Abstract
Using integrative taxonomy, we describe Macrobiotus kosmali sp. nov. from Ribeira Brava, Madeira (Portugal). Based on morphological and morphometric data from both phase contrast light microscopy (PCM), as well as, from scanning electron microscopy (SEM), description of the new species is provided. Additionally, four DNA markers, three nuclear (18S rRNA, 28S rRNA, ITS-2) and one mitochondrial (COI) were used to calculate the p-distances between Macrobiotus kosmali sp. nov. and other species of the genus Macrobiotus. The uncorrected genetic distances indicated that the new species is the most similar to its four congeners, namely Mac. cf. recens and morphologically, new species is most similar to Mac. dariae, Mac. glebkai, Mac. noemiae, Mac. recens, Mac. rybaki and Mac. scoticus, but differs from them mainly in details of its egg chorion and egg processes.
http://urn:lsid:zoobank.org:pub:B371F633-B35C-4578-8C75-9FA2B5E80588
Introduction
The archipelago of Madeira is located in the North Atlantic, approximately 630 km northwest from the West African coast (Casablanca, Morocco) and 900 km southwest from Europe (Lisbon, Portugal). It comprises the islands of Madeira, the largest island with an area of 741 km2, Porto Santo and the Desertas and together with the Selvagens, Canary Islands, Azores and Cape Verde archipelagos, makes up the biogeographical zone of Macaronesia. The Madeira climate is Mediterranean and deeply influenced by the northeast trade wind system, with weather conditions varying considerably between the south and north coasts and according to altitude (Gonçalves Silva & Ferreira Citation2019).
Species of the phylum Tardigrada, commonly called water bears, inhabit terrestrial and aquatic (freshwater and marine) environments. They are found in mosses, lichens, soil, leaf litter, sediments and on aquatic plants (Ramazzotti & Maucci Citation1983 with translation by Beasley Citation1995; Nelson et al. Citation2020a). Till date, more than ca. 1400 species of tardigrades have been described throughout the world (Guidetti & Bertolani Citation2005; Degma & Guidetti Citation2007, Citation2022; Vicente & Bertolani Citation2013). Phylum Tardigrada is divided into two classes i.e., Heterotardigrada and Eutardigrada (Nelson et al. Citation2020b). The class Eutardigrada is later divided into two limnoterrestrial orders, Apochela and Parachela. The order Parachela, comprises the widespread, common and species rich superfamily Macrobiotoidea. Presence of a rigid buccal tube with a straight ventral bar lacking a ventral hook, pharynx with two macroplacoids and one microplacoid, 10 peribuccal lamellae, symmetrical diploclaws, pores in the cuticle, and freely laid ornamented eggs are main characteristics of nominal genus for the superfamily Macrobiotus C.A.S. Schultze Citation1834 (Stec et al. Citation2021a). The Macrobiotus genus is one of the most species-rich and widespread genera in entire the phylum Tardigrada, and it was also the first formally described tardigrade genus (Greven Citation2018). Till date, only four Macrobiotus species i.e., Macrobiotus echinogenitus Richters, 1903, Macrobiotus hufelandi C.A.S. Schultze, Citation1834, Macrobiotus occidentalis Murray, 1910 and Macrobiotus recens Cuénot, Citation1932 have been reported from the island of Madeira (Da Cunha & Do Nascimento Ribeiro Citation1962).
In this study, we applied integrative taxonomy in order to describe a new species of the genus Macrobiotus from the Island of Madeira.
Material and methods
Sampling
Moss sample was collected from riverbed of Ribeira Brava, Madeira (32°44ʹ29.8''N 17°01ʹ00.5''W), 517 metres asl in September 2019. The sample was then packed in a paper envelope, dried at a temperature of ca. 20°C, and delivered to the Department of Animal Taxonomy and Ecology at the Faculty of Biology, Adam Mickiewicz University in Poznań (Poland). The tardigrade extraction and mounting techniques followed the protocol of Stec et al. (Citation2015).
Microscopy and imaging
In total, 87 animals, 8 exuvium, 4 simplex and 31 eggs were mounted on microscope slides in the Hoyer’s medium and then examined under Olympus BX41 Phase-contrast light Microscope (PCM) associated with Olympus SC50 digital camera (Olympus Corporation, Shinjuku-ku, Japan). Thirty animals and 30 eggs were prepared for Scanning Electron Microscope (SEM) analysis according to the protocol in Roszkowska et al. (Citation2018), and examined under high vacuum in Hitachi S3000N SEM (Hitachi, Japan). The tardigrade exoskeleton was extracted from a pellet containing Chelex beads on the bottom of each tube and obtained exoskeletons were mounted on a microscope slide in Hoyer’s medium for further morphological analysis Cesari et al. (Citation2011).
All figures were assembled in Corel Photo-Paint 2017. For deep structures that could not be fully focused on a single photograph, a series of 2–10 images were taken every ca. 0.5 micrometres [μm] and then manually assembled into a single deep-focus image in Corel Photo-Paint 2017.
Morphometrics and morphological nomenclature
All measurements are given in μm. Structures were measured only if their orientation was suitable. Body length was measured from the anterior extremity to the end of the body, excluding hind legs. The types of bucco-pharyngeal apparatuses and claws were classified according to Pilato and Binda (Citation2010). All measurements and terminology of adults and eggs of Macrobiotus were prepared according to Kaczmarek and Michalczyk (Citation2017). Terminology describing the oral cavity armature (OCA) follows Michalczyk and Kaczmarek (Citation2003) and OCA morphotypes are given according to Kaczmarek and Michalczyk (Citation2017). The macroplacoid length sequence was indicated according to Kaczmarek et al. (Citation2014a). The pt ratio is the ratio of the length of a given structure to the length of the buccal tube expressed as a percentage (Pilato Citation1981). The terminology of cuticular bars in macrobiotid legs follows Kiosya et al. (Citation2021). Genus abbreviations follow Perry et al. (Citation2019).
Morphometric data were handled using the “Parachela” ver. 1.8 template available from the Tardigrada Register (Michalczyk & Kaczmarek Citation2013). Raw morphometric data for the new species are given in Supplementry Materials (SM.05).
Comparative material
For identification of the new species, we used the key by Kaczmarek and Michalczyk (Citation2017) for genus Macrobiotus. All the new species description after Kaczmarek and Michalczyk (Citation2017) were also checked.
Genotyping
Before molecular analysis, each tardigrade specimen was examined in vivo under a light microscope (LM). In order to obtain voucher specimens, genomic DNA was extracted from individual animals following a Chelex 100 resin (BioRad) extraction method provided by Casquet et al. (Citation2012) with modifications described in Stec et al. (Citation2020a).
We amplified four DNA fragments, i.e., one mitochondrial (COI) and three nuclear (18S rRNA, 28S rRNA, ITS-2). Two conservative nuclear ribosomal subunit genes, i.e., 18S rRNA and 28S rRNA genes as well as the nuclear internal transcribed spacer ITS-2 were amplified using the following primers: 18S_Tar_Ff1 (5ʹ–AGGCGAAACCGCGAATGGCTC–3ʹ) and 18S_Tar_Rr1 (5ʹ–GCCGCAGGCTCCACTCCTGG–3ʹ; Stec et al. (Citation2017b)) for the 18S rRNA gene fragment; 28SF0001 (5ʹ–ACCCvCynAATTTAAGCATAT–3ʹ) and 28SR 0990 (5ʹ–CCTTGGTCCGTGTTTCAAGAC–3ʹ; (Mironov et al. Citation2012)) for the 28S rRNA gene fragment; ITS-3 (5′–GCATCGATGAAGAACGCAGC–3′) and ITS-4 (5′–TCCTCCGCTTATTGATATGC–3′; White et al. Citation1990) for the ITS-2 gene fragment. In turn, the COI mitochondrial sequences were amplified using universal primers: HCO2198 (5ʹ–TAAACTTCAGGGTGACCAAAAAATCA–3ʹ) and LCO1490 (5ʹ–GGTCAACAAATCATAAAGATATTGG–3ʹ (Folmer et al. Citation1994)). Three molecular markers, i.e., 28S rRNA, ITS-2 and COI were amplified according to the protocols described in Kaczmarek et al. (Citation2020). In turn, 18S rRNA molecular marker was amplified according to the protocol described in Stec et al. (Citation2017b). The PCR products were cleaned using alkaline phosphatase FastAP (1 U/μl, Thermo Scientific) and exonuclease I (20 U/μl, Thermo Scientific) and sequenced directly using the BigDye™ terminator cycle method and ABI Prism 3130xl genetic analyser (Life Technologies).
Molecular data analysis
The BLAST (Basic Local Alignment Search Tool; Altschul et al. (Citation1990)) search at NCBI was used to verify the identity and homology of the amplified molecular markers with sequences deposited in the GenBank database. Obtained nuclear and mitochonrial DNA sequences were checked for quality and trimmed to the same length in BioEdit v. 7.2.5 (Hall Citation1999). For each individual was then created consensus sequence. The sequences of nuclear molecular markers were aligned using the ClustalW Multiple Alignment tool (Thompson et al. Citation1994), implemented in BioEdit v. 7.2.5 using default settings. In turn, the sequences of mitochondrial gene fragment were translated into amino acid sequences using the EMBOSS- TRANSEQ application (Rice et al. Citation2000; Goujon et al. Citation2010) to test against pseudogenes and check for indels, as well as internal stop codons and unambiguously aligned without inserting gaps. The COI haplotypes were retrieved using DNASP v.5.10.01 software (Librado & Rozas Citation2009).
Genetic comparisons between obtained and available in the GenBank molecular markers of the genus Macrobiotus were performed to supplement phenotypic description of the new species. Single sequence of molecular markers representing each Macrobiotus species were applied. Sequences which were too short after genetic comparisons or represented different fragments of DNA markers due to the application of various primers for the amplification were not used and as a result not all species were represented in all data sets. Overall, aligned sequences were trimmed to 554 (36 species), 651 (32 species), 258 bp (34 species) and 433 bp (39 species) for 18S rRNA, 28S rRNA, ITS-2 and COI molecular markers, respectively.
Mega X (Kumar et al. Citation2018) was applied to calculate the uncorrected genetic distances (p-distance) for each DNA fragment and to perform the nucleotide sequence composition of the analysed molecular markers. Genetic distances computed between species of the Macrobiotus species and the GenBank accession numbers of applied sequences provided in the Supplementary Materials (SM.1-SM.4).
Results
Taxonomic account
Phylum: Tardigrada (Spallanzani, Citation1777)
Class: Eutardigrada Richters, Citation1926
Order: Macrobiotoidea Thulin, Citation1928 (Guil et al. 2018)
Family: Macrobiotidae Thulin, Citation1928
Genus: Macrobiotus C.A.S. Schultze, Citation1834
Macrobiotus kosmali sp. nov.
(, )
Table I. Measurements [in µm] and pt values of selected morphological structures of individuals of Macrobiotus kosmali sp. nov. mounted in Hoyer’s medium (N–number of specimens/structures measured; RANGE refers to the smallest and the largest structure among all measured specimens; SD–standard deviation, pt–ratio of the length of a given structure to the length of the buccal tube expressed as a percentage).
Figure 1. Macrobiotus kosmali sp. nov.: a – dorso-ventral projection (holotype, PCM); b – dorso-ventral projection (paratype, SEM) c – d – cuticular pores on dorsal side of the body (paratype, PCM and SEM, respectively). Scale bars in µm.
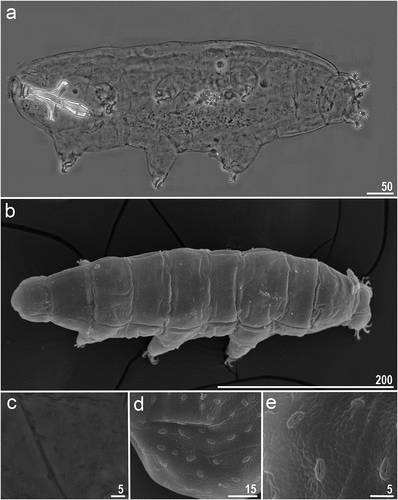
Figure 2. Macrobiotus kosmali sp. nov.: a – oral cavity armature (paratype, PCM) from dorsal side; b – oral cavity armature (paratype, PCM) from ventral side; c – mouth with ten peribuccal lamella (paratype, SEM); d – f– oral cavity armature (paratype, SEM). Filled unindented arrowhead represents first band of teeth, empty unindented arrowhead represents second band of teeth, filled indented arrowhead represents third band of teeth from dorsal side and empty indented arrowhead represents third band of teeth from ventral side. Scale bars in µm.
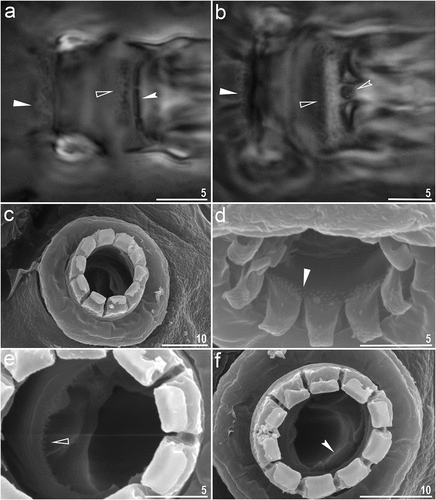
Figure 3. Macrobiotus kosmali sp. nov.: bucco-pharyngeal apparatus (dorso–ventral projection): a – general view (paratype); b – placoid morphology in dorsal view (paratype) c – ventral placoids (paratype). Filled unindented arrowhead represents first macroplacoid with central constriction, empty unindented arrowhead represents second macroplacoid with sub-terminal constriction and filled arrowhead indicates a first macroplacoid with central constriction in ventral side. All PCM. Scale bars in µm.
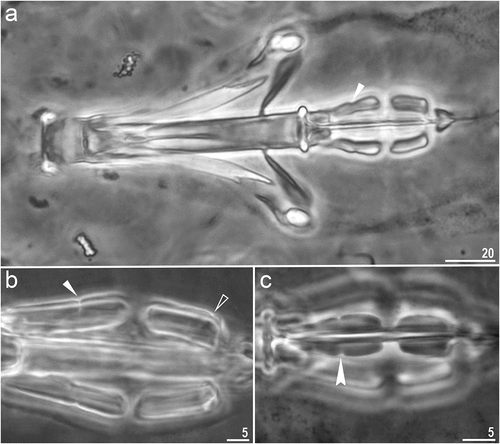
Figure 4. Macrobiotus kosmali sp. nov.: a – claws II (paratype, PCM); b – claws I (paratype, PCM) with pulvunis like structure; c – claws IV (paratype, PCM); d – claws III (paratype, SEM); e – claws IV (paratype, SEM). Filled indented arrowhead represents smooth lunulas, empty indented arrowhead represents divided cuticular bars, filled unindented arrowhead represents pulvinus-like structure, empty arrow represents doubled muscle attachments and filled arrow represents granulations present on legs. Scale bars in µm.
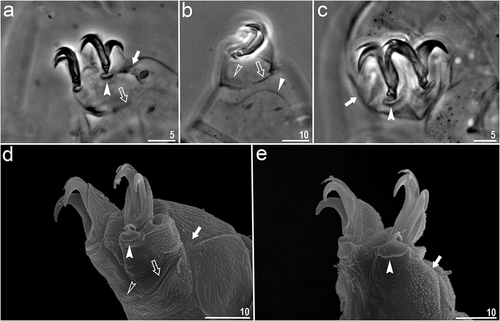
Figure 5. Macrobiotus kosmali sp. nov.: eggs: a – b – egg chorion (paratype, PCM and SEM respectively); c – f – the surface between egg processes (paratype, PCM and SEM respectively); g – egg processes with small terminal discs (paratype, PCM); h – egg processes with bifurcation at top (paratype, PCM); i – j – egg processes with cap like disc and trunks covered with tiny granules (paratype, SEM). Filled indented arrowhead represents bubbles inside egg processes trunk, empty indented arrowhead represents pores on egg chorion surface, filled unindented arrowhead represents bifurcated egg processes, empty unindented arrowhead represents tiny granules on egg processes trunk. Scale bars in µm.
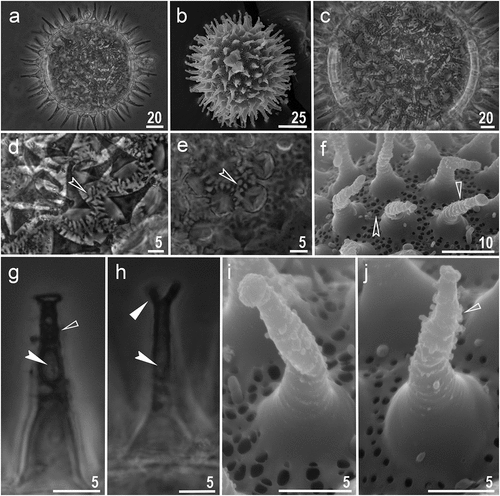
Table II. Measurements [in µm] of selected morphological structures of eggs of Macrobiotus kosmali sp. nov. mounted in Hoyer’s medium (N – number of specimens/structures measured, RANGE refers to the smallest and the largest structure among all measured eggs; SD – standard deviation).
Type Locality
Ribeira Brava, Madeira (32°4429.8 N 17°0100.5 W) moss from riverbed, September 2019, leg. Łukasz Sługocki, Ricardo Araújo and Juan J.G. Silva.
Material examined
The 131 animals, i.e., holotype and 130 paratypes (animals: 86, exuvium: 8, simplex: 4 and eggs: 31) mounted on microscope slides in Hoyer’s medium, 30 animals and 30 eggs prepared for SEM and ten animals prepared for molecular analyses. However, DNA sequences were obtained from two adult specimens (exoskeleton: 1/S, 2/S) which were later mounted on microscope slide in Hoyer’s medium and included into type series.
Type depositories
Holotype (M8/7) and 120 paratypes (animals: 81, exuvium: 6, simplex: 4 and eggs: 29) (slides: M8/*, where the asterisk can be substituted by any of the following numbers: 1, 4–16, 18–26, 1/S, 2/S) were deposited at the Department of Animal Taxonomy and Ecology, Institute of Environmental Biology, Adam Mickiewicz University in Poznań, Uniwersytetu Poznańskiego 6, 61–614 Poznań, Poland, 5 paratypes (slides: M8/17 and M8/27 (3 adults, 1 exuvium and 1 eggs)) were deposited at Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Sławkowska 17, 31–016, Kraków, Poland and (slides: M8/14 and M8/24 (3 adults, 1 exuvium and 1 eggs)) were deposited at Museu de História Natural do Funchal, Rua da Mouraria, 31, 9004–546 Funchal, Madeira – Portugal.
Etymology
The authors would like to dedicate this species to Roman Kosmala, one of the most recognizable Polish sculptors. Author of many monuments, obelisks and commemorative plaques.
Description of the new species
Adults (measurements and statistics in )
Body transparent after fixation in Hoyer’s medium, eyes present in all fixed specimens (. Entire cuticle covered with elliptical pores with (1.4–2.9 µm in length and 0.9–1.8 µm in width) distributed throughout the body and clearly visible in both PCM and SEM (. The edges of cuticular pores are evidently thicker compared with surrounding cuticle in the SEM. Tiny granules inside pores absent. Bucco–pharyngeal apparatus of the Macrobiotus type, with ventral lamina and ten peribuccal lamellae (. Mouth antero-ventral. Oral cavity armature of the hufelandi type under PCM with all three bands of teeth visible. First band of teeth made of numerous small cones below peribuccal lamellae. The second band composed of numerous minute teeth and consists of 3–4 rows of teeth visible in PCM. The third band of teeth comprises with a system of three dorsal () and three ventral transverse ridges (). Pharyngeal bulb spherical with triangular apophyses, two rod–shaped macroplacoids and a triangular microplacoid. Macroplacoid length sequence 2 < 1 (. The first macroplacoid with central constriction (, the filled unindented arrowhead). The second macroplacoid with sub-terminal constriction (, the empty unindented arrowhead). Claws Y– shaped of the hufelandi type, stout (. Primary branches with distinct accessory points and a stalk connecting lunula and claw. Lunules under all claws smooth (, filled indented arrowhead). Easily visible granulations present on legs I–IV (, filled arrow), dorsal side entirely covered with them. A pulvinus like structure is present on the internal surface of all legs I–III ()), visible only if the legs are fully extended and well oriented. Also, under claws I–III, a divided cuticular bars (, empty indented arrowhead) and doubled muscle attachments (, empty arrow) present and visible in both PCM and SEM.
Eggs (measurements and statistics in )
Eggs spherical, white, ornamented and laid freely (. Egg chorion surface between processes porous and reticulated (. Pores of egg surface mesh small i.e., 0.3–1.1 μm in diameter with roundish and/or irregular shapes. In PCM, pores visible in the form of reticulation around the egg processes base whereas in SEM, clearly visible pores around the base and entire chorion surface (. The bases of egg processes are surrounded by a crown of evident thickenings visible as dark extensions/short striae radiated from the processes bases in PCM, but they are also distinct as wider bars of the “reticulum” extending from the processes bases in SEM ()). Processes in the shape of long and thin cones, sometimes with small terminal discs ( and sometimes bifurcated (). Egg processes, from the middle to the top, covered with tiny granules which are clearly visible in SEM only (. The granulation present on distal portion of egg processes walls starts in the middle with several singular rows of tiny granulation resembling faint annulation and then the granulation increases in size toward the top of the process loosing verticillar arrangement. In the eggs processes midsections bubble-like structures are clearly visible in PCM (.
DNA sequences
We obtained good quality sequences for the applied molecular markers:
18S rRNA: GenBank: OP142472-73 (species voucher numbers: M8.1/S-M8.2/S); 950 and 1000 bp long;
28S rRNA: GenBank: OP143765-66 (species voucher numbers: M8.1/S -M8.2/S); 752 and 756 bp long;
ITS-2: GenBank: OP153786 (species voucher number: M8.1/S); 454 bp long;
COI: GenBank: OP141639-40 (species voucher numbers: M8.1/S -M8.2/S); 683 bp long.
Differential diagnosis
By having porous/reticulated egg surface between processes, reduced/absent goblet-shape terminal discs and normal (not elongated) claw branches the new species is most similar to Mac. dariae Pilato & Bertolani, Citation2004, Mac. glebkai Biserov Citation1990, Mac. noemiae Roszkowska & Kaczmarek Citation2019, Mac. recens Cuénot Citation1932, Mac. rybaki Vecchi & Stec Citation2021 and Mac. scoticus Stec, Morek, Gąsiorek, Blagden & Michalczyk 2017 but differs specifically from:
Mac. dariae, known only from the type locality in Cyprus (Pilato & Bertolani Citation2004), by: a different oral cavity armature type (hufelandi type in the new species vs patagonicus type in the Mac. dariae), presence of smooth lunules on IV pair of legs, longer buccal tube (47.0–73.0 µm in the new species vs 46.5–46.8 µm in Mac. dariae), higher pt of stylet support insertion point (80.1–83.6 in the new species vs 77.2–77.2 in Mac. dariae), longer placoid row (30.3–54.8 µm in the new species vs 29.4–29.6 µm in Mac. dariae), lower number of processes on egg circumference (23–26 in the new species vs 34–38 in Mac. dariae), longer egg processes (15.6–25.0 µm in the new species vs 7.0–7.5 µm in Mac. dariae) and wider egg processes bases (7.6–12.3 µm in the new species vs 6.3–7.3 µm in Mac. dariae).
Mac. glebkai known from type locality in Ulyanovsk (Biserov Citation1990) by: a different oral cavity armature type (hufelandi type in the new species vs patagonicus type in the Mac. glebkai), presence of thicker cuticular pores edges compared with surrounding cuticle, larger egg bare diameter (99.1–111.0 µm in the new species vs 87–89 µm in Mac. glebkai) and narrower process base (7.6–12.3 µm in the new species vs 15–18 µm in Mac. glebkai).
Mac. noemiae known from type locality in Spain (Roszkowska & Kaczmarek Citation2019) by: a different oral cavity armature type (hufelandi type in the new species vs patagonicus type in the Mac. noemiae), smooth lunules on claw on leg IV, bigger elliptical cuticular pores (1.4–2.9 µm in the new species vs 0.8–1.1 µm in Mac. noemiae), longer ventral lamina (29.3–47.5 µm in the new species vs 19–29 µm in Mac. noemiae), presence of terminal disc, absence of long, thin, hair-like, and flexible filaments on apical part of egg processes, larger egg full diameter (128.9–154.0 µm in the new species vs 118.5–123.5 µm in Mac. noemiae), longer egg processes (15.6–25.0 µm in the new species vs 7.4–9.1 µm in Mac. noemiae), longer distance between processes (2.8–5.1 µm in the new species vs 1.5–2.2 µm in Mac. noemiae) and lower number of processes on egg circumference (23–26 in the new species vs 35–36 in Mac. noemiae).
Mac. scoticus known from type locality in Scotland (Stec et al. Citation2017a) by: a different oral cavity armature type (hufelandi type in the new species vs maculatus type in the Mac. scoticus), smooth lunules on claw on leg IV, bigger cuticular pores (1.4–2.9 µm in the new species vs 0.4–0.9 µm in Mac. scoticus), longer body and buccal tube (413–673 µm and 47.0–73.0 µm respectively in the new species vs 220–406 µm and 24.7–34.9 µm respectively in Mac. scoticus), longer stylet support insertion point and higher pt of stylet support insertion point (38.2–61.0 µ m and 80.1–83.6 respectively in the new species vs 16.5–24.1 µm and 65.1–71.5 respectively in Mac. scoticus), wider buccal tube external and internal width (7.1–12.5 µm and 4.6–9.2 µm respectively in new species vs 2.6–4.6 µm and 1.3–2.8 µm respectively in Mac. scoticus), higher pt of buccal tube internal width (9.7–15.0 in new species vs 4.7–8.8 in Mac. scoticus), longer ventral lamina length and higher pt of ventral lamina length (29.3–47.5 µm and 62.3–67.9 respectively in the new species vs 9.8–14.6 µm and 38.4–48.2 respectively in Mac. scoticus), longer macroplacoid 1, macroplacoid 2 and microplacoid lengths (13.7–26.3 µm, 8.6–17.0 µm and 3.7–7.3 µm respectively in the new species vs 5.1–8.7 µm, 2.6–5.7 µm and 1.1–2.3 µm respectively in Mac. scoticus), higher pt of macroplacoid 1 and macroplacoid 2 (29.1–36.7 and 17.0–24.3 respectively in the new species vs 18.1–27.1 and 10.2–16.9 respectively in Mac. scoticus), longer macroplacoid and placoid row (24.7–45.4 µm and 30.3–54.8 µm respectively in the new species vs 9.0–15.9 µm and 10.5–19.1 µm respectively in Mac. scoticus), higher pt of macroplacoid and placoid row (50.4–62.8 and 62.3–76.6 respectively in the new species vs 34.7–47.0 and 40.9–56.5 respectively in Mac. scoticus), longer claws on all legs (see in this paper for new species and in Stec et al. 2017 for Mac. scoticus) and presence of granules from middle to top on egg processes.
Mac. recens, known from different localities like China, Cyprus, France, Greece, Italy, Romania, Spain (Cuénot Citation1932), by: larger egg bare diameter (99.1–111.0 µm in the new species vs 78–85 µm in Mac. recens), higher number of processes on egg circumference (23–26 in new species vs 21–22 in Mac. recens) and longer egg processes (15.6–25.0 µm in the new species vs 12.5–13.8 µm in Mac. recens).
Mac. rybaki, known only from type locality in Greece (Vecchi & Stec Citation2021) by: longer buccal tube length (47.0–73.0 µm in the new species vs 34.9–44.4 µm in Mac. rybaki), longer stylet support insertion point (38.2–61.0 µm in the new species vs 25.8–33.1 µm in Mac. rybaki), wider buccal tube external width (7.1–12.5 µm in new species vs 4.4–6.6 µm in Mac. rybaki), longer ventral lamina length (29.3–47.5 µm in new species vs 21.5–28.9 µm in Mac. rybaki), higher pt of stylet support insertion point (80.1–83.6 in new species vs 73.0–75.4 in Mac. rybaki), larger macroplacoid 1 and macroplacoid 2 lengths (13.7–26.3 µm and 8.6–17.0 µm respectively in the new species vs 8.2–13.1 µm and 5.8–8.0 µm respectively in Mac. rybaki), longer macroplacoid and placoid row (24.7–45.4 µm and 30.3–54.8 µm respectively in the new species vs 15.4–22.1 µm and 18.2–25.2 µm respectively in Mac. rybaki), smooth lunules on IV pair of legs, presence of granulation in the distal portion of egg processes, different egg surface between processes, larger egg bare and full diameter (99.1–111.0 µm and 128.9–154.0 µm respectively in the new species vs 68.7–93.4 µm and 83.6–107.9 µm respectively in Mac. rybaki), and longer egg processes (15.6–25.0 µm in the new species vs 6.7–13.0 µm in Mac. rybaki).
Genetic distances
The ranges of uncorrected genetic p-distances between Macrobiotus kosmali sp. nov. and other species of the genus Macrobiotus are as follows (see SM.1–SM.4):
18S rRNA: 0.0–3.3% (1.5% on average), with the most similar being Mac. glebkai Biserov Citation1990 (GenBank: MW247177) and Mac. cf. recens (GenBank: MH063927), and the least similar being Mac. polonicus Pilato, Kaczmarek, Michalczyk & Lisi Citation2003 (GenBank: HM187580).
28S rRNA: 0.2–11.8% (5.5% on average), with the most similar being Mac. cf. recens (GenBank: MH063936), and the least similar being Mac. polypiformis Roszkowska, Ostrowska, Stec, Janko & Kaczmarek Citation2017 (GenBank: KX810009).
ITS-2: 1.7–27.2% (14.4% on average), with the most similar being Mac. cf. recens (GenBank: MH063936), and the least similar being Mac. scoticus Stec, Morek, Gąsiorek, Blagden & Michalczyk 2017 (GenBank: KY797268).
COI: 10.3–27.5% (22.9% on average), with the most similar being Mac. cf. recens (GenBank: MH063936), and the least similar being Mac. kamilae Coughlan & Stec Citation2019 (GenBank: MK737920).
Conclusion
Macrobiotus is most diverse and largest taxon within the family Macrobiotidae and if GenBank sequences labelled as “Mac. echinogenitus” represent a species of the family Richtersiidae, Macrobiotus genus is monophyletic (Stec et al. Citation2021a). Inside the genus the Mac. hufelandi group is the most studied species group in this genus (e.g., Bertolani & Rebecchi Citation1993; Cesari et al. Citation2009; Cesari et al. Citation2022; Bertolani et al. Citation2011a, Citation2011b; Guidetti et al. Citation2013; Kaczmarek & Michalczyk Citation2017; Stec et al. Citation2017a, Citation2018a, Citation2018b, Citation2018c; Coughlan et al. Citation2019; Coughlan & Stec Citation2019; Kayastha et al. Citation2020, Citation2021; Marnissi et al. Citation2021; Kiosya et al. Citation2021; Vecchi & Stec Citation2021, Citation2021a, Citation2021b, Citation2022; Vecchi et al. Citation2022). The genus has cosmopolitan distribution with records from all continents (e.g., Kaczmarek et al. Citation2014b, Citation2015, Citation2016; Kaczmarek & Michalczyk Citation2017; McInnes et al. Citation2017). However, most of the species have very narrow distributions with only few species reported from larger areas (see e.g., McInnes Citation1994; Meyer Citation2013; Kaczmarek et al. Citation2014b, Citation2015, Citation2016; McInnes et al. Citation2017; Michalczyk et al. Citation2022).
With the discovery of Mac. kosmali sp. nov. the Macrobiotus species identified in the Island of Madeira increased to 5. However, Mac. kosmali sp. nov. is very similar to Mac. recens reported from Madeira by Da Cunha and Do Nascimento Ribeiro (Citation1962) in the past. Based on this we should probably consider that specimens reported in the past also belong to Mac. kosmali sp nov. instead of Mac. recens. We should also treat with extreme caution all past records of Mac. recens which are outside the original type locality. Moreover, other Macrobiotus species reported from Madeira i.e., Mac. echinogenitus, Mac. hufelandi and Mac. occidentalis are burdened with major taxonomic problems and should be considered as doubtful reports which need a confirmation in further studies.
Supplemental Material
Download MS Excel (99.5 KB)Supplemental Material
Download MS Excel (129.5 KB)Acknowledgements
Studies have been partially conducted in the framework of activities of BARg (Biodiversity and Astrobiology Research group). Pushpalata Kayastha is scholarship holders of Passport to the future - Interdisciplinary doctoral studies at the Faculty of Biology, Adam Mickiewicz University, Poznań POWR.03.02.00-00-I006/17 and her study was also supported by Minigrant Edition II, contract number POWER6/2021/2ed, Adam Mickiewicz University, POWR.03.02.00-00- I006/17. The work of Monika Mioduchowska was partially supported by grant no. 2021/43/D/NZ8/00344 from the National Science Centre, Poland and grant no. 1220/146/2021 from the Small Grants Programme of the University of Gdansk (i.e., UGrants-first competition). We would like to thank Alan Nóbrega for logistic support during field trips.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2022.2163312
Additional information
Funding
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology 215(3):403–410. DOI: 10.1016/S0022-2836(05)80360-2.
- Beasley CW. 1995. The phylum Tardigrada. By G. Ramazzotti and W. Maucci. English Translation. Published by the Translator at McMurry University, Abilene, Texas, USA, pp. 1–1014.
- Ben Marnissi J, Cesari M, Rebecchi L, Bertolani R. 2021. Integrative description of a new Tunisian tardigrade species, Macrobiotus azzunae sp. nov. (Eutardigrada, Macrobiotidae, hufelandi group). European Journal of Taxonomy 758:122–146. DOI: 10.5852/ejt.2021.758.1429.
- Bertolani R, Biserov V, Rebecchi L, Cesari M. 2011a. Taxonomy and biogeography of tardigrades using an integrated approach: New results on species of the Macrobiotus hufelandi group. Invertebrate Zoology 8(1):23–36. DOI: 10.15298/invertzool.08.1.05.
- Bertolani R, Rebecchi L. 1993. A revision of the Macrobiotus hufelandi group (Tardigrada, Macrobiotidae), with some observations on the taxonomic characters of eutardigrades. Zoologica scripta 22(2):127–152. DOI:10.1111/j.1755-0998.2009.02538.x.
- Bertolani R, Rebecchi L, Giovannini I, Cesari M. 2011b. DNA barcoding and integrative taxonomy of Macrobiotus hufelandi C.A.S. Schultze 1834, the first tardigrade species to be described, and some related species. Zootaxa 2997(1):19–36. DOI: 10.11646/zootaxa.2997.1.2.
- Biserov VI. 1990. New species of Tardigrada in the USSR fauna. Zoologichesky Zhurnal 69(5):17–25.
- Casquet J, Thebaud C, Gillespie RG. 2012. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol‐stored spiders. Molecular Ecology Resources 12(1):136–141. DOI: 10.1111/j.1755-0998.2011.03073.x.
- Cesari M, Bertolani R, Rebecchi L, Guidetti R. 2009. DNA barcoding in Tardigrada: The first case study on Macrobiotus macrocalix Bertolani & Rebecchi 1993 (Eutardigrada, Macrobiotidae). Molecular Ecology Resources 9(3):699–706. DOI: 10.1111/j.1755-0998.2009.02538.x.
- Cesari M, Giovannini I, Altiero T, Guidetti R, Cornette R, Kikawada T, Rebecchi L. 2022. Resistance to extreme stresses by a newly discovered Japanese Tardigrade Species, Macrobiotus kyoukenus (Eutardigrada, Macrobiotidae). Insects 13(7):634. DOI: 10.3390/insects13070634.
- Cesari M, Giovannini I, Bertolani R, Rebecchi L. 2011. An example of problems associated with DNA barcoding in tardigrades: A novel method for obtaining voucher specimens. Zootaxa 3104(1):42–51. DOI: 10.11646/zootaxa.3104.1.3.
- Coughlan K, Michalczyk Ł, Stec D. 2019. Macrobiotus caelestis sp. nov., a new tardigrade species (Macrobiotidae: hufelandi group) from the Tien Shan Mountains (Kyrgyzstan). In: Annales Zoologici. Museum and Institute of Zoology, Polish Academy of Sciences. Vol. 69, No. 3, pp. 499–513.
- Coughlan K, Stec D. 2019. Two new species of the Macrobiotus hufelandi complex (Tardigrada: Eutardigrada: Macrobiotidae) from Australia and India, with notes on their phylogenetic position. European Journal of Taxonomy 573:1–38. DOI: 10.5852/ejt.2019.573.
- Cuénot L. 1932. Tardigrades. Faune de France 24:1–96.
- Da Cunha AX, Do Nascimento Ribeiro F. 1962. A fauna de tardígrados da Ilha da Madeira. Memórias e Estudos do Museu Zoológico da Universidade de Coimbra 279:1–24.
- Degma P, Guidetti R. 2007. Notes to the current checklist of Tardigrada. Zootaxa 1579(1):41–53. DOI: 10.11646/zootaxa.1579.1.2.
- Degma P, Guidetti R. 2022. Actual checklist of Tardigrada species [ 13 Sept 2022]. Università di Modena e Reggio Emilia. DOI: 10.25431/11380_1178608.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from metazoan invertebrates. Molecular Marine Biology and Biotechnology 3(5):294–299.
- Gonçalves Silva JJ, Ferreira JP. 2019. First record of the Rose Evening Primrose Oenothera rosea L’ Hér. ex Aiton (Onagraceae) on the island of Madeira (Portugal). Boletim Do Museu de História Natural Do Funchal 69(355):33–38.
- Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R. 2010. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Research 38(Web Server):W695–W699. DOI: 10.1093/nar/gkq313.
- Greven H. 2018. From Johann August Ephraim Goeze to Ernst Marcus: A ramble through the history of early tardigrade research (1773 until 1929). In: Schill RO, editor. Water bears: The biology of tardigrades. Cham: Springer International Publishing. Vol. 2, pp. 1–55.
- Guidetti R, Bertolani RB. 2005. Tardigrade taxonomy: An updated check list of the taxa and a list of characters for their identification. Zootaxa 845(1):1. DOI: 10.11646/zootaxa.845.1.1.
- Guidetti R, Peluffo JR, Rocha AM, Cesari M, de Peluffo MCM. 2013. The morphological and molecular analyses of a new South American urban tardigrade offer new insights on the biological meaning of the Macrobiotus hufelandi group of species (Tardigrada: Macrobiotidae). Journal of Natural History 47(37–38):2409–2426. DOI: 10.1080/00222933.2013.800610.
- Hall TA. 1999. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In: Nucleic acids symposium series. London: Information Retrieval Ltd. Vol. 41, No. 41, pp. 95–98. c1979–c2000.
- Kaczmarek Ł, Cytan J, Zawierucha K, Diduszko D, Michalczyk Ł. 2014a. Tardigrades from Peru (South America), with descriptions of three new species of Parachela. Zootaxa 3790(2):357. DOI: 10.11646/zootaxa.3790.2.5.
- Kaczmarek Ł, Michalczyk Ł. 2017. The Macrobiotus hufelandi group (Tardigrada) revisited. Zootaxa 4363(1):101. DOI: 10.11646/zootaxa.4363.1.4.
- Kaczmarek Ł, Michalczyk Ł, McInnes SJ. 2014b. Annotated zoogeography of non-marine Tardigrada. Part i: Central America. Zootaxa 3763(1):1. DOI: 10.11646/zootaxa.3763.1.1.
- Kaczmarek Ł, Michalczyk Ł, Mcinnes SJ. 2015. Annotated zoogeography of non-marine Tardigrada. Part ii: South America. Zootaxa 3923(1):1. DOI: 10.11646/zootaxa.3923.1.1.
- Kaczmarek Ł, Michalczyk Ł, Mcinnes SJ. 2016. Annotated zoogeography of non-marine Tardigrada. Part iii: North America and Greenland. Zootaxa 4203(1):1. DOI: 10.11646/zootaxa.4203.1.1.
- Kaczmarek Ł, Roszkowska M, Poprawa I, Janelt K, Kmita H, Gawlak M, Fiałkowska E, Mioduchowska M. 2020. Integrative description of bisexual Paramacrobiotus experimentalis sp. nov. (Macrobiotidae) from republic of Madagascar (Africa) with microbiome analysis. Molecular Phylogenetics and Evolution 145:106730. DOI: 10.1016/j.ympev.2019.106730.
- Kayastha P, Berdi D, Mioduchowska M, Gawlak M, Łukasiewicz A, Gołdyn B, Kaczmarek Ł. 2020. Some Tardigrades from Nepal (Asia) with integrative description of Macrobiotus wandae sp. nov. (Macrobiotidae: hufelandi group). Annales Zoologici 70(1):121. DOI: 10.3161/00034541ANZ2020.70.1.007.
- Kayastha P, Roszkowska M, Mioduchowska M, Gawlak M, Kaczmarek Ł. 2021. Integrative descriptions of two new tardigrade species along with the new record of Mesobiotus skorackii Kaczmarek et al, 2018 from Canada. Diversity 13(8):394. DOI: 10.3390/d13080394.
- Kiosya Y, Pogwizd J, Matsko Y, Vecchi M, Stec D. 2021. Phylogenetic position of two Macrobiotus species with a revisional note on Macrobiotus sottilei Pilato, Kiosya, Lisi & Sabella, 2012 (Tardigrada: Eutardigrada: Macrobiotidae). Zootaxa 4933(1):113–135. DOI: 10.11646/zootaxa.4933.1.5.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. Mega X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35(6):1547–1549. DOI: 10.1093/molbev/msy096.
- Kuzdrowska KA, Mioduchowska M, Gawlak M, Bartylak T, Kepel A, Kepel M, Kaczmarek Ł. 2021. Integrative description of Macrobiotus porifini sp. nov. (Macrobiotidae) from Madagascar and its phylogenetic position within the hufelandi group. The European Zoological Journal 88(1):375–389. DOI: 10.1080/24750263.2021.1883752.
- Librado P, Rozas J. 2009. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25(11):1451–1452. DOI: 10.1093/bioinformatics/btp187.
- McInnes SJ. 1994. Zoogeographic distribution of terrestrial/freshwater tardigrades from current literature. Journal of Natural History 28(2):257–352. DOI: 10.1080/00222939400770131.
- McInnes SJ, Michalczyk Ł, Kaczmarek Ł. 2017. Annotated zoogeography of non-marine Tardigrada. Part iv: Africa. Zootaxa 4284(1):1. DOI: 10.11646/zootaxa.4284.1.1.
- Meyer HA. 2013. Terrestrial and freshwater Tardigrada of the Americas. Zootaxa 3747(1):1. DOI: 10.11646/zootaxa.3747.1.1.
- Michalczyk Ł, Kaczmarek Ł. 2003. A description of the new tardigrade Macrobiotus reinhardti (Eutardigrada: Macrobiotidae, harmsworthi group) with some remarks on the oral cavity armature within the genus Macrobiotus Schultze. Zootaxa 331(1):1. DOI: 10.11646/zootaxa.331.1.1.
- Michalczyk Ł, Kaczmarek Ł. 2013. The Tardigrada Register: A comprehensive online data repository for tardigrade taxonomy. Journal of Limnology 72(1s):e22. DOI: 10.4081/jlimnol.2013.s1.e22.
- Michalczyk Ł, Kaczmarek Ł, Mcinnes SJ. 2022. Annotated zoogeography of non-marine Tardigrada. Part v: Australasia. Zootaxa 5107(1):1–119. DOI: 10.11646/zootaxa.5107.1.1.
- Mironov SV, Dabert J, Dabert M. 2012. A new feather mite species of the genus Proctophyllodes Robin, 1877 (Astigmata: Proctophyllodidae) from the Long-tailed Tit Aegithalos caudatus (Passeriformes: Aegithalidae) –morphological description with DNA barcode data. Zootaxa 3253(1):54. DOI: 10.11646/zootaxa.3253.1.2.
- Nelson DR, Adkins Fletcher R, Guidetti R, Roszkowska M, Grobys D, Kaczmarek Ł. 2020a. Two new species of Tardigrada from moss cushions (grimmia sp.) in a xerothermic habitat in northeast Tennessee, (USA, North America) with the first identification of males in the genus Viridiscus. PeerJ 8:e10251. DOI: 10.7717/peerj.10251.
- Nelson DR, Guidetti R, Rebecchi L, Kaczmarek Ł, McInnes S. 2020b. Phylum Tardigrada. In: Thorp and Covich’s Freshwater Invertebrates. Elsevier. pp. 505–522. DOI: 10.1016/B978-0-12-804225-0.00015-0.
- Nowak B, Stec D. 2018. An integrative description of Macrobiotus hannae sp. nov. (Tardigrada: Eutardigrada: Macrobiotidae: hufelandi group) from Poland. Turkish Journal of Zoology 42(3). DOI: 10.3906/zoo-1712-31.
- Perry E, Miller WR, Kaczmarek Ł. 2019. Recommended abbreviations for the names of genera of the phylum Tardigrada. Zootaxa 4608(1):145. DOI: 10.11646/zootaxa.4608.1.8.
- Pilato G. 1981. Analisi di nuovi caratteri nello studio degli Eutardigradi. Animalia 8:51–57.
- Pilato G, Bertolani R. 2004. Macrobiotus dariae sp. nov., a new species of eutardigrade (Eutardigrada, Macrobiotidae) from Cyprus. Zootaxa 638(1):1–7. DOI: 10.11646/zootaxa.638.1.1.
- Pilato G, Binda MG. 2010. Definition of families, subfamilies, genera and subgenera of the Eutardigrada, and keys to their identification. Zootaxa 2404(1):1–54. DOI: 10.11646/zootaxa.2404.1.1.
- Pilato G, Kaczmarek Ł, Michalczyk Ł, Lisi O. 2003. Macrobiotus polonicus, a new species of Tardigrada from Poland (Eutardigrada: Macrobiotidae, ‘hufelandi group’). Zootaxa 258(1):1–8. DOI: 10.11646/zootaxa.258.1.1.
- Ramazzotti G, Maucci W. 1983. Il Phylum Tardigrada. III edizione riveduta e aggiornata. Memorie dell’Istituto Italiano di Idrobiologia 41:1–1012.
- Rice P, Longden I, Bleasby A. 2000. Emboss: The European molecular biology open software suite. Trends in Genetics 16(6):276–277. DOI: 10.1016/S0168-9525(00)02024-2.
- Richters F. 1926. Tardigrada. Handbuch Der Zoologie 3:1–68.
- Roszkowska M, Kaczmarek L. 2019. Macrobiotus noemiae sp. nov., a new tardigrade species (Macrobiotidae: Hufelandi group) from Spain. Turkish Journal of Zoology 43(4):331–348. DOI: 10.3906/zoo-1902-5.
- Roszkowska M, Ostrowska M, Stec D, Janko K, Kaczmarek Ł. 2017. Macrobiotus polypiformis sp. nov., a new tardigrade (Macrobiotidae; hufelandi group) from the Ecuadorian Pacific coast, with remarks on the claw abnormalities in eutardigrades. European Journal of Taxonomy 327:1–19. DOI: 10.5852/ejt.2017.327.
- Roszkowska M, Stec D, Gawlak M, Kaczmarek Ł. 2018. An integrative description of a new tardigrade species Mesobiotus romani sp. nov. (Macrobiotidae: harmsworthi group) from the Ecuadorian Pacific coast. Zootaxa 4450(5):550–564. DOI: 10.11646/zootaxa.4450.5.2.
- Schill RO, Steinbrück G. 2007. Identification and differentiation of Heterotardigrada and Eutardigrada species by riboprinting. Journal of Zoological Systematics and Evolutionary Research 45(3):184–190. DOI: 10.1111/j.1439-0469.2007.00409.x.
- Schultze CAS. 1834. Macrobiotus hufelandii, animal e crustaceorum classe novum, reviviscendi post diuturnam asphyxiam et ariditatem potens. Berlin, Germany: Berolini Apud Carolum Curths. pp. 1–8.
- Spallanzani L. 1777. Opuscoli di fisica animale, e vegetabile. Vol. 2. Modena, Italy: Il Tardigrado etc., Opusc. 4, sez, spec., pp. 181–253.
- Stec D, Arakawa K, Michalczyk Ł. 2018a. An integrative description of Macrobiotus shonaicus sp. nov. (Tardigrada: Macrobiotidae) from Japan with notes on its phylogenetic position within the hufelandi group. PLOS ONE 13(2):e0192210. DOI: 10.1371/journal.pone.0192210.
- Stec D, Kristensen RM, Michalczyk Ł. 2018b. Integrative taxonomy identifies Macrobiotus papei, a new tardigrade species of the Macrobiotus hufelandi complex (Eutardigrada: Macrobiotidae) from the Udzungwa Mountains National Park (Tanzania). Zootaxa 4446(2):273–291. DOI: 10.11646/zootaxa.4446.2.7.
- Stec D, Kristensen RM, Michalczyk Ł. 2020a. An integrative description of Minibiotus ioculator sp. nov. from the Republic of South Africa with notes on Minibiotus pentannulatus Londoño et al., 2017 (Tardigrada: Macrobiotidae). Zoologischer Anzeiger 286:117–134. DOI: 10.1016/j.jcz.2020.03.007.
- Stec D, Krzywański Ł, Michalczyk Ł. 2018c. Integrative description of Macrobiotus canaricus sp. nov. with notes on M. recens (Eutardigrada: Macrobiotidae). European Journal of Taxonomy 452:1–36. DOI: 10.5852/ejt.2018.452.
- Stec D, Morek W, Gąsiorek P, Blagden B, Michalczyk Ł. 2017a. Description of Macrobiotus scoticus sp. nov. (Tardigrada: Macrobiotidae: hufelandi group) from Scotland by means of integrative taxonomy. Annales Zoologici 67(2):181–197. DOI: 10.3161/00034541ANZ2017.67.2.001.
- Stec D, Smolak R, Kaczmarek Ł, Michalczyk Ł. 2015. An integrative description of Macrobiotus paulinae sp. nov. (Tardigrada: Eutardigrada: Macrobiotidae: hufelandi group) from Kenya. Zootaxa 4052(5):501–526. DOI: 10.11646/zootaxa.4052.5.1.
- Stec D, Tumanov DT, Kristensen RM. 2020b. Integrative taxonomy identifies two new tardigrade species (Eutardigrada: Macrobiotidae) from Greenland. European Journal of Taxonomy 614:1–40. DOI: 10.5852/ejt.2020.614.
- Stec D, Vecchi M, Calhim S, Michalczyk Ł. 2021a. New multilocus phylogeny reorganises the family Macrobiotidae (Eutardigrada) and unveils complex morphological evolution of the Macrobiotus hufelandi group. Molecular Phylogenetics and Evolution 160:106987. DOI: 10.1016/j.ympev.2020.106987.
- Stec D, Vecchi M, Dudziak M, Bartels PJ, Calhim S, Michalczyk Ł. 2021b. Integrative taxonomy resolves species identities within the Macrobiotus pallarii complex (Eutardigrada: Macrobiotidae). Zoological Letters 7(1):1–45. DOI: 10.1186/s40851-021-00176-w.
- Stec D, Vončina K, Møbjerg Kristensen R, Michalczyk Ł. 2022. The Macrobiotus ariekammensis species complex provides evidence for parallel evolution of claw elongation in Macrobiotid tardigrades. Zoological Journal of the Linnean Society 195(4):1067–1099. DOI: 10.1093/zoolinnean/zlab101.
- Stec D, Zawierucha K, Michalczyk Ł. 2017b. An integrative description of Ramazzottius subanomalus (Biserov, 1985 (Tardigrada) from Poland. Zootaxa 4300(3):403–420. DOI: 10.11646/zootaxa.4300.3.4.
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22(22):4673–4680. DOI: 10.1093/nar/22.22.4673.
- Thulin G. 1928. Über die phylogenie und das system der tardigraden. Hereditas 11(2–3):207–266. DOI: 10.1111/j.1601-5223.1928.tb02488.x.
- Vecchi M, Stec D. 2021. Integrative descriptions of two new Macrobiotus species (Tardigrada, Eutardigrada, Macrobiotidae) from Mississippi (USA) and Crete (Greece). Zoosystematics and Evolution 97(1):281–306. DOI: 10.3897/zse.97.65280.
- Vecchi M, Stec D, Vuori T, Ryndov S, Chartrain J, Calhim S. 2022. Macrobiotus naginae sp. nov., a new Xerophilous Tardigrade Species from Rokua Sand Dunes (Finland). Zoological Studies 61(22). DOI: 10.6620/ZS.2022.61-22.
- Vicente F, Bertolani R. 2013. Considerations on the taxonomy of the phylum Tardigrada. Zootaxa 3626(2):245–248. DOI: 10.11646/zootaxa.3626.2.2.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and application. Academic Press, San Diego, California 315–322. DOI: 10.1016/B978-0-12-372180-8.50042-1.
