?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The Clouded Apollo, Parnassius mnemosyne is a charismatic West Palaearctic butterfly, threatened in some European countries and listed in Appendix IV of the Habitats’ Directive. We investigated a large lowland population of the species inhabiting a complex of irregularly coppiced light forest and adjacent wet meadows in the anthropogenic landscape in the valley of the Narew river in NE Poland. A mark-release-recapture (MRR) method was used over 4 years to estimate population size, adult catchability, daily survival and lifespan. The beginning and length of the flight period (which lasted 4–6 weeks) differed over the years and was clearly related to weather conditions (temperature, sunshine) in April, i.e. the most important period for larval development. The seasonal population size varied from 555 to 942 adults. In contrast to some previous studies, the sex ratio turned out to be well balanced, although the catchability of males was significantly higher than that of females in each year. The quality of the wings deteriorated during the season in both sexes, but it was generally better in the case of females, which indicates that they are less active. Inter-seasonal variation in the lifespan (6.36–12.45 days) is more difficult to interpret, but it is worth noting that individuals lived the longest in the coldest and least sunlit flight period. The temporal fragmentation index (i.e. the ratio of flight-period length to adult lifespan) also varied (3.37–5.97) but was generally relatively low, which suggests that there is no threat of reduction of the effective population size at the moment. Nevertheless, the investigated population may be at risk of decline due to the observed intensification of meadow use and especially the possible conversion of grasslands into cornfields.
Introduction
Butterflies are a model group of insects for studies of adult demography and dispersal. Their daily activity and usually conspicuous behaviour make them especially convenient subjects. A large amount of data can be obtained with the quite simple and cheap mark-release-recapture method, usually consisting of drawing a unique number or code on a wing (Haddad et al. Citation2008; Nowicki et al. Citation2008; Kral et al. Citation2018).
Among the parameters which can be inferred from such studies are estimates of adult numbers and their longevity. Studies of European butterflies indicate that their average lifespan varies from 2 to 15 days, showing variation among sites and seasons (Bubová et al. Citation2016). Intra-specific variation may also concern the difference between the two sexes, and some studies indicate that the lifespan of females is longer than that reported for males (Sielezniew et al. Citation2019).
As far as vulnerable species are concerned, knowledge on population size is even more important. Most species show high temporal fluctuations related to seasonal weather conditions and habitat quality as well as survival of prematures (Wilson & Roy Citation2009). Parasitoids which could decimate premature instars contribute to this instability (Shaw et al. Citation2009). Population dynamics may also be related to some species-specific traits such as diet specialisation, mobility, length of flight period, habitat area, and distance to the edge of the species’ distribution (Franzén et al. Citation2013; Nowicki Citation2017). Species with larger fluctuations generally face higher extinction risks (Hanski Citation2005).
Local abundance is an important factor in the dynamics, genetics, and evolution of populations (Gyllenberg et al. Citation1997). The effect of population size may be altered by a distorted sex ratio, e.g. by enhancing the level of competition among individuals of the prevalent sex (Casula & Nichols Citation2003). According to Fisher’s principle (Hamilton Citation1967) one may expect that males and females are present in a butterfly population in the same numbers, but cases of extraordinary sex ratios are also reported. A bias may be primary, e.g. the ratio of eggs of different sexes is different from 1:1 or there may be differences in survival rates between male and female prematures (Frey & Leong Citation1993; Underwood & Shapiro Citation1999). Infection with certain strains of Wolbachia could also be the cause of sex ratio distortion, and the most well-known examples of female-biased populations have been affected by this male-killing bacterium (Dyson & Hurst Citation2004; Sakamoto et al. Citation2011).
However, in natural adult butterfly populations, male-biased sex ratios are most often observed (Ehrlich et al. Citation1984). Capture-recapture models (Nichols Citation1992) can help to distinguish whether differences in abundance are not just artefacts of sampling arising from the different “catchabilities” of males and females in the studied area (Stoks Citation2001). In the case of butterflies, the latter phenomenon usually follows sex-related differences in behaviour, and males are more conspicuous, especially when using a patrolling strategy to find mates. Hence, they are recorded and captured more often than females (Frey & Leong Citation1993; Casula & Nichols Citation2003; Zimmermann et al. Citation2011; Čelik et al. Citation2012).
Typical contradictions concerning sex ratio are related to representatives of the charismatic genus Parnassius, and some data analyses indicate that surpluses in abundance of males are not apparent but real (Brommer & Fred Citation1999; Matter & Roland Citation2002; Vlasanek et al. Citation2009). One of the most intensively studied species is the Clouded Apollo Parnassius mnemosyne, which is an interesting research object because of its conservation status as well as its life history. Larvae hatch in early spring, and they develop remarkably quickly to produce adults locally even as early as April (Pásztor et al. Citation2022).
The Clouded Apollo, despite its wide Eurosiberian range, is considered as near threatened in Europe and listed in Appendix II of the Bern Convention, as well as in Appendix IV of the Habitats’ Directive (Van Swaay et al. Citation2010). In Europe, it is reported from 32 countries and in 28 of them it is considered as a species of conservation concern (Maes et al. Citation2019). Habitat loss, i.e. the disappearance of traditionally managed flower-rich meadows and pastures accompanied with open deciduous forest or open grasslands, is probably the main threat, as suggested by studies in Finland and Sweden (Kuussaari et al. Citation2015; Johansson et al. Citation2017). On a continental scale, P. mnemosyne is also potentially threatened by climate change (Settele et al. Citation2008), which could be one of the reasons for the recent decline of the Clouded Apollo in the Italian Alps (Cini et al. Citation2020). On the other hand, in the north (e.g. in Estonia), warming has possibly contributed to species expansion (Liivamägi et al. Citation2013).
In the present paper, we studied the demography of a large lowland population of P. mnemosyne for 4 years, which differed significantly in weather conditions. Each year we aimed to estimate the abundance and sex ratio, as well as the lifespan of adults, and to look for the possible relationships of these traits with temperature and insolation.
Materials and methods
Study species
The Clouded Apollo, Parnassius mnemosyne (Linnaeus, 1758) is a West Palaearctic butterfly associated with sparse woodlands, clearings, wooded meadows and forest steppes of the temperate vegetation belt. In Central and Southern Europe it occurs in mountain and submontane regions while in the northeast it inhabits lowlands (Tolman & Lewington Citation2009; Kudrna Citation2011). Poland could be a typical example of this distribution type, with two main areas of P. mnemosyne occurrence, i.e. the Carpathians and the lowlands in the north-eastern part of the country. Isolated populations occur in the Sudety mountains and in Central Poland (Gromek et al. Citation2021). Genetic studies have revealed the existence of several spatially segregated lineages in Europe, indicating postglacial recolonization from different refugial areas (Gratton et al. Citation2008).
The Clouded Apollo is a univoltine species, which flies from late April to August depending on locality and season (Tolman & Lewington Citation2009). It is not specific towards nectar plants and can adjust its foraging to varying resource availability, although it shows some inter-specific variation in preferences (Szigeti et al. Citation2019, Citation2020). Males use a patrolling strategy when looking for mates. During copulation, a sperm plug (sphragis) is deposited on the female abdomen by a male to prevent subsequent matings (Vlašánek et al. Citation2009). Females usually lay their eggs in the vicinity of larval food plants i.e. Corydalis spp. (C. solida, C. cava and C. intermedia), since in the flight period they are already mostly dried up as typical geophytes (Bergström Citation2005). Caterpillars overwinter in egg shells to emerge in early spring when they develop very quickly, moulting three times, and finally pupate in dried spun tree leaves. The transition zone between deciduous light forest and extensively used grasslands is preferred for development (Habel et al. Citation2022).
Study site
We conducted our study on a lowland site of P. mnemosyne localized near Tykocin (53°13ʹN; 22°50ʹE, 105–108 m a.s.l.) in the Narew Valley (NE Poland), within the Natura 2000 Site “Ostoja Narwiańska” PLH200024. The investigated population was part of a metapopulation system consisting of about 20 occupied patches, of which the focal site was the largest, and well known for at least 25 years. The current landscape was created in the 1970s when this section of the Narew river was regulated and former swamps were converted into grasslands. The inhabited biotope was heterogeneous, and its central part was an elongated fragment of raised land stretching from west to east, 570 m long and 50–100 m wide. This area of about 4 ha was covered by light deciduous forest (mostly oaks, limes, hornbeams and aspens) with some small clearings, surrounded by wetter hay meadows (usually mowed twice a year). The forest was irregularly coppiced, several larger trees were usually cut down every year during the winter time. The only larval food plant of P. mnemosyne at the site, i.e. Corydalis solida, was patchily distributed and locally very abundant. Caterpillars could be found in April, mostly in sun-exposed patches. Adult butterflies were most often observed along the forest edge and in the clearings, as well as in meadows adjacent to the forest, usually up to about 50 m from the forest boundary ().
Data collection
We sampled the population through a mark-release-recapture (MRR) study over 4 years (2017, 2019–2021), on 17–21 occasions (74 in total). Each year the sampling covered the entire flight period of the focal species, and the site was visited every second day on average if the weather was favourable (i.e. dry and not very cloudy or windy), between 9 am and 5 pm CEST. One or two people spent about 2–4 hours on the site during each sampling day. Butterflies were captured with an entomological net, marked on the underside of their hind-wings with unique identity numbers () using a fine-tipped waterproof pen, and then immediately released at the place of capture. Date, time of each (re)capture and ID number of each butterfly were recorded, as well as sex and for females also the presence/absence of a mating plug (sphragis). Recaptures of individuals on the same day were not recorded. Additionally, in two seasons (2019 and 2021) the wing wear of each individual was assessed on a 4-point scale (0 = fresh, 1 = the least worn, 2 = moderately worn, 3 = heavily worn).
Analysis
We analysed our data with the Jolly-Seber model (Arnason & Schwarz Citation1999) using Program MARK, version 8.0 (White & Burnham Citation1999). The model represents a well-established standard for estimating population size in open populations, and it has been frequently applied in butterfly studies (Schtickzelle et al. Citation2002; Nowicki and Vrabec Citation2011; Osváth-Ferencz et al. Citation2017). Based on the lowest value of the Akaike Information Criterion corrected for small sample size (AICc) (Hurvich & Tsai Citation1989), the model variant φ(.)p(s + t)B(s*t) turned out to perform the best for each year. This best-performing model assumed a constant (and equal for both sexes) survival rate (φ), but differences as far as other parameters were concerned, such as sex-dependent and time-varying (thus differing between capture days) recruitments of new individuals into the population (B), and sex-dependent and time-varying capture probabilities (p) with a constant difference between sexes. The model was used to obtain the estimates of daily numbers of males and females as well as their seasonal population sizes. Subsequently, we estimated the total population size as the sum of the male and female population sizes estimated from the model. Based on survival estimates we calculated the mean adult lifespans as (Nowicki et al. Citation2005). For comparative purposes, we also derived mean capture probabilities for males and females across the entire season. Additionally, we calculated the temporal fragmentation index, i.e., ratio of flight period length to adult lifespan, which is considered as one of the indicators of species vulnerability (Bubová et al. Citation2016).
Weather data for the characterization of the sampling seasons were acquired from the Białystok weather station located approximately 23 km south-east of the study site and run by the Polish Institute of Meteorology and Water Management - National Research Institute (IMGW-PIB).
Results
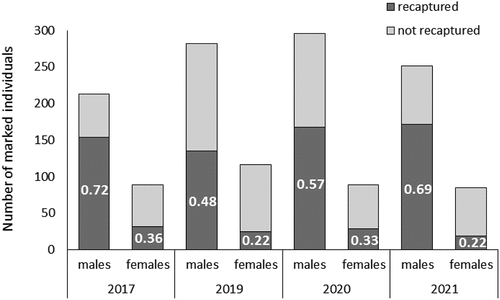
Sampling years differed significantly as far as the phenology of Parnassius mnemosyne was concerned and there were also substantial weather variations (). In 2019, the first butterflies were already on the wing on May 3, while in 2021 they emerged nearly 3 weeks later. Then, the flight period lasted from only 4 weeks in 2017 to six in 2020. Overall, 1,421 individuals (1,042 males and 379 females) were captured and marked in four seasons (302–398 in each one). The sex ratio of marked individuals was strongly male biased (2.8:1) and ranged from 2.4:1 in 2017 to 3.3:1 in 2020. The presence of sphragis was recorded in the case of 85.4% females, with relatively small inter-year variability ().
Table I. General information for the study years. Weather conditions in the flight period refer to daily means recorded between 8 a.m. and 5 p.m., i.e. the period of butterfly activity in favourable conditions. Weather conditions in April (i.e. the month most important in the context of larval development) refer to daylight time. Data were acquired from the IMGW-PIB Białystok.
Table II. Summary of mark-recapture surveys conducted.
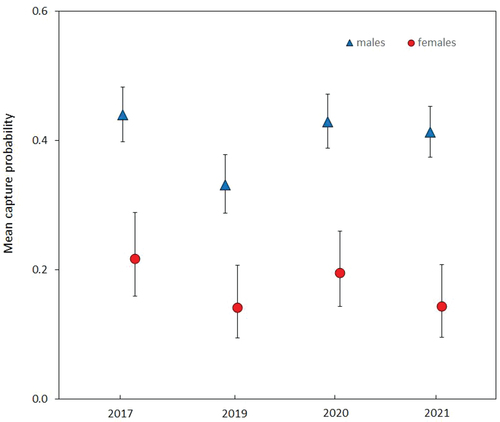
About 50% of the marked individuals were recaptured at least once. The proportion of recaptured individuals differed somewhat between years (0.4–0.6) and was substantially higher (1.7–3.1 times) for males than for females (). Correspondingly, the average capture probability was 2–2.9 higher for males than for females in all years ().
Figure 4. Mean capture probability in the four sampling years derived for males (triangles) and females (dots) as estimated with the Jolly-Seber model. Error bars represent 95% confidence intervals. Significant intersexual differences were detected for all years.
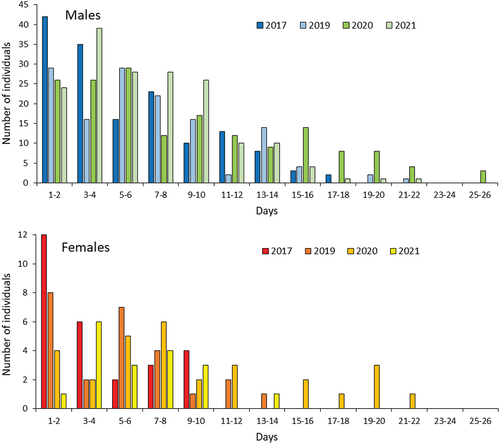
The maximum duration between captures of an individual reached 22 days for males and 25 days for females in 2020 (). The mean number of days between the first and last captures ranged between 5.65 in 2017 to 8.89 days in 2020, and in all years except 2020 was longer for males than for females (). The estimated population size of adults ranged between 555 individuals in 2017 and 942 in 2019. The estimated sex ratio was fairly well balanced in all seasons, with a slight predominance of males in three of the years and females only in 2019 () The distortion was not significant in any year (Χ2 test, P = 0.73–0.85). The estimated figures were 2–3 times lower compared to direct observations, i.e. proportions of marked individuals.
Table III. The mean numbers of days between the first and last captures in the four sampling seasons.
Table IV. Estimated population sizes for the four investigated seasons; the values in parentheses represent 95% confidence intervals.
Figure 5. Distribution of recaptured individuals according to maximum temporal distance between the first and the last capture.
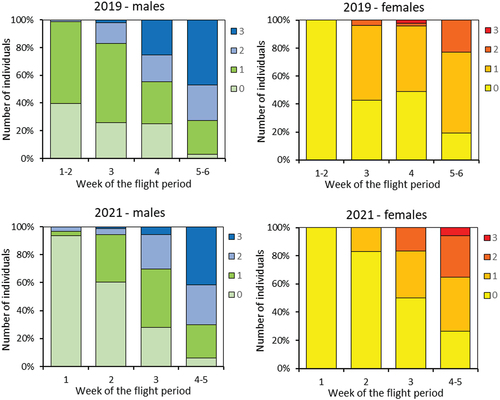
The wing condition of captured individuals decreased continuously during the flight period in both of the seasons in which this assessment was made. In the case of females, wings were generally of better quality compared to males throughout the studies and even in the final weeks they were given mostly 0 or 1 ().
Figure 6. Wing wear of captured individuals (recaptures included) in two investigated years. Colours of bars correspond to wing wear on a 4-point scale (0 – very fresh, 1 - the least worn, 2 - moderately worn, 3 - heavily worn). Data for weeks 1–2 and 5–6 in 2019 and for weeks 4–5 in 2021 were pooled, due to very few individuals being captured in the beginning/end of the flight period.
The daily survival rate obtained with the Jolly-Seber model varied between 0.854 and 0.923, which corresponded to an estimated adult lifespan of 6.36–12.45 days (). Taking into consideration the recorded flight periods in particular years, the temporal fragmentation index was estimated at 3.37–5.97.
Table V. Estimates of daily survival rate and lifespan for the four investigated seasons; the values in parentheses represent 95% confidence intervals.
Discussion
Variation concerning both the timing and length of the flight period is not surprising when compared with other observations of P. mnemosyne, e.g. from long-term studies in Finland (Kuussaari et al. Citation2016), and can be easily explained by weather conditions. In 2017 and 2021, when butterflies were on the wing for only about 4 weeks, mean temperatures and sunshine percentages were higher compared to 2019 and 2020, when the appearance of the species was extended. The beginning of adult emergence was clearly earlier in years with warmer and more sunny conditions in early spring, i.e. the relevant time for larval development. Analyses of long-term data for 20 univoltine butterfly species indicate that temperature in the months immediately preceding adult emergence has the strongest relationship with phenology (Gutiérrez & Wilson Citation2021). Phenological shifts related to weather conditions are generally well-documented, especially in the context of global warming, and some life history traits as well as habitat types may influence specific responses (Altermatt Citation2010, Citation2012; Brooks et al. Citation2017, Fric et al. Citation2020; Zografou et al. Citation2021).
Variation in the duration of the flight period recorded in our study was similar to that observed by Pásztor et al. (Citation2022) for a small population in Hungary over seven seasons (26–45 days). Even greater phenological shifts and variation in the duration of the flight period were reported for montane populations (Adamski et al. Citation2019).
Adult population size was relatively stable when compared to some other butterfly species (e.g. Nowicki et al. Citation2009). Long-term observations over 25 years suggest that P. mnemosyne has been numerous on the site every year. In our study, the maximum abundance was recorded in 2019, when the weather conditions for the quick development of caterpillars were probably the best. Larvae are thermophilic, switching between feeding and basking. Weather may also act indirectly by affecting the interactions between prematures and parasitoids (Wilson & Roy Citation2009), but in the case of P. mnemosyne, data on natural enemies of this kind are lacking (Shaw et al. Citation2009), which could be related to difficulties in finding larvae (Vlasánek et al. Citation2017).
Butterfly populations are sensitive to weather, which affects numbers and flight behaviour. Generally, their activity increases with temperature and decreases with cloudiness, which can translate into dispersal abilities (Cormont et al. Citation2011; Kuussaari et al. Citation2016). Relationships between weather and numbers are usually more complicated, and often concern previous seasons and/or not only the conditions during the flight period but also those experienced during larval development, and sensitivity may vary across distribution ranges (Mills et al. Citation2017). Case studies on the alpine Parnassius smintheus indicate that snow cover in early winter is the best predictor of annual adult population change (Roland et al. Citation2021).
A combination of climate and interspecific interactions also affects longevity. Estimation of lifespan in our studies showed considerable inter-seasonal variation. Previous assessments concerning P. mnemosyne also suggest similar variation (5–11 days), but it is worth noting that the estimates were for different sites (Konvička & Kuras Citation1999, Bubová et al. Citation2016). In the case of our studies, the most puzzling was 2020, when adults lived on average twice as long as in the previous year. Both mean temperatures and sunshine percentages were the lowest during the flight period in 2020, but no correlation could be found when only the four studied years were taken into consideration.
Little is known about factors affecting adult mortality, which could explain differences in adult longevity among the years. During our studies, only a few cases of spider predation were recorded. Adamski (Citation2013) suggests that ants can be important predators of Parnassius butterflies. Adults are active only in sunny weather, and they sit to rest on plants almost immediately when the sun hides behind the clouds. Therefore, they can be attacked by ants while resting. Formica spp. are common on the presently-studied site, especially near trees, i.e. where most adults emerge. The pressure from ants is probably lower in surrounding open grasslands, so the most affected are adults emerging from pupae on cloudy days and waiting for conditions enabling flight. However, our anecdotal observations suggest that individuals often successfully spend nights near ant hills, so the impact of this kind of pressure is not clear. Butterflies basking in the early morning could also be prey to small mammals as is indicated for Pieris butterflies showing similar colouration (Kingsolver Citation1987). Little is known about the impact of birds. The beak-mark frequency can be used to estimate the pressure of birds (Shapiro Citation1974; Ota et al. Citation2014). However, in the case of the present studies, clear symptoms which could be attributed to such events were not recorded, and no direct observations were made either. The question of wing colouration of P. mnemosyne, which could be an aposematic signal, remains open (Lyytinen et al. Citation1999).
Konvička and Kuras (Citation1999) found male-biased sex ratio of captured individuals in a population studied in the Czech Republic. Our data are only partially consistent with those findings. While we marked far more males than females and the mean capture probability was twice as high for males, our estimates clearly indicate no bias in real numbers of individuals of both sexes. However, studies carried out in the Czech Republic by Vlasanek et al. (Citation2009) designed especially to eliminate possible biases show that males are really more abundant (a 1.6 bias towards males). A very similar ratio (1.5–1.6) is reported from two sites in Hungary (Meglécz et al. Citation1999). Vlasanek et al. (Citation2009) suggest that these phenomena could be related to larval development, but it has not been confirmed (see Vlasánek et al. Citation2017). Moreover, data concerning the congeneric Apollo butterfly P. apollo based on captive breeding indicate no bias in sex ratio (Adamski Citation2004).
The better detectability of males is not surprising taking into consideration their conspicuous patrolling behavior. Females just after emergence are probably not very active, since at least 85% of them had already mated (as proved by the presence of sphragis) when they were captured for the first time. In fact, the actual proportion of females that mated soon after emergence is likely to be even higher, as a very recent research indicated that in some cases previous copulation is marked with some inconspicuous structures (that might have been overlooked in our field study) rather than with a typical sphragis (Gór et al. Citation2023). Ovipositing females could also be difficult to detect in the turf, especially since during the flight period vegetation is relatively high. The higher catchability of males is typical for butterflies (see e.g. Zimmermann et al. Citation2011; Sielezniew et al. Citation2019). The reverse pattern is rare and may occur for less conspicuous species inhabiting biotopes dominated by tall herbs and young trees, where patrolling males flying low could be more difficult to detect (Sielezniew & Nowicki Citation2017).
We also found that the quality of wings in males deteriorated much more than in females. The most likely explanation is related to the fact that they spend a higher proportion of time flying than females. Pásztor et al. (Citation2022) assessed phenotypic senescence using different measurements, i.e. the body mass and thorax, and found that their values decreased in both sexes. A steeper decline was observed in the case of females, and it was suggested that the females may use more nutrients from muscle breakdown for reproduction than males. Therefore, wing quality probably better reflects flight activity, and this is why females are generally ‘less worn’.
The Clouded Apollo is a relatively long-lived butterfly in its adult stage. Some individuals may live for up to 3 weeks, so for the most of the flight period. The calculated values of the temporal fragmentation index showed some variability but were relatively low compared to previous studies on both P. mnemosyne and the congeneric relative P. apollo. Species of conservation concern are rather characterized by higher values of this index and, on average, a shorter adult lifespan (see review by Bubová et al. Citation2016).
The investigated population is potentially one of the largest in the region and therefore seasonal fluctuations are not really a threat to it. The current coppicing management of the breeding habitat, i.e. light deciduous forest with Corydalis, seems to be optimal for the butterfly, because it creates the microhabitats preferred for larval development (Välimäki & Itämies Citation2005). Some concerns may be related to the surrounding meadows. The use of fertilizers contributes to the reduction of wildflowers, which constitute nectar resources for the focal butterfly, and some of them are cut during the flight period. However, its relatively early emergence makes the butterfly relatively unaffected by this factor. The Clouded Apollo is the most common butterfly species in the end of May at the site. Most meadow species cannot survive the twice yearly mowing, which is the typical local mowing regime. In the future, possible further threats may be related to the conversion of meadows into corn fields, as observed in the area. Unfortunately, P. mnemosyne is listed only in Appendix IV of the Habitat Directive, and thus it is not a target of conservation actions within Natura 2000 sites. Hence, appropriate management of meadows seems to be the most important measure for species preservation. Mowing should be delayed until the end of the flight period, i.e. mid-June, and the use of fertilizers should be excluded at least in places neighbouring the breeding habitats of P. mnemosyne. Extensive grazing would likely be a more favourable alternative use (Johansson et al. Citation2017). Finally, some measures should be also taken to prevent possible deforestation of the forest sites occupied by P. mnemosyne.
Acknowledgements
Piotr Ambroziak, Monika Bystrowska, Urszula Jabłońska, Sylwia Piekutowska, Aurelia Sielezniew, Gustaw Sielezniew and Adam Suprunowicz helped in the field studies. The Regional Director for Environmental Protection in Białystok issued relevant permissions for the mark-release-recapture sampling of P. mnemosyne. Sarah Luczaj made linguistic improvements on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adamski P. 2004. Sex ratio of apollo butterfly Parnassius apollo (Lepidoptera: Papilionidae) - facts and artifacts. European Journal of Entomology 101(2):341–344. DOI: 10.14411/eje.2004.046.
- Adamski P. 2013. Preferencje siedliskowe i biologia rozrodu niepylaka mnemozyny Parnassius mnemosyne oraz ich znaczenie dla ochrony gatunku. Studia Naturae 61:1–65.
- Adamski P, Ćmiel AM, Lipińska AM. 2019. Intraseasonal asynchrony as a factor boosting isolation within a metapopulation: The case of the clouded apollo. Insect Science 26(5):911–922. DOI: 10.1111/1744-7917.12589.
- Altermatt F. 2010. Tell me what you eat and I’ll tell you when you fly: Diet can predict phenological changes in response to climate change: Phenological change and diet breadth. Ecology Letters 13(12):1475–1484. DOI: 10.1111/j.1461-0248.2010.01534.x.
- Altermatt F. 2012. Temperature-related shifts in butterfly phenology depend on the habitat. Global Change Biology 18(8):2429–2438. DOI: 10.1111/j.1365-2486.2012.02727.x.
- Arnason AN, Schwarz CJ. 1999. Using POPAN-5 to analyse banding data. Bird Study 46(157–168):S157–S168. DOI: 10.1080/00063659909477242.
- Bergström A. 2005. Oviposition site preferences of the threatened butterfly Parnassius mnemosyne - implications for conservation. Journal of Insect Conservation 9(1):21–27. DOI: 10.1007/s10841-004-3204-4.
- Brommer JE, Fred MS. 1999. Movement of the Apollo butterfly Parnassius apollo related to host plant and nectar plant patches. Ecological Entomology 24(2):125–131. DOI: 10.1046/j.1365-2311.1999.00190.x.
- Brooks SJ, Self A, Powney GD, Pearse WD, Penn M, Paterson GLJ. 2017. The influence of life history traits on the phenological response of British butterflies to climate variability since the late-19th century. Ecography 40(10):1152–1165. DOI: 10.1111/ecog.02658.
- Bubová T, Kulma M, Vrabec V, Nowicki P. 2016. Adult longevity and its relationship with conservation status in European butterflies. Journal of Insect Conservation 20(6):1021–1032. DOI: 10.1007/s10841-016-9936-0.
- Casula P, Nichols JD. 2003. Temporal variability of local abundance, sex ratio and activity in the Sardinian chalk hill blue butterfly. Oecologia 136(3):374–382. DOI: 10.1007/s00442-003-1288-2.
- Čelik T. 2012. Adult demography, spatial distribution and movements of Zerynthia polyxena (Lepidoptera: Papilionidae) in a dense network of permanent habitats. European Journal of Entomology 109(2):217–227. DOI: 10.14411/eje.2012.028.
- Cini A, Barbero F, Bonelli S, Bruschini C, Casacci LP, Piazzini S, Scalercio S, Dapporto L. 2020. The decline of the charismatic Parnassius mnemosyne (L.) (Lepidoptera: Papilionidae) in a Central Italy National Park: A call for urgent actions. Journal of Insect Biodiversity 16(2):47–54. DOI: 10.12976/jib/2020.16.2.2.
- Cormont A, Malinowska AH, Kostenko O, Radchuk V, Hemerik L, WallisDeVries MF, Verboom J. 2011. Effect of local weather on butterfly flight behaviour, movement, and colonization: Significance for dispersal under climate change. Biodiversity and Conservation 20(3):483–503. DOI: 10.1007/s10531-010-9960-4.
- Dyson EA, Hurst GDD. 2004. Persistence of an extreme sex-ratio bias in a natural population. Proceedings of the National Academy of Sciences 101(17):6520–6523. DOI: 10.1073/pnas.030406810135.
- Ehrlich PR, Launer AE, Murphy DD. 1984. Can sex ratio be defined or determined? The case of a population of checkerspot butterflies. The American Naturalist 124(4):527–539. DOI: 10.1086/284292.
- Franzén M, Nilsson SG, Johansson V, Ranius T. 2013. Population fluctuations and synchrony of grassland butterflies in relation to species traits. PLoS One 8(10):e78233. DOI: 10.1371/journal.pone.0078233.
- Frey DF, Leong KLH. 1993. Can microhabitat selection or differences in “catchability” explain male-biased sex ratios in overwintering populations of monarch butterflies? Animal Behaviour 45(5):1025–1027. DOI: 10.1006/anbe.1993.1120.
- Fric ZF, Rindoš M, Konvička M. 2020. Phenology responses of temperate butterflies to latitude depend on ecological traits. Ecology Letters 23(1):172–180. DOI: 10.1111/ele.13419.
- Gór Á, Fónagy A, Pásztor K, Szigeti V, Lang Z, Kis J. 2023. Facultative male investment in prolonged mate-guarding in a butterfly. Behaviour 160(6):515–557. DOI: 10.1163/1568539X-bja10219.
- Gratton P, Konopiński MK, Sbordoni V. 2008. Pleistocene evolutionary history of the Clouded Apollo (Parnassius mnemosyne): Genetic signatures of climate cycles and a ‘time-dependent’ mitochondrial substitution rate. Molecular Ecology 17(19):4248–4262. DOI: 10.1111/j.1365-294X.2008.03901.x.
- Gromek M, Ł S, Sielezniew M, Jaworski T. 2021. Pierwsze stwierdzenie niepylaka mnemozyny Parnassius mnemosyne (Linnaeus, 1758). (Lepidoptera: Papilionidae) w środkowej Polsce. Acta entomologica silesiana 29(27):1–8.
- Gutiérrez D, Wilson RJ. 2021. Intra‐ and interspecific variation in the responses of insect phenology to climate. Journal of Animal Ecology 90(1):248–259. DOI: 10.1111/1365-2656.13348.
- Gyllenberg M, Hastings A, Hanski I. 1997. Structured metapopulation models. In: Hanski I, Gilpin M, editors. Metapopulation Biology: Ecology, genetics and evolution. London: Academic Press. pp. 93–122.
- Habel JC, Angerer V, Gros P, Teucher M, Eberle J. 2022. The relevance of transition habitats for butterfly conservation. Biodiversity and Conservation 31(5–6):1577–1590. DOI: 10.1007/s10531-022-02411-y.
- Haddad NM, Hudgens B, Damiani C, Gross K, Kuefler D, Pollock K. 2008. Determining Optimal Population Monitoring for Rare Butterflies. Conservation Biology 22(4):929–940. DOI: 10.1111/j.1523-1739.2008.00932.x.
- Hamilton WD. 1967. Extraordinary sex ratios: A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology. Science 156(3774):477–488. DOI: 10.1126/science.156.3774.477.
- Hanski I. 2005. Metapopulation ecology. New York: Oxford University Press.
- Hurvich CM, Tsai C-L. 1989. Regression and time series model selection in small samples. Biometrika 76(2):297–307. DOI: 10.1093/biomet/76.2.297.
- Johansson V, Knape J, Franzén M. 2017. Population dynamics and future persistence of the clouded Apollo butterfly in southern Scandinavia: The importance of low intensity grazing and creation of habitat patches. Biological Conservation 206:120–131. DOI: 10.1016/j.biocon.2016.12.029.
- Kingsolver JG. 1987. Predation, thermoregulation, and wing color in pierid butterflies. Oecologia 73(2):301–306. DOI: 10.1007/BF00377522.
- Konvička M, Kuras T. 1999. Population structure, behaviour and selection of oviposition sites of an endangered butterfly, Parnassius mnemosyne, in Litovelské Pomoravíl. Czech Republic. Journal of Insect Conservation 3(3):211–223. DOI: 10.1023/A:1009641618795.
- Kral K, Harmon J, Limb R, Hovick T. 2018. Improving our science: The evolution of butterfly sampling and surveying methods over time. Journal of Insect Conservation 22(1):1–14. DOI: 10.1007/s10841-018-0046-z.
- Kudrna O. 2011. Distribution atlas of butterflies in Europe. Halle, Germany: Gesellschaft für Schmetterlingsschutz.
- Kuussaari M, Heikkinen RK, Heliölä J, Luoto M, Mayer M, Rytteri S, von Bagh P. 2015. Successful translocation of the threatened Clouded Apollo butterfly (Parnassius mnemosyne) and metapopulation establishment in southern Finland. Biological Conservation 190:51–59. DOI: 10.1016/j.biocon.2015.05.011.
- Kuussaari M, Rytteri S, Heikkinen RK, Heliölä J, von Bagh P 2016. Weather explains high annual variation in butterfly dispersal. Proceedings of the Royal Society B: Biological Sciences 283(1835):20160413. DOI: 10.1098/rspb.2016.0413
- Liivamägi A, Kuusemets V, Luig J, Kask K. 2013. Changes in the distribution of Clouded Apollo Parnassius mnemosyne (Lepidoptera: Papilionidae) in Estonia. Entomologica Fennica 24(3):186–192. DOI: 10.33338/ef.8985.
- Lyytinen A, Alatalo RV, Lindström L, Mappes J. 1999. Are European white butterflies aposematic? Evolutionary Ecology 13(7–8):709. DOI: 10.1023/A:1011081800202.
- Maes D, Verovnik R, Wiemers M, Brosens D, Beshkov S, Bonelli S, Buszko J, Cantú-Salazar L, Cassar L-F, Collins S, Dincă V, Djuric M, Dušej G, Elven H, Franeta F, Garcia-Pereira P, Geryak Y, Goffart P, Á G, Hiermann U, Höttinger H, Huemer P, Jakšić P, John E, Kalivoda H, Kati V, Kirkland P, Komac B, Á K, Kulak A, Kuussaari M, L’Hoste L, Lelo S, Mestdagh X, Micevski N, Mihoci I, Mihut S, Monasterio-León Y, Morgun DV, Munguira ML, Murray T, Nielsen PS, Ólafsson E, Õunap E, Pamperis LN, Pavlíčko A, Pettersson LB, Popov S, Popović M, Pöyry J, Prentice M, Reyserhove L, Ryrholm N, Šašić M, Savenkov N, Settele J, Sielezniew M, Sinev S, Stefanescu C, Švitra G, Tammaru T, Tiitsaar A, Tzirkalli E, Tzortzakaki O, van Swaay CAM, Viborg AL, Wynhoff I, Zografou K, Warren MS. 2019. Integrating national red lists for prioritising conservation actions for European butterflies. Journal of Insect Conservation 23(2):301–330. DOI: 10.1007/s10841-019-00127-z.
- Matter SF, Roland J. 2002. An experimental examination of the effects of habitat quality on the dispersal and local abundance of the butterfly Parnassius smintheus: Habitat quality and dispersal. Ecological Entomology 27(3):308–316. DOI: 10.1046/j.1365-2311.2002.00407.x.
- Meglécz E, Nève G, Pecsenye K, Varga Z. 1999. Genetic variations in space and time in Parnassius mnemosyne (L.) (Lepidoptera) populations in north-east Hungary: Implications for conservation. Biological Conservation 89(3):251–259. DOI: 10.1016/S0006-3207(99)00006-3.
- Mills SC, Oliver TH, Bradbury RB, Gregory RD, Brereton T, Kühn E, Kuussaari M, Musche M, Roy DB, Schmucki R, Stefanescu C, van Swaay C, Evans KL. 2017. European butterfly populations vary in sensitivity to weather across their geographical ranges. Global Ecology and Biogeography 26(12):1374–1385. DOI: 10.1111/geb.12659.
- Nichols JD. 1992. Capture-Recapture Models. BioScience 42(2):94–102. DOI: 10.2307/1311650.
- Nowicki P. 2017. Survey precision moderates the relationship between population size and stability. Biological Conservation 212:310–315. DOI: 10.1016/j.biocon.2017.06.041.
- Nowicki P, Bonelli S, Barbero F, Balletto E. 2009. Relative importance of density-dependent regulation and environmental stochasticity for butterfly population dynamics. Oecologia 161:227–239. DOI: 10.1007/s00442-009-1373-2.
- Nowicki P, Settele J, Henry P-Y, Woyciechowski M. 2008. Butterfly monitoring methods: The ideal and the real World. Israel Journal of Ecology & Evolution 54(1):69–88. DOI: 10.1560/IJEE.54.1.69.
- Nowicki P, Vrabec V. 2011. Evidence for positive density-dependent emigration in butterfly metapopulations. Oecologia 167(3):657–665. DOI: 10.1007/s00442-011-2025-x.
- Nowicki P, Witek M, Skórka P, Settele J, Woyciechowski M. 2005. Population ecology of the endangered butterflies Maculinea teleius and M. nausithous and the implications for conservation. Population Ecology 47(3):193–202. DOI: 10.1007/s10144-005-0222-3.
- Osváth-Ferencz M, Bonelli S, Nowicki P, Peregovits L, Rákosy L, Sielezniew M, Kostro-Ambroziak A, Dziekańska I, Á K. 2017. Population demography of the endangered large blue butterfly Maculinea arion in Europe. Journal of Insect Conservation 21(3):411–422. DOI: 10.1007/s10841-016-9944-0.
- Ota M, Yuma M, Mitsuo Y, Togo Y. 2014. Beak marks on the wings of butterflies and predation pressure in the field: Beak mark and predation pressure. Entomological Science 17(4):371–375. DOI: 10.1111/ens.12076.
- Pásztor K, Kőrösi Á, Gór Á, Szigeti V, Vajna F, Kis J. 2022. Phenotypic senescence in a natural insect population. Ecology and Evolution 12(12). DOI: 10.1002/ece3.9668.
- Roland J, Filazzola A, Matter SF. 2021. Spatial variation in early‐winter snow cover determines local dynamics in a network of alpine butterfly populations. Ecography 44(2):334–343. DOI: 10.1111/ecog.05407.
- Sakamoto Y, Hirai N, Tanikawa T, Yago M, Ishii M. 2011. Infection by two strains of wolbachia and sex ratio distortion in a population of the endangered butterfly zizina emelina (Lepidoptera: Lycaenidae) in Northern Osaka Prefecture, Central Japan. Annals of the Entomological Society of America 104(3):483–487. DOI: 10.1603/AN09168.
- Schtickzelle N, Le Boulengé E, Baguette M. 2002. Metapopulation dynamics of the bog fritillary butterfly: Demographic processes in a patchy population. Oikos 97(3):349–360. DOI: 10.1034/j.1600-0706.2002.970305.x.
- Settele J, Kudrna O, Harpke A, Kühn I, van Swaay C, Verovnik R, Warren M, Wiemers M, Hanspach J, Hickler T, Kühn E, van Halder I, Veling K, Vliegenthart A, Wynhoff I, Schweiger O. 2008. Climatic risk atlas of European butterflies. Biorisk 1:1–712. DOI: 10.3897/biorisk.1.
- Shapiro AM. 1974. Beak-mark frequency as an index of seasonal predation intensity on common butterflies. The American Naturalist 108(960):229–232. DOI:10.1086/282901.
- Shaw MR, Stefanescu C, van Nouhuys S. 2009. Parasitoids of European butterflies. In: Settele J, Shreeve T, Konvička M, van Dyck H, editors. Ecology of Butterflies in Europe. Cambridge: Cambridge University Press. pp. 130–156.
- Sielezniew M, Kostro‐Ambroziak A, Klimczuk P, Deoniziak K, Pałka K, Nowicki P. 2019. Habitat-related differences in the adult longevity of two ecotypes of a specialized butterfly. Journal of Zoology 307(2):93–103. DOI: 10.1111/jzo.12625.
- Sielezniew M, Nowicki P. 2017. Adult demography of an isolated population of the threatened butterfly scarce heath Coenonympha hero and its conservation implications. Journal of Insect Conservation 21(4):737–742. DOI: 10.1007/s10841-017-0014-z.
- Stoks R. 2001. Male-biased sex ratios in mature damselfly populations: Real or artefact?: Biased sex ratios: Real or artefact? Ecological Entomology 26(2):181–187. DOI: 10.1046/j.1365-2311.2001.00301.x.
- Szigeti V, Kőrösi Á, Harnos A, Kis J. 2019. Lifelong foraging and individual specialisation are influenced by temporal changes of resource availability. Oikos 128(5):649–658. DOI: 10.1111/oik.05400.
- Szigeti V, Vajna F, Kőrösi Á, Kis J. 2020. Are all butterflies equal? Population-wise proboscis length variation predicts flower choice in a butterfly. Animal Behaviour 163:135–143. DOI: 10.1016/j.anbehav.2020.03.008.
- Tolman T, Lewington R. 2009. Collins butterfly guide. The most complete guide to the butterflies of Britain and Europe. London: Harper Collins Publishers.
- Underwood DL, Shapiro AM. 1999. A male-biased primary sex ratio and larval mortality in Eucheira socialis (Lepidoptera: Pieridae). Evolutionary Ecology Research 1(6):703–717.
- Välimäki P, Itämies J. 2005. Effects of canopy coverage on the immature stages of the Clouded Apollo butterfly [Parnassius mnemosyne (L.)] with observations on larval behaviour. Entomologica Fennica 16(2). DOI: 10.33338/ef.84244.
- Van Swaay C, Cuttelod A, Collins S, Maes D, Munguira ML, Šašić M, Settele J, Verovnik R, Verstrael T, Warren M, Wiemers M, Wynhoff. 2010. European red list of Butterflies. Luxembourg: Publication Office of the European Union.
- Vlasánek P, Bartonová A, Marec F, Konvička M. 2017. Elusive Parnassius mnemosyne (Linnaeus, 1758. larvae: habitat selection, sex determination and sex ratio (Lepidoptera: Papilionidae). SHILAP Revista de lepidopterología 45(180):561–569.
- Vlasanek P, Hauck D, Konvička M. 2009. Adult sex ratio in the Parnassius mnemosyne butterfly: Effects of survival, migration, and weather. Israel Journal of Ecology & Evolution 55(3):233–252. DOI: 10.1560/IJEE.55.3.233.
- White GC, Burnham KP. 1999. Program MARK: Survival estimation from populations of marked animals. Bird Study 46(sup1):120–139. DOI: 10.1080/00063659909477239.
- Wilson RJ, Roy D. 2009. Butterfly population structure and dynamics. In: Settele J, Shreeve M, Konvička H, van Dyck H, editors. Ecology of Butterflies in Europe. Cambridge: Cambridge University Press. pp. 81–96.
- Zimmermann K, Blazkova P, Cizek O, Fric Z, Hula V, Kepka P, Novotny D, Slamova I, Konvička M. 2011. Demography of adults of the Marsh fritillary butterfly, Euphydryas aurinia (Lepidoptera: Nymphalidae) in the Czech Republic: Patterns across sites and seasons. European Journal of Entomology 108(2):243–254. DOI: 10.14411/eje.2011.033.
- Zografou K, Swartz MT, Adamidis GC, Tilden VP, McKinney EN, Sewall BJ. 2021. Species traits affect phenological responses to climate change in a butterfly community. Scientific Reports 11(1):3283. DOI: 10.1038/s41598-021-82723-1.