Abstract
The biology of Mimumesa littoralis was studied on sandy soil (Sierakowo) and wasteland with hard substrate (Kowalewo Pomorskie) in northern Poland. The study presents information on the bionomics of the rare digger wasp Mimumesa littoralis (Bondroit,1934). Females of Mimumesa littoralis nested both on sandy soil and wasteland with hard substrate. Their nests consisted of 2–3 cells. One cell contained up to 11 prey items belonging to species from five genera: Megadelphax spp., Dicranotropis hamata, Javesella spp., Laodelphax striatella, Javesella pellucida (Hemiptera: Delphacidae), and Streptanus sp. (Hemiptera: Cicadellidae), which had not been previously reported in the literature. The frequency with which the female brought prey to the nest varied from 12 to 21 min. In addition, the kleptoparasite Senotainia conica (Diptera: Sarcophagidae) was found in the nest. Adult individuals were observed feeding on the flowers of Pimpinella sp.
Introduction
The genus Mimumesa Malloch, 1933 includes black, “slender” digger wasps that closely resemble the genera Psen Latreille, 1796 and Pseneo Malloch, 1933. Represented by 32 species, including eight species found in Europe, Mimumesa is distributed mainly in the Holarctic and Indo-Australian regions (Pulawski Citation2022).
The species build their nests in wood, in plant stems, in clay cliffs or on steep, bare slopes (Kazenas Citation2001). The nests are multicellular, linear or branched; the cells are located in tunnels one behind the other and are separated by partitions composed of soil particles (Kazenas Citation2001).
The diet of larvae consists of small leafhoppers and planthoppers (Hemiptera: Auchenorrhyncha) (Blösch Citation2000; Kazenas Citation2001). The prey captured by the female is supported in flight by her middle legs, as in M. atratina (F. Morawitz, 1891). Occasionally, very small prey items are carried only by the mandibles, as in M. dahlbomi (Wesmael, 1852) (Blösch Citation2000). After feeding, the larva produces a dense silk cocoon of a light brown colour (Gurney Citation1951; Kazenas Citation2001). Notozus, Elampus, Chrysis, Omalus, Hedychridium and Ноlоруgа (Hymenoptera: Chrysididae) species are known nest parasites (Bohart & Menke Citation1976; Kazenas Citation2001).
M. littoralis is widespread in the Palaearctic and has been recorded from the south coast of England (Archer Citation2015), North Devon, North West England, Wales, and Dublin Bay in Ireland (J.P. Early, personal communication) as well as from isolated sites in most of Northern and Central Europe (Lomholdt Citation1984). Little is known about the biology of M. littoralis, and the existing information refers mainly to the study by Spooner (Citation1948), who reported that the species reproduces on sandy sites near the coast, in locations similar to those of Pompilus plumbeus Fabr. (Pompilidae), such as white dunes. Tsuneki (Citation1959) described the structure of the nests on a steep loamy slope and showed that the main burrow can have one or more branches, with each branch having either a single terminal cell or two cells. Lomholdt (Citation1984) speculated that this digger wasp may nest in dead stems or roots. At a site in Charmouth (Dorset county, southwest England), Else and Felton (Citation1994) described a female entering a burrow in the ground that had been dug by another insect. Females observed at this site were bringing a female of Chloriona (Hemiptera: Delphacidae) and a nymph of the genus Macrosteles (Hemiptera: Cicadellidae) to their nests as prey.
Mimumesa littoralis is morphologically very similar to the closely related and more widespread M. unicolor (Vander Linden, 1829). The nesting biology of the two species is also similar. Females can be distinguished by rather variable characters of the head sculpture and the colour of the antennal flagellum (Dollfuss & Bitsch Citation2001), or by applying the DNA barcoding (Schmid-Egger et al. Citation2018). The species is included on the red list of endangered invertebrates in Poland with the status Endangered (Olszewski et al. Citation2021c).
The objective of this study was to supplement the existing data on the nesting biology of M. littoralis: (1) nesting behaviour of females, (2) frequency of bringing prey to the nest, (3) prey range, (4) phenology, and (5) accompanying kleptoparasites.
Material and methods
Study area
The research was conducted at two sites in Poland. One site was located in the village of Sierakowo (53°10ʹ18.8 ''N; 18°52'22.2''E) on a sandy loam esker overgrown with grassland vegetation containing some segetal and ruderal species. The site (with an area of about 500 m2) was surrounded by cultivated fields and meadows, as well as farm buildings.
The second site was an agricultural wasteland with segetal and ruderal species located in the town of Kowalewo Pomorskie (53°10'06.6''N; 18°52'17.3''E). The site (with an area of about 500 m2) was surrounded by cultivated fields and meadows, as well as farm buildings.
The sites in Sierakowo ()) and Kowalewo Pomorskie ()) were previously the subject of research on the nesting biology of other digger wasp species: Dryudella stigma (Panzer, 1809), Alysson spinosus (Panzer, 1801), Lindenius albilabris (Fabricius, 1793), Oxybelus variegatus Wesmael, 1852 (Olszewski et al. Citation2021a, Citation2021b, Citation2022a, Citation2022b).
Sampling
The nesting biology (i.e., nest digging and prey transport) was investigated based on direct observations and on-site notes. Observations were made during the activity of the females (July to late September), at a minimum ambient temperature of 18°C. During the day, observation times varied from 3 to 9 h, depending on the weather conditions.
Photographs were taken with an EOS M50 camera; in addition, a Raynox M-250 macro converter was used. The range of prey, the presence of kleptoparasites and the nest structure were documented by visual inspection after detaching the nest from the substrate.
DNA barcoding
Females were caught at the nest using a sweep net and, given the similarity of M. littoralis to M. unicolor (Vander Linden, 1829), identified by applying the DNA barcoding. The DNA was extracted from thoracic muscle tissues, using the GeneJet Genome Purification Kit protocol (Thermo Fisher Scientific Baltics, Vilnius, Lithuania). For the polymerase chain reaction (PCR), the primers T3Lep-f (5'-attaaccctcactaaagtchacwaaycayaaarayatygg-3') and T7Nancy-r (5'-aatacgactcactataggdaraattaraatrtaracytcwg-3') were used (Budrys et al. Citation2019). The 25 μL PCR mixture consisted of 12.5 μL of 2× polymerase chain reaction buffer (Thermo Fisher Scientific Baltics), 2.5 μL of 10 pmol of each primer, 7.5 μL of deionised water, and 1 μL of genomic DNA. The PCR conditions were as follows: initial denaturation at 95°C for 5 min, 40 cycles of denaturation at 94°C for 40s, annealing at 45°C for 40s, and extension at 68°C for 60s; final extension at 68°C for 7 min. The success of the PCR was assessed by electrophoresis on 1.5% agarose gel (Thermo Fisher Scientific Baltics). Polymerase chain reaction products were purified following the protocol of Exonuclease I/FastAP Thermosensitive Alkaline Phosphatase (Thermo Fisher Scientific Baltics). Synthesis of primers and sequencing were performed at Macrogen (Seoul, South Korea). Universal primers T3 (5'-attaaccctcactaaag-3') and T7 (5'-aatacgactcactatag-3') were used for sequencing.
Results
All examined nesting Mimumesa females were representatives of M. littoralis, with the exception of a single individual of M. unicolor in Sierakowo (), which was caught near the nesting site of M. littoralis. The latter specimen confirmed the similarity in habitat preferences between the two species. The nesting data of M. unicolor were excluded from the analysis.
The largest number of females (above four) was observed in mid-August (12 August 2021). Single nests of M. littoralis were found in sparsely vegetated areas in Sierakowo and Kowalewo Pomorskie. A total of four nests were found, but no digging females were observed at any of them. Interestingly, a female was observed with prey (supporting the prey with her middle legs – ; two observations), and a female without prey (); three observations), probably searching for a suitable nest site, entering the open nest tunnels of other Aculeata (; ), e.g., Halictus tumulorum (Linnaeus.1758) – one of the most abundant nesting wild bees at the site in Kowalewo Pomorskie. One nest (with one prey item) of M. littoralis was abandoned for unknown reasons (at the site in Sierakowo). The females would land in front of the entrance to the nest tunnel and then quickly carry the prey inside. The frequency with which the female brought the prey to the nest varied from 12 to 21 min (an average of 15 min, observations of four nests). During the excavation of two nests, it was noted that the main burrow led to two and three cells. Unfortunately, it was not possible to determine the exact structure of the nest (the angle of the main burrow) during the excavation.
Figure 2. Nest of Mimumesa littoralis. (a–d) Top view of the nest entrance; (e) habitat of Kowalewo Pomorskie; (f) habitat of Sierakowo.
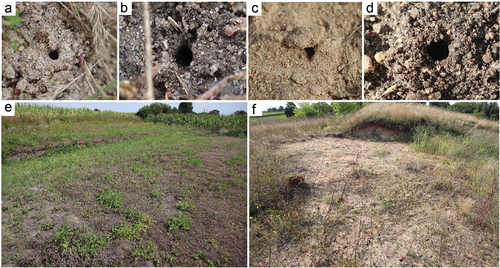
Figure 3. Adult specimens of Mimumesa littoralis. (a, b) Female with prey; (c) female in flight near the nest; (d) specimen on Pimpinella sp.; (e) female in nest.
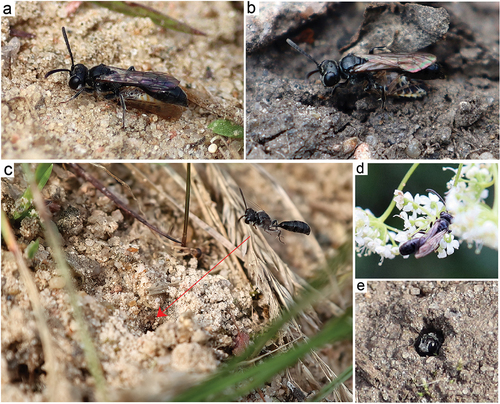
The nest cells contained a puparium of Senotainia conica (which was reared to adulthood and subsequently identified to species), a larva of M. littoralis ()) with two prey items, a fragment of a cocoon (the walls of which were formed by fragments of prey; ()) with three prey items, seven prey items (including one with an egg of M. littoralis); two other cells contained nine and 11 prey items. It was found that the food of M. littoralis larvae consisted of species from the family of Cicadellidae and the genus Streptanus, as well as the family of Delphacidae ()) belonging to five genera: Megadelphax spp., Dicranotropis hamata, Javesella spp., Laodelphax striatella, and Javesella pellucida (). Females provisioned the cells most frequently between 10 a.m. and 4 p.m. No female hunting for prey was observed. Adult individuals of M. littoralis were observed on the flowers of Pimpinella sp. plants ()).
Figure 4. Mimumesa littoralis. (a) Prey; (b) fragment of a cocoon formed by fragments of prey; (c, d) larvae. Ruler gradation (in (a) and (b)) = 1 mm.

Table I. Prey of Mimumesa littoralis from the four observed nests.
Discussion
According to Spooner (Citation1948), M. littoralis reproduces in sandy places near the coasts (white dunes). Recently, however, an increasing diversity in M. littoralis habitats has been observed. Recent data on the populations of this species have been reported from the central and southern Ural Mountains (Rudoiskatel Citation2010), from the Tegel Airport and the Flughafensee Protected Area (Saure Citation2010), from a former basalt quarry (Frommer Citation2016) and from sandy lake shores and pine heath (observations by Jacobs described in Frommer Citation2016) in Germany, as well as from anthropogenic sites in the Czech Republic (Tropek et al. Citation2013; Bogusch & Straka Citation2011; the latter – information about nesting in a postindustrial habitat). Our observations of nests on the wasteland confirm this trend. Tsuneki (Citation1959) observed this species in Japan and reported that its nests were located on a steep loamy slope and consisted of one or more main burrows, with each burrow terminating in a single nest cell or two nest cells located one behind the other. This was not confirmed in our study, as all the nests were observed only on flat terrain. The steep slopes at both sites were occupied by other digger wasps, mainly by Diodontus minutus (Fabricius, 1793) and D. tristis (Vander Linden, 1829) in Sierakowo, and by D. minutus in Kowalewo Pomorskie. Tsuneki (Citation1959) reported that the female lays her egg by attaching it to the outer base of the hind coxa, and that the placement of the egg on the hind coxa may be the rule for egg laying in the Mimumesa genus. Unfortunately, we were unable to establish this pattern due to the fact that the egg was detached from the prey of M. littoralis.
At the site in Charmouth, Else and Felton (Citation1994) described a female entering an abandoned burrow in the ground. This report is consistent with our observations at both sites, in Sierakowo and Kowalewo Pomorskie. The lack of previous data on M. littorails digging nests may suggest that this species takes over the nests of other insect species. Spooner (Citation1948) reports that Mimumesa does not nest in the ground, but occupies already existing burrows in wood, including decayed wood and other types of cavities. It appears that a morphologically very similar species, M. unicolor, may have similar habitat preferences and nesting biology as M. littoralis. However, a female of M. unicolor was observed entering a nest tunnel in the ground at a site in Emer Bog, Hampshire and Thorney Island, Sussex, and another female was observed burrowing into the ground at a site in Blackgang Chine, the Isle of Wight (Else & Felton Citation1994).
Among the prey used as food by M. littoralis larvae, one representative of the genus Streptanus in the family Cicadellidae was found. The other collected prey belonged to four genera of the Delphacidae family (). It can be assumed that the number of prey items was determined by factors such as the species of prey, the size of individuals and their availability in the habitat of M. littoralis. All identified genera and species (one leafhopper, three species and four genera of planthoppers) are common and abundant in their habitats in Poland. The insects listed in the table mainly inhabit grasslands, often disturbed places: roadsides, ruderal sites, forest clearings, abandoned cultivated fields, as well as fertilised meadows and pastures. As insects with piercing-sucking mouthparts, they feed on plant juices and they are responsible for damage and growth inhibition (stunting) in crop plants, and, when present in large numbers, can cause the death of entire plants, resulting in crop damage. Prey of M. littoralis also includes insects that can indirectly damage crop plants by transmitting plant pathogens (viruses and bacteria and phytoplasmas), often causing even greater damage to crops than damage caused by direct foraging (Nickel Citation2003). In Japan, M. littoralis was reported to prey on common pests found in rice fields near the nests of this wasp. The prey included rice leafhoppers – Nephotettix nigropictus (Stål) (Cicadellidae) and white-backed planthopper – Sogata furcifera (Horv.) (Delphacidae) (Tsuneki Citation1959). In the United Kingdom, M. littoralis was observed to prey on leafhopper species of the Cicadellidae family – a nymph of the genus Macrosteles, and the Delphacidae family – an imago of the genus Chloriona, which is trophically associated with the common reed (Phragmites australis (Cav.)Trin. ex Steud) (Else & Felton Citation1994). In our opinion, the genus Mimumesa should be thoroughly studied in terms of nesting biology (and also molecular biology) to establish basic facts about the biology of its representatives.
Acknowledgements
We thank Karolina and Łukasz Musiał for the opportunity to conduct our research in Kowalewo Pomorskie and Kamila Ludwik for the opportunity to conduct our research in Sierakowo. We also thank Jeremy Early for his critical review of the manuscript and Krzysztof Szpila (Nicolaus Copernicus University) for identifying the kleptoparasitic fly. We are also grateful to the three anonymous reviewers for their helpful comments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Archer ME. 2015. The solitary wasps and bees (Hymenoptera: Aculeata) of English coastal sand dunes. British Journal of Entomology and Natural History 28:193–203.
- Blösch M. 2000. Die Grabwespen Deutschlands. Sphecidae s. str., Crabronidae. Lebensweise, Verhalten, Verbreitung. In: Blank SM, Taeger A, editors. Die Tierwelt Deutschlands. 71. Teil. Keltern, Germany: Goecke & Evers. pp. 480.
- Bogusch P, Straka J. 2011. Žahadloví blanokřídlí. In: Tropek R, Řehounek J, editors. Bezobratlí postindustriálních stanovišť: Význam, ochrana a management. Calla: České Budejovice. pp. 152.
- Bohart RM, Menke AS. 1976. Sphecid wasps of the world. A generic revision. Berkeley: University of California Press. pp. ix + 695.
- Budrys E, Budrienė A, Orlovskytė S, Soon V. 2019. Two new species of Diodontus (Hymenoptera: Pemphredonidae) from Western Mediterranean and their phylogenetic relationships. The Canadian Entomologist 151:558–583. DOI:10.4039/tce.2019.46.
- Dollfuss H, Bitsch J. 2001. Psenini. In: Bitsch J et al., editors. Faune de France 86. Hyménoptères Sphecidae d’Europe Occidentale. Vol. 3. Paris: Fédération française des sociétés de sciences naturelles. pp. 14–55, 459.
- Else GR, Felton JC. 1994. Mimumesa unicolor (Vander Linden, 1829) (Hymenoptera: Sphecidae), a wasp new to the British list, with observations on related species. Entomologist’s Gazette 45:107–114.
- Frommer U. 2016. Erstnachweis der Grabwespenarten Mimumesa littoralis (Bondroit, 1934) und Trypoxylon kolazyi Kohl, 1893 (Hymenoptera: Crabronidae) für Hessen mit Hinweisen zum Lebensraum und Rote Liste Status. Hessische Faunistische Briefe 35:1–6.
- Gurney AB 1951. The nesting habits of Mimesa (Mimumesa) nigra (Packard) (Hymenoptera, Sphecidae). Proceedings of the Entomological Society of Washington 53:280
- Kazenas VL. 2001. Fauna and Biology of Sphecid Wasps (Hymenoptera, Sphecidae) of Kazakhstan and Central Asia. Kazgos INTI: Almaty, Kazakhstan. pp. 333.
- Lomholdt O. 1984. The Sphecidae (Hymenoptera) of Fennoscandia and Denmark. Fauna Entomologica Scandinavica. 4. 2nd ed. Leiden, Copenhagen: E.J. Brill/Scandinavian Science Press. pp. 452.
- Nickel H. 2003 he Leafhoppers and Planthoppers of Germany (Hemiptera, Auchenorrhyncha): Patterns and Strategies in a Highly Diverse Group of Phytophagous Insects. Annals of the Entomological Society of America 99(1):187–188. https://doi.org/10.1603/0013-8746(2006)099[0187:TLAPOG]2.0.CO;2.
- Olszewski P, Bogusch P, Hebda G, Budrys E. 2022b. Nesting behaviour and description of mature larva of Lindenius albilabris (Fabricius, 1793) (Hymenoptera: Crabronidae). The European Zoological Journal 89(1):1040–1048. DOI:10.1080/24750263.2022.2105962.
- Olszewski P, Bogusch P, Klejdysz T, Szpila K. 2022a. Nesting behaviour and description of the larva of Alysson spinosus (Panzer, 1801) (Hymenoptera: Bembicidae). The European Zoological Journal 89:957–965. DOI:10.1080/24750263.2022.2100494.
- Olszewski P, Bogusch P, Mięsikowski M, Baños-Picon L, Puchałka R. 2021b. Behavioural and ecological data on Dryudella stigma (Panzer,180 (Hymenoptera: Crabronidae) with the first description of the mature larva. Journal of Hymenoptera Research 82:305–316. DOI:10.3897/jhr.82.63594.
- Olszewski P, Bogusch P, Szpila K. 2021a. Life history of Oxybelus variegatus Wesmael, 1852 (Hymenoptera: Crabronidae) with a Description of the Mature Larva. Insects 12:100. DOI:10.3390/insects12020100.
- Olszewski P, Wiśniowski B, Ljubomirov T. 2021c. Current list of the Polish digger wasps (Hymenoptera: Spheciformes). Spixiana 44(1):81–107.
- Pulawski W 2022. Catalog of Sphecidae. California Academy of Sciences, San Francisco. Available: https://researcharchive.calacademy.org/research/entomology/entomology_resources/hymenoptera/sphecidae/genera/Mimumesa.pdf. Accessed on 2022 Nov 8.
- Rudoiskatel PV 2010. Fauna royushchikh os (Hymenoptera: Apoidea: Sphecidae, Crabronidae) srednego i yuzhnogo Urala. In Ekologiya ot yuzhnukh gor do severnykh morey. Materialy Vserossiyskoy Konferentsii Molodykh Uchenykh Posvyashchennoy 90-letiyu so Dnya Rozhdeniya Akademika P. L. Gorchakovskogo. Yekaterinburg: Rossiyskaya Akademiya Nauk, Uralskoye Otdeleniye, Institut Ekologii Rasteniy i Zhivotnykh. Goshchitskiy. pp. 145–161.
- Saure C. 2010. Bienen und Wespen in den Gebieten Flughafen Tegel und Flughafensee in Berlin-Reinickendorf (Hymenoptera) – Bees and wasps in the areas Airport Tegel und Flughavensee in Berlin-Reinickendorf (Hymenoptera). Märkische Entomologische Nachrichten 12:165–193.
- Schmid-Egger C, Straka J, Ljubomirov T, Blagoev GA, Morinière J, Schmidt S. 2018. DNA barcodes identify 99 per cent of apoid wasp species (Hymenoptera: Ampulicidae, Crabronidae, Sphecidae) from the Western Palearctic. Molecular Ecology Resources 19:476–484. DOI:10.1111/1755-0998.12963.
- Spooner GM. 1948. The British species of psenine wasps (Hymenoptera: Sphecidae). The Transactions of the Royal Entomological Society of London 99(part 3):129–172. DOI:10.1111/j.1365-2311.1948.tb01234.x.
- Tropek R, Černá I, Straka J, Čížek O, Konvička M. 2013. Is coal combustion the last chance for vanishing insects of inland drift sand dunes in Europe? Biological Conservation 162:60–64. DOI:10.1016/j.biocon.2013.03.027.
- Tsuneki K. 1959. Contributions to the knowledge of the Cleptinae and Pseninae Faunae of Japan and Korea (Hymenoptera, Chrysididae and Sphecidae). Memoirs of the Faculty of Liberal Arts, Fukui University (Series II, Natural Science) 9:1–78.

