, (g) ultrastructure of the cuticle of ABD VII and VIII, (h) ultrastructure of trichoid sensilla socket and projections on the cuticle, (i) ultrastructure of dorsal cuticle with and without waxy secretion.
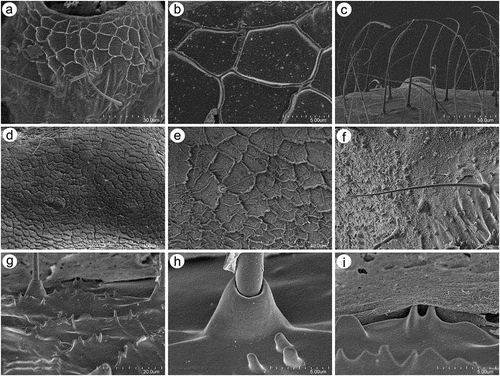
Figure 7. SEM of general morphological characters of alatoid nymph of S. yushanensis: (a) head, (b) dorsal side of pedicel with campaniform sensillum (star), (c) compound eye with larger dorsal edge and triommatidium, (d) dorsal area of eye cuticle with small openings (arrow), (e, f) ultrastructure of the possible ampulla sensilla, (g) trichoid sensilla of ANT III, (h) small multiporous placoid sensilla (secondary rhinaria) on ANT IV, (i) ultrastructure of the secondary rhinarium, (j) big multiporous placoid sensillum (primary rhinarium) on ANT V, (k) ultrastructure of porous membrane of the sensillum, (l) ANT VI sensilla, (m) ultrastructure of small multiporous placoid sensillum on ANT VI, (n) ultrastructure of type II trichoid sensilla on the tip of PT, (o) ultrastrusture of sunken coeloconic sensilla.
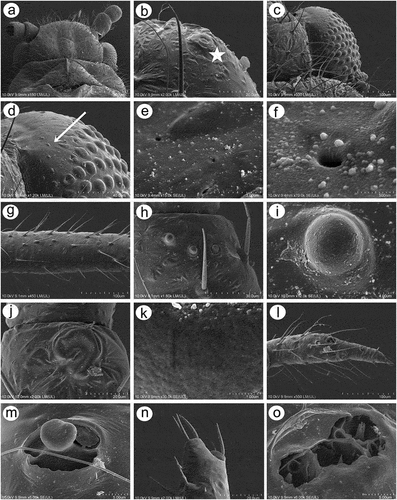
Figure 8. SEM of body surfaces and characters of alatoid nymph of S. yushanensis: (a) dorsal side of thorax with wing buds, (b) thoracic cuticle and trichoid sensilla, (c) dorso-lateral side of proximal part of fore wing bud with numerous openings in the cuticle, (d, e) ultrastructure of the openings in the cuticle, (f) lateral side of fore wing bud, (g) trichoid sensilla on the wing bud, (h) cuticle of the middle part of fore wing bud, (i, j) cuticle and trichoid sensilla of ABD I–III and ABD VII–VIII, (k, l) cuticle and trichoid sensilla of ABD IV–VII, (m) siphunculus, (n, o)perianal structures showing ABD VIII (VIII), short cauda (c), anal plate (ap) and genital plate bud (arrow).
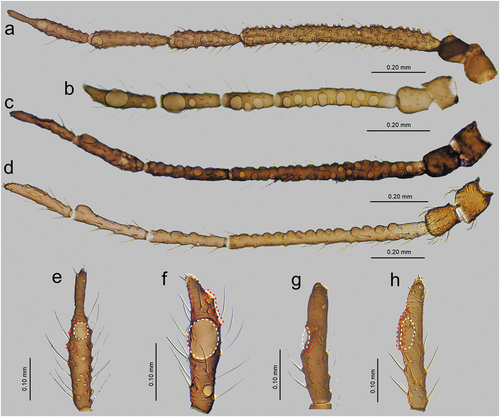
3.1.2. Antennal sensilla
In apterous viviparous females, the antennae bear several types of sensilla of which type I trichoid sensilla are the most numerous and in the form of long, very fine, hair-like setae with almost filamentous, pointed apices (; 10(a)). Type I trichoid sensilla are distributed on all antennomeres except the terminal process and have protuberant and trapezoid sockets (). On its basal portion, the surface of the sensilla is smooth, graduating to a visibly ribbed surface ()). On ANT VI terminal process apex, a second type of trichoid sensilla can be found: type II. Those forming the apical and subapical setae, are short, stiff with rounded sockets and ribbed on the whole length (). In contrast to type I trichoid sensilla the type II sensilla are characterized by blunt or slightly rounded apices with a granulose surface ()). During the examination of the apterous viviparous antennal flagellum besides type I trichoid sensilla, small multiporous placoid sensilla, big multiporous placoid sensilla and sunken coeloconic sensilla have been found (). In the apterae of S. yushanensis, small multiporous placoid sensilla have been found on ANT IV and V as secondary rhinaria and on ANT VI BASE as primary (accessory) rhinaria. Small placoid multiporous sensilla on ANT IV and V are rather small, rounded, slightly protuberant and lie in depressions of the cuticle (). Antennomere V bears also big placoid sensillum on the distal end (); 11(b)). The sensillum is visibly protuberant without any sclerotized collar or projections. The sensillum surface is covered by numerous minute pores (). Most types of sensilla on ANT VI are distributed on the BASE and consist of big multiporous placoid sensillum (major rhinarium) with almost all accessory rhinaria lying on the lateral side of the major rhinarium and one small placoid sensillum moved far from the other ones to the terminal process ()). The structure and porous surface of the big placoid sensillum (major rhinarium) are the same as on ANT V. The rest of the accessory rhinaria are forming by two small multiporous placoid sensilla in polar positions, between which four sunken coeloconic sensilla are distributed ()). Accessory rhinaria lying at the major rhinarium are located separately deep in cuticle depressions and are surrounded by cuticular collars ()). In contrast to small multiporous placoid sensilla on ANT IV and V those ones on ANT VI present quite different and unique morphological structures as spheres or club-shaped projections, and the one sensillum on the terminal process is the more visibly protuberant and exposed on a rounded basal part (). Sunken coeloconic sensilla can be divided into two types: type I with short projections and type II with long projections (). In alate viviparous females, we were able to examine sensilla of the pedicel and three types of sensilla have been found. On the ventral side of the pedicel one highly visible rhinariolum has been found in the form of exposed coeloconic sensillum type I with short projections and a very low sclerotic collar (). The dorsal side of the pedicel bears a clearly visible, rounded campaniform sensillum () and a third type of very small sensillum in the form of cuticle opening close to the articulation with ANT III which could be an ampullaceal sensillum (). Alate viviparous females of S. yushanensis and all other Sinolachnus species are exceptional among all aphids owing to their numerous small rounded and very protuberant small multiporous placoid sensilla on all segments of the antennal flagellum ()). The sensilla (secondary rhinaria) are very characteristically protuberant and distributed slightly irregularly or in irregular lines on the whole surface of antennomeres (). They are also of different sizes and some of them may fused into larger ones which are very similar to the large placoid multiporous sensilla (). Each of the small multiporous placoid sensilla extends far from the antennomere cuticle and is composed of the robust sclerotic collar, an extension of the cuticle and semispherical multiporous membrane with densely distributed pores (). The large placoid sensillum on ANT V, bearing numerous pores on the membrane, is characterized by larger openings of the sensillum membrane which are rather regularly distributed on the sensillum plate (). The type I trichoid sensilla on alate viviparous females are characterized by a more ribbed structure () but overall they are similar as in apterous morphs. Beyond all the types of sensilla mentioned in apterous females, the antennal segment VI of alate viviparous females bears small placoid sensilla which are the secondary rhinaria on the proximal part of the BASE (). The arrangement of the sensilla forming primary rhinaria is similar to that of the apterous morphs with the large multiporous placoid sensillum (major rhinarium) and accessory rhinaria consist of two bulb-shaped small multiporous placoid sensilla in the polar position (and one moved to the PT) and sunken coeloconic sensilla between them (). The structure and ultrastructure of the sensilla are very similar to those in apterous females (). Examination of the antennae of the alatoid nymph revealed that besides type I and type II trichoid sensilla, small multiporous placoid sensilla (treated as secondary rhinaria) are present on ANT III () and are similar as small multiporous placoid sensilla of the alate females ()). The large multiporous placoid sensilla on ANT V and VI are small in comparison to those of the adult alate, but numerous pores are visible on the membrane (). The structure and arrangement of ANT VI sensilla are similar to ANT VI of adult apterous and alate viviparous females ()
Figure 9. SEM of antennal sensilla of apterous viviparous female of S. yushanensis: (a) antennal flagellum general view, (b) ANT III with type I trichoid sensilla, (c) ANT IV with type I trichoid sensilla and one small multiporous placoid sensillum (Orange) – secondary rhinarium, (d) ANT V with type I trichoid sensilla, one small multiporous placoid sensillum (Orange) – secondary rhinarium and big multiporous placoid sensillum (yellow) primary rhinarium, (e) ANT VI with type I trichoid sensilla, type II trichoid sensilla (violet), big multiporous placoid sensillum (yellow) – major rhinarium, small multiporous placoid sensilla (green) and sunken coeloconic sensilla (pink) – accessory rhinaria.
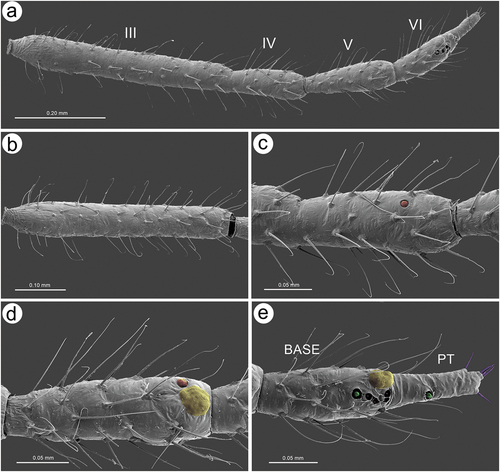
Figure 10. SEM of antennal trichoid sensilla of apterous viviparous female of S. yushanensis: (a) structure of type I trichoid sensilla with very fine often curved apices, (b) ultrastructure of type I trichoid sensillum and its socket, (c) ANT VI PT with small multiporous placoid sensillum and type II trichoid sensilla on the tip, (d) type II trichoid sensilla on the tip of ANT VI PT as apical (ap) and subapical (sap)setae, (e) structure of type II trichoid sensilla, (f) ultrastructure of type II trichoid sensilla apices.
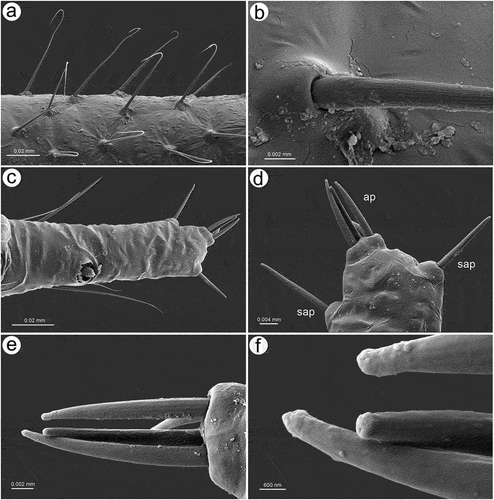
Figure 11. SEM of antennal placoid and coeloconic sensilla of apterous viviparous female of S. yushanensis: (a), ultrastructure of small multiporous placoid sensillum on ANT IV, (b) small and big multiporous placoid sensilla on ANT V, (c) ultrastructure of small multiporous placoid sensillum on ANT V, (d) ultrastructure membrane of small multiporous placoid sensillum on ANT V, (e) sensilla types on ANT VI BASE: type I trichoid sensilla (blue), big multiporous placoid sensillum (yellow) – major rhinarium, small multiporous placiod sensillum (green), two kinds of sunken coeloconic sensilla (pink) – accessory rhinaria, (f) group of two types of sunken coeloconic sensilla, (g) ultrastructure of small multiporous placoid sensillum on the BASE, (h) ultrastructure of small multiporous placoid sensillum on the PT, (i) type I sunken coeloconic sensillum (with short projections) and type II sunken coeloconic sensillum (with long projections).
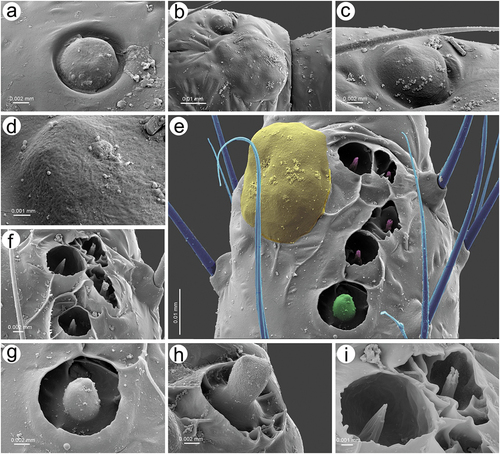
Figure 12. SEM of pedicel sensilla of alate viviparous female of S. yushanensis: (a) ventral side of pedicel with visible bare rhinariolum, (b) ultrastructure of the rhinariolum coeloconic-like sensillum with short projections, (c) dorso-lateral side of pedicel with campaniform sensillum (star) and a probable second type of sensillum on the edge (arrowhead), (d) ultrastructure of campaniform sensillum with small pore (asterisk), (e) edge of latero-dorsal side of pedicel with visible campaniform sensillum and the opening of a second type sensillum, (f) ultrastructure of the second type sensillum opening.
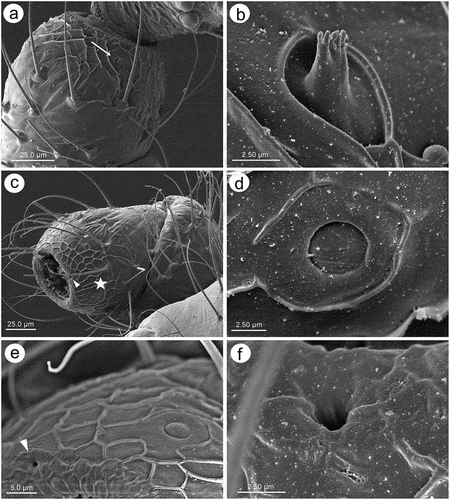
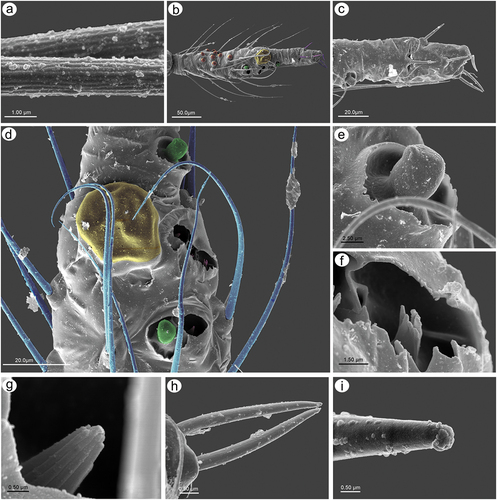
Figure 13. SEM of antennal flagellum sensilla of alate viviparous female of S. yushanensis: (a) fragment of ANT III with numerous small multiporous placoid sensilla (secondary rhinaria), (b, c) structure of secondary rhinaria and type I trichoid sensilla on ANT III, (d) different sizes of small multiporous placoid sensilla on ANT III with one of a size of big multiporous placoid sensillum (star), (e) small multiporous placoid sensilla n ANT IV od different sizes (star), (f) small multiporous placoid sensilla (secondary rhinaria) and big multiporous placoid sensilla (primary rhinarium) on ANT VI, (g, h) ultrastructure of small multiporous placoid sensillum with well visible sclerotic collar. Arrowhead showing the border between the cuticle and porous membrane, (i) ultrastructure of the porous membrane of small sensilla, (j) big multiporous sensillum with openings on the membrane (arrows), (k) structure of the sensillum membrane with the openings, (l) ultrastructure of porous membrane and the additional opening.
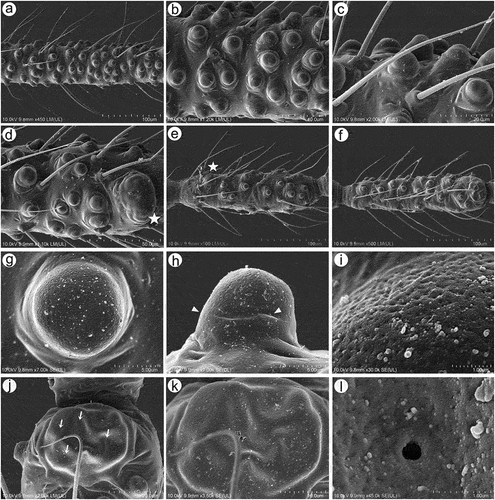
Figure 14. SEM of antennal flagellum sensilla of alate viviparous female of S. yushanensis: (a) ultrastructure of type I trichoid sensilla, (b) sensilla types on ANT VI: type I trichoid sensilla (unpigmented), small multiporous placoid sensilla (Orange) – secondary rhinaria, big multiporous placoid sensillum (major rhinarium), small multiporous placoid sensilla (green) – accessory rhinaria, sunken coeloconic sensilla (pink) – accessory rhinaria, type II trichoid sensilla (violet), (c) structure of type II trichoid sensilla, (d) topography of primary rhinaria on ANT VI. The largest is big multiporous placoid sensillum – major rhinarium (yellow), accessor rhinaria are divided into two general groups, sunken coeloconic sensilla in one group (pink) between two small multiporous placoid sensilla (green) in polar position, all are surrounded by type I trichoid sensilla (blue), (e) ultrastructure of small multiporous sensillum (accessory rhinarium), (f) ultrastructure of type I sunken coeloconic sensillum, (g) ultrastructure of type II coeloconic sensillum, (h) ultrastructure of type II trichoid sensilla, (i) ultrastructure of type II trichoid sensillum apex.
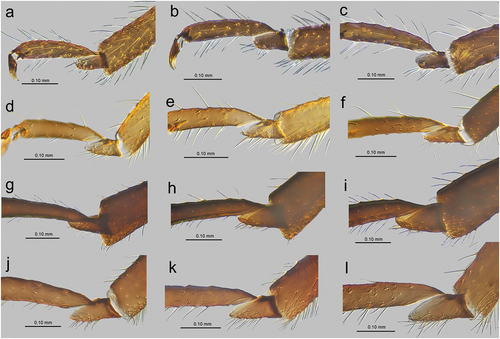
3.1.3. Mouthparts sensilla
The labium (rostrum) both in the apterous and alate viviparous females is long. The first segment is short, membranous and without sensilla. The second segment is the longest, sclerotized and covered by trichoid sensilla. Third segment is short and bulbous. The ultimate rostral segments (IV and V) are tapered and pointed ()). In apterous viviparous females, ultimate rostral segments (URS) from the ventral, lateral and dorsal sides are in the form of more or less regular triangles and are densely covered by long, fine trichoid sensilla (). The sensilla are characterized by rounded sockets and their surface is smooth (). On the proximal part of RIV, occur a pair of type II basiconic sensilla (sometimes hidden) which are much shorter than trichoid sensilla, with the apices more blunt (). The last fifth segment is quite well separated from the fourth one and bears seven pairs of long, stiff and rounded apices type III basiconic sensilla on the very tip (). In alate viviparous female mouthparts, the ultimate rostral segments in particular share similar features as in apterous viviparous females (with some exceptions). In alate viviparous females, the trichoid sensilla sockets appear more protuberant and trapezoid (), strongly ribbed and the extreme tip is slightly blunt (). Also the cuticle of the segments seems to be more sclerotized and covered by longitudinal reinforcement (). Type II basiconic sensilla are slightly longer, stiff with evident sockets, visibly ribbed and with very slightly blunt apices (). There are eight pairs of type III basiconic sensilla which are, similar to those of apterous viviparous females, long and clearly visible even under low magnification (), stiff () and with more or less rounded apices (). On the basal part of the sensilla, a well-developed molting pore can also be found ()).
Figure 15. SEM of mouthparts sensilla of apterous viviparous female of S. yushanensis: (a) ventral side of ultimate rostral segments (URS) with numerous trochoid sensilla (arrow) on RIV and type III basiconic sensilla on the tip of RV (asterisk), (b) lateral side of URS with numerous trichoid sensilla (solid arrow), type II basiconic sensilla (dotted arrow) on RIV and type III basiconic sensilla on the tip of RV (asterisk), (c) dorsal side of URS with numerous trochoid sensilla (arrow) on RIV and type III basiconic sensilla on the tip of RV (asterisk), (d) structure of trichoid sensilla, (e) ultrastructure of basal part of trichoid sensillum and its socket, (f, g) ultrastructure of type II basiconic sensilla, (h) RV with type III basiconic sensilla and visible styletes (s), (i) four pairs of type III basiconic sensilla visible on the dorsal side of RV, (j) seven pairs of type III basiconic sensilla visible on the lateral side of RV, (k) six pairs of type III basiconic sensilla visible on the ventral side of RV, (l) ultrastructure of type III basiconic sensilla with molting pore on the basal part (star).
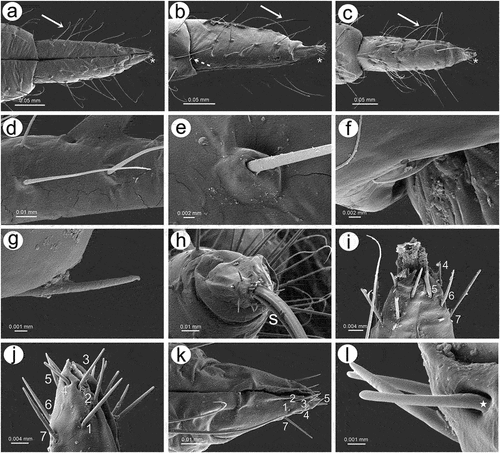
Figure 16. SEM of mouthparts characters and sensilla of alate viviparous female of S. yushanensis: (a) labium (rostrum) general view showing all five rostral segments, (b) trichoid sensilla on RII, (c) ultrastructure of socket and basal part of trichoid sensillum, (d) ultrastructure of middle part of trichoid sensillum, (e) ultrastructure of apical part of trichoid sensillum, (f) cuticle of the labial segment, (g) ventral side of ultimate rostral segments (URS) with numerous long trichoid sensilla and basiconic sensilla (asterisk), (h) proximal part of RIV with one pair of type II basiconic sensilla (arrows), (i) structure of type II basiconic sensillum, (j) ultrastructure of the socket of type II basiconic sensillum, (k) ultrastructure of the middle part of type II basiconic sensillum, (l) ultrastructure of the apical part of type II basiconic sensillum, (m) ultrastructure of sockets of trichoid sensilla, (n) RV with type III basiconis sensilla on the very apex, (o) dorso-lateral side of the apical part of RV with 7 visible pairs of basiconic sensilla, (p) ultrastructure of the basal part of type III basiconic sensillum with molting pore (star), (q) ultrastructure of apical part of basiconic sensillum with flat apex, (r) dorsal side of URS with numerous long trichoid sensilla and basiconic sensilla (asterisk), (s) lateral side of RV with type III basiconis sensilla on the very apex, (t), lateral side of the apical part of RV with 8 pairs of basiconic sensilla (u), ultrastructure of apical part of basiconic sensillum with rounded apex.
3.1.4. Wing morphology and sensilla
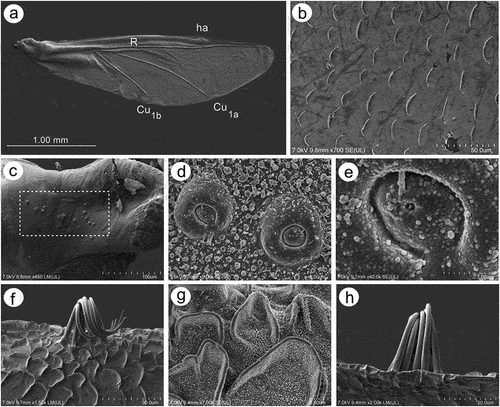
Fore wings in alate viviparous females are characterized by morphology rather typical for most Lachninae. The pterostigma is clearly visible and covered by numerous scale-like elements. The wing venation is well marked, with protuberant radial sector, media and concave cubital veins ()) which are concave (radial sector and media) and protuberant (cubital veins) on the dorsal side of the wing membrane (). The ventral side of the wing is also characterized by the claval fold near Cu1b ()). On the basal part of the wing, numerous campaniform sensilla can be found (). The campaniform sensilla are protuberant, with a rounded outer collar and two forms of internal structure: rounded ()) and indented in the outer collar ()). In both types of sensilla, pores in the central part of the inner part are visible. Rounded campaniform sensilla are arranged along the concave Sc+R + M+ Cu veins ()). The wing membrane is covered by numerous crescent-shaped scale elements and a very thin wax layer (). The scale-like elements become more semicircular or almost circular near the edges of the wings ()). The convex veins are (especially near the wing edges) formed by single ruffled structures, which appear to be the wing membrane protuberances (). The hind wings are smaller, shorter and narrower, characterized by very similar membrane with numerous crescent-shaped scale-like elements, and covered by very thin wax powder (). On the basal part of the hind wings, a large group of campaniform sensilla can be found, but they are always fewer in number than those on fore wing ()). As on the fore wing, they are protuberant, rounded and the pore in the center of the inner portion is quite visible (). On the front of the hind wings five curved hamuli can be found and the area around them is generously covered by robust semicircular scale-like elements ().
Figure 17. SEM of fore wings of alate viviparous female of S. yushanensis: (a) general view of the ventral side of fore wing with convex media (M1, M2), concave cubital veins (Cu1a, Cu1b) and visible claval fold (arrow), (b) general view of dorsal side of fore wing with concave media and convex cubital veins, (c) distal part of pterostigma and radial sector, (d, e) numerous campaniform sensilla near to the wing articulation, (f, g) ultrastructure of campaniform sensilla, (h) campaniform sensilla along inner side of Sc+R + M+ Cu (arrows), (i) scale-like elements on wing membrane on the inner surface, (j) ultrastructure of scale-like element, (k) scale like elements on the outer surface, (l) ruffled structures forming convex veins, (m) ultrastructure of the ruffled structures, (n) ultrastructure of scale-like element and membrane covered by wax secretion.
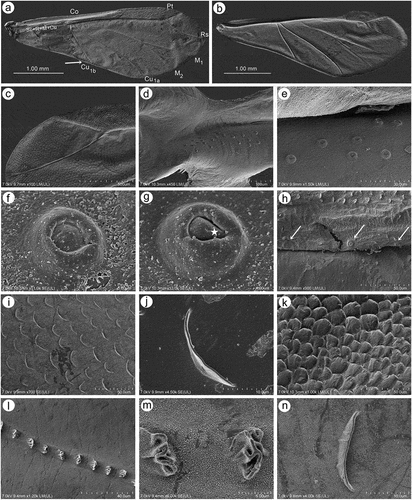
Figure 18. SEM of hind wings of alate viviparous female of S. yushanensis: (a) general view of the hind wing, (b) membrane and scale-like elements structure, (c) numerous small campaniform sensilla near the wing articulation (dotted rectangle), (d) ultrastructure of campaniform sensilla, (e) ultrastructure of the central part of campaniform sensilla with well-visible pore, (f) ventral side of claval area with five hamuli, (g) scale-like elements in the claval area, (h) dorsal side of claval area and hamuli.
3.1.5. Legs and tarsi morphology and sensilla
Legs in apterous and alate viviparous females are of the same morphological features and sensilla. On the ventro-lateral sides of trochantera and basal parts of femora different number of clearly visible campaniform sensilla can be found which in detailed structure are very similar to those on antennae or wings (; 20(a)–(g)). Interestingly, on the lateral side of the basal part of hind femora of alate viviparous females (in the region with campaniform sensilla), a very small and rounded opening in the cuticula has been found which is similar to those of the sensilla ampullaceal (). The legs are densely covered by numerous long, fine, hire-like in the distal part and with pointed apices trichoid sensilla (; 20(i)–(k)). As on the antennae and mouthparts, their sockets are protuberant, oval or rounded from the top and trapezoid from the lateral side and their surface is smooth in the basal part but later becomes prominently ribbed (). Tarsi are characterized by the first segment with short dorsal length (); 20(l)), normal-shaped claws and greatly reduced parempodia (empodial setae) (; 20(n)–(p)) strictly in form of very short pegs (a bit longer in alate viviparous females). First segments of tarsi are characterized by two kinds of ventral setae: long, more fine and pointed; and short, peg-like ones which are stiffer and with less pointed apices (); 20(m)). Also, the sockets of the sensilla are of different sizes – larger in the long setae and smaller in the peg-like setae. The ratio of the number of short, peg-like setae to the long ones is the largest on the first segment of prothoracic tarsi (; 20(q)), later on the first segment the mesothoracic tarsi (; 20(r)) and the lowest on the first segment of metathoracic tarsi (; 20(s)).
Figure 19. SEM of legs sensilla of apterous viviparous female of S. yushanensis: (a) campaniform sensilla on the inner side of hind trochanter (solid arrow) and hind femur (dotted arrow), (b) ultrastructure of campaniform sensillum on trochanter with visible pore (star), (c) trichoid sensilla on hind femur, (d) ultrastructure of tibial trichoid sensilla and their sockets, (e) trichoid sensilla on hind tibia, (f) ultrastructure of tibial trichoid sensillum and its socket, (g) hind tarsus, (h) two kinds of sensilla on ventral side of HTI – long sensilla (dotted arrow) and short, peg-like sensilla (solid arrow), (i) apical part of HT II with claws and residual, almost invisible parempodia (empodial setae) (arrow), (j) first segment of fore tarsus with largest number of peg-like sensilla, (k) first segment of middle tarsus with medium number of peg-like sensilla, (l) first segment of hind tarsus with smallest number of peg-like sensilla, HT I-first segment of hind tarsus, HT II-second segment of hind tarsus, cl-claws.
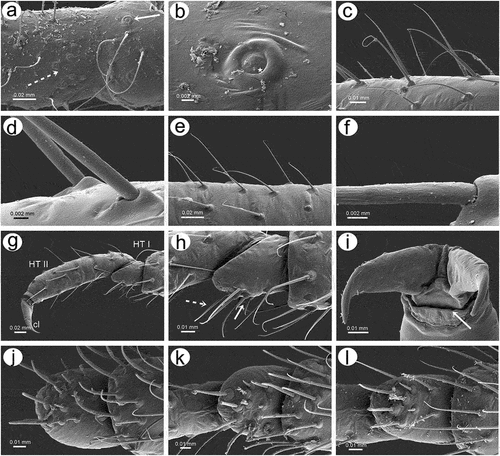
Figure 20. SEM of legs sensilla of alate viviparous female of S. yushanensis: (a) campaniform sensillum on hind trochanter, (b) ultrastructure of trochanteral campaniform sensillum with visible pore (star), (c) campaniform sensilla on hind femur, (d) ultrastructure of femoral campaniform sensillum with visible pore (star), (e, f) most probable ampulla sensillum (arrowhead) on the inner side of hind femora in the vicinity of campaniform sensilla, (g) ultrastructure of campaniform sensillum on the inner side of hind femora, (h) ultrastructure of probable ampulla sensillum, (i) trichoid sensilla on hind tibia, (j) ultrastructure of tibial trichoid sensilla, (k) trichoid sensilla on hind tibia, (l) hind tarsus, (m) first segment of hind tarsus with two kinds of sensilla on the ventral side, (n, o, p) apical part of HT II with very short almost residual parempodia (arrow) (empodial setae), (q) first segment of fore tarsus with largest number of peg-like sensilla, (r) first segment of middle tarsus with medium number of peg-like sensilla, (s) first segment of hind tarsus with smallest number of peg-like sensilla.
3.2. Taxonomy
3.2.1. Diagnosis of the genus Sinolachnus
Sinolachnus aphids may be distinguished from all other Tuberolachnini and Lachninae representatives by the alate viviparous females with uniformly light brown or hyaline wings and all segments of antennal flagellum bearing extremely large numbers of very small and protuberant secondary rhinaria (e.g. 66–290 on ANT III and 14–95 on ANT IV), while in other Lachninae species alate viviparous females are characterized by larger, flatter and much less numerous secondary rhinaria (not more than 50 in Tramini). Secondary rhinaria with the exception of some Tramini are absent on the BASE of ANT VI. Apterous viviparous females can be separated from all other Lachninae species by sclerotization of the thorax and abdomen, with small denticles and a large number of “sense pegs” on first segments of especially fore tarsi (6–12), middle tarsi (2–5) and hind tarsi (2–3), while in all other Lachninae species the sclerotized part of the thorax and abdomen is smooth or at most wrinkled and the first segments of tarsi bear not more than 3 “sense pegs” (often only one). Apterous and alate viviparous females of Sinolachnus differ from other Lachninae species and genera by the characters of ANT VI accessory rhinaria arrangement. One of the accessory rhinaria lies singly at the border between BASE and PT or on the basal part of PT while the remaining accessory rhinaria are in a single group together near the major rhinarium.
3.2.2. Checklist of species of the genus Sinolachnus
Family: Aphididae Latreille, 1802
Subfamily: Lachninae Herrich-Schaeffer, 1854
Tribe: Tramini Herrich-Schaeffer, 1854
Genus Sinolachnus Hille Ris Lambers, Citation1956: 472
1. S. elaeagnensis Chakrabarti & Das, Citation2015
2. S. niitakayamensis (Takahashi, Citation1925) typus generis
3. S. nipponicus sp. nov.
4. S. plurisensoriatus (Zhang, Citation1988) comb. nov. = Cinara plurisensoriata Zhang, Citation1988
5. S. rubi (Ghosh & Raychaudhuri, Citation1972) comb. nov. = Maculolachnus rubi Ghosh & Raychaudhuri, Citation1972
6. S. taiwanus Tao, Citation1989
7. S. takahashii sp. nov.
8. S. yushanensis sp. nov.
3.2.3. Key to alate viviparous females of the genus Sinolachnus
Media of fore wing one-branched …………… 4
- Media of fore wing twice-branched ……………2
BL about 2.0 mm. Wings hyaline. Scale elements on fore wings mostly in the distal part. ANT IV/ANT V about 0.90 … S. taiwanus Tao
- BL more than 3.0 mm. Wings uniformly pigmented, brown. Scale elements on the whole surface of the wing. ANT IV/ANT V 0.96-1.13 … 3
ANT VI BASE with 24-28 setae, ABD VIII with 21 setae, URS 0.76-0.78 × ANT VI and 0.85-0.88 × HT II … S. plurisensoriatus (Zhang) comb. nov.
- ANT VI BASE with 43-38 setae, ABD VIII with 20-37 setae, URS 0.68-0.71 × ANT VI and 0.66-0.71 × HT II … S. elaeagnensis Chakrabarti & Das
ANT III with 220-255 secondary rhinaria. ANT IV with 50-70 secondary rhinaria. ANT V with 30-60 secondary rhinaria, HW 0.35-0.40 × ANT, URS 0.25-0.27 × ANT III. Fore wings with scale elements only in the distal part … S. takahashii sp. nov.
- ANT III with 66-200 secondary rhinaria. ANT IV with fewer than 40 secondary rhinaria. ANT V with 14-40 secondary rhinaria, HW 0.41-0.44 × ANT, URS 0.29-0.40 × ANT III. Fore wings with scale elements on the whole surface … 5
ANT VI with 7-17 secondary rhinaria. Hind wings with 17-19 sensilla on the basal part. PT/BASE 0.35-0.42. ANT VI/ANT III 0.36-0.38. ANT IV/ANT III 0.28-0.32 … S. yushanensis sp. nov.
- ANT VI with 4-6 secondary rhinaria. Hind wings with 9-11 sensilla on the basal part. PT/BASE 0.57-0.83. ANT VI/ANT III 0.41-0.50. ANT IV/ANT III 0.34-0.38 … 6
Sclerotized part of rostrum groove 0.75 mm. ANT VI with basal setae and additional long setae on the PT. ANT IV with 14-16, ANT V with 12 secondary rhinaria, PT/BASE 0.73-0.83 … S. nipponicus sp. nov.
- Sclerotized part of rostrum groove 1.00-1.05 mm. ANT VI with basal setae only (PT without additional long setae). ANT IV with 36-40, ANT V with 35-40 secondary rhinaria, PT/BASE 0.57-0.62 … S. niitakayamensis (Takahashii)
3.2.4. Key to known apterous viviparous females of the genus Sinolachnus
Body about 2.50 mm. ANT about 1.20 mm. III TIBIAE about 1.30 mm. ANT III and IV rarely with 0-2 secondary rhinaria … S. niitakayamensis (Takahashi)
- Body larger than 2.75 mm (mostly more than 3.00 mm). ANT 1.50-1.59 mm. III TIBIAE 1.77-1.92. ANT III always with 1-6 secondary rhinaria. ANT IV always with 2-6 secondary rhinaria … 2
HW/ANT 0.42-0.45. ANT VI with 2-3 secondary rhinaria. ANT VI BASE with 22-25 setae … S. rubi comb. nov.
-HW/ANT 0.50-0.51. ANT VI without secondary rhinaria. ANT VI BASE with 30-33 setae ...…S. yushanensis sp. nov.
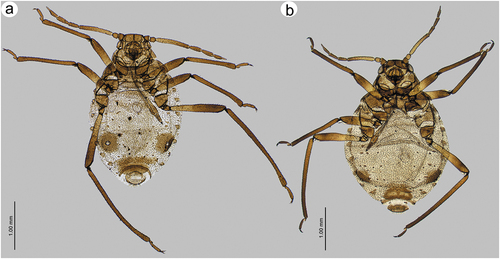
3.2.5. Description – shared characters of representatives of Sinolachnus ()
Apterous viviparous females
The body is almost rounded or slightly pear-shaped. Head with well-developed compound eyes and triommatidia. Epicranial suture distinct. Head and the rest of body densely covered by numerous long, fine and pointed setae. Head cuticle sclerotized and smooth. Antennae 6-segmented. ANT I square-shaped, ANT II with flat base and bulbous distal part. ANT III longest, as long as or shorter than ANT IV+ANT V+ ANT VI. ANT segments with small to very small rounded and protuberant secondary rhinaria on ANT III-Ant VI, often in the distal part. Primary rhinaria with sclerotic rosette. Accessory rhinaria on ANT VI often divided into two groups, with one moved to the PT and the remaining ones near the major rhinarium. PT only with five apical in two groups (2 + 3) and two subapical setae. Rostrum reaching beyond hind coxae. URS triangular. Thorax sclerotized, covered by numerous minute denticles, arranged more or less linearly. Legs normal. First segments of tarsi with different number of “sense-pegs”, with the most numerous on FT I. Abdomen membranous, often with more or less developed sclerotization in the form of sclerosis and sclerites. SIPH on sclerites with mostly irregular edges and well-developed flange. ABD VII and VIII with spino-pleural sclerotized cross-bars. Abdominal cuticle wrinkled with polygonal sculpture. Cauda semi-circular. GP densely covered by setae.
Alate viviparous females
Head with large compound eyes and visible, well-developed triommatidia. Epicranial suture clearly visible. Head and the remainder of body densely covered by long, fine and pointed setae. ANT 6-segmented with numerous (tens or hundreds) secondary rhinaria on ANT III–VI. Secondary rhinaria small to very small, rounded and extremely protuberant. ANT I square-shaped, ANT II with flat base and bulbous distal part. ANT III longest, as long as or shorter than ANT IV+ANT V+ ANT VI. Antenna covered by numerous long, fine and pointed setae. Primary rhinaria with sclerotic rosette. Accessory rhinaria on ANT VI divided into two groups with one located on the PT and the rest together on the lateral side of the major rhinarium. PT with five spical setae in two groups (2 + 3) and 1–3 subapical setae. Rostrum long, reaching over hind coxae. URS narrow, triangular. Thorax sclerotized. Fore wings hyaline or uniformly brown pigmented. Pterostigma brown of normal width and rounded apex. RS slightly curved. Both Cu veins raised separately directly from Sc+R + M+ Cu. Media poorly visible, one or twice-branched. Scale elements covering whole surface or only distal part of the wing membrane. Legs normal, densely covered by long, fine and pointed setae. HT I with different number of “sense-pegs” with the most numerous on FT I. Abdomen membranous with sclerotization in form of sclerites, scleroites and sclerotic plates. SIPH on sclerites with mostly irregular edges and well-developed flange. ABD VII and ABD VIII with spino-pleural cross-bars. Cauda semi-circular. GP densely covered by setae.
Nymphs
I instar larvae of apterous viviparous females are very small, only slightly sclerotized on head and thorax (prothorax especially). Siphunculi are small and on very small sclerites. Alatoid nymphs have well-developed thorax and wing buds. The abdomen is as developed as in apterous and alate viviparous females but the siphunculi are characterized by smaller sclerites.
3.2.6. Review of species
Sinolachnus elaeagnensis Chakrabarti & Das, Citation2015
Chakrabarti & Das Citation2015: 328(; )
Table I. Measurements (in mm) of the examined apterous viviparous females of Sinolachnus.
Table II. Measurements (in mm) of known alate viviparous females of Sinolachnus.
Material examined HOLOTYPE: Bhutan: Trongsa, Apterous viviparous female, 10.X.1999 from Elaeagnus sp., D. Das leg. (coll. no. 10049 (1) VCK). PARATYPES: 2 alate viviparous females, (coll. no. 10049 (2), 10049 (3), VCK), other data as in holotype.
Alate viviparous female – redescription
Colour in life: unknown. Pigmentation of mounted specimens: ANT dark brown to black, head and thorax dark brown, legs dark brown to black with slightly paler proximal parts of femora, wings uniformly brown, abdomen yellow or light brown with brown sclerotization ()). Morphometric characters. HW 0.33–0.37 × ANT. Head setae 0.11–0.13 mm long. ANT 0.54–0.56 × BL. ANT III shorter that the remaining segments with 275–280 rhinaria of different size (), ANT IV slightly longer than ANT V with 87–95 rhinaria, ANT V shorter than ANT VI with 60–65 rhinaria ()). ANT VI with 15–20 rhinaria ()). PT 0.45–0.75 × BASE. Other antennal ratios: ANT VI/ANT III 0.39–0.40, ANT V/ ANT III 0.33–0.36, ANT IV/ANT III 0.37–0.38, ANT IV/ANT V 1.03–1.13. LS III 1.57 × BD III. ANT VI with 34–38 basal setae. URS 0.27–0.28 × ANT III, 0.68–0.71 × ANT VI, and 0.66–0.71 × HT II, with 10–12 accessory setae ()). Fore wings uniformly brown pigmented with 10–12 sensilla near the base, media twice-branched and scale elements on the whole surface of the wing membrane ()). Pterostigma with 24–30 fine, hair-like setae, 0.034–0.052 mm long. Hind wings with 16–18 sensilla near base ()). III FEMORA setae 0.075–0.100 mm long. III TIBIAE setae 0.080–0.110 mm long. FT I with 8–9 ()), MT I with 6 ()) and HT I with 3 sense pegs ()). HT Ib 3.00–3.25 × HT Id, 0.57–0.65 × HT Iv and 0.70–0.81 × HT Iv. HT II 0.39–0.41 × ANT III and 1.00–1.02 × ANT VI. Dorsal abdominal cuticle with poorly visible polygonal sculpture and very small, rounded scleroites at setal bases (). SIPH sclerites with regular edges ()). Dorsal abdominal setae 0.090–0.100 mm long ()). ABD VIII with 30–37 setae. Genital plate with irregular edges of the proximal and smooth edges of the distal part ()).
Diagnosis
Sinolachnus elaeagnensis with its twice branched media is similar to S. plurisensoriatus and S. taiwanus (and differs from all other known species). Both S. elaeagnensis and S. plurisensoriatus differ from S. taiwanus especially by its much larger body size – more than 3 mm (body about 2 mm in S. taiwanus), and ANT/BL ratio that is 0.54–0.57 (about 0.68 in S. taiwanus). Sinolachnus elaeagnensis differs from S. plurisensoriatus by number of rhinaria on ANT IV (87–95) and ANT V (60–65) (70–74 and 70–75 in S. plurisensoriatus respectively), number of ANT VI basal setae – 34-38 (24–28 in S. plurisensoriatus), number of ABD VIII setae – 30-37 (21 in S. plurisensoriatus) and the ratio of URS/HT II – 0.66–0.71 (0.85–0.88 in S. plurisensoriatus). Both species differ from each other also by the differences in ANT VI/ANT III, ANT IV/ANT III and URS/ANT VI ratios.
Host plant
This species has been found on an unidentified Elaeagnus species on which according to Chakrabarti and Das (Citation2015) the aphids infest young stems.
Distribution
S. elaeagnensis is known so far only from Trongsa in central Bhutan ().
Remarks
Superficially, this species is very similar to S. plurisensoriatus, which is known from Tibet, but detailed analyses have shown undoubted distinctiveness of both species.
Apterous viviparous females as well as the sexual generation remain still unknown.
Sinolachnus niitakayamensis (Takahashi, Citation1925)
Lachnus niitakayamensis Takahashi, Citation1925: 36
(; )
Material examined
LECTOTYPE (present designation): Taiwan: Kanko, alate viviparous female with the letter “L”, 17.IX.1924, Sonan leg. (coll. no. MNHN (EH)23025, MNHN). PARALECTOTYPE: alate viviparous female on the same slide.
Apterous viviparous female – redescription (based on Takahashi Citation1927)
Colour in life: Yellowish brown, blackish on the dorsal side of abdomen. Antennae yellowish brown, with black apices (Takahashi Citation1927). Pigmentation of mounted specimens: unknown. Morphometric characters. BL about 2.5 mm long, ANT about 1.2 mm long 0.48 × BL. ANT III shorter that the remaining segments with 0–2 rhinaria, ANT IV slightly longer than ANT V with one rhinarium near the distal end, ANT V shorter than ANT VI with one rhinarium in the middle. ANT VI PT 0.56 × BASE. Other antennal ratios: ANT VI/ANT III 0.44, ANT V/ ANT III 0.32, ANT IV/ANT III 0.33, ANT IV/ANT V 1.03. Fore femur about 1.3 mm long, longer than ANT III. Hind tarsus slightly longer than ANT VI.
Alate viviparous female - redescription
Colour in life: head antennae and thorax black. Legs black with paler proximal parts of femora. Abdomen greenish (Takahashi Citation1925). Pigmentation of mounted specimens: ANT brown with slightly paler basal part of ANT III, legs head and pronotum brown, legs brown with paler proximal parts of femora, abdomen yellow with light brown sclerotization ()). Morphometric characters. HW 0.35–0.40 × ANT. Head setae 0.085–0.110 mm long. ANT 0.50–0.56 × BL. ANT III as long as or slightly shorter that the remaining segments with 220–255 rhinaria of similar size (), ANT IV slightly longer than ANT V with 50–70 rhinaria, ANT V as long as or shorter than ANT VI with 30–56 rhinaria ()). ANT VI with 4–12 rhinaria ()). PT 0.42–0.50 × BASE. Other antennal ratios: ANT VI/ANT III 0.34–0.36, ANT V/ ANT III 0.29–0.35, ANT IV/ANT III 0.32–0.37, ANT IV/ANT V 0.96–1.25. LS III 2.10–2.44 × BD III. ANT VI with 21–24 basal setae. URS 0.25–0.27 × ANT III, 0.73–0.77 × ANT VI, and 0.74–0.80 × HT II, with 14–16 accessory setae ()). Fore wings uniformly brown pigmented with paler basal parts, with 9–12 sensilla near the base, media twice-branched and scale elements on the whole surface of the wing membrane with smooth basal region ()). Pterostigma with 22–29 fine, hair-like setae, 0.035–0.062 mm long. Hind wings with 12–18 sensilla near base ()). III FEMORA setae 0.060–0.110 mm long. III TIBIAE setae 0.070–0.120 mm long. FT I with 5–6 ()), MT I with 4 ()) and HT I with 2 sense pegs ()). HT Ib 1.90–2.25 × HT Id, 0.40–0.45 × HT Iv and 0.66–0.75 × HT Ii. HT II 0.32–0.36 × ANT III and 0.92–1.01 × ANT VI. Dorsal abdominal cuticle with poorly visible polygonal sculpture and very small, rounded scleroites at setal bases (). SIPH sclerites with rather regular edges ()). Dorsal abdominal setae 0.060–0.125 mm. long. Abdomen with marginal sclerotic plates on ABD I–IV and cross-bands on ABD VII–VIII. ABD VIII with 23–27 setae. Genital plate with regular edges and very small symmetric indentation in the middle of the proximal portion ()).
Diagnosis
Sinolachnus niitakayamensis is most similar to two other new species (S. nipponicus and S. yushanensis) owing to the similar number of antennal sensilla and one-branched media of forewings, by which they can be distinguished from other congeners (S. elaeagnensis, S. plurisensoriatus and S. takahashii). Together with S. nipponicus, S. niitakayamensis can be easily distinguished from S. yushanensis by the smaller number of secondary rhinaria on ANT VI – 4-6 (7–16 in S. yushanensis), smaller (9–11) number of sensilla on the basal part of hind wings (17–19 in S. yushanensis), and ratios of different body parts such as PT/BASE, ANT VI/ANT III, ANT IV/ANT III. On the other hand, S. niitakayamensis can be distinguished from S. nipponicus especially by PT with apical and subapical setae only (PT of S. nipponicus is characterized by additional long setae), also by lower ratio of PT/BASE – 0.57–0.62 (0.73–0.83 in S. nipponicus), lower ratio ANT VI/ANT III – 0.41–0.43 (0.47–0.50 in S. nipponicus), greater number of secondary rhinaria on ANT IV (36–40) and ANT V (35–40) (14–16 and 12 in S. nipponicus respectively) as well as by much longer sclerotized part of rostrum groove – 1.00–1.05 (0.75 in S. nipponicus).
Host plant
Representatives of this species feed on Elaeagnus oldchamii (Takahashi, 1926)
Distribution
The species is known from Kanko in Taiwan ().
Remarks
During the description of the species, Takahashi (Citation1925) described only alate viviparous female of this species (“two winged females”) and later (Takahashi Citation1927) described briefly the apterous viviparous female. Unfortunately, no material of apterous viviparous females nor the type material is left in the TARI collection. The slide with two alate viviparous females collected on 17 September, 1924 at Kanko has been found in the collection of Muséum national d’Histoire naturelle and we recognize and treat those two samples as the type material of S. niitakayamensis (most probably the slide having been sent to G. Remaudière from TARI as the handwriting on the label is the same as on other slides of R. Takahashii). As during the description of the species no holotype has been indicated, we treat this type material as syntypes and according to the International Code of Zoological Nomenclature lectotype and paralectotype are here designated.
There is no information on the life cycle and sexual morphs of this species.
Sinolachnus nipponicus sp. nov.
(; )
http://urn:lsid:zoobank.org:act:FEFF84BF-1922-41AC-9E5C-449602885B72
Material examined
HOLOTYPE: Japan: Kyôto Prefecture, Kyôtamba-chô, alate viviparous female, 20.X.1939, R. Yoshii leg. (coll. no. 31-1-1-5, TARIIC).
Alate viviparous female – description
Colour in life: unknown. Pigmentation of mounted specimens: Head and thorax brown, ANT brown. Legs brown with paler proximal end of femora. SIPH, abdominal sclerotization and genital plate brown ()). Morphometric characters. HW 0.43–0.44 × ANT. Head setae 0.070–0.075 mm long. ANT 0.55–0.56 × BL. ANT III shorter than the remaining segments with 66–70 secondary rhinaria (), ANT IV almost as long as ANT V with 14–16 secondary rhinaria, ANT V shorter than ANT VI with 12 secondary rhinaria ()). ANT VI with 4 secondary rhinaria ()) and PT 0.73–0.83 × BASE. Other antennal ratios: ANT VI/ANT III 0.47–0.50, ANT V/ ANT III 0.36–0.37, ANT IV/ANT III 0.34–0.35, ANT IV/ANT V 0.95. LS III 2.83–3.60 × BD III. ANT VI with 21–23 basal setae and 6–8 extra setae on the PT. URS 0.40 × ANT III, 0.80–0.84 × ANT VI, and 1.02 × HT II, with 11 accessory setae ()). Fore wings with scale elements on entire membrane, media once-branched ()) and 15–16 campaniform sensilla in the area of articulation. Pterostigma with 17–18 fine, hair-like setae, 0.030–0.038 mm long. Hind wings with 9–11 campaniform sensilla ()). III FEMORA setae 0.050–0.080 mm long. III TIBIAE setae 0.070–0.075 mm long. FT I with 5 ()), MT I with 3 ()) and HT I with 2 sense pegs ()). HT Ib 2.33 × HT Id, 0.53 × HT Iv and 0.87 × HT Ii. HT II 0.39 × ANT III and 0.82 × ANT VI. Dorsal abdominal cuticle with visible polygonal sculpture and very small, rounded scleroites at setal bases (). SIPH sclerites with irregular edges ()). Dorsal abdominal setae 0.060–0.100 mm long. Abdomen with marginal sclerotic plates on ABD I–III and cross-band on ABD VIII. ABD VIII with 23 setae. Genital plate with irregular and divided proximal part and numerous setae ()).
Diagnosis
This very distinct species can be easily separated from all other Sinolachnus species by the fewest antennal sensilla – only 66–70 on ANT III, 14–16 on ANT IV, 12 on ANT V and 4 on ANT VI (165 and more on ANT III, 25 and more on ANT IV, 22 and more on ANT V and 7–16 on ANT VI in all remaining species of Sinolachnus). Sinolachnus nipponicus differs moreover from all other species by the presence of long, fine and pointed setae – type I trichoid sensilla (of the same features as the basal setae) on the ANT VI PT (in all remaining species the PT bears only apical and subapical setae – type II trichoid sensilla) and the largest ratio of PT/BASE – 0.73–0.83 (0.33–0.46 in remaining species).
Etymology
The name of the new species is derived from the name of Japan in Japanese – “Nippon” and the suffix “ensis” for species names related to places.
Host plant
Most probably any species of Elaeagnus. This species has been collected in a cave by Japanese Collembola researcher R. Yoshii.
Distribution
Japan ().
Remarks
This is the first record of the genus Sinolachnus in Japan. This single alate has been collected during work by R. Yoshi (well known as a cave biologist) and most probably sent to R. Takahashi, who designated it as S. niitakayamensis (Lachnus on the slide). So far no other morphs, including the sexual generation, are known.
Sinolachnus plurisensoriatus (Zhang, Citation1988) comb. nov.
Cinara plurisensoriata Zhang, Citation1988: 169(; )
Material examined
HOLOTYPE: China, Tibet: Medog County, 2000 m, alate viviparous female, 23.IX.1982, Han Yin-heng leg. (coll. no. 7786-1-1-I, NZMC).
Alate viviparous female – redescription
Colour in life: unknown. Pigmentation of mounted specimens: Head and thorax brown, ANT brown. Legs brown with slightly paler proximal end of femora. SIPH, abdominal sclerotization and genital plate brown ()). Morphometric characters. HW 0.35 × ANT. Head setae 0.090–0.110 mm long. ANT 0.56–0.57 × BL. ANT III much shorter that the remaining segments with 271–290 secondary rhinaria (), ANT IV as long as ANT V with 70–74 secondary rhinaria, ANT V shorter than ANT VI with 70–75 secondary rhinaria ()). ANT VI with 23–32 secondary rhinaria ()) and PT 0.50–0.59 × BASE. Other antennal ratios: ANT VI/ANT III 0.42–0.43, ANT V/ ANT III 0.33–0.34, ANT IV/ANT III 0.32–0.33, ANT IV/ANT V 0.96–1.00. LS III 2.00 × BD III. ANT VI with 24–28 basal setae. URS 0.33 × ANT III, 0.76–0.78 × ANT VI, and 0.85–0.88 × HT II, with 12 accessory setae ()). Fore wings uniformly light brown pigmented with paler basal parts, with 12–14 sensilla near the base, media twice-branched and scale elements on the whole surface of the wing membrane with smooth basal portion ()). Pterostigma with 25–30 long, thick and stiff setae, 0.077–0.102 mm long. Hind wings with 13–15 sensilla near base ()). III FEMORA setae 0.050–0.100 mm long. III TIBIAE setae 0.065–0.125 mm long. FT I with 8 ()), MT I with 5 ()) and HT I with 2 sense pegs ()). HT Ib 2.75 × HT Id, 0.55 × HT Iv and 0.73 × HT Ii. HT II 0.37–0.39 × ANT III and 0.88 × ANT VI. Dorsal abdominal cuticle with poorly visible polygonal sculpture and very small, rounded scleroites at setal bases (). SIPH sclerites with rather regular edges ()). Dorsal abdominal setae 0.035–0.130 mm long. Abdomen with marginal sclerotic plates on ABD I–IV and cross-bands on ABD VII–VIII. ABD VIII with 21 setae. Genital plate with slightly irregular edges and symmetric indentations in the middle of distal and proximal parts ()).
Diagnosis. As with S. elaeagnensis and S. taiwanus, S. plurisensoriatus can be easily distinguished from other species by the twice-branched media of the fore wings. Together with S. elaeagnensis, this species differs from S. taiwanus by its larger body size – more than 3 mm (body about 2 mm in S. taiwanus) and higher ratio of PT/BASE – 0.45–0.75 (about 0.25 in S. taiwanus). From all other species of Sinolachnus of which the material was available to us, S. plurisensoriatus and S. elaeagnensis are characterized by the highest (an almost enormous) number of antennal sensilla – 271-280 on ANT III, 70–95 on ANT IV, 60–75 on ANT V and 15–32 on ANT VI (55–255 on ANT III, 14–70 on ANT IV, 12–60 on ANT V and 4–16 on ANT VI in remaining species). Sinolachnus plurisensoriatus differs from S. elaeagnensis by: higher ratio of URS/HT II – 0.85–0.88 (0.66–0.71 in S. elaeagnensis), higher ratio of URS/ANT VI – 0.76–0.78 (0.68–0.71 in S. elaeagnensis), more sensilla on ANT VI – 23-32 (15–20 in S. elaeagnensis), 24–28 basal setae (34–38 in S. elaeagnensis) and ABD VIII with 21 setae (30–37 in S. elaeagnensis).
Host plant
Unknown. Most probably species of Elaeagnus as in most other species.
Distribution
Tibet, China ().
Remarks
Sinolachnus plurisensoriatus for several years remained unrecognized and described in the genus Cinara mostly due to the character of the pointed structure of URS (especially RV). This was most probably due to the very poor level of the knowledge of Sinolachnus as a whole. Apterous viviparous females, as well as the sexual generation, are so far unknown.
Sinolachnus rubi (Ghosh & Raychaudhuri, Citation1972) comb. nov.
Maculolachnus rubi Ghosh & Raychaudhuri, Citation1972: 101
(; )
Material examined
PARATYPES: India, Meghalaya, Shillong, Assam, 19.XI.1970, apterous viviparous female, Rubus sp., Sarkar leg., BM 1984–340 (1) BMNH; apterous viviparous female, BM 1984–340 (2) BMNH; apterous viviparous female, BM 1984–340 (3) BMNH
Apterous viviparous female – redescription
Colour in life: dark brown (Ghosh & Raychaudhuri Citation1972). Pigmentation of mounted specimens: Head and thorax brown, ANT brown with paler basal part of ANT III. Legs brown with paler proximal parts of femora. SIPH, abdominal sclerotization and genital plate brown ()). Morphometric characters. HW 0.42–0.45 × ANT. Head setae 0.08–0.10 mm long. ANT 0.52–0.54 × BL. ANT III shorter than the remaining segments with 3–6 secondary rhinaria, ANT IV shorter or slightly longer than ANT V with 5–6 secondary rhinaria ()), ANT V shorter than ANT VI with 3–4 secondary rhinaria ()). ANT VI with 2–3 secondary rhinaria ()) and PT 0.56–0.62 × BASE. Other antennal ratios: ANT VI/ANT III 0.40–0.42, ANT V/ ANT III 0.35–0.36, ANT IV/ANT III 0.31–0.38, ANT IV/ANT V 0.86–1.09. LS III 2.12–2.75 × BD III. ANT VI with 22–25 basal setae. URS 0.36 × ANT III, 0.85–0.92 × ANT VI, and 0.83–0.92 × HT II, with 11–12 accessory setae ()). Mesosternal furca wide, fused at base with two pointed protuberances ()). Thorax with minute denticles on sclerites ()). III FEMORA setae 0.050–0.110 mm long. III TIBIAE setae 0.050–0.110 mm long. Tarsi with very short dorsal length ()). FT I with 10–12 ()), MT I with 8–10 ()) and HT I with 2–3 sense pegs ()). HT Ib 1.80–2.00 × HT Id, 0.42–0.50 × HT Iv and 0.57–0.61 × HT Ii. HT II 0.40–0.42 × ANT III and 1.00–1.06 × ANT VI. SIPH sclerites with irregular edges ()). Dorsal abdominal setae 0.100–0.110 mm. long ()). Abdomen with spinal sclerotic cross-bar on ABD I, spino-pleural cross-bar on ABD VIII and cross-band on ABD VIII. Abdominal cuticle with or without sclerites, with polygonal sculpture ()). ABD VIII with 19–22 setae.
Diagnosis
Apterous viviparous females are the only known morphs of this species and by the extremely small number of apterae of other morphs, we were able only to compare S. rubi with S. niitakayamensis (after Takahashi, 1926) and S. yushanensis. Apterous viviparous females of both S. rubi and S. yushanensis differ from S. niitakayamensis by their larger body size – more than 3 mm (body about 2.50 mm in S. niitakayamensis), longer antennae – 1.50–1.90 (about 1.20 in S. niitakayamensis) and longer tibiae – 1.77–1.92 (about 1.30 in S. niitakayamensis). Sinolachnus rubi differs from S. yushanensis especially by the presence of secondary rhinaria on ANT VI (absent in S. yushanensis), 22–25 basal setae (30–30 in S. yushanensis) and the lower ratio of HW/ANT – 0.42–0.45 (0.50–0.51 in S. yushanensis).
Host plant
This species is associated with undetermined Rubus species from which it has been collected at the altitude of 1598 m and is always attended by ants.
Distribution
So far this species is known only from Shillong in Meghalaya (India) from the type series ().
Remarks
The geographic proximity to Bhutan may raise some questions if apterous viviparous females of this species and alate viviparous females of S. elaeagnensis are conspecific but we know of no study (morphological of the same morphs or molecular) to determine this and so we treat these species as separate. This species was firstly described in the genus Maculolachnus Gaumont most probably due to some superficial similarity with M. submacula apterae. The character of dorsal cuticle, presence of secondary rhinaria on ANT VI and the arrangement of accessory rhinaria in respect to the major rhinarium and finally the number of sense-pegs of particular first segments of tarsi undoubtedly indicate that the species should be treated as Sinolachnus member. There is no information on the alate viviparous females, or the sexual generation, of this species.
Sinolachnus taiwanus Tao, Citation1989
Tao Citation1989: 27
())
Alate viviparous female – redescription (based on Tao Citation1989)
Pigmentation
Head, antennae, two last rostrum segments, thorax, legs, stigma and veins (except media) pale. Siphunculi, ABD VII and VIII, cauda and anal plate blackish brown; the remaining anatomy white (Tao Citation1989) – most probably this description refers to living or alcohol-preserved samples rather than to mounted specimen. Available morphometric characters. ANT 0.68 × BL. ANT III shorter than the remaining segments, ANT IV slightly longer than ANT V, ANT V shorter than ANT VI. PT 0.25 × BASE. Other antennal ratios: ANT VI/ANT III 0.42, ANT V/ ANT III 0.33 (~ 0.34), ANT IV/ANT III 0.30, ANT IV/ANT V 0.90. URS with eight pairs of setae. Fore wings with media twice-branched and scale elements sparsely distributed only on the margin of wing membrane ()).
Diagnosis
We considered carefully whether to include this species in the key for alate viviparous females of this poorly known genus, for obtaining reliable results. In accordance with the description and robust comparative analyses of the remaining species with the available material, we now compare this species together with S. elaeagnensis and S. plurisensoriatus regarding the twice branched media of fore wing. Sinolachnus taiwanus differs by its much smaller body size (~ 2 mm), its hyaline wings with scale elements sparsely distributed and visible only in the distal margins, as well as in the ratio of ANT IV/ ANT V ~ 0.90. In both S. elaeagnensis and S. plurisensoriatus the alates are larger, wings are pigmented with scale elements of the whole surface of the wing, and the ratio of ANT IV/ ANT V is 0.96-.1.13.
Host plant
Unknown (collected from air samples)
Distribution
Nantou Hsien, Meinfeng, 2150 m, Taiwan ().
Remarks
Sinolachnus taiwanus is the most poorly known and enigmatic species in this genus. We were not able to examine any material as this species has been described from only one sample (not exceptional in Sinolachnus) which moreover must be recognized as lost, according to Chi-Feng Lee (Taiwan Agricultural Research Institute, TARIIC); hence, material of this species is absent in the collection. Other morphs including oviparous female and male are unknown.
Sinolachnus takahashii sp. nov.
(; )
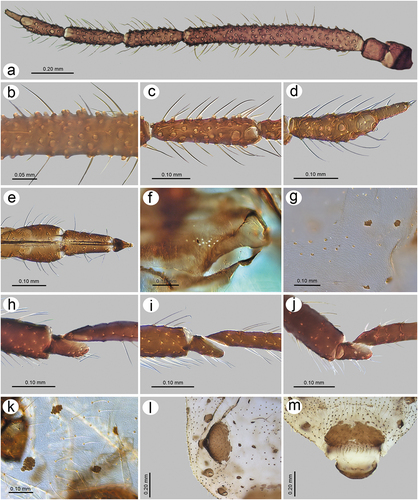
Figure 23. Alate viviparous females of Sinolachnus: (a) S. elaeagnensis, (b) S. nipponicus sp. nov., (c) S. niitakayamensis, (d) S. plurisensoriatus comb. nov., (e) S. takahashii sp. nov., (f) S. yushanensis sp. nov.
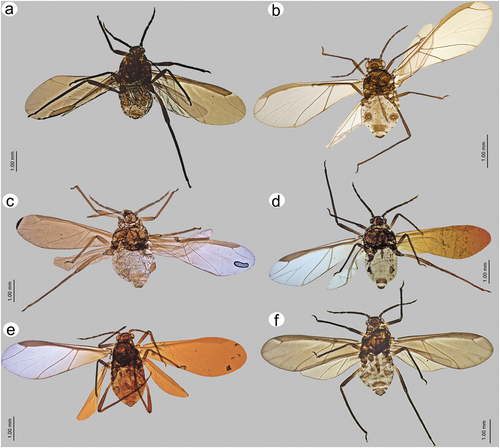
Figure 24. Morphological features of alate viviparous female of S. elaeagnensis: (a) antenna, (b) sensilla structure on ANT III, (c) ANT V with sensilla, (d) ANT VI with sensilla, (e) ultimate rostral segments, (f) hind wing sensilla, (g) dorsal abdominal cuticle, (h) first segment of fore tarsus, (i) first segment of middle tarsus, (j) first segment of hind tarsus, (k) dorsal abdominal chaetotaxy, (l) SIPH, (m) genital plate.
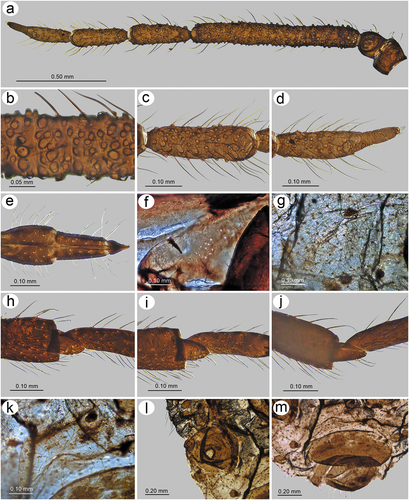
Figure 25. Morphological features of alate viviparous female of S. niitakayamensis: (a) antenna, (b) sensilla structure on ANT III, (c) ANT V with sensilla, (d) ANT VI with sensilla, (e) ultimate rostral segments, (f) hind wing sensilla, (g) dorsal abdominal cuticle, (h) first segment of fore tarsus, (i) first segment of middle tarsus, (j) first segment of hind tarsus, (k) dorsal abdominal chaetotaxy, (l) SIPH, (m) genital plate.
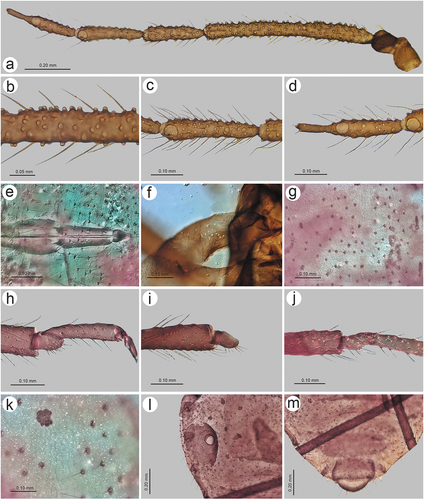
Figure 26. Morphological features of alate viviparous female of S. nipponicus sp. nov.: (a) antenna, (b) sensilla structure on ANT III, (c) ANT V with sensilla, (d) ANT VI with sensilla, (e) ultimate rostral segments, (f) hind wing sensilla, (g) dorsal abdominal cuticle, (h) first segment of fore tarsus, (i) first segment of middle tarsus, (j) first segment of hind tarsus, (k) dorsal abdominal chaetotaxy, (l) SIPH, (m) genital plate.
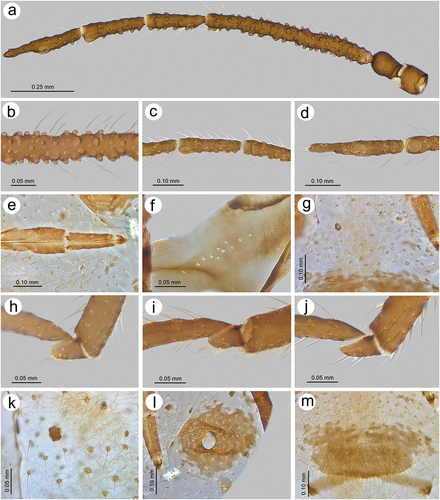
Figure 27. Morphological features of alate viviparous female of S. plurisensoriatus comb. nov.: (a) antenna, (b) sensilla structure on ANT III, (c) ANT V with sensilla, (d) ANT VI with sensilla, (e) ultimate rostral segments, (f) hind wing sensilla, (g) dorsal abdominal cuticle, (h) first segment of fore tarsus, (i) first segment of middle tarsus, (j) first segment of hind tarsus, (k) dorsal abdominal chaetotaxy, (l) SIPH, (m) genital plate.
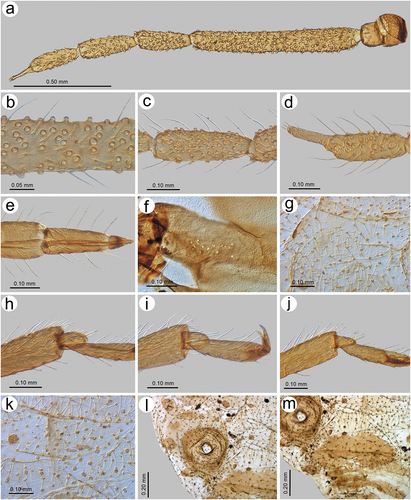
Figure 28. Morphological features of apterous viviparous female of S. rubi comb. nov.: (a) antenna, (b) sensilla on ANT IV, V and VI, (c) ANT VI with sensilla, (d) ultimate rostral segments, (e) mesosternal furca, (f) dorsal thoracic cuticle, (g) HT II, (h) first segment of fore tarsus, (i) first segment of middle tarsus, (j) first segment of hind tarsus showing the peg-like sensillum, (k) SIPH, (l) dorsal abdominal chaetotaxy, (m) dorsal abdominal cuticle.
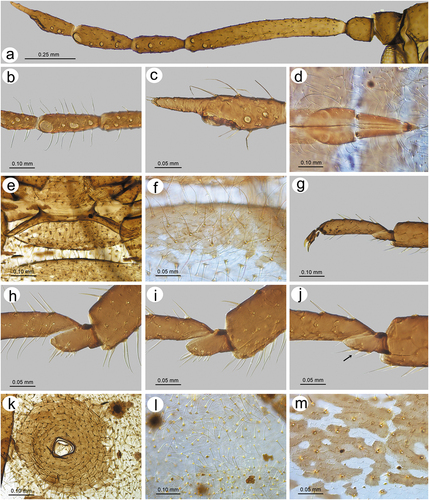
Figure 29. Morphological features of alate viviparous female of S. takahashii sp. nov.: (a) antenna, (b) sensilla structure on ANT III, (c) ANT V with sensilla, (d) ANT VI with sensilla, (e) ultimate rostral segments, (f) hind wing sensilla, (g) dorsal abdominal cuticle, (h) first segment of fore tarsus, (i) first segment of middle tarsus, (j) first segment of hind tarsus, (k) dorsal abdominal chaetotaxy, (l) SIPH, (m) genital plate.
http://urn:lsid:zoobank.org:act:A8A4CCB2-3434-4ADF-A61C-665111F2D5E6
Material examined
HOLOTYPE: Taiwan: Domon, Karenko, alate viviparous female with the letter “L”, 13.XI.1925, Takahashi leg. (coll. no. 31-1-1-3, TARIIC). PARTYPES: Three alate viviparous female on the same slide, data the same as the holotype; two alate viviparous females, other data the same as in the holotype (coll. no. 31-1-1-4, TARIIC); alate viviparous female, data the same as in the holotype with Elaeagnus oldhamii as the host plant (coll. no. 1984–340 NHMUK).
Alate viviparous female - redescription
Colour in life: unknown. Pigmentation of mounted specimens: ANT dark brown, head and thorax brown, legs brown with paler proximal parts of femora. Wings hyaline. Abdomen pale brown with brown sclerotization ()). Morphometric characters. HW 0.35–0.40 × ANT. Head setae 0.085–0.110 mm long. ANT 0.50–0.56 × BL. ANT III as long as or slightly shorter than the remaining segments with 220–255 rhinaria (), ANT IV slightly longer than ANT V with 50–70 rhinaria, ANT V as long as or shorter than ANT VI with 30–56 rhinaria ()). ANT VI with 4–12 rhinaria (Fig. 29ad). PT 0.42–0.50 × BASE. Other antennal ratios: ANT VI/ANT III 0.34–0.36, ANT V/ ANT III 0.29–0.35, ANT IV/ANT III 0.32–0.37, ANT IV/ANT V 0.96–1.25. LS III 2.10–2.44 × BD III. ANT VI with 21–24 basal setae. URS 0.25–0.27 × ANT III, 0.73–0.77 × ANT VI, and 0.74–0.80 × HT II, with 14–16 accessory setae ()). Fore wings with 9–12 sensilla near the base, media twice-branched and scale elements mostly in the distal part and smooth basal part ()). Pterostigma with 26–30 fine, hair-like setae, 0.040–0.050 mm long. Hind wings with 12–18 sensilla near base ()). III FEMORA setae 0.060–0.110 mm long. III TIBIAE setae 0.070–0.120 mm long. FT I with 5–6 ()), MT I with 4 ()) and HT I with 2 sense pegs ()). HT Ib 1.90–2.25 × HT Id, 0.40–0.45 × HT Iv and 0.66–0.75 × HT Ii. HT II 0.32–0.36 × ANT III and 0.92–1.01 × ANT VI. Dorsal abdominal cuticle with well-visible polygonal sculpture and very small, rounded scleroites at setal bases (). SIPH sclerites with scattered edges ()). Dorsal abdominal setae 0.060–0.125 mm long. Abdomen with marginal sclerotic plates on ABD I–IV and cross-bands on ABD VII–VIII. ABD VIII with 23–27 setae. Genital plate with scattered edges and symmetric indentations in the middle of distal and proximal parts ()).
Diagnosis
Alate viviparous females of S. takahashii with large number of secondary rhinaria on ANT III, ANT IV and V, lower ratio of HT/ANT, lower ratio of URS/ HT II and URS/ANT VI similar to that of S. elaeagnensis and S. plurisensoriatus. From both species, S. takahashii can be distinguished especially by the singly branched media of wings, lower ratio of ANT VI/ANT III (0.34–0.36), and smaller number of secondary rhinaria on ANT IV (50–70) and ANT V (30–60), while in S. elaeagnensis and S. plurisensoriatus the media of wings is twice-branched; also, the ANT VI/ANT III ratio is 0.39–0.40 and 0.42–0.44. The number of secondary rhinaria on ANT IV and ANT V is 87–95 and 60–65 in S. elaeagnensis and 70–74 and 70–75 in S. plurisensoriatus.
Etymology
We are pleased to name the new species to honour Riochi Takahashi, an outstanding Japanese aphidologist who has worked for several years on the aphid fauna of Taiwan and was the collector of the specimens.
Host plant
Alate viviparous females of this species have been collected from an undetermined Elaeagnus species and E. oldhamii.
Distribution
This species is known from Domon in Taiwan ().
Remarks
Slides of the alate viviparous female and the final-instar larvae of the apterous viviparous female of this species was certainly examined by Hille Ris Lambers (the collection data are not the same as of the data of alate viviparous females of S. niitakayamensis lectotype and paralectotype), deposited in his collection and, after his death, moved with all other slides to the Natural History Museum of London. In fact Hille Ris Lambers (Citation1956) erected and described the genus Sinolachnus on the basis of S. takahashii. Apterous viviparous females, as well as the sexual generation, remain unknown.
Sinolachnus yushanensis sp. nov.
(, 30–32; )
Figure 30. Morphological features of apterous viviparous female of S. yushanensis sp. nov.: (a) antenna, (b) sensilla on ANT IV, V and VI, (c) ANT VI with sensilla, (d) ultimate rostral segments, (e) mesosternal furca, (f) dorsal thoracic cuticle, (g) HT II, (h) first segment of fore tarsus, (i) first segment of middle tarsus, (j) first segment of hind tarsus showing the peg-like sensillum, (k) SIPH, (l) dorsal abdominal chaetotaxy, (m) dorsal abdominal cuticle.
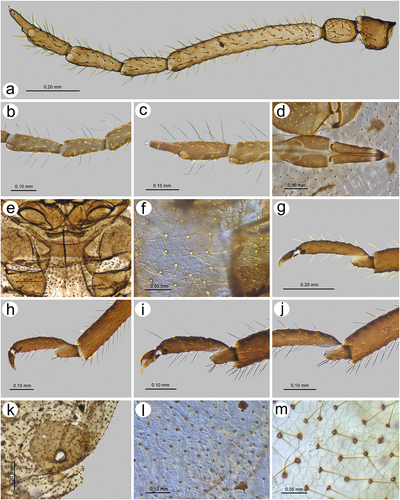
Figure 31. Morphological features of alate viviparous female of S. yushanensis sp. nov.: (a) antenna, (b) sensilla structure on ANT III, (c) ANT V with sensilla, (d) ANT VI with sensilla, (e) ultimate rostral segments, (f) hind wing sensilla, (g) dorsal abdominal cuticle, (h) first segment of fore tarsus, (i) first segment of middle tarsus, (j) first segment of hind tarsus, (k) dorsal abdominal chaetotaxy, (l) SIPH, (m) genital plate.

Figure 32. Biology of S. yushanensis: (a, b) colony of numerous apterous viviparous females and one alate viviparous female showing the pigmentation. Freshly molted individuals are characterized by shiny dorsum in contrast to the matt ones, (c, d) freshly molted apterous viviparous female and alate viviparous female visited by ants, (e) colony of mostly larvae of apterous viviparous females and nymphs of alate viviparous females and a freshly molted alate viviparous females with pale wings, (f) adult apterous viviparous female attacked by a neuropteran larva.
http://urn:lsid:zoobank.org:act:A47559D9-3715-4DC5-9696-C6C76E0AA631
Material examined
HOLOTYPE: Taiwan: Dongpu, Xinyi Township, Nantou County, apterous viviparous female, 10.IV.2020, Elaeagnus oldhamii, H-T Yeh leg. (coll. no. 281–1, DZUS). PARATYPES: apterous viviparous female, data the same as in holotype (coll. no. 281–2, DZUS); apterous viviparous female, data the same as in holotype (coll. no. 281–3, DZUS); apterous viviparous female, 17.XII.2020, (coll. no. 292–1, DZUS); alate viviparous female, 17.XII.2020, (coll. no. 292–2, DZUS); alate viviparous female, 17.XII.2020, (coll. no. 292–3, DZUS); alate viviparous female, 17.XII.2020, (coll. no. 292–4, DZUS); alate viviparous female, 17.XII.2020, (coll. no. 292–5, DZUS); alate viviparous female, 17.XII.2020, (coll. no. 292–6, DZUS); two apterous viviparous females, 10.V.2016, (coll. no. 142–1, DZUS); apterous and alate viviparous female, (coll. no. 142–2, DZUS) – all other data as in the holotype.
Apterous viviparous female – description
Colour in life: Body (head, thorax and abdomen) generally black, sometimes dark brown (different shades of brown in older larvae instars and nymphs), first instar larvae yellowish-orange. Head sometimes slightly paler than the rest of the body. From pronotum to ABD IV a narrow whitish stripe runs in the spinal area. Antennae yellow with darker ANT V and VI. Legs yellow or light brown with darker distal part of tibiae and tarsi. Freshly molted specimens are shiny, turning to a dull colour (). Pigmentation of mounted specimens: Head and thorax brown. ANT brown with yellow pedicel and proximal half of ANT III. Legs brown with darker distal parts of femora, tibiae and tarsi. Abdomen pale brown with brown sclerotization ()). Morphometric characters. HW 0.50–0.51 × ANT. Head setae 0.09–0.13 mm long. ANT 0.44–0.55 × BL. ANT III shorter that the remaining segments with 1–3 rhinaria ()), ANT IV shorter than ANT V with 2–6 rhinaria ()), ANT V shorter than ANT VI with 0–4 rhinaria. ANT VI PT 0.50–0.58 × BASE. Other antennal ratios: ANT VI/ANT III 0.39–0.44, ANT V/ANT III 0.34–0.37, ANT IV/ANT III 0.29–0.35, ANT IV/ANT V 0.80–0.95. LS III 2.37–2.85 × BD III. ANT VI with 30–33 basal setae ()). URS 0.37–0.43 × ANT III, 0.92–0.96 × ANT VI, and 0.92–1.02 × HT II, with 20–24 accessory setae ()). Mesosternal furca wide, fused at base with large, robust stem ()). Thorax with well-visible minute denticles on sclerites forming longitudinal groups ()). III FEMORA setae 0.080–0.110 mm long. III TIBIAE setae 0.070–0.090 mm long. Dorsal aspect of tarsi very short ()). FT I with 6–8 ()), MT I with 4–5 ()) and HT I with 1–2 sense pegs ()). HT Ib 2.00–2.25 × HT Id, 0.47–0.52 × HT Iv and 0.60–0.69 × HT Ii. HT II 0.40–0.42 × ANT III and 0.92–1.01 × ANT VI. SIPH sclerites with only slightly scattered edges ()). Dorsal abdominal setae 0.065–0.130 mm long ()). Abdomen with broken spinal sclerite on ABD VII and a cross-band on ABD VIII. Abdominal cuticle without sclerites, with very well-developed polygonal sculpturing and small, rounded sclerites at setal bases ()). ABD VIII with 22–25 setae.
Alate viviparous female - description
Colour in life: Head and antennae black. A light brown stripe lies between the head and prothorax. Thorax black. Wings uniformly dusky. Legs dark brown to black with orange proximal parts or halves of femora. Abdomen brownish-black. Freshly molted specimens with light brown head and thorax and yellow legs and wings, shiny, turning dull in older specimens (). Pigmentation of mounted specimens: Head and thorax brown, ANT uniformly dark brown. Wings uniformly brown pigmented. Legs brown with slightly paler proximal parts of femora. Abdomen pale brown with brown sclerotization ()). Morphometric characters. HW 0.42–0.46 × ANT. Head setae 0.075–0.120 mm long. ANT 0.51–0.53 × BL. ANT III only slightly shorter that the remaining segments with 165–200 secondary rhinaria (), ANT IV shorter than ANT V with 25–40 secondary rhinaria, ANT V shorter than ANT VI with 22–30 secondary rhinaria ()). ANT VI with 7–16 secondary rhinaria ()) and PT 0.35–0.45 × BASE. Additional antennal ratios: ANT VI/ANT III 0.34–0.38, ANT V/ ANT III 0.31–0.35, ANT IV/ANT III 0.28–0.32, ANT IV/ANT V 0.80–0.96. LS III 2.50–2.85 × BD III. ANT VI with 30–23 basal setae. URS 0.29–0.33 × ANT III, 0.80–0.88 × ANT VI, and 0.88–0.96 × HT II, with 19–20 accessory setae ()). Fore wings with 17–25 sensilla near the base, media one-branched and scale elements on the whole surface of the wing membrane with smooth basal portion ()). Pterostigma with 11–19 fine, hair-like setae, 0.037–0.045 mm long. Hind wings with 17–19 sensilla near base ()). III FEMORA setae 0.070–0.110 mm long. III TIBIAE setae 0.055–0.105 mm long. FT I with 6–8 ()), MT I with 2–5 ()) and HT I with 2 sense pegs ()). HT Ib 2.25–3.00 × HT Id, 0.45–0.52 × HT Iv and 0.69–0.81 × HT Ii. HT II 0.32–0.35 × ANT III and 0.89–0.94 × ANT VI. Dorsal abdominal cuticle with well-visible polygonal sculpturing and small, rounded scleroites at setal bases (). SIPH sclerites with rather regular and smooth edges ()). Dorsal abdominal setae 0.040–0.110 mm long. Abdomen with marginal sclerotic plates on ABD I–III, some scattered spinal sclerites on ABD VI, spino-pleural cross-bar on ABD VII and cross-banding on ANT VIII. ABD VIII with 24–28 setae. Genital plate with slightly irregular edges and symmetric indentations in the middle of distal and proximal parts ()).
Figure 33. Fore wings comparison of alate viviparous females of Sinolachnus: (a) S. elaeagnensis, (b) S. niitakayamensis, (c) S. nipponicus sp. nov., (d) S. plurisensoriatus omb. nov., (e) S. takahashii sp. nov., (f) S. yushanensis sp. nov., (g) S. taiwanus (redrawn from Tao Citation1989).
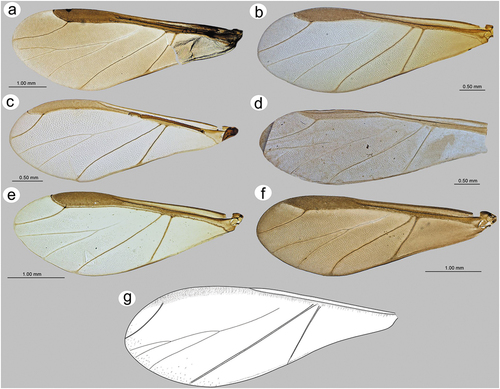
Diagnosis
Apterous viviparous females of S. yushanensis and S. rubi differ from those of S. niitakayamensis by their larger body size, longer antennae and tibiae (see the diagnosis of S. rubi). From S. rubi, apterae differ by ANT VI without secondary rhinaria and 30–33 basal setae (ANT VI with secondary rhinaria and 22–25 basal setae in S. rubi). Alate viviparous females of the new species are most similar to S. nipponicus and S. niitakayamensis especially by the singly branched media in the wings. Alate viviparous females of S. nipponicus have very distinct features which separate them from all other known Sinolachnus species (see diagnosis for S. nipponicus): hence it is critical to include here the differences between S. yushanensis and S. niitakayamensis. Alate viviparous females of S. yushanensis differ from those of S. niitakayamensis by the distinct number of antennal sensilla: 165–200 on ANT III, 22–30 on ANT V and 7–16 on ANT VI (130–160 on ANT III, 35–40 on ANT V and 5–6 on ANT VI in S. niitakayamensis). In S. yushanensis, in the area between the wing articulation and Cu1b, the scale-like elements occupy mostly the proximal part of the membrane, whereas in S. niitakayamensis the same area is occupied by the scale-like elements on the portion near Cu1b. The species are distinguished by:
ANT/BL ratio, 0.51–0.53 in S. yushanensis and 0.58–0.61 in S. niitakayamensis
PT/BASE ratio, 0.35–0.45 in S. yushanensis and 0.57–0.62 in S. niitakayamensis
ANT VI/ANT III ratio, 0.34–0.38 in S. yushanensis and 0.41–0.43 in S. niitakayamensis
URS/HT II ratio, 0.88–0.96 in S. yushanensis and 0.79–0.82 in S. niitakayamensis
URS/ANT VI ratio, 0.80–0.88 in S. yushanensis and 0.70–0.76 in S. niitakayamensis
Etymology
The name of the species is derived from the place of occurrence on Mount Yushan Mountains and the suffix “ensis” for species names related to places.
Host plant and notes on biology
The new species occurs on Elaeagnus oldhamii on which it feeds on the woody parts and is always attended by ants. During observations of S. yushanensis colonies the apterous viviparous females were the most dominant morphs with some alate viviparous females. Colonies of S. yushanensis are often kept by ants in soil shelters (). The ages of particular stages and morphs are revealed in the colour and dorsal abdominal site characters of particular individuals; e.g., young larvae are orange or brownish-yellow, and the final instars and nymphs are similar to the adults. Young, fully pigmented individuals are shiny, turning dull () but the very freshly molted specimens are pale ()). Some individuals serve as prey, syrphid fly larvae (Diptera), for example, which furthermore are cryptic and nearly invisible ()). During the growing season only viviparous generations have been observed (as late as December), suggesting this species is anholocyclic.
Distribution
The new species is known so far from Yushan Mountains (at Dongpu, an altitude of 1.130 m) in Taiwan ().
4. Discussion
4.1. Taxonomy of Sinolachnus
Until now, Sinolachnus has, indeed, been the genus most recently analyzed within Lachninae and even in Aphididae as a whole. Previously our only knowledge about Sinolachnus was based on the first species description of Takahashi (Citation1925, Citation1927), the genus description of Hille Ris Lambers (Citation1956) and the second species description of Chakrabarti and Das (Citation2015). The description of S. taiwanus by Tao (Citation1989) cannot be regarded as an important contribution as it was too general and lacking in the detail necessary for further analysis. To date, only three species are known in the genus Sinolachnus: S. niitakayamensis, S. taiwanus from Taiwan and S. elaeagnensis from Bhutan. Sinolachnus niitakayamensis was the first species identified in this genus but, as with many other Lachninae described by Takahashi (Citation1925), it was placed in the genus Lachnus. More recently, Hille Ris Lambers (Citation1956) decided that the unusually numerous sensilla on all flagellar segments and the many “sense-pegs” on the first segments of the tarsi were good features to erect a separate genus for this species. From its earliest recognition, members of Sinolachnus have been found to be rare, possibly lacking sexual morphs (maybe the very small first instar larvae serve as the persistent stages), and can be rather difficult to find especially when hidden under soil shelters constructed by ants. These may be the primary reasons why so little was known about Sinolachnus for almost 100 years despite an otherwise good understanding of the aphid fauna of Taiwan. Potential confusion may have arisen during determination of the collected material, which was automatically assigned by Takahashi to S. niitakayamensis (evidently a large number of antennal sensilla was for him rather a specific feature). The second as good as possible described species – S. elaeagnensis has been reported by Chakrabarti and Das (Citation2015) in which the authors found several morphological differences from the type species. The poor level of knowledge and extremely small quantity of existing material of these entities may have contributed to the failure to recognize other Sinolachnus species (and thus determined as S. niitakayamensis), or described in other Lachninae genera. Sinolachnus rubi, described from apterous viviparous females from Meghalaya (India), has been placed in Maculolachnus (Ghosh & Raychaudhuri, Citation1972) most probably owing to the superficial similarity in general morphology to M. submacula in its numerous, scattered sclerites on the dorsal abdomen. These authors did not take into consideration some very discreet but important characteristics, e.g., the presence of secondary rhinaria on all flagellar segments (in Maculolachnus the rhinaria are placed on ANT III and IV), the primary rhinarium without any sclerotic collar or rosette (in Maculolachnus primary rhinaria are always surrounded by an evident sclerotic collar or rosette), numerous peg-like sensilla (“sense pegs”) on first segments of tarsi, especially of fore and middle legs (in Maculolachnus first segments of all tarsi always with only one peg-like sensillum) and the dorsal cuticle with minute scales or denticles (in Maculolachnus the cuticle even sclerotized is smooth). A similar situation involves the alate viviparous female of S. plurisensoriatus from Tibet, which has not been recognized even after erection of the genus by Hille Ris Lambers (on the basis of alate viviparous females) and its placement in Cinara (Zhang & Zhong, Citation1988). Quite likely, contemporary authors still had serious problems with the access to all papers on aphid taxonomy and the lack of awareness of the host plant was an additional obstacle. Zhang (Zhang & Zhong, Citation1988) decided on the identity of the new species on the basis of the excessive number of small sensilla on the flagellum, placing it within Cinara most probably due to the shape of ultimate rostral segments and the well-separated RV from RIV. All morphology mentioned in the diagnosis and description of Sinolachnus fully supports the placement of this species in the genus. Moreover, all Cinara species like remaining Eulachnini (e.g. Eulachnus and Pseudessigella) are characterized by only one peg-like sensillum on the first segments of tarsi (Kanturski et al. Citation2017a, Citation2017c, Citation2017d). As a result of our detailed comparative studies on all representatives of Sinolachnus, we propose a new and greatly improved list of eight species of this genus. Three species: S. elaeagnensis, S. niitakayamensis and S. taiwanus are the existing ones in the literature and databases (Remaudière & Remaudière Citation1997; Favret Citation2022); three new species: S. nipponicus, S. takahashii and S. yushanensis, and two species for several years treated as members of other Lachninae genera: S. plurisensoriatus (=Cinara plurisensoriata) and S. rubi (=Maculolachnus rubi). Also, the distribution of the genus has been extended from two countries (Bhutan and Taiwan) to include India, China (Tibet), and Japan.
As mentioned previously, the antennal features of especially the alate viviparous females in Sinolachnus were most probably responsible for confusion in the recognition of particular species in this genus. Chakrabarti and Das (Citation2015) provided detailed differences between S. elaeagmensis and S. niitakayamensis additionally pointing out (outside the evident differences, e.g. wing venation) differences in the number of sensilla on particular antennomeres. The new species (S. nipponicus, S. takahashii and S. yushanensis) also are characterized by several differences in the features of wings (pigmentation, distribution of scale-like elements, number of pterostigma setae) and antennae (number of sensilla on particular antennal segments and ANT VI chaetotaxy). Taking into account the morphological similarities of Sinolachnus species, it is clear that they form two quite distinct groups, based on the number of sensilla on flagellar segments. In this regard, S. elaeagnensis, S. plurisenaoriatus and S. takahashii are one group of species with antennae bearing the largest number of sensilla (220–290 on ANT III, 50–95 on ANT IV, 30–75 on ANT V and 4–32 on ANT VI). Considering other, morphometric results, those three species moreover have similar ratios of: HT/ANT (0.33–0.40), ANT IV/ANT V (0.96–1.25), URS/HT II (0.66–0.88) and URS/ANT VI (0.68–0.78) and within this group, S. takahashii is the most distinctive. The second group of species (in terms of antennal characters) comprises S. niitakayamensis, S. nipponicus and S. yushanensis. These species are characterized by a lower number of antennal sensilla on particular antennal segments (66–200 on ANT III, 14–40 on ANT IV, 12–40 on ANT V and 4–16 on ANT VI). in contrast to the first group, there are differences of morphometric ratios (S. elaeagnensis, S. plurisensoriatus and S. takahashii) such as HT/ANT (0.41–0.46), ANT IV/ANT V (0.80–1.00), URS/HT II (0.79–1.02) and URS/ANT VI (0.80–0.88 for S. nipponicus and S. yushanensis). Although the number of antennal sensilla is a good character to distinguish these two species groups, the group “niitakayamensis/nipponicus/yushanensis” is more inclusive. The most distinctive species is S. nipponicus which is, in fact, the most outstanding within the whole genus. Alate viviparous females of S. nipponicus have quite unique features like small body size, the lowest number of antennal sensilla (60–70 on ANT III, 14–16 on ANT IV, 12 on ANT V and 4 on ANT VI), and the sensilla moreover seems to be larger than in other species. Besides the mentioned characters, S. nipponicus stands out from other species by the presence of normal long setae not only on the ANT VI BASE but also on the terminal process. This species differs from other ones in this group by the twice-branched media of the wings. Sinolachnus yushanensis differs from S. niitakayamensis not only by the number of antennal sensilla and morphometric ratios but also by the number of setae on pterostigma (11–19) which additionally is the smallest number within all examined species.
In this revision, S. taiwanus appears to be still the most poorly known and enigmatic. During the description, Tao (Citation1989) gave some information but most of it is not useful for assigning identity to this species (and there is no type material in TARIIC, where according to the introduction of the paper it is supposed to be deposited). Instead, the species description concerns generic characters common to most Lachninae. Tao (Citation1989) did not give detailed information of sensilla even on ANT V or VI (if the number of ANT was too high) and besides the relative lengths of antennal segments, he did not provide measurements for the URS and HT II which could be extremely useful. Even the diagnosis from the type species is rather poor and there are only two differences given by Tao: the body length and the information on the twice-branched media. Ultimately, the relative length of antennal segments allows us to obtain the ratios of particular antennomeres. Further, we have determined that the information on wing venation and a small number of scale-like elements (“sparsely imbricated”) and ratios of antennal segments should be enough to maintain the species status of S. taiwanus as nothing more can be done without examination of the types material or other material similar to that in the description. It is puzzling that Tao described a second Sinolachnus species from the material collected in 1985 in 1989 (manuscript accepted in June, 1989) but did not discuss S. taiwanus in the “Aphid fauna of Taiwan Province” (Tao Citation1991), and on pages 95–96 only S. niitakayamensis is given as the representative of this genus from Taiwan. Sinolachnus taiwanus has been added to the list of species in “List of Aphidoidea (Homoptera) of China” (Tao Citation1999).
Studies on this genus have shown and confirmed the importance of correct indication of relevant and valid features in morphological analyses in species recognition. Our results reinforced previous ones that detailed analyses have to be done as in other Lachninae genera like the European Lachnus species which, although well placed within the genus, may be confusing in species recognition despite the validity of morphological traits (Mróz et al. Citation2015).
4.2. Sinolachnus and other Tuberolachnini genera
Sinolachnus was previously a member of the tribe Lachnini when only three tribes of Lachninae (Lachnini, Cinarini and Tramini) were recognized (Remaudière & Remaudière Citation1997 and all papers until 2016). Chen et al. (Citation2016) confirmed Normark’s (Citation2000) first results and proposed five tribes within Lachninae, with Tuberolachnini and Stomaphidini as independent taxa. As a result of their molecular research, the authors left in Lachnini the genera Lachnus, Maculolachnus, Longistigma and Pterochloroides and proposed four genera: Nippolachnus, Pyrolachnus, Sinolachnus and Tuberolachnus as members of the tribe Tuberolachnini. In fact, Sinolachnus was absent in their analyses and transferring this genus to Tuberolachnini was, potentially, arbitrary as the authors did not provide any comparison nor comment about the similarities or differences between the Tuberolachnini genera. On the other hand, it should be emphasized that Tuberolachnini is a group of genera characterized by some divergent features. The representatives of Nippolachnus are medium in size to large with very characteristic alate viviparous females (; 35(b)). Moreover, Nippolachnus species feed only on the leaves and never on stems or branches like the other Tuberolachnini and most Lachninae genera (Kanturski et al. Citation2018b). Pyrolachnus and Tuberolachnus species (; 35(c) and (d)) constitute some of the largest Lachninae (and aphids as a whole right after Stomaphis and Longistigma). Additionally, apterous viviparous females in Tuberolachnus are characterized by a dorsal abdominal tubercle and alate viviparous females of Pyrolachnus are easy to recognize due to the dusky basal part of fore wings ()). On this background, Sinolachnus species ()) are the smallest Tuberolachnini (additionally with the shortest tibiae) and well distinguished by their often uniformly brown pigmented wings of alate viviparous females ()). The wings of Sinolachnus differ from other Tuberolachnini by shorter and wider pterostigma with gentle edges, evidently curved Cu1a and media which arises directly from the Sc+R + M+ Cu (; 35(a)). The wings of alate viviparous females in Nippolachnus, Pyrolachnus and Tuberolachnus wings are characterized by a long and narrow pterostigma with pointed tip, straight or only slightly (in Tuberolachnus) curved Cu1a and media which does not reach the Sc+R + M+ Cu ().
Figure 34. Comparison of Sinolachnus and remaining Tuberolachnini apterous viviparous females: (a) Sinolachnus yushanensis, (b) Nippolachnus piri, (c) Pyrolachnus pyri, (d) Tuberolachnus salignus.
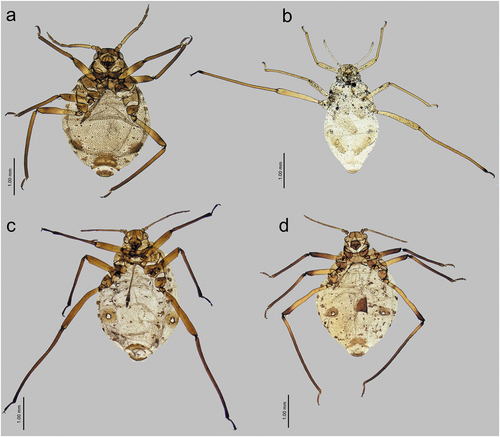
Figure 35. Comparison of Sinolachnus and remaining Tuberolachnini alate viviparous females: (a) Sinolachnus yushanensis, (b) Nippolachnus piri, (c) Pyrolachnus pyri, (d) Tuberolachnus salignus.
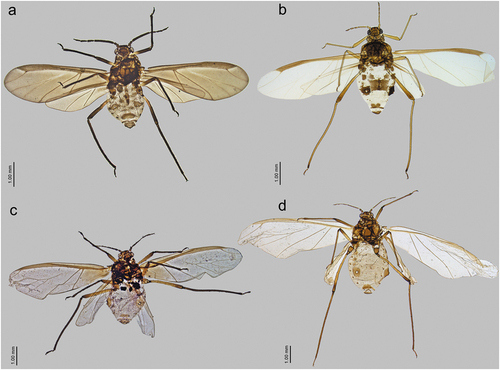
Antennae in Sinolachnus alate viviparous females are robust and their hundreds of sensilla are the best distinguishing feature ()). The presence of numerous secondary rhinaria on ANT V and VI in alate viviparous females is a distinctive feature in aphids and often varies among species within a given genus. In Sinolachnus, all known alate viviparous females bear secondary rhinaria on ANT V and especially VI, whereas in Nippolachnus only some individuals (and only on one antennal segment) of N. piri bear secondary rhinaria. Beyond this feature, the antennae are characteristized by the very large rhinaria on the remaining segments ()). In alate viviparous female of Pyrolachnus pyri the rhinaria are numerous but the number never reaches even the half of the number of sensilla in Sinolachnus () and they never appear on ANT VI. Large, flat (as in Nippolachnus and Pyrolachnus) secondary rhinaria can be found also in Tuberolachnus from which they are always absent on ANT V and ANT VI ()). In the case of the arrangement of sensilla on ANT VI (position of accessory rhinaria relative to the major rhinarium), Sinolachnus species are quite distinguished from other Tuberolachnini especially by the accessory rhinaria occurring as almost singular structures, of which one (small placoid sensillum) is situated on the PT (); 36(e)). In the remaining genera, the accessory rhinaria always form one group of tightly adjoined structures near the major rhinarium (even in Nippolachnus in which they are almost all moved to the PT) (). Sinolachnus differ from all other Tuberolachnini also in terms of the first segment of tarsi and the number of peg-like setae. In Sinolachnus first segment of tarsi is characterized by its extremely short dorsal length and rounded distal portion of the ventral aspect (). Also, the number of peg-like setae is the largest, especially on the fore and middle tarsi. The remaining Tuberolachnini are characterized by first segments of tarsi with long dorsal length and pointed distal part of ventral length (). Moreover, Nippolachnus species are characterized always by only one () and Tuberolachnus by 2 or 3 peg-like setae on the first segments of tarsi (). Only Pyrolachnus is characterized by a larger (4 or 5) number of peg-like setae on the first segment of fore tarsi and 2 or 3 on middle and hind tarsi ().
4.3. Sinolachnus and Tramini genera
Our attention has been focused not only on Tuberolachnini genera but also especially on one genus from the tribe Tramini – Eotrama and the species E. moerickei. Eotrama has been described by Hille Ris Lambers (Citation1969) as “a link between two aphid groups” – “the relatives of Lachnus” and “the relatives of Lachnini”. Hille Ris Lambers also pointed out that Eotrama is very closely related to Sinolachnus, and after extensive examination of the morphology of representatives of this genus, this is also our position. Sinolachnus apterous viviparous females are more similar to those of E. moerickei especially by the shorter tibiae and a similar ratio of antennae and body lengths (). The alate viviparous females share similarly short and wide pterostigma of blunt edges and robust antennae (). Antennal sensilla in Sinolachnus are numerous and very protuberant ()) which is very similar to characters of antennal sensilla of Protrama radicis ()) and E. moerickei ()). Further, Sinolachnus, Protrama and Eotrama share the presence of secondary rhinaria on ANT V and ANT VI (). This is how a major difference between Sinolachnus and Tuberolachnini in the case of Tramini becomes a similarity – the position and arrangement of accessory rhinaria relative to the major rhinarium. In Protrama (also in Trama) the secondary rhinaria, as in Sinolachnus, are in linear position and occur as singlular structures partly on the ANT VI BASE and spanning the PT (this character of ANT VI antennal sensilla is also present in Stomaphis) (). In this case, E. moerickei is the most outstanding case with secondary rhinaria rather as one group and also rather on the BASE ()). What is very noticeable is the very similar morphology of the first segments of especially fore and middle (Protrama and Trama) and all tarsi (Eotrama) with first segments of tarsi in Sinolachnus representatives. As in Sinolachnus ()), Tramini members are characterized by short dorsal length and rounded distal part of the ventral side and numerous evident peg-like setae on middle and fore tarsi (). Apterous viviparous females of Sinolachnus are obligatory myrmecophilous as Tramini and they are well adapted to this relationship: they can live in soil shelters made by the ants and they have the same morphological adaptations in the form of the trophobiotic organ () recently discovered in Tramini and almost the same as in Protrama (Kanturski et al. Citation2017b; Kaszyca-Taszakowska & Yamamoto Citation2020).
Figure 38. Comparison of Sinolachnus and Eotrama: (a) apterous viviparous female of E. moerickei, (b) alate viviparous female of E. moerickei, (c) apterous viviparous female of S. yushanensis, (d) alate viviparous female of S. yushanensis.
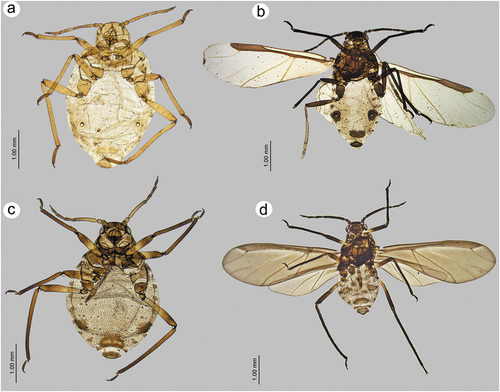
Figure 39. Comparison of Sinolachnus and Tramini: (a) antenna of Sinolachnus nipponicus, (b) antenna of Protrama radicis, (c) antenna of Eotrama moerickei, (d) ANT VI of S. nipponicus, (e) ANT VI of P. radicis, (f) E. moerickei, (g) first segment of fore tarsus of S. yushanensis, (h) first segment of fore tarsus of E. moerickei, (i) first segment of middle tarsus of E. moerickei, (j) first segment of fore tarsus of P. radicis, (k) first segment of middle tarsus of P. radicis, (l) first segment of fore tarsus of Trama troglodytes.

The old division of Lachninae (e.g. Remaudière & Remaudière Citation1997) was based mostly on the basis of ecological features of particular groups: Cinarini (representatives feed on conifers), Lachnini (representatives feed on stems, branches and trunks of deciduous shrubs and trees) and Tramini (representatives feed underground on the roots). Owing to the unique biology of Tramini, their monophyly has never been in doubt, and has been established firmly in the minds of aphidologists. This historical precedent may hinder our understanding of this tribe as occupying habitats above ground. According to Chen et al. (Citation2016), at least five transitions from thick bark to other feeding sites took place during the evolution of Lachninae on angiosperms (including once in Tramini). The closeness of Sinolachnus and Eotrama has been confirmed by Chen et al. (Citation2016), following Hille Ris Lambers (Citation1969), but the authors decided to put it in Tuberolachnini due to the lack of root feeding and its distribution which is similar to Pyrolachnus, Nippolachnus and Tuberolachnus, although the latter have a cosmopolitan distribution.
As a result of the presented similarities and differences, we are of the opinion that Sinolachnus should be a member of this tribe as members of this genus share more similarities with Eotrama and other Tramini than with Tuberolachnini genera. The strong biological argument has been given by Chen et al. (Citation2016) in the case of Tramini after the results of their research were found no longer relevant in the case of Stomaphidini (which feed on trunks and earlier were treated as Lachnini members which feed on deciduous trees), which lost its relevance in the case of Tuberolachnini in which Nippolachnus species feed only on leaves, whereas Pyrolachnus and Tuberolachnus members feed on the woody parts. Treating Sinolachnus as a link between Tramini allows us to advance a novel hypothesis. Maybe in the distant evolutionary past, an ancestor of this group was heteroecious and host alternating with forms which were feeding on trees and with generations on roots (common in Anoeciinae and Eriosomatinae) or other plants (Hormaphidinae, Macrosiphini). This already has been shown in Lachninae on the example of Stomaphis japonica (Yamamoto et al. Citation2020). Certain factors may have caused the separation of the ancient taxa of which two biological distinct lineages started diverging along separate paths. The above questions may be solved even partially by future molecular studies including Sinolachnus and other Lachninae.
Acknowledgements
The first author is sincerely grateful to Paul A. Brown (Natural History Museum, London, UK), Prof. Thierry Bourgoin, dr Adeline Soulier and Laurent Fauvre (Muséum national d’Histoire naturelle, Paris, France) for their friendly assistance and help during several visits and loan of numerous Tuberolachnini and Lachninae samples. We are grateful to Dr Chi-Feng Lee (Taiwan Agricultural Research Institute, Taichung, Taiwan), Prof. Ge-Xia Qiao (Chinese Academy of Sciences, Beijing, China), and Prof. Samiran Chakrabarti (Kalyani University, Kolkata, India) for the type and every available material of Sinolachnus. Special thanks go to Daisuke Sasaki (Hokkaido Research Organization, Japan) for linguistic help with the slides), dr Agnieszka Bugaj-Nawrocka (University of Silesia in Katowice, Poland) for preparing the distributional map and to Professor Donald G.Miller (California State University, Chico, USA) for linguistic correction of the MS. We are grateful to the four anonymous reviewers and Editor for all comments and suggestions to the earlier versions of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Blackman RL, Eastop VF. 2006. Aphids on the World’s herbaceous plants and shrubs. West Sussex: John Wiley & Sons, Ltd. pp. 1439.
- Chakrabarti S, Das D. 2015. A new species and new records of aphids (Hemiptera) from Bhutan. Oriental Insects 48:3–4, 327–336. DOI: 10.1080/00305316.2015.1013183.
- Chakrabarti S, Medda PK, Kanturski M. 2020. Conifer-feeding aphids (Insecta: Hemiptera: Aphididae) of India, Bhutan and Nepal with descriptions of three new species of the genus Cinara. The European Zoological Journal 87(1):659–687. DOI: 10.1080/24750263.2020.1831086.
- Chen R, Favret C, Jiang L, Wang Z, Qiao G. 2016. An aphid lineage maintains a bark-feeding niche while switching to and diversifying on conifers. Cladistics 32(5):555–572. DOI: 10.1111/cla.12141.
- Chen R, Wang Z, Chen J, Jiang L-Y, Qiao G-X. 2017. Insect-bacteria parallel evolution in multiple-co-obligate-aphid association: A case in Lachninae (Hemiptera: Aphididae). Scientific Reports 7(1):10204. DOI: 10.1038/s41598-017-10761-9.
- Favret C. 2022. Aphid Species File. Version 5.0/5.0. Available: http://Aphid.SpeciesFile.org. Accessed May 2022 03.
- Ghosh AK, Raychaudhuri DN. 1972. Studies on the aphids (Homoptera: Aphididae) from Eastern India. Proceedings of the Zoological Society, Calcutta 25:93–107.
- Google Inc. 2022. Google Earth, version 7.3.2. Mountain View, CA. Available: http://www.google.com/earth/index.html. Accessed Sep 2022 20.
- Hille Ris Lambers D. 1956. Lachnids from Elaeagnaceae (Hom. Aph.). Zeitschrift für Angewandte Entomologie 39(4):467–473. DOI: 10.1111/j.1439-0418.1956.tb01264.x.
- Hille Ris Lambers D. 1969. Eotrama gen. nov. (Aphididae, Homoptera), a link between two aphid groups. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 42(3):181–184.
- Ilharco FA, van Harten A. 1987. Systematics. In: Minks AK, Harrewijn P, editors. Aphids: Their biology, natural enemies and control. Amsterdam: Elsevier Science Publishers. pp. 51–77.
- Jousselin E, Cruaud A, Genson G, Chevenet F, Foottit RG, Cœur D’acier A. 2013. Is ecological speciation a major trend in aphids? Insights from a molecular phylogeny of the conifer-feeding genus Cinara. Frontiers in Zoology 10(1):1–18. DOI: 10.1186/1742-9994-10-56.
- Kanturski M, Akbar S-A, Favret C. 2017d. The Bhutan pine aphid Pseudessigella brachychaeta Hille Ris Lambers (Hemiptera: Aphididae: Lachninae) from India reveals the hitherto unknown oviparous female and dwarfish male. Zoological Studies 56:12. DOI: 10.6620/ZS.2017.56-12.
- Kanturski M, Ali Akbar S, Favret C. 2017c. Morphology and sensilla of the enigmatic Bhutan pine aphid Pseudessigella brachychaeta Hille Ris Lambers (Hemiptera: Aphididae)- a SEM study. Zoologischer Anzeiger 266:1–13. DOI: 10.1016/j.jcz.2016.10.007.
- Kanturski M, Bugaj-Nawrocka A, Wieczorek K. 2016. Pine pest aphids of the genus Eulachnus (Hemiptera: Aphididae: Lachninae). How far can their range extend? Agricultural and Forest Entomology 18(4):398–408. DOI: 10.1111/afe.12171.
- Kanturski M, Karcz J, Kaszyca N, Ł D. 2017b. Perianal structures in myrmecophilous subterranean aphids (Insecta: Hemiptera: Aphididae) – Comparative morphology of trophobiotic organ with its first description in Lachninae. Arthropod Structure & Development 46(4):496–507. DOI: 10.1016/j.asd.2017.06.001.
- Kanturski M, Lee Y, Choi J, Lee S. 2018a. First record of Lachnus chosoni (Hemiptera: Aphididae: Lachninae) in the Republic of Korea with description of sexual morphs. Zoological Studies 57:20. DOI: 10.6620/ZS.2018.57-20.
- Kanturski M, Lee Y, Choi J, Lee S. 2018b. DNA barcoding and a precise morphological comparison revealed a cryptic species in the Nippolachnus piri complex (Hemiptera: Aphididae: Lachninae). Scientific Reports 8(1):8998. DOI: 10.1038/s41598-018-27218-2.
- Kanturski M, Ł K, Wieczorek K. 2017a. European species of the aphid genus Eulachnus Del Guercio, 1909 (Hemiptera: Aphididae: Lachninae): revision and molecular phylogeny. Zootaxa Monograph 4356(1):001–08. DOI: 10.11646/zootaxa.4356.1.1.
- Kanturski M, Wieczorek K. 2014. Systematic position of Eulachnus cembrae Börner, with description of hitherto unknown sexual morphs of E. pumilae Inouye (Hemiptera: Aphididae: Lachninae). Deutsche Entomologische Zeitschrift 61(2):123–132. DOI: 10.3897/dez.61.8048.
- Kanturski M, Zia A, Rafi MA. 2017e. The Lachnus of Pakistan with description of a new species (Hemiptera: Aphididae: Lachninae). Journal of Asia-Pacific Entomology 20(4):1219–1227. DOI: 10.1016/j.aspen.2017.09.001.
- Kaszyca-Taszakowska N, Yamamoto TDŁ. 2020. Trophobiotic organ and perianal structures in aphid genus Stomaphis WALKER (Aphididae, Lachninae). Micron 138:102930. DOI: 10.1016/j.micron.2020.102930.
- Mróz E, Trela J, Ł D. 2015. Taxonomic analysis of Lachnus pallipes/longirostris–roboris complex(Hemiptera, Aphididae, Lachninae), with the redescription of sexual morphs and new synonimy. Zoologischer Anzaiger 254:51–61. DOI: 10.1016/j.jcz.2014.11.002.
- Normark B. 2000. Molecular systematics and evolution of the aphid family lachnidae. Molecular Phylogenetics and Evolution 14(1):131–140. DOI: 10.1006/mpev.1999.0699.
- The Plant List. 2013. Version 1.1. Published on the Internet. Available: http://www.theplantlist.org/. Accessed Apr 2022 04.
- QGIS Development Team. 2022. QGIS Geographic Information System. Open-Source Geospatial Foundation Project. Available: http://qgis.osgeo.org. Accessed Sep 2022 20.
- Remaudière G, Remaudière M. 1997. Catalogue des Aphididae du Monde. Paris: INRA. pp. 473.
- Takahashi R. 1925. Aphidididae of Formosa. Part 4. Reports of Departments of Agriculture Government Research Institute Formosa, Japan.
- Takahashi R. 1927. Aphididae of Formosa 5. Reports of Departments of Agriculture Government Research Institute Formosa 22:1–22.
- Takahashi R. 1931. Aphidididae of Formosa. Part 6. Reports of Departments of Agriculture Government Research Institute Formosa, Japan 53:1–127.
- Tao CC. 1989. Aphids from Taiwan collected by Malaise Trap (Homoptera: Aphididae). Journal of Taiwan Museum 42(2):21–30.
- Tao CC. 1991. Aphid fauna of Taiwan Province, China. Taibei: Taiwan Provincial Museum Press. pp. 327.
- Tao CC. 1999. List of Aphidoidea (Homoptera) of China. Taiwan Agricultural Research Institute 77:1–144.
- Théry T, Kanturski M, Favret C. 2018. Molecular Phylogenetic Analysis and Species Delimitation in the Pine Needle-feeding Aphid Genus Essigella (Hemiptera, Sternorrhyncha, Aphididae). Insect Systematics and Diversity 2(4):1; 1–15. DOI: 10.1093/isd/ixy006.
- Yamamoto T, Hattori M, Matsumoto Y, Ueda S, Itino T. 2020. Evolutionary diversification of Japanese Stomaphis aphids (Aphididae, Lachninae) in relation to their host plant use and ant association. The Science of Nature 107(2):14. DOI: 10.1007/s00114-020-1671-4.
- Zhang G, Zhong T. 1988. Homoptera Aphidoides. In: Huang F, editor. Insects of Mt. Namjagbawra Region of Xizang. Science Press, Beijing. pp. 167–171.