Abstract
The European honey bee, Apis mellifera L., is the bee species with the largest geographic distribution in the world. It is considered a pollinator generalist of wild plants and agricultural crops, as well as honey bee products as honey, beeswax, and propolis. For this reason, it plays an extremely important role in the world’s ecosystems, economy, and food security. However, Apis mellifera is facing population declines due to biotic and abiotic factors, such as climate change, habitat loss, agrochemical use, and emerging pests and diseases. Pests and diseases are directly associated with a phenomenon known as colony collapse disorder (CCD), which is linked with the loss of millions of bee colonies annually worldwide. One particularly important pest is the varroa mite, which is already widely distributed and is considered the major threat to apiculture. In this review, we compile documentation of the presence and distribution of pests and diseases reported to affect Apis mellifera in Mexico. Surveys demonstrate that the following pests are present: Varroa destructor, Acarapis woodi, Aethina tumida, Galleria mellonella, as well as the diseases caused by Melissococcus plutonius, Paenibacillus larvae, Vairimorpha (Nosema) apis, V. ceranae, Ascosphaera apis and several viruses. It should be noted that the distribution and presence of European bee pathogens throughout the beekeeping states of Mexico is not very well defined, due to the lack of current bee health studies in apiaries or nationwide databases to quickly update information on their distribution and incidence.
1. Introduction
Between 60% and 90% of plants species on the planet require visitation by pollinators to reproduce (Kremen et al. Citation2007). Given that many agricultural crops are dependent on pollinators, bees play a very important role in the ecology, economy, and food production of all countries (García-Anaya et al. Citation2016; Hamadache et al. Citation2017). Pollination is also critical to maintain plant biodiversity. In addition to their pollination services, pollinators like honey bees also provide products of economic value such as honey, pollen, beeswax, and propolis (Hamadache et al. Citation2017).
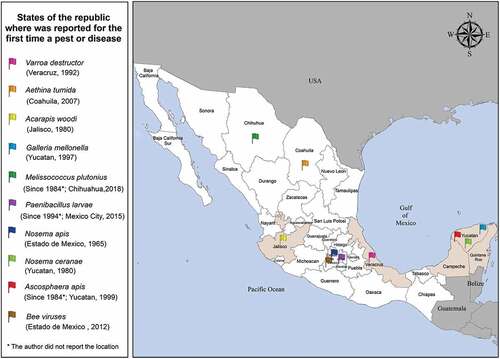
Mexico is composed of 32 states and shares land borders with the United States of America to its north and Guatemala and Belize to its south (Central America) (). The country’s climate is highly diverse with tropical, subtropical and temperate climatic zones (Vergara-Castaneda et al. Citation2012). This diversity of climates has allowed for the development of a beekeeping industry. The regions in Mexico with the highest honey production are Campeche, Yucatán and Quintana Roo on the Yucatan Peninsula, as well as the states of Jalisco and Veracruz () (Baena-Díaz et al. Citation2022). Beekeeping has great socio-economic importance in Mexico, as it is a major source of employment and income from the sale of different bee-derived products. In this context, Mexico contributes an annual production of 62,320 tons of honey (Zavala-Beltrán et al. Citation2021), ranking sixth in terms of honey production and third in exports worldwide, generating a foreign exchange of $93,725 million USD (Gallardo-López et al. Citation2021).
Figure 1. Mexico country consists of 32 states and shares land borders with USA to its north and with Guatemala and Belize to its south (Central America). The regions in Mexico with more production of honey are the Yucatan peninsula (Campeche, Yucatan and Quintana Roo), as well as the states of Jalisco and Veracruz (highlighted in beige).
However, in recent years the European honey bee has experienced a collapse in its populations, a phenomenon directly related to the increase pests and diseases, and anthropogenic activities such as agrochemical use, habitat loss, climate change (Potts et al. Citation2010; Paudel et al. Citation2015; Toledo-Hernández et al. Citation2021;). Bee pests and diseases affect A. mellifera populations by causing an abrupt collapse in their populations. The presence of pests and diseases in A. mellifera has been a triggering factor for the phenomenon known as colony collapse disorder (CCD), which has been shown to significantly compromise the survival of A. mellifera (Oldroyd, Citation2007; VanEngelsdorp et al. Citation2009). To date, there are some published works regarding pests and diseases affecting A. mellifera worldwide (Bradbear Citation1988; Allen & Ball Citation1996; Matheson Citation1996; Ellis & Munn Citation2005). However, further studies on the regional distribution of pests and diseases may help to avoid the spillover of the pathogens among states and counties within the same country, as well as safeguarding the borders with other countries where the pathogens are not present.
The sanitary status and problems of A. mellifera in Mexico are poorly known. Reports of pests and diseases are general and/or focused only on certain states within the country. The objective of this review was therefore to compile the current knowledge of the presence and distribution of pests and diseases that affect A. mellifera in Mexico. This information will be useful to help understand the dynamics of spread or migration of these pathogens. The scientific literature analyzed showed that the most serious zoosanitary problems faced by honey bees in Mexico are the pests Varroa destructor, Acarapis woodi, Aethina tumida, Galleria mellonella, as well as diseases caused by Melissococcus plutonius, Paenibacillus larvae, Vairimorpha (Nosema) apis, V. ceranae, Ascosphaera apis, and different viruses such as Black Queen Cell Virus, Kashmir Bee Virus, Deformed Wing Virus among others (Medina-Flores et al. Citation2014; Martínez-Cesáreo et al. Citation2016; Beaurepaire et al. Citation2020). This information may help the animal and health authorities to promote management programs for these pathogens, as well as in the generation of strategies to prevent their spread to other states of the republic in order to safeguard the country’s beekeeping activity.
2. Pests of A. mellifera reported in Mexico
2.1 Varroa destructor (Mesostigmata: Varroidae)
The mite Varroa destructor (Anderson & Trueman), erroneously identified in Mexico as Varroa jacobsoni until 2000, is the etiological agent of the disease known as Varroosis and the most important threat to honey bees worldwide (Traynor et al. Citation2020). The natural host of Varroa destructor is the Asian honey bee Apis cerana; however during the 1950ʹs it was recorded in A. mellifera hives in northeastern Asia, then spread rapidly to all continents through the global honey bee trade (Ramsey et al. Citation2019; Traynor et al. Citation2020). The life cycle of this ectoparasite is closely related to that of its host, and its reproductive cycle is synchronized with the development of bee broods (Rosenkranz et al. Citation2010). It feeds on the fatty tissue of pupal and adult bees (Ramsey et al. Citation2019). However, the lethality of V. destructor is mainly due to the fact that it is a vector of entomopathogenic viruses including Acute Bee Paralysis Virus (ABPV), Kashmir Bee Virus (KBV), Deformed Wing Virus (DWV), and Israeli Acute Paralysis Virus (IAPV) (Bakonyi et al. Citation2002; Chen et al. Citation2004; Yue & Genersch Citation2005; Di Prisco et al. Citation2011), thus contributing to the abrupt decline of A. mellifera populations. The presence of V. destructor was first reported in Mexico by Chihu-Amparan et al. (Citation1992) in A. mellifera hives in the city of Xalapa in the State of Veracruz. In the same year, Rodriguez-Dehaibes et al. (Citation1992) reported it in other apiaries in the same state. Later, it was reported in three municipalities of the neighboring state of Tamaulipas (Ambriz et al. Citation1995). The absence of an effective management program for V. destructor, together with the exponential growth of the mite population and the presence of viruses in A. mellifera, lead to the collapse of a colony in 2 or 3 years (Levin et al. Citation2019; Mondet et al. Citation2020). To date, this mite is distributed throughout the Mexican Republic () and is one of the most widespread and aggressive pests in apiaries, making it one of the main causes of damage to bee health in Mexico.
Table I. Geographical distribution of A. mellifera pests in Mexico.
2.2 Aethina tumida (Coleoptera: Nitidulidae)
Aethina tumida (Murray) or small hive beetle (SHB) discovered in 1867 (Murray Citation1867), is a parasitic insect native to sub-Saharan Africa; it is now distributed worldwide and has become a devastating pest of A. mellifera hives outside its natural range (Neumann & Elzen Citation2004). The beetles infest the inside of hives, where they feed, develop and oviposit. Both adults and larvae of A. tumida feed primarily on bee broods (larvae and pupae), as well as honey, pollen, and dead bees (Hood Citation2004). However, the larval stage of A. tumida causes the most damage to the colony due to the formation of galleries in the panels and contamination of honey with Kodamaea ohmeri, a yeast associated with SHB. The rapid decline of the population size within A. mellifera colonies is due to hive destruction and honey fermentation, which compromises the bees’ capacity to store food and generate a new brood, thereby causing colony collapse (Ramírez & Calderón Citation2018; Tucuch-Haas et al. Citation2020).
In Mexico, A. tumida was first recorded in 2007 in apiaries in the northern region of the state of Coahuila (Del Valle-Molina Citation2007). Since then, it has been reported in more than 14 states, including the main honey producers in the country, such as Veracruz, Quintana Roo, Yucatán and Campeche (Saldaña-Loza et al. Citation2014; Hernández-Torres et al. Citation2021). The high incidence of A. tumida recently reported in different states () demonstrates the need for a coordinated, nationwide effort to combat this threat to bee health and beekeeping.
2.3 Acarapis woodi (Acari: Tarsonemidae)
Acarapis woodi (Rennie) is a tracheal endoparasite of honey bees that has spread rapidly to all continents since it was first identified on the Isle of Wight, England (Rennie Citation1921). The mite parasitizes mainly the prothoracic trachea of bees, which it perforates to feed on hemolymph (Loeza-Concha et al. Citation2020). However, mites can also infest the air sacs of the bees’ abdomen and head (Sammataro et al. Citation2013). The disease produces nonspecific clinical symptomatology that is associated with an inability to thermoregulate in hives during winter, causing a decrease in brood area, lower honey production, and increased adult mortality (McMullan & Brown Citation2009; Szawarski et al. Citation2017). In addition, there is evidence of synergistic interactions between tracheal mite and other pathogens, such as V. destructor, resulting in a rapid weakening of the colonies (McMullan & Brown Citation2009). Acarapis woodi was first discovered in Mexico in 1980, in apiaries near the city of Guadalajara, Jalisco (Wilson & Nunamaker Citation1982). Subsequently, studies revealed a high incidence of acarapisosis in the states of Nuevo Leon and Tamaulipas (Guzman-Novoa & Zozaya-Rubio Citation1984), even though it was believed that areas further south and northwest of Mexico were likely to remain free of this parasite (Wilson & Nunamaker Citation1985). Later reports throughout the country suggest that acarapisosis had already spread rapidly despite the efforts of animal health authorities to prevent its spread in the beekeeping regions of the country ().
2.4 Galleria mellonella (Lepidotera: Pyralidae)
The greater wax moth, Galleria mellonella (Linnaeus) is a pest of the two honey bee species, A. mellifera and A. cerana. It was first reported affecting honey bee colonies of Asian honey bee Apis cerana, but later spread to northern Africa, Great Britain, some parts of Europe, Northern America, and New Zealand (Paddock Citation1918; Akratanakul Citation1987). Wax moths feed only while they are in the larval life stage; they feed on wax combs, cast skins of bee larvae, and honey in the weakened colonies, casing the majority of their damage in stored combs (Ellis et al. Citation2013). The presence of G. mellonella larvae is identified by the presence of silk threads when the larvae create tunnels in the comb and leave masses of threads on the frame (Sohail et al. Citation2017). The feeding habits of the larvae of G. mellonella reduce the combs to a pile of wax debris. In addition, the silken threads produced by the larvae can trap developing honey bee broods inside the cells and entangle emerging bees, causing death by starvation; this phenomenon is described as galleriasis (Ellis et al. Citation2013). In Mexico, there are no reports that record this pest for the first time, however, it is well known that it is spreading in the beekeeping states of the republic. Since 1997, it has already been reported their presence in honey bee hives of Yucatan state, Mexico (Echazarreta et al. Citation1997).
3. Diseases of A. mellifera reported in Mexico
3.1 Melissococcus plutonius (Lactobacillales: Enterococcaceae)
Before Varroa destructor jumped from A. cerana to A. mellifera, the most important threats to European honey bees were the bacterial diseases European foulbrood (EFB) and American foulbrood (AFB) (Genersch Citation2008). European foulbrood (EFB), an important disease of honey bee brood, is caused by the bacterium Melissococcus plutonius, a Gram-positive, lanceolate, microaerophilic coccus (Bailey & Collins Citation1982). The infectious cycle begins when M. plutonius is introduced into the digestive tract of larvae through contaminated food provided by adult bees. The pathogen reaches the midgut of the larvae, where it multiplies vigorously until it colonizes completely (Nakamura et al. Citation2020). Diseased larvae change from white to yellowish, then brown, become flaccid, give off a foul or sour odor, and die 4–5 days after infection and before reaching the pupal stage (Budge et al. Citation2010; Grossar et al. Citation2020). Melissococcus plutonius is considered to kill its host before it can successfully invade body tissues (Nakamura et al. Citation2020). Massive brood loss due to infection weakens the colony and in severe cases, can lead to its collapse (Djukic et al. Citation2018). Because there is no effective treatment and given the severity of outbreaks, it is considered a notifiable disease in Mexico and many countries worldwide (Diario Oficial de la Federación (DOF) Citation2018; Grossar et al. Citation2020). In Mexico, there are few reports about the distribution of this pathogen. Wilson et al. (Citation1984) reported the presence of this bacterium in the country for the first time in 1984, but it was not reported again until 2018, in honey bee colonies in the state of Chihuahua (de León-Door et al. Citation2018). Although to date there are no other reports in the scientific literature, anecdotal evidence suggests that bacterial infection by M. plutonius is common in Mexican hives, leading some beekeepers resort to the application of antibiotics of the tetracycline group (personal communication) despite their use being banned by animal health agencies due to the contamination of products such as honey, pollen, wax, and propolis, making it even more difficult to control this pathogen.
3.2 Paenibacillus larvae (Bacillales: Paenibacillaceae)
One of the most damaging pathogens of honey bee broods is the gram-positive, facultative anaerobic, spore-forming bacterium Paenibacillus larvae, which causes the disease known as American foulbrood (AFB) (Genersch et al., Citation2006). Endospores are the only infectious form of this organism, which are acquired by larvae through ingestion of contaminated food. The vegetative bacteria colonize the midgut of the larvae where they proliferate massively, then penetrate the epithelium and invade the hemocoel, causing death by septicemia (Yue et al. Citation2008; Genersch Citation2010). Detection of AFB in the field is based on the clinical symptoms of diseased brood, including the decomposition of the larval body into a brownish and viscous mass (the basis for the “ropiness” test), which later dry into hard scales that bees cannot removed from brood cells (Sarwar Citation2016). These scales are then a constant focus of reinfection, since they contain millions of environmentally stable spores (Ueno et al. Citation2018).
Although AFB occurs worldwide and cases have been reported on all continents (Antúnez et al. Citation2004; Ueno et al. Citation2018), in Mexico there is poor documentation of the distribution of P. larvae within the country, including the states with important beekeeping industries. However, the presence of this bacterium in the country has been reported since 1994 (Djordjevic et al. Citation1994), and more recently it was isolated from European honey bee larvae in the Xochimilco municipality of Mexico City (Peréz de la Rosa et al. Citation2015). In addition, some cases of AFB have been detected and isolated by the Mexican Ministry of Agriculture, but no further data were provided on the incidence or distribution of P. larvae in the country (Peréz de la Rosa et al. Citation2015). The detection of this pathogen plays an important role in the efficient control of its dissemination, so it is necessary to update this information. Mexico is one of the countries where AFB is considered a notifiable disease, and the destruction of diseased hives by fire is the only viable control measure. This causes considerable economic losses to beekeepers, which is one of the reasons for the lack of reporting in the country.
3.3 Vairimorpha (Nosema) apis (Dissociodihaplophasida: Nosematidae)
Vairimorpha (Nosema) apis was the first microsporidian parasite described in A. mellifera. It was thought to be the only etiological agent of nosemosis, however, in 2007 the species V. ceranae was also identified affecting the European honey bee (Paxton et al. Citation2007). Vairimorpha spp. carries out its life cycle inside the epithelial cells of the bee midgut. Infection in bees occurs by fecal-oral transmission, through ingestion of spores during fecal cleansing activities in the comb and the consumption of contaminated food and water (Martín-Hernández et al. Citation2011). During the foraging activity of infected bees, they contaminate floral resources with their droppings, as well as the collected pollen and the hive, thus contributing to the infection cycle (Grupe & Quandt Citation2020). Infection with N. apis is considered a low virulence disease whose typical characteristics are dysentery, lethargy, reduced longevity of workers, inefficient foraging, lower honey yields, and increased mortality during winter (Grupe & Quandt Citation2020; Higes et al. Citation2020). However, there is evidence that concurrent exposure to pesticides such as neonicotinoids during Vairimorpha (Nosema) infection exacerbates the disease and accelerates bee death (Alaux et al. Citation2010). Zozaya reported the presence of this disease in Mexico for the first time affecting A. mellifera hives in the state of Mexico in 1965 (Tapia-González et al. Citation2017). Later, in 1980, Wilson & Nunamaker reported the presence of this etiological agent in the states of Sonora, Oaxaca, and Tamaulipas (Wilson & Nunamaker Citation1983). To date, there are few studies on the presence of this pathogen in apiaries in Mexico (), so little is known about its current distribution in the states of the country. It is important to learn more about the spread of this disease in order to help beekeepers in regions with severe problems by information campaigns and recommendations of hygienic hive management practices.
Table II. Geographical distribution of A. mellifera diseases in Mexico.
3.4 Vairimorpha (Nosema) ceranae (Dissociodihaplophasida: Nosematidae)
Vairimorpha (Nosema) ceranae is another microsporidian intestinal parasite of Apis mellifera, considered the etiological agent of Nosemosis type C (Martín-Hernández et al. Citation2018). This fungus was originally described as a pathogen of the Asian honey bee Apis cerana, but research over the past few decades has shown that N. ceranae has replaced N. apis as the main cause of Nosemosis in honey bees, and that it exhibits higher virulence than N. apis (Fleites-Ayil et al., Citation2018). Compared to infection caused by N. apis, which presents apparent symptoms and seasonality, N. ceranae infection in honey bees results in high mortality, causing population decline and colony collapse during all seasons of the year (Grupe & Quandt Citation2020; Higes et al. Citation2020). Vairimorpha ceranae affects the immune response, produces nutritional stress, increases sugar consumption, and induces early foraging (Pașca et al. Citation2019). Nosemosis (Vairimorpha spp) has been known to affect A. mellifera in Mexico since the 1960s; however, samples of Africanized honey bees (A. mellifera scutellata) collected on the Yucatan Peninsula in 1980 and later analyzed by Guzman-Novoa et al. (Citation2011) demonstrated the presence of N. ceranae for the first time in Mexico. In a similar case, Africanized bees collected between 1995 and 1996 in the State of Mexico and later analyzed by Guerrero-Molina et al. (Citation2016) were found to have this pathogen. Vairimorpha ceranae was then confirmed in other hives from Mexico State as well as other states in central Mexico, including Morelos, Hidalgo, and Mexico City (Guzman-Novoa et al. Citation2011). To date, there is little information on the overall geographic distribution of this pathogen in the beekeeping states of Mexico (), but like N. apis, the presence of N. ceranae may be common in apiaries throughout the country. Nosemosis, caused by V. apis and V. ceranae, is recognized as one of the most widespread diseases worldwide in European honey bees. These pathogens contribute to the reduction of populations, putting the survival of A. mellifera at risk (Cueto-González et al. Citation2020).
3.5 Ascosphaera apis (Onygenales: Ascosphaeraceae)
Ascospherosis or chalkbrood disease is a mycosis caused by the fungus Ascosphaera apis, which affects the brood of all castes of A. mellifera. The two main routes of infection are by germination of fungal spores on the cuticle of the larva and by ingestion of food contaminated with spores of the fungus. Both forms of infection produce mycelia that pass through the intestinal wall of the larvae and spread throughout the body, causing their death and generating a mummified appearance (Reynaldi et al. Citation2015). Ascosphaera apis is characterized as an opportunistic pathogen; it has been shown that its development requires susceptible brood, which is stressed by the presence of other pathogens, such that A. apis generally established in colonies affected by other diseases, thus aggravating larval mortality (Castagnino et al. Citation2020). In Mexico,A. apis was reported before the introduction of V. destructor, so it was considered the most widespread brood disease among honey bees in different beekeeping regions, with the exception of the state of Yucatan (Wilson et al. Citation1984). Later, Medina-Medina and Vicario-Mejia (Citation1999) reported the presence of A. apis in hives in the state of Yucatan. It is important to note that there are currently few reports and studies related to the incidence and distribution of this fungus in the beekeeping states of Mexico ().
3.6 Bee viruses
Honey bees are also infected by different viruses that often persist as covert asymptomatic infections, then act synergistically with other biotic and abiotic stressors, causing the collapse of host colonies (DeGrandi-Hoffman & Chen Citation2015). Currently, about 18 viruses have been identified in honey bees; the most frequent are single-stranded RNA viruses, such as Sacbrood Virus (SBV), Deformed Wing Virus (DWV), Israeli Acute Paralysis Virus (IAPV), Acute Bee Paralysis Virus (ABPV), Kashmir Bee Virus (KBV), and Black Queen Cell Virus (BQCV) (De Miranda et al. Citation2013; Wang et al. Citation2016). All viruses are asymptomatic at low levels of infection, and most significantly decrease bee longevity by affecting various morphological, physiological, and behavioral traits (Beaurepaire et al. Citation2020). Some viruses produce symptoms that are clearly identifiable by beekeepers at high viral titers, allowing a simple and direct diagnosis. However, many other viruses do not generate apparent symptoms and their association with A. mellifera colony mortality remains unexplained (De Miranda et al. Citation2013; Wang et al. Citation2016). According to epidemiological data, the distribution of viruses in A. mellifera appears to be worldwide (Beaurepaire et al. Citation2020). In Mexico, the first viral disease in honey bees was reported by Bailey (Citation1967), caused by Chronic Bee Paralysis Virus (CBPV). However, the author does not provide a specific description of the geographic distribution of this virus in Mexico. It was not until 2012 that Guzman-Novoa et al. (Citation2012), reported the presence of SBV, DWV, IAPV, and ABPV in colonies of A. mellifera in the State of Mexico. Subsequently, the presence of BQCV and A. mellifera Filamentous Virus (AmFV) was also reported in other beekeeping regions of the country ().
4. Conclusions and perspectives
The honey bee (Apis mellifera L.) is an extremely important species for ecosystems; its role as a pollinator contributes to the conservation of plant biodiversity and increases the productivity of agricultural crops (Larsen et al. Citation2019). In addition, it is important for international trade due to its products. Honey bees live in an environment in which they can be exposed to different biotic and abiotic stress factors, which affect all castes and are related to the phenomenon known as colony collapse disorder (CCD). Pests and diseases are part of the complex of factors that A. mellifera faces and that put its survival at risk. They can cause important population declines, leading directly to the socio-economic issues of the beekeeping sector in Mexico and around the world. In order to identify the health problems of honey bees in Mexico, researchers and health agencies have been conducting studies on A. mellifera populations in the beekeeping states for the last decades. These studies have shown the presence of aggressive pests and diseases such as parasites, bacteria, fungi and viruses, which is why beekeeping is at risk. The information presented in this work shows evidence of spillover of the pathogens of A. mellifera among states with apiculture activity in Mexico. Pests and diseases can furthermore spread across international borders to the USA and Central American countries where these pathogens are not present, which is a real threat for their beekeeping activities. Alger et al. (Citation2018) claimed that other threats to beekeeping include the migration of swarms of Africanized honey bees between countries, facilitating the introduction and spillover of pests and diseases to healthy colonies of A. mellifera. So too, the legal or illegal introduction of new races of A. mellifera, as well as contaminated products such as honey, wax, and pollen have contributed to the arrival of pathogens to other regions (Mutinelli Citation2011).
There is currently limited and heterogeneous information and little communication among Latin American and Caribbean countries with respect to pests and diseases affecting beekeeping, and more regional surveys are needed to improve this situation (Olate-Olave et al. Citation2021). For instance, Vandame and Palacio (Citation2010) claimed that in Latin America there are no records of massive losses of honey bee colonies, but just a few years later, honey bee colony collapse and mortality were reported (Maggi et al. Citation2016; Requier et al. Citation2018). It is necessary to carry out studies to determine the current health status of the honey bees as well as the distribution of pests and diseases of A. mellifera throughout the Americas. This information could help in pathogen identification and surveillance to understand their dynamics of migration in the region. This would help to establish pathogen management policies and generate pertinent bee health programs to safeguard beekeeping in Mexico and other Latin America countries such as Argentina, Brazil, Chile, Uruguay, and Venezuela, among others, where beekeeping has high economic and ecological relevance (Maggi et al. Citation2016; Gallardo-López et al. Citation2021).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aguirre JL, Demedio J, Espaine LC. 1999. Pesquisaje de Varroa jacobsoni Oud. y Acarapis woodi Ren. en apiarios de Baja California Sur, México. Revista de Producción Animal 11:81–83.
- Akratanakul P. 1987. Honeybee diseases and enemies in Asia: A practical guide. Rome, Italy: food & agriculture organization.
- Alaux C, Brunet JL, Dussaubat C, Mondet F, Tchamitchan S, Cousin M, Brillard J, Baldy A, Belzunces L, Le Conte Y. 2010. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environmental Microbiology 12(3):774–782. DOI: 10.1111/j.1462-2920.2009.02123.x.
- Alger SA, Burnham PA, Lamas ZS, Brody AK, Richardson LL. 2018. Home sick: Impacts of migratory beekeeping on honey bee (Apis mellifera) pests, pathogens, and colony size. PeerJ 6:e5812. DOI: 10.7717/peerj.5812.
- Allen M, Ball B. 1996. The incidence and world distribution of honey bee viruses. Bee World 77(3):141–162. DOI: 10.1080/0005772X.1996.11099306.
- Álvarez-Ramírez AL, Jiménez-González E, Ortiz-Muñoz E, Ruíz-Garcia I, Orozco-Hernández R. 2017. Influence of the weather conditions on the ascospherosis (Ascosphaera apis) or chalkbrood on the Apis mellifera (bee). Abanico Veterinario 7(3):37–46.
- Ambriz JAJ, Martínez MTQ, Poumián AM. 1995. Frecuencia de varroasis en apiarios de veinticinco municipios del estado de Tamaulipas en 1992. Veterinaria México 26(2):141–144.
- Anguiano-Baez R, Guzman-Novoa E, Md. Hamiduzzaman M, Espinosa-Montano LG, Correa-Benítez A. 2016. Varroa destructor (Mesostigmata: Varroidae) parasitism and climate differentially influence the prevalence, levels, and overt infections of deformed Wing Virus in honey bees (Hymenoptera: Apidae). Journal of Insect Science 16(1):1–7. DOI: 10.1093/jisesa/iew029.
- Antúnez K, D’Alessandro B, Piccini C, Corbella E, Zunino P. 2004. Paenibacillus larvae larvae spores in honey samples from Uruguay: A nationwide survey. Journal of Invertebrate Pathology 86(1–2):56–58. DOI: 10.1016/j.jip.2004.03.011.
- Baena-Díaz F, Chévez E, Ruiz de la Merced F, Porter-Bolland L. 2022. Apis mellifera in Mexico: Honey production, melliferous flora and pollination aspects. Review. Revista Mexicana de Ciencias Pecuarias 13(2):525–548. DOI: 10.22319/rmcp.v13i2.5960.
- Bailey L. 1967. The incidence of virus diseases in the honey bee. Annals of Applied Biology 60(1):43–48. DOI: 10.1111/j.1744-7348.1967.tb05920.x.
- Bailey L, Collins MD. 1982. Reclassification of ‘Streptococcus pluton’ (White) in a new genus Melissococcus, as Melissococcus pluton nom. rev.; comb. nov. Journal of Applied Bacteriology 53(2):215–217. DOI: 10.1111/j.1365-2672.1982.tb04679.x.
- Bakonyi T, Farkas R, Szendröi A, Dobos-Kovács M, Rusvai M. 2002. Detection of acute bee paralysis virus by RT-PCR in honey bee and Varroa destructor field samples: Rapid screening of representative Hungarian apiaries. Apidologie 33(1):63–74. DOI: 10.1051/apido:2001004.
- Bayona-Celis A, Valdovinos-Flores C, Dorantes-Ugalde JA, and Saldaña-Loza LM. 2018. Land suitability potentials and major reproduction risk of the Small Hive Beetle, Aethina tumida Murray (Coleoptera, Nitidulidae) in Mexico. Reality, Data and Space International Journal of Statistics and Geography 9(2):4–13.
- Beaurepaire A, Piot N, Doublet V, Antunez K, Campbell E, Chantawannakul P, … Dalmon A. 2020. Diversity and global distribution of viruses of the western honey bee. Apis Mellifera. Insects 11(4):239. DOI: 10.3390/insects11040239.
- Bradbear N. 1988. World distribution of major honeybee diseases and pests. Bee World 69(1):15–39. DOI: 10.1080/0005772X.1988.11098943.
- Budge GE, Barrett B, Jones B, Pietravalle S, Marris G, Chantawannakul P, Thwaites R, Hall J, Cuthbertson AG, Brown MA. 2010. The occurrence of Melissococcus plutonius in healthy colonies of Apis mellifera and the efficacy of European foulbrood control measures. Journal of Invertebrate Pathology 105(2):164–170. DOI: 10.1016/j.jip.2010.06.004.
- Canto A, Medina-Medina LA, Chan E, Rodríguez R. 2020. Presence of the yeast Kodamaea ohmeri associated with Aethina tumida (Coleoptera: Nitidulidae) collected in Africanized honey bee colonies from two apiaries of Yucatan, Mexico. Revista Mexicana de Ciencias Pecuarias 11(4):1101–1112. DOI: 10.22319/rmcp.v11i4.5301.
- Castagnino GLB, Mateos A, Meana A, Montejo L, Zamorano Iturralde LV, Cutuli de Simón MT. 2020. Etiology, symptoms and prevention of chalkbrood disease: A literature review. Revista Brasileira de Saúde e Produção Animal 21:1–16. DOI: 10.1590/S1519-9940210332020.
- Chen Y, Pettis JS, Evans JD, Kramer M, Feldlaufer MF. 2004. Transmission of Kashmir bee virus by the ectoparasitic mite Varroa destructor. Apidologie 35(4):441–448. DOI: 10.1051/apido:2004031.
- Chihu-Amparan D, Rojas-Avalos L, Rodriguez-Dehaibes S. 1992. Presencia en Veracruz, México del Acaro Varroa Jacobsoni, causante de la Varroasis de la abeja melífera (Apis mellifera L.). Técnica Pecuaria en México 30:2.
- Clark WH, Clark MH, Rhodes HA. 1986. First record of Acarapis woodi Rennie in the honey bee from Baja California, Mexico. American Bee Journal 126(2):123–124.
- Contreras-Ramírez DN, Pérez León MI, Payró-de la Cruz E, Rodríguez-Ortiz G, Castañeda-Hidalgo E, Gómez-Ugalde RM. 2016. Defense, hygiene and production behavior of Apis mellifera L. ecotypes in Tabasco, Mexico. Revista Mexicana de Ciencias Agrícolas 7(8):1867–1877. DOI: 10.29312/remexca.v7i8.98.
- Cueto-González SA, López-Valencia G, Orozco-Cabrera C, Gómez-Gómez SD, Moreno-Torres K, Espinoza-Blandón KO, Guerrero-Velázquez JG, Silva-Paz LE, Trasviña-Muñoz E, Monge-Navarro FJ. 2020. Prevalence and geographical distribution of Nosema apis and Nosema ceranae in apiaries of Northwest Mexico using a duplex real-time PCR with melting-curve analysis. Journal of Apicultural Research 59(2):195–203. DOI: 10.1080/00218839.2019.1676999.
- DeGrandi-Hoffman G, Chen Y. 2015. Nutrition, immunity and viral infections in honey bees. Current Opinion in Insect Science 10:170–176. DOI: 10.1016/j.cois.2015.05.007.
- de Guzman LI, Rinderer TE, Stelzer JA. 1999. Occurrence of two genotypes of Varroa jacobsoni Oud. in North America. Apidologie 30(1):31–36. DOI: 10.1051/apido:19990104.
- de León-Door AP, Romo-Chacón A, Rios-Velasco C, Zamudio-Flores PB, de Jesús Ornelas-Paz J, Acosta-Muñiz CH. 2018. Prevalence, typing and phylogenetic analysis of Melissococcus plutonius strains from bee colonies of the state of Chihuahua, Mexico. Journal of Invertebrate Pathology 159:71–77. DOI: 10.1016/j.jip.2018.10.006.
- Del Valle-Molina J (2007). Small hive beetle infestation (Aethina tumida) in Mexico: Immediate notification report (Report No. OIE: 6397). Animal World Organisation for Animal Health (OIE). http://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/statusdetail
- De Miranda JR, Bailey L, Ball BV, Blanchard P, Budge GE, Chejanovsky N, Chen Y, Gauthier L, Genersch E, de Graaf DC, Ribière M, Ryabov E, De Smet L, Van Der Steen JJ. 2013. Standard methods for virus research in Apis mellifera. Journal of Apicultural Research 52(4):1–56. DOI: 10.3896/IBRA.1.52.4.22.
- Diario Oficial de la Federación (DOF). 2018. Acuerdo mediante el cual se dan a conocer en los Estados Unidos Mexicanos las enfermedades y plagas exóticas y endémicas de notificación obligatoria de los animales terrestres y acuáticos. Mexico: DOF - Diario Oficial de la Federación.
- Di Prisco G, Pennacchio F, Caprio E, Boncristiani Jr HF, Evans JD, Chen Y. 2011. Varroa destructor is an effective vector of Israeli Acute Paralysis Virus in the honeybee, Apis mellifera. Journal of General Virology 92(1):151–155. DOI: 10.1099/vir.0.023853-0.
- Djordjevic S, Ho-Shon M, Hornitzky M. 1994. DNA restriction endonuclease profiles and typing of geographically diverse isolates of Bacillus larvae. Journal of Apicultural Research 33(2):95–103. DOI: 10.1080/00218839.1994.11100856.
- Djukic M, Erler S, Leimbach A, Grossar D, Charrière JD, Gauthier L, Hartken D, Dietrich S, Nacke H, Daniel R, Poehlein A. 2018. Comparative genomics and description of putative virulence factors of Melissococcus plutonius, the causative agent of European foulbrood disease in honey bees. Genes 9(8):419. DOI: 10.3390/genes9080419.
- Duarte-Chávez JMA, Martínez-Puc JM, Cetzal-Ix W, Basu NGVSK. 2017. The small hive beetle (Aethina tumida Murray) and its actual situation in the beekeeping of the Yucatan Peninsula, Mexico. In Basu SK, Zandi P, Chalaras SK editors. Environment at crossroads: Challenges, dynamics and solutions. 1st ed. Guilan Province, Iran: Haghshenass Publishing.
- Echazarreta CM, Quezada-Euán JJ, Medina LM, Pasteur KL. 1997. Beekeeping in the Yucatan peninsula: Development and current status. Bee World 78(3):115–127. DOI: 10.1080/0005772X.1997.11099346.
- Eischen FA. 1987. Overwintering performance of honey bee colonies heavily infested with Acarapis woodi (Rennie). Apidologie 18(4):293–304. DOI: 10.1051/apido:19870401.
- Eischen FA, Wilson WT, Pettis JS, Suarez A, Cardoso-Tamez D, Maki DL, Dietz A, Vargas J, de Estrada CG, Rubink WL. 1990. The spread of Acarapis woodi (Acari: Tarsonemidae) in Northeastern Mexico. Journal of the Kansas Entomological Society 63(3):375–384.
- Ellis JD, Graham JR, Mortensen A. 2013. Standard methods for wax moth research. Journal of Apicultural Research 52(1):1–17. DOI: 10.3896/IBRA.1.52.1.10.
- Ellis JD, Munn PA. 2005. The worldwide health status of honey bees. Bee World 86(4):88–101. DOI: 10.1080/0005772X.2005.11417323.
- Fleites-Ayil FA, Quezada-Euán JJG, Medina-Medina LA. 2018. Onset of foraging and lifespan of Africanized honey bees (Apis mellifera) infected with different levels of Nosema ceranae spores in Neotropical Mexico. Apidologie 49(6):781–788. DOI: 10.1007/s13592-018-0602-2.
- Gallardo-López F, Castellanos-Potenciano BP, Díaz-Padilla G, Pérez-Vásquez A, Landeros-Sánchez C, Sol-Sánchez Á. 2021. Cognitive dissonance in the face of climate change in beekeepers: A case study in Mexico. Revista Mexicana de Ciencias Pecuarias 12(1):238–255. DOI: 10.22319/rmcp.v12i1.5213.
- García-Anaya MC, Romo-Chacón A, Sáenz-Mendoza AI, Pérez-Ordoñez G, Acosta-Muñiz CH. 2018. Detection of Israeli Acute Paralysis Virus (IAPV) and Filamentous Virus (AmFV) in Honey Bees in Mexico. Journal of Apicultural Science 62(1):141–144. DOI: 10.2478/JAS-2018-0009.
- García-Anaya MC, Romo-Chacón A, Zamudio-Flores PB, Ríos-Velasco C, Acosta-Muñiz CH. 2016. Detection of viruses in colonies of honey bees (Apis mellifera L.) in the state of Chihuahua, Mexico. Journal of Apicultural Research 55(3):240–242. DOI: 10.1080/00218839.2016.1226605.
- García-Figueroa C, Arechavaleta-Velasco ME. 2018. Prevalence of honeybee tracheal mite disease and infestation levels of Acarapis woodi in honeybee colonies of Morelos, Mexico. Revista Mexicana de Ciencias Pecuarias 9(3):567–575. DOI: 10.22319/rmcp.v9i3.4194.
- García-Millán M, Quezada-Euán JJ. 1993. Distribución de la Nosemosis en apiarios comerciales del estado de Yucatán. Apicultura Moderna 5:22–24.
- Genersch E. 2008. Paenibacillus larvae and American foulbrood–long since known and still surprising. Journal für Verbraucherschutz und Lebensmittelsicherheit 3(4):429–434. DOI: 10.1007/s00003-008-0379-8.
- Genersch E. 2010. American Foulbrood in honeybees and its causative agent Paenibacillus Larvae. Journal of Invertebrate Pathology 103:S10–S19. DOI: 10.1016/j.jip.2009.06.015.
- Genersch E, Forsgren E, Pentikäinen J, Ashiralieva A, Rauch S, Kilwinski J, and Fries I. 2006. Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. International Journal of Systematic and Evolutionary Microbiology 56(3):501–511. DOI:10.1099/ijs.0.63928-0.
- Grossar D, Kilchenmann V, Forsgren E, Charrière JD, Gauthier L, Chapuisat M, Dietemann V. 2020. Putative determinants of virulence in Melissococcus plutonius, the bacterial agent causing European foulbrood in honey bees. Virulence 11(1):554–567. DOI: 10.1080/21505594.2020.1768338.
- Grupe AC, Quandt CA. 2020. A growing pandemic: A review of Nosema parasites in globally distributed domesticated and native bees. PLoS Pathogens 16(6):e1008580. DOI: 10.1371/journal.ppat.1008580.
- Guerrero-Molina C, Correa-Benítez A, Hamiduzzaman MM, Guzman-Novoa E. 2016. Nosema ceranae is an old resident of honey bee (Apis mellifera) colonies in Mexico, causing infection levels of one million spores per bee or higher during summer and fall. Journal of Invertebrate Pathology 141:38–40. DOI: 10.1016/j.jip.2016.11.001.
- Gutiérrez RV, Collí-Ucán W. 1996. La apicultura en la Península de Yucatán, México y sus perspectivas. Folia Entomológica Mexicana 97:55–70.
- Guzman-Novoa E, Hamiduzzaman MM, Arechavaleta-Velasco ME, Koleoglu G, Valizadeh P, Correa-Benítez A. 2011. Nosema ceranae has parasitized Africanized honey bees in Mexico since at least 2004. Journal of Apicultural Research 50(2):167–169. DOI: 10.3896/IBRA.1.50.2.09.
- Guzman-Novoa E, Hamiduzzaman MM, Correa-Benítez A, Espinosa-Montaño LG, Uribe-Rubio JL. 2013. A scientific note on the first detection of Black Queen Cell Virus in honey bees (Apis mellifera) in Mexico. Apidologie 44(4):382–384. DOI: 10.1007/s13592-012-0191-4.
- Guzman-Novoa E, Hamiduzzaman MM, Espinosa-Montaño LG, Correa-Benítez A, Anguiano-Baez R, Ponce-Vázquez R. 2012. First detection of four viruses in honey bee (Apis mellifera) workers with and without deformed wings and Varroa destructor in Mexico. Journal of Apicultural Research 51(4):342–346. DOI: 10.3896/IBRA.1.51.4.08.
- Guzman-Novoa E, Zozaya-Rubio A. 1984. The effects of chemotherapy on the level of infestation and production of honey in colonies of honey bees with acariosis. American Bee Journal 124:669–672.
- Hamadache M, Benkortbi O, Hanini S, Amrane A. 2017. QSAR modeling in ecotoxicological risk assessment: Application to the prediction of acute contact toxicity of pesticides on bees (Apis mellifera L.). Environmental Science and Pollution Research 25(1):896–907. DOI: 10.1007/s11356-017-0498-9.
- Hernández-Torres H, García-Martínez O, Romero-Nápoles J, Sánchez-Valdez VM, Aguirre-Uribe LA, Sánchez-Peña SR. 2018. Sap beetles of Coahuila, Mexico and effective collecting attractants. Southwestern Entomologist 43(1):151–166. DOI: 10.3958/059.043.0107.
- Hernández-Torres H, Georgievich-Kirejtshuk A, Núñez-Vázquez C, García-Martínez O. 2021. On Aethina tumida Murray (Coleoptera: Nitidulidae: Nitidulinae) in hives of Apis mellifera Linnaeus (Hymenoptera: Apidae) in Campeche, Mexico. Journal of Apicultural Research:1–4. http://doi.org/10.1080/00218839.2021.1889223.
- Higes M, García-Palencia P, Urbieta A, Nanetti A, Martín-Hernández R. 2020. Nosema apis and Nosema ceranae tissue tropism in worker honey bees (Apis mellifera). Veterinary Pathology 57(1):132–138. DOI: 10.1177/0300985819864302.
- Hood WM. 2004. The small hive beetle, Aethina tumida: A review. Bee World 85(3):51–59. DOI: 10.1080/0005772X.2004.11099624.
- Kremen C, Williams NM, Aizen MA, Gemmill‐Herren B, LeBuhn G, Minckley R, … Ricketts TH. 2007. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land‐use change. Ecology Letters 10(4):299–314. DOI: 10.1111/j.1461-0248.2007.01018.x.
- Larsen A, Reynaldi FJ, Guzman-Novoa E. 2019. Fundaments of the honey bee (Apis mellifera) immune system. Review. Revista Mexicana de Ciencias Pecuarias 10(3):705–728. DOI: 10.22319/rmcp.v10i3.4785.
- Levin S, Sela N, Erez T, Nestel D, Pettis J, Neumann P, Chejanovsky N. 2019. New viruses from the ectoparasite mite Varroa destructor infesting Apis mellifera and Apis cerana. Viruses 11(2):94. DOI: 10.3390/v11020094.
- Loeza-Concha H, Salgado-Moreno S, Avila-Ramos F, Escalera-Valente F, Lemus-Flores C, Domínguez-Rebolledo Á, Carmona-Gasca CA. 2020. Seasonal variation in the prevalence of Varroa, Nosema and Acarapis in hives from which queen bee mating nuclei are produced. Journal of Apicultural Research 59(4):558–563. DOI: 10.1080/00218839.2020.1717060.
- Lozano LG, Moffett JO, Campos PB, Guillen M,M, Perez E ON, Maki DL, Wilson WT. 1989. Tracheal mite Acarapis woodi (Rennie) (Acari: Tarsonemidae) infestations in the honey bee, Apis mellifera L. (Hymenoptera: Apidae) in Tamaulipas, Mexico. Journal of Entomological Science 24(1):40–46. DOI: 10.18474/0749-8004-24.1.40.
- Maggi M, Antúnez K, Invernizzi C, Aldea P, Vargas M, Negri P, … Eguaras M. 2016. Honeybee health in South America. Apidologie 47(6):835–854. DOI: 10.1007/s13592-016-0445-7.
- Martínez-Cesáreo M, Rosas-Córdoba J, Prieto-Merlos D, Carmona-Gasca A, Peña-Parra B, Ávila-Ramos F. 2016. Presence of Varroa destructor, Nosema apis, and Acarapis woodi, in honey bee (Apis mellifera) of the east region in the State of Mexico. Abanico Veterinario 6(2):30–38. DOI: 10.21929/abavet2016.62.1.
- Martínez-Puc JF, Cetzal-Ix W, González-Valdivia NA, Casanova-Lugo F, Saikat-Kumar B. 2018. Characterization of beekeeping activity in the main municipalities of honey production in Campeche, Mexico. Journal of the Selva Andina Animal Science 5(1):44–53. DOI: 10.36610/j.jsaas.2018.050100044.
- Martínez-Puc JF, Medina-Medina LA, Catzín-Ventura GA. 2011. Frequency of Varroa destructor, Nosema apis and Acarapis woodi in managed colonies and wild swarms of honey bees (Apis mellifera) in Merida, Yucatan, Mexico. Revista Mexicana de Ciencias Pecuarias 2(1):25–38.
- Martín-Hernández R, Bartolomé C, Chejanovsky N, Le Conte Y, Dalmon A, Dussaubat C, García-Palencia P, Meana A, Pinto MA, Soroker V, Higes M. 2018. Nosema ceranae in Apis mellifera: A 12 years postdetection perspective. Environmental Microbiology 20(4):1302–1329. DOI: 10.1111/1462-2920.14103.
- Martín-Hernández R, Botías C, Barrios L, Martínez-Salvador A, Meana A, Mayack C, Higes M. 2011. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitology Research 109(3):605–612. DOI: 10.1007/s00436-011-2292-9.
- Matheson A. 1996. World bee health update 1996. Bee World 77(1):45–51. DOI: 10.1080/0005772X.1996.11099281.
- McMullan JB, Brown MJ. 2009. A qualitative model of mortality in honey bee (Apis mellifera) colonies infested with tracheal mites (Acarapis woodi). Experimental and Applied Acarology 47(3):225–234. DOI: 10.1007/s10493-008-9213-3.
- Medina-Flores CA, Guzman-Novoa E, Aréchiga-Flores CF, Aguilera-Soto JI, Gutiérrez-Piña FJ. 2011. Effect of Varroa destructor infestations on honey yields of Apis mellifera colonies in Mexico’s semiarid high plateau. Revista Mexicana de Ciencias Pecuarias 2(3):313–317.
- Medina-Flores CA, Guzman-Novoa E, Espinosa-Montaño LG, Uribe-Rubio JL, Gutiérrez-Luna R, Gutiérrez-Piña FJ. 2014. Frequency of Varroosis and Nosemosis in honeybee (Apis mellifera) colonies in the state of Zacatecas, Mexico. Revista Chapingo Serie Ciencias Forestales y del Ambiente 20(3):159–167. DOI: 10.5154/r.rchscfa.2013.08.028.
- Medina-Medina L. 1997. Frequency and infestation levels of the mite Varroa jacobsoni Oud. in managed honey bee (Apis mellifera L.) colonies in Yucatan, Mexico. American Bee Journal 138(2):125–127.
- Medina-Medina L, Vicario-Mejia E. 1999. The Presence of Varroa jacobsoni mite and Ascosphaera apis fungi in collapsing and normal honey bee (Apis mellifera L.) colonies in Yucatan, Mexico. American Bee Journal 139(10):794–796.
- Mondet F, Beaurepaire AL, McAfee A, Locke B, Alaux C, Blanchard S, Danka B, Le Conte YL. 2020. Honey bee survival mechanisms against the parasite Varroa destructor: A systematic review of phenotypic and genomic research efforts. International Journal for Parasitology 50(6–7):433–447. DOI: 10.1016/j.ijpara.2020.03.005.
- Mondragón L, Spivak M, Vandame R. 2005. A multifactorial study of the resistance of honeybees Apis mellifera to the mite Varroa destructor over one year in Mexico. Apidologie 36(3):345–358. DOI: 10.1051/apido:2005022.
- Morfin N, Gashout HA, Macías-Macías JO, De la Mora A, Tapia-Rivera JC, Tapia-González JM, Contreras-Escareño F, Guzman-Novoa E. 2020. Detection, replication and quantification of deformed Wing Virus-A, deformed Wing Virus-B, and Black Queen Cell Virus in the endemic stingless bee, Melipona colimana, from Jalisco, Mexico. International Journal of Tropical Insect Science 41(2):1285–1292. DOI: 10.1007/s42690-020-00320-7.
- Murray A. 1867. XLI-list of Coleoptera received from Old Calabar, on the west coast of Africa. Annals and Magazine of Natural History London 20(119):314–323. DOI: 10.1080/00222936708646336.
- Mutinelli F. 2011. The spread of pathogens through trade in honey bees and their products (including queen bees and semen): Overview and recent developments. Revue Scientifique Et Technique De L Office International Des Epizooties 30(1):257–271. DOI: 10.20506/rst.30.1.2033.
- Nakamura K, Okumura K, Harada M, Okamoto M, Okura M, Takamatsu D. 2020. Different impacts of pMP19 on the virulence of Melissococcus plutonius strains with different genetic backgrounds. Environmental Microbiology 22(7):2756–2770. DOI: 10.1111/1462-2920.14999.
- Neumann P, Elzen PJ. 2004. The biology of the small hive beetle (Aethina tumida, Coleoptera: Nitidulidae): Gaps in our knowledge of an invasive species. Apidologie 35(3):229–247. DOI: 10.1051/apido:2004010.
- Olate-Olave VR, Verde M, Vallejos L, Perez Raymonda L, Cortese MC, Doorn M. 2021. Bee health and productivity in Apis mellifera, a consequence of multiple factors. Veterinary Sciences 8(5):76. DOI: 10.3390/vetsci8050076.
- Oldroyd BP. 2007. What’s killing American honey bees? PLoS Biology 5(6):e168. DOI: 10.1371/journal.pbio.0050168.
- Paddock FB. 1918. The beemoth or waxworm. College Station, TX, USA: Texas agricultural experiment stations.
- Pașca C, Mărghitaș LA, Șonea C, Bobiș O, Buzura-Matei IA, Dezmirean DS. 2019. A review of Nosema ceranae and Nosema apis: Characterization and impact for beekeeping. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Animal Science and Biotechnologies 76(2):77–87. DOI: 10.15835/buasvmcn-asb:0018.19.
- Paudel YP, Mackereth R, Hanley R, Qin W. 2015. Honey bees (Apis mellifera L.) and pollination issues: Current status, impacts, and potential drivers of decline. Journal of Agricultural Science 7(6):93–109. DOI: 10.5539/jas.v7n6p93.
- Paxton RJ, Klee J, Korpela S, Fries I. 2007. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 38(6):558–565. DOI: 10.1051/apido:2007037.
- Peréz de la Rosa D, Pérez de la Rosa JJ, Cossio-Bayugar R, Miranda-Miranda E, Lozano L, Bravo-Díaz MA, Rocha-Martínez MK, Sachman-Ruiz B. 2015. Complete genome sequence of Paenibacillus larvae MEX14, isolated from honey bee larvae from the Xochimilco quarter in Mexico City. Genome Announcements 3(4):e00968–15. DOI: 10.1128/genomea.00968-15.
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: Trends, impacts and drivers. Trends in Ecology & Evolution 25(6):345–353. DOI: 10.1016/j.tree.2010.01.007.
- Ramírez M, Calderón RA. 2018. Small hive beetle, Aethina tumida, situation in Africanized honey bees (Apis mellifera) colonies in Costa Rica: Sampling of apiaries 2014-2017. Ciencias Veterinarias 36(1):19–26. DOI: 10.15359/rcv.36-1.2.
- Ramírez NC, Correa BA, Romero LJ, Martínez MJ. 2012. Frequency and both spatial and temporal distribution of Acarapis woodi in honey bees (Apis mellifera) in five Mexico City localities from 2002 to 2005. Journal of Apicultural Research 51(1):139–141. DOI: 10.3896/IBRA.1.51.1.18.
- Ramsey SD, Ochoa R, Bauchan G, Gulbronson C, Mowery JD, Cohen A, Lim D, Joklik J, Cicero JM, Ellis JD, Hawthorne D, vanEngelsdorp D. (2019). Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proceedings of the National Academy of Sciences 116(5):1792–1801. DOI:10.1073/pnas.1818371116
- Rennie J. 1921. Isle of Wight disease in hive bees—acarine disease: The organism associated with the disease—Tarsonemus woodi, n. sp. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 52(4):768–779. DOI: 10.5962/bhl.title.55870.
- Requier F, Antúnez K, Morales CL, Aldea Sánchez P, Castilhos D, Garrido PM, … Garibaldi LA. 2018. Trends in beekeeping and honey bee colony losses in Latin America. Journal of Apicultural Research 57(5):657–662. DOI: 10.1080/00218839.2018.1494919.
- Reyna-Fuentes JH, Martínez González JC, López Aguirre D, Silva Contreras A. 2021. The small beetle of the hive, a plague that threatens Tamaulipas, Mexico. Zootecnia Tropical 39:e4567456. DOI: 10.5281/zenodo.4567456.
- Reynaldi FJ, Lucia M, Garcia MLG. 2015. Ascosphaera apis, the entomopathogenic fungus affecting larvae of native bees (Xylocopa augusti): First report in South America. Ibero-American Journal of Mycology 32(4):261–264. DOI: 10.1016/j.riam.2015.01.001.
- Rodriguez-Dehaibes SR, Mendez JM, Colina GO. 1992. Varroa found in Mexico. American Bee Journal 132:728.
- Rosenkranz P, Aumeier P, Ziegelmann B. 2010. Biology and control of Varroa destructor. Journal of Invertebrate Pathology 103:S96–S119. DOI: 10.1016/j.jip.2009.07.016.
- Ruíz-Flores A, Ramírez-Hernández E, Maldonado-Simán E, Palafox-Guillén J, Ochoa-Torres E, López-Ordaz R. 2012. Incidence and infestation level of varroatosis in honeybees (Apis mellifera) at the bee identification and diagnosis laboratory from 2002 to 2006. Revista Chapingo Serie Ciencias Forestales y del Ambiente 18(2):175–182. DOI: 10.5154/r.rchscfa.2011.03.023.
- Saldaña-Loza LM, Lara-Alvarez LG, Dorantes-Ugalde JA. 2014. Manual nuevos manejos de la apicultura para el control del pequeño escarabajo de la colmena Aethina tumida Murray. 1st ed. México: SAGARPA.
- Sammataro D, de Guzman L, George S, Ochoa R, Otis G. 2013. Standard methods for tracheal mite research. Journal of Apicultural Research 52(4):1–20. DOI: 10.3896/IBRA.1.52.4.20.
- Sarwar M. 2016. Challenges due to bacterial infections of the honey bees and contributions to manage pest problems. International Journal of Entomology Research 1(1):4–10.
- Sohail M, Aqueel MA, Ellis JD, Afzal M, Raza AM. 2017. Seasonal abundance of greater wax moths (Galleria mellonella L.) in hives of western honey bees (Apis mellifera L.) correlates with minimum and maximum ambient temperature. Journal of Apicultural Research 56(4):416–420. DOI: 10.1080/00218839.2017.1335824.
- Szawarski N, Quintana S, Levy E, Lucía M, Abrahamovich A, Porrini M, Brasesco C, Negri P, Sarlo G, Eguaras M, Maggi M. 2017. Is Acarapis woodi mite currently infesting Apis mellifera colonies in Argentina? Journal of Apicultural Research 56(4):387–393. DOI: 10.1080/00218839.2017.1339519.
- Tapia-González JM, Alcazar-Oceguera G, Macías-Macías JO, Contreras-Escareño F, Tapia-Rivera JC, Chavoya-Moreno FJ, Martínez-González JC. 2017. Nosemosis in worker bees and their relationship with environmental factors in Jalisco, Mexico. Revista Mexicana de Ciencias Pecuarias 8(3):325–330. DOI: 10.22319/rmcp.v8i3.4510.
- Tapia-González JM, Alcazar-Oceguera G, Macías-Macías JO, Contreras-Escareño F, Tapia-Rivera JC, Petukhova T, Guzman-Novoa E. 2019a. Varroosis in honey bees in different environmental and regional conditions of Jalisco, Mexico. Ecosistemas y Recursos Agropecuarios 6(17):243–251. DOI: 10.19136/era.a6n17.2018.
- Tapia-González JM, Alcazar-Oceguera G, Macías-Macías JO, Contreras-Escareño F, Tapia-Rivera JC, Petukhova T, Guzman-Novoa E. 2020. Ascospherosis in honey bees and its relationship to environmental factors in Jalisco, Mexico. Revista Mexicana de Ciencias Pecuarias 11(2):468–478. DOI: 10.22319/rmcp.v11i2.4926.
- Tapia-González JM, Morfin N, Macías-Macías JO, De la Mora A, Tapia-Rivera JC, Ayala R, Contreras-Escareño F, Gashout HA, Guzman-Novoa E. 2019b. Evidence of presence and replication of honey bee viruses among wild bee pollinators in subtropical environments. Journal of Invertebrate Pathology 168:107256. DOI: 10.1016/j.jip.2019.107256.
- Toledo-Hernández E, Hernández-Flores J, Sotelo-Leyva C, Alvear-García A, Peña-Chora G. 2021. A new enemy of Apis mellifera (Hymenoptera: Apidae): First report of Nomamyrmex esenbeckii (Hymenoptera: Formicidae) attacking honey bee colonies. Journal of Apicultural Research:1–2. DOI: 10.1080/00218839.2021.1987740.
- Toledo-Hernández E, Sotelo-Leyva C, Ortega-Acosta SA, Palemón-Alberto F, Moraga-Cáceres EU, Alvear-García A, Peña-Chora G. 2022. Growing threat to Apis mellifera in North America; arrival of parasitic small hive beetle, Aethina tumida, to the State of Morelos, Mexico. Southwestern Entomologist 47(4):935–937. DOI: 10.3958/059.047.0417.
- Traynor KS, Mondet F, de Miranda JR, Techer M, Kowallik V, Oddie MA, Chantawannakul P, McAfee A. 2020. Varroa destructor: A complex parasite, crippling honey bees worldwide. Trends in Parasitology 36(7):592–606. DOI: 10.1016/j.pt.2020.04.004.
- Tucuch-Haas JI, Rangel-Fajardo MA, Casanova-Lugo F, Ruíz-Sánchez E, Utrera-Quintana F, Tucuch-Haas CJ. 2020. Control alternativo de Aethina tumida Murray (Coleoptera: Nitidulidae) con polvos vegetales. Acta Agrícola y Pecuaria 6:1. DOI: 10.30973/aap.
- Ueno Y, Yoshida E, Misumi W, Watando E, Suzuki K, Hirai Y, Okura M, Osaki M, Katsuda K, Takamatsu D. 2018. Population structure and antimicrobial susceptibility of Paenibacillus larvae isolates from American foulbrood cases in Apis mellifera in Japan. Environmental Microbiology Reports 10(2):210–216. DOI: 10.1111/1758-2229.12623.
- Vandame R, Palacio MA. 2010. Preserved honeybee health in Latin America: A fragile equilibrium due to low intensity agriculture and beekeeping? Apidologie 41(3):243–255. DOI: 10.1051/apido/2010025.
- VanEngelsdorp D, Evans JD, Saegerman C, Mullin C, Haubruge E, Nguyen BK, … Pettis JS. 2009. Colony collapse disorder: A descriptive study. PloS one 4(8):e6481. DOI: 10.1371/journal.pone.0006481.
- Vargas-Valero A, Reyes-Carrillo JL, Gaspar-Ramírez O, Moreno-Reséndez A. 2021. Parasitosis and pesticide residues in honey and wax in bee colonies. Ecosistemas y Recursos Agropecuarios 8(2):e2827. DOI: 10.19136/era.a8n2.2827.
- Vergara-Castaneda A, Escobar-Gutiérrez A, Ruiz-Tovar K, Sotelo J, Ordonez G, Cruz-Rivera MY, … Vaughan G. 2012. Epidemiology of varicella in Mexico. Journal of Clinical Virology 55(1):51–57. DOI: 10.1016/j.jcv.2012.06.004.
- Wang H, Meeus I, Smagghe G. 2016. Israeli Acute Paralysis Virus associated paralysis symptoms, viral tissue distribution and Dicer-2 induction in bumblebee workers (Bombus terrestris). Journal of General Virology 97(8):1981–1989. DOI: 10.1099/jgv.0.000516.
- Wilson W, Nunamaker R. 1982. The infestation of honey in Mexico with. Acarapis Woodi. American Bee Journal 122(7):503–505.
- Wilson W, Nunamaker R. 1983. The incidence of Nosema apis in honeybees in Mexico. Bee World 64(3):132–136. DOI: 10.1080/0005772X.1983.11097930.
- Wilson W, Nunamaker R. 1985. Further distribution of Acarapis woodi in Mexico. American Bee Journal 125(2):109–111.
- Wilson WT, Nunamaker RA, Maki D. 1984. The occurrence of brood diseases and the absence of the Varroa mite in honey bees from Mexico. American Bee Journal 124:51–53.
- Yue C, Genersch E. 2005. RT-PCR analysis of deformed Wing Virus in honeybees (Apis mellifera) and mites (Varroa destructor). Journal of General Virology 86(12):3419–3424. DOI: 10.1099/vir.0.81401-0.
- Yue D, Nordhoff M, Wieler LH, Genersch E. 2008. Fluorescence in situ hybridization (FISH) analysis of the interactions between honeybee larvae and Paenibacillus larvae, the causative agent of American foulbrood of honeybees (Apis mellifera). Environmental Microbiology 10(6):1612–1620. DOI: 10.1111/j.1462-2920.2008.01579.x.
- Zavala-Beltrán JI, López-Santiago MA, Valdivia-Alcalá R, Montiel-Batalla BM. 2021. Analysis of beekeeping profitability by strata in Aguascalientes, Mexico. Revista Mexicana de Ciencias Pecuarias 12(2):453–468. DOI: 10.22319/rmcp.v12i2.5652.
