Abstract
Based on newly acquired inclusions in Taimyr amber, we have described new taxa (Sidorchukaphis katyae Ogłaza & Wegierek gen. et sp. nov.; Baikuraphis abdominalis gen. et sp. nov.; Canadaphis ugolyaki Ogłaza & Wegierek sp. nov.) of aphids or redescribed previously reported forms (Ambaraphis baikurensis Palaeoaphis incognita Tajmyraphis zherichini; Retinaphis glandulosa). The presented species belong to both extinct and extant families from the Late Cretaceous stage of aphid evolution. A cladistic analysis of the morphological characters of the Tajmyraphidoidea has been performed. We assign Burmitaphididae, as the subfamily Burmitaphidinae, to the Tajmyraphididae, which now comprises the subfamilies Tajmyraphidinae and Burmitaphidinae. We also suggest elevating the Mongolaphidinae to family rank (Mongolaphididae).
http://www.zoobank.org/urn:lsid:zoobank.org:pub:2140429C-686C-4EE2-BF7A-67A368C1FFAC
Introduction
The earliest information on aphids from the end of the Late Cretaceous, based on Canadian amber inclusions, was published by E.O. Essig (Carpenter et al. Citation1937) and Richards (Citation1966). Canadian amber remains one of the richest sources of data on Late Cretaceous fauna (Heie & Wegierek Citation2011a). Taimyr amber proves similarly rich in aphid inclusions. Based on these materials, it has been possible to describe several new taxa. The name of this variously aged amber comes from the Taimyr Peninsula, Asia’s northernmost peninsula, located in the north of Russia (Perkovsky & Vasilenko Citation2019).
The amber collected by expeditions to northern Siberia between 1970 and 1976 performed by the Arthropoda Laboratory of the Palaeontological Institute of the Russian Academy of Sciences (PIN) yielded over a dozen aphid inclusions, which were examined and described by E. L. Kononova (Citation1975, Citation1976, Citation1977). In total, 13 new genera containing 17 new species have been recorded (). Most of them belong to extinct aphid families, whereas six species represent extant families and subfamilies. In 2017, the list of species described on the basis of that collection was supplemented by one more aphid species (Ambaraphis baikurensis Wegierek & Perkovsky, Citation2017). A subsequent expedition to Taimyr Peninsula, which aimed at obtaining new amber nuggets, was conducted by researchers of PIN in 2012. Although they stayed only 20 days in Yantardakh, more than 3000 new insect inclusions, including aphids, were acquired (Rasnitsyn et al. Citation2016; Perkovsky & Vasilenko Citation2019; Makarov & Perkovsky Citation2020; Colombo et al. Citation2021; Melnitsky & Ivanov Citation2021). During preliminary examination of the materials, two species have already been redescribed (Ogłaza et al. Citation2022a, Citation2022b).
Table I. Taimyr amber localities with aphid inclusions.
The family Tajmyraphididae is the most abundantly represented in Taimyr amber, while the remaining families/subfamilies are rare and each is represented by a single species. The aforementioned family was originally described by Kononova (Citation1975) and initially recognised as endemic to Taimyr amber. It comprised four genera and seven species. In 1996, O. E. Heie extended the family in order to include a new genus and species (Grassyaphis pikei Heie, Citation1996) observed from Canadian amber (Medicine Hat, Alberta, Canada) and later, after describing two more new species (Lebanaphis minor Heie, Citation2000 and Megarostrum azari Heie, Citation2000) from Lebanese amber, he revised the family once more (Heie & Azar Citation2000). In Heie’s new definition, the family was divided into five subfamilies which later, in “A list of fossil aphids (Hemiptera, Sternorrhyncha, Aphidomorpha)” (Heie & Wegierek Citation2011a), were each elevated to family rank. In 2020, Żyła and Wegierek described the representatives of the family (three genera, seven species) based on imprints from Khotont (Mongolia, Late Jurassic/Early Cretaceous) for the first time. At present, the superfamily Tajmyraphidoidea Kononova, Citation1975 contains two families: Burmitaphididae and Tajmyraphididae. The latter is divided into three subfamilies: Lebanaphidinae, Mongolaphidinae and Tajmyraphidinae. The subfamily Tajmyraphidinae contains all the species described from Taimyr amber by Kononova. Currently, the representatives of the family are known from both the Early and the Late Cretaceous. The systematic position of Tajmyraphididae and the relationships within the family have not been examined prior to this study.
The primary aims of this study are to examine and describe the new inclusions of aphids in the Taimyr amber collection, report new species and redescribe known taxa, since most materials previously examined by Kononova have been destroyed.
Additionally, this paper aims to recognise morphological characters for their possible use in cladistic analysis and to aid in the identification of synapomorphic characters in order to define the taxonomic status of the Tajmyraphidoidea.
Material and methods
Specimen examination
The Taimyr Peninsula is located in the north of Russia, in the Krasnoyarsk Krai: Taimyrsky Dolgano-Nenetsky District. This territory is known for several variously dated Cretaceous amber sites from which aphids have been described (). The oldest ambers originated from the latest Early Cretaceous and the youngest ones from mid-Late Cretaceous deposits (Rasnitsyn et al. Citation2016; Perkovsky & Vasilenko Citation2019). The materials described by Kononova, including the holotypes and paratypes, were nearly completely destroyed as a result of storing them in castor oil (see Perkovsky & Vasilenko Citation2019). The present study aims to compensate for the aforementioned loss as much as possible, via the examination and description of additional materials, including those collected during the Taimyr expedition of 2012.
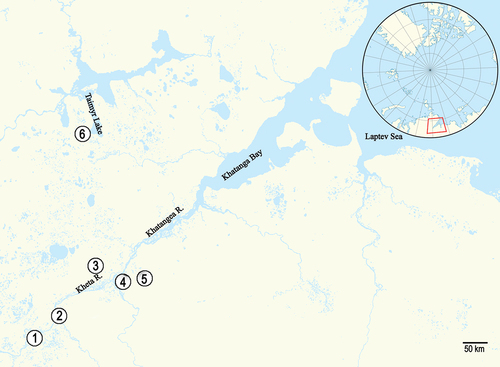
Figure 1. Map of Taimyr amber sites from which aphids were described: (1) Romanikha, (2) Yantardakh, (3) Ugolyak, (4) Kresty, (5) Zhdanikha, (6) Baikura; 1, 2, 3: Late Santonian; 4, 5: Albian–Late Cenomanian; 6: Albian–Early Cenomanian.
The specimens were observed under a Leica M205C and a Nikon SMZ1500 stereomicroscope, a Nikon Eclipse-E600 light microscope, and an Olympus IX83 fluorescence microscope. Photographs were taken under the Nikon SMZ1500 stereomicroscope with an attached Nikon DS Fi3 camera (resolution: 2560 × 1920 pixels). For the Leica M205C stereomicroscope, an attached Leica flexcam C3 camera was used. For the Nikon Eclipse-E600 light microscope, photographs were taken with an attached Nikon DS Fi2 camera. The program NIS Elements D 4.13.05, 64-bit, was used. Fluorescence photographs were taken using an Olympus IX83 fluorescence microscope, and the scanning electron micrographs were taken with a Phenom XL.
All measurements are reported in millimetres. The fossil specimens described here are housed in PIN. Systematics follow Heie and Wegierek (Citation2011b).
Programs used for cladistic analysis
The data matrix (Table S1) was constructed using the table in Mesquite v. 3.81 (Maddison & Maddison Citation2023) and saved in TNT and Nexus Format. The matrix includes 31 characters scored for 26 taxa. Unknown character states were coded with “?”. The dataset includes the studied taxon sampling data, and all morphological characteristics were treated as ordered (). The cladistic analysis was conducted with the program TNT v. 1.1 (Goloboff et al. Citation2008) using maximum parsimony (traditional search) as the optimality criterion.
Table II. List of characters.
Parsimonious trees were searched based on prior character weights (Goloboff et al. Citation2008), with concavity indices (K) ranging from 3 to 8. All trees obtained in TNT were visualised using ASADO v. 1.00.08 for Windows (Nixon Citation2004), with acctran (Fast), deltran (Slow), and unambiguous optimisations. The tree with the highest mean of non-homoplastic characters was selected as the working hypothesis tree with slow optimisation. The slow optimisations use delayed transformation (deltran), where changes are assigned along branches as close to the tips as possible. In deltran optimisation, the total numbers of character state changes are more unevenly scattered over the whole tree than in acctran (fast optimisations). However, deltran may be more appropriate in cases where rate constancy is unlikely, as may occur with morphological characters showing character state asymmetries, because it preserves homology better (Agnarsson & Miller Citation2008).
For the analysis, one of the shortest trees with the most significant number of synapomorphies was chosen to indicate support for the nodes in the clades. Non-homoplasy and homoplasy on cladograms were searched using slow optimisation, allowing the map of all characters and states simultaneously. Non-homoplasy apomorphy is presented in green, reversion is shown in white and homoplasy is marked with a red circle in the figures; for confirmation of strict synapomorphic characters, the diagnosed characters’ “slow optimisation” in the ASADO was used.
Outgroup selection. Archeoviparosiphum baissense (Shaposhnikov & Wegierek, Citation1989) has been chosen as an outgroup in the present study. This species is an Early Cretaceous representative of the family Oviparosiphidae. It is abundant and very well preserved, so its morphological description is complete.
The set of morphological characters was compiled for particular in-group and outgroup taxa. All characters were coded based on first-hand examination of specimens and information derived from the literature. This provided improved characteristics, which could be combined to achieve a more precise coding of all groups/taxa to be analysed from a phylogenetic perspective.
Results
Systematic palaeontology
Order Hemiptera Linnaeus, 1758
Suborder Sternorrhyncha Amyot and Audinet-Serville, 1843
Infraorder Aphidomorpha Becker-Migdisova and Aizenberg, 1962
Family Eriosomatidae Kirkaldy, 1905
Type genus Eriosoma Leach, 1818
Pemphigidae Herrich-Schaeffer in Koch, 1845
Type genus Pemphigus Hartig, 1839
Genus Sidorchukaphis Ogłaza and Wegierek gen. nov.
Type species Sidorchukaphis katyae Ogłaza and Wegierek sp. nov., by present designation.
Etymology
The new genus was named in honour of the late Dr Ekaterina (Katya) Sidorchuk, who was an important participant in an expedition for amber to Taimyr Peninsula; she introduced breakthrough techniques in studying fossil specimens and described a large number of important taxa.
Diagnosis
Head capsule and pronotum separated. Terminal process shorter than the basal half of the ultimate antennal segment. Cauda broadly rounded. Apical segment of rostrum not distinctly subdivided. Wax glands present, in the basal part of abdomen arranged in two rows, in the apical part additional glands clustered on lateral margins of tagma. Wax gland plates with central facets surrounded by a ring. Siphuncular pores absent.
Remarks
The structure of the thorax, the shape of cauda and the occurrence of wax glands indicate that Sidorchukaphis gen. nov. should be placed within the family Eriosomatidae. In turn, the structure of wax glands indicates that it belongs to the subfamily Eriosomatinae. It differs from the extant genera in the arrangement of wax glands. The genera of Eriosomatinae have 4–6 longitudinal rows of wax gland plates on the dorsum of the whole abdomen. The newly described genus has only 2 longitudinal rows of wax gland plates along the first abdominal segments, and apically clusters of several glands occur only on the margins of tergites.
Prior to this study, no apterous morph with such a combination of characters had been described from the Late Cretaceous amber.
Sidorchukaphis katyae Ogłaza and Wegierek sp. nov.
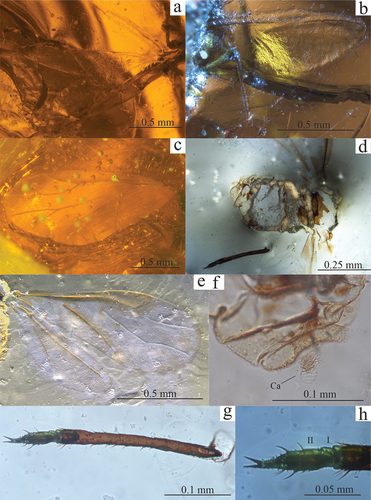
Figure 2. (a) Sidorchukaphis katyai sp. nov., dorsal side of the body; (b) S. katyae sp. nov., ventral side of the body (photo by Dmitry Vorontsov); (c) S. katyae sp. nov., head with right antenna (photo by Dmitry Vorontsov); (d) S. katyae sp. nov., abdomen, Ca – cauda, Wgm – wax glands marginal, Wgp – wax glands pleural (photo by Dmitry Vorontsov); (e) S. katyae sp. nov., larva (photo by Dmitry Vorontsov); (f) Baikuraphis abdominalis sp. nov., habitus; (g) B. abdominalis sp. nov., apical part of abdomen, Ca – cauda, Ap – anal plate; (h) Canadaphis ugolyaki sp. nov., fore and hind wings; (i) C. ugolyaki sp. nov., fore wing, scanning electron microscope view; (j) Ambaraphis baikurensis, specimen PIN 3730/109b, habitus. (Dmitry Vorontsov gave verbal consent for the use of photos taken by him in this publication).

Etymology
The new species was named in honour of the late Dr Ekaterina (Katya) Sidorchuk.
Diagnosis
As for the genus because of monotypy.
Material
Holotype PIN 3311/1352a, paratype PIN 3311/1352b; occur in the same piece of amber. Right bank of the Maimecha River, 3 km upstream from its confluence with the Kheta River (a left tributary of the Khatanga River), Yantardakh Hill (Yantardakh). An apterous morph (PIN 3311/1352a – ) and larva (PIN 3311/1352b – ) preserved.
Description
Body length – 0.47. Eyes composed of only 3 ommatidia. Antennae short – 0.09, 5-segmented (I – 0.01, II – 0.02, III – 0.02, IV – 0.02, V – 0.03), with a very short terminal process (). Secondary rhinaria absent. Rostrum long – 0.31 reaching termination of the body, segment III – 0.02, IV – 0.03. Legs short, tarsi 2-segmented. Fore femur – 0.05; middle femur – 0.04, middle tibia – 0.03 and tarsus – 0.03. Abdomen length – 0.22, boundaries between segments broad. Wax gland plates on tergites V–VII are clustered in groups of 3–4 at marginal areas; on the remaining segments, single glands occupy pleural areas of dorsum. Abdomen covered laterally with short, stout setae. Cauda broad – 0.05 and short – 0.03 with 6 short, stout setae – 0.01 ().
Larva (). Almost no morphological details are visible, but the larva was assigned to the same species because it was found in the same pieces of amber. Body length – 0.24, width at the widest point – 0.13. Head width – 0.06. Antennae 4-segmented. Length of antennomeres – I – 0.007, II – 0.02, III – 0.028, IV – 0.031. Short narrowing in apical part of the last segment with 3 setae at the end. Length of the fore coxa – 0.012, fore femur – 0.052, middle femur – 0.054.
Family Aphididae Latreille, 1802
Type genus Aphis Linnaeus, 1758
Genus Baikuraphis Ogłaza and Wegierek gen. nov.
Type species Baikuraphis abdominalis sp. nov., by present designation.
Etymology
After the Baikura locality.
Diagnosis
Rostrum short. Siphunculi invisible. Long, lingulate cauda without overweighting, anal plate not divided. Single, long seta only in apical part of cauda.
Remarks
Despite the incompleteness of the specimen, the presence of lingulate cauda and an undivided anal plate is a unique combination among Aphididae.
Presently the family Aphididae is divided into the following two subfamilies: Pterocommatinae (cauda shorter than 1.1 × its width at base) and Aphidinae (cauda longer than 1.2 × its width at base). The latter (Aphidinae) contains two tribes, Aphidini and Macrosiphini. In some representatives of both tribes there are genera with a very long cauda similar to that in Baikuraphis gen. nov. However, in contrast to Baikuraphis, all representatives of those genera show well-developed siphunculi. Already at the tribe level, taxonomic differentiation depends on a combination of characters that can be used for analyses and identification of specimens only after microscopic slides have been prepared. Prior to this study only one species representing this family was known from the Late Cretaceous Taimyr amber. In Aphidocallis the rostrum and body are subequal in length; the cauda is lingulate and covered with dense setae. In the newly described genus the rostrum is shorter and setae on cauda are sparse.
Baikuraphis abdominalis Ogłaza and Wegierek sp. nov.
Etymology
After the body part that was found: the abdomen.
Material
Holotype PIN 3730/405. Southern bank of Lake Taimyr near the southern tip of Baikura-Neru Bay. A fragment of thorax with legs and abdomen preserved ().
Diagnosis
As for the genus because of monotypy.
Description
Rostrum reaching hind coxa (segment IV – 0.05). Legs slender, elongate – fore: tibia – 0.27, tarsal segment II – 0.06; middle: femur – 0.25, tibia – 0.31, tarsal segment I – 0.02, II – 0.07; hind: coxa – 0.07, femur – 0.31, tibia – 0.52, tarsal segment I – 0.02, II – 0.1. Abdomen 0.33 long and 0.25 wide, with distinct sternites. Terminal tergite of abdomen lingulate, strongly elongate, cauda length – 0.09, width at base – 0.02, width at broadest point – 0.03, rounded distally. Subapical part of cauda with 3 setae, the longest equal to half length of cauda. Anal plate in lateral view triangular (height at base – 0.05; length – 0.04), almost reaching half length of cauda, covered with dense setae both dorsally and ventrally. Longest seta, at end of plate, as long as plate itself. Gonapophyses located above genital plate ().
Remarks
Up to now, two species with unpaired cauda representing the extant families Drepanosiphidae and Aphididae have been described from Taimyr amber. However, in Aniferella sibirica the cauda is flask-shaped and the anal plate is incised; in Aphidocallis caudatus the cauda is similar in shape to that in Baikuraphis abdominalis sp. nov. but the chaetotaxy is completely different.
Family Canadaphididae Richards, Citation1966
Type genus Canadaphis Essig, Citation1937
Genus Canadaphis Essig, Citation1937
Type species Canadaphis carpenteri Essig, Citation1937, by original designation and monotypy.
Canadaphis ugolyaki Ogłaza and Wegierek sp. nov.
Etymology
After Ugolyak, the locality where the inclusion was found.
Material
Holotype PIN 3631/22. The Ugolyak site on the left bank of the Severny Ugolyak River, 9.5 km upstream of its mouth, was examined in 1977. Fore- and hind wings preserved ().
Diagnosis
Pterostigma on fore wing broad, 3.1× as long as broad. Vein Rs separating from proximal part of Pt. Vein M directed towards the main stem at the base of Pt; M branched above the point where Rs separates from Pt.
Description
Fore wing, length – 1.2, width – 0.55. Pterostigma lentiform, quite short – 0.36 and wide – 0.1. Vein Rs leaving pterostigma in proximal part, at approximately 1/3 of its length. Vein M three-branched, not connected with main stem. Angle between M1+2 and M3 – 48°, between M1 and M2 – 40°. Common stem of M equal to M1+2 – 0.21. M1 – 0.19 reaching apical part of wing. M2 – 0.14 M3+4 – 0.27 long. Veins CuA departing from main stem clearly apart, distance between them 0.05. Proximal vein – 0.39 separating from common stem (at an angle of 77°) at about 2/3 of length (without pterostigma). CuA1 – 0.55, distinctly bent, 1.41x longer than CuA2. Hind wings reaching apical part of CuA1, quite long (0.87–0.73× fore wing length) and narrow (0.29). Main stem halfway along its length at point where CuA2 separates, curved towards middle inside of wing, 2 separate veins arising from it, proximal (0.21) perpendicularly, distal (0.28) at an angle of about 45°.
Remarks
Differs from other species of the genus by the following combination of characters: Rs separating from the proximal part of Pt (like C. kovalevi but unlike C. mordvilkoi); M directed towards the main stem at the base of Pt (like C. kovalevi but unlike C. carpenteri); M branched below the point where Rs separates from Pt (like C. carpenteri but unlike C. mordvilkoi). M3+4 almost equals M1+2 (like C. mordvilkoi but unlike C. kovalevi). M3+4 almost equals M1+2 (like C. mordvilkoi but unlike C. kovalevi).
Family Palaeoaphididae Richards, Citation1966
Type genus. Palaeoaphis Richards, Citation1966, by original designation and monotypy.
Genus Ambaraphis Richards, Citation1966
Type species Ambaraphis costalis Richards, Citation1966, by original designation and monotypy.
Ambaraphis baikurensis Wegierek and Perkovsky, Citation2017
Additional material
PIN 3730/109b. Southern bank of Lake Taimyr near the southern tip of Baikura-Neru Bay. Collected in 1976 during a PIN expedition. Hind wing preserved ().
Diagnosis
Pterostigma short, wide, tapering towards apex. Vein M bifurcated. Hind wing with single transverse vein extending well beyond half length of wing.
Specimen description
Hind wing: length – 1.32, width – 0.43 (). Single transverse vein initially running along anterior wing margin and at ¼ of its length curving towards posterior wing margin; beyond half of its length approaching posterior margin but not reaching it.
Remarks
Hind wings have not been recorded in most of the Late Cretaceous species of Palaeoaphididae. In Jersaphis Wegierek, Citation2000 transverse veins are lacking in the hind wings; in Palaeoaphis incognita and in Ambaraphis kotejai Kania and Wegierek, Citation2005 the vein does not exceed or only slightly exceeds half the length of the wing. In Ambaraphis costalis it seems hardly possible to determine the course of the vein in detail (Richards Citation1966).
Unlike the holotype condition, well-preserved hind wings should be regarded as a diagnostic character of the species.
Genus Palaeoaphis Richards, Citation1966
Type species Palaeoaphis archimedia Richards, Citation1966, by original designation and monotypy.
Palaeoaphis incognita Kononova, Citation1976
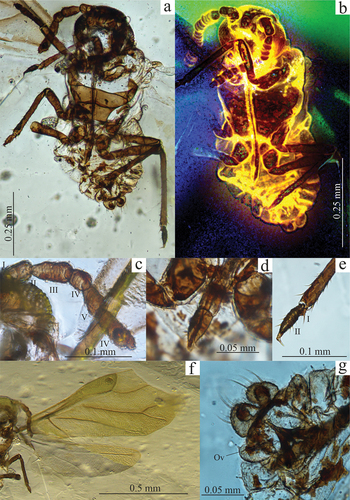
Figure 3. (a) Palaeoaphis incognita specimen PIN 3311/1108, habitus; (b) P. incognita specimen PIN 3311/1108, hind wing; (c) P. incognita specimen PIN 3311/2313, habitus; (d) Tajmyraphis zherichini specimen PIN 3311/1145b, dorsal side of the body; (e) T. zherichini specimen PIN 3311/1145b, fore and hind wing; (f) T. zherichini specimen PIN 3311/1145b, apical part of abdomen, Ca – cauda; (g) T. zherichini specimen PIN 3311/1145b, hind leg; (h) T. zherichini specimen PIN 3311/1145b, hind tarsus.
Additional material
PIN 3311/2313, PIN 3311/1108. The right bank of the Maimecha River, 3 km upstream from its confluence with the Kheta River (a left tributary of the Khatanga River), Yantardakh Hill (Yantardakh). Collected in 2012 during a PIN expedition. 3311/1108 – a fragment of fore wing, hind wing (); 3311/2313 – fore wing (). Same locality, collected 1971.
Diagnosis
Vein M directed towards base of CuA (projection of M base reaching CuA base). Rs arises at basal 1/3 of Pt length.
Redescription
Fore wing, length – 1.8, width – 0.77. Distance from base of wing to end of pterostigma – 1.36. Pterostigma spindle-shaped, short; length – 0.63, width – 0.15. Vein Rs departing from proximal part of Pt at angle of 46°. M not connected with main stem. M bifurcating at 2/3 of Pt length at angle of 34°. Common part of M – 0.47 long, M1+2 – 0.48, M3+4 – 0.33. Common part of CuA, – 0.26–0.28, departing from main stem at angle of 57–60°, branching at angle of 45–57°, CuA1 – 0.63–0.37; CuA2 – 0.27–0.30. Hind wing (), length – 1.08, width – 0.4. CuA separating from main vein at – 0.32, forming a bowed curve towards base and not exceeding half length of wing.
Remarks
Palaeoaphis incognita, which was previously described based only on a wing fragment, differs from typical species of the genera Ambaraphis and Longiradius in having a short Pt. In Palaeoaphis archimedia CuA1 is only twice as long as the common stem of CuA, whereas in Palaeoaphis incognita it is at least 2.5× longer.
The description of the holotype specimen includes only tentative sizes of the wing and the description of its basal part. The redescription presents additionally the shape of the pterostigma, the course of Rs, the shape of M and the structure of the hind wings.
Family Tajmyraphididae Kononova, Citation1975
Type genus Tajmyraphis Kononova, Citation1975
Genus Tajmyraphis Kononova, Citation1975
Type species Tajmyraphis zherichini Kononova, Citation1975, by original designation.
Tajmyraphis zherichini Kononova, Citation1975
Additional material
PIN 3311/1145b. The right bank of the Maimecha River 3 km upstream from its confluence with the Kheta River (a left tributary of the Khatanga River), Yantardakh Hill (Yantardakh). Collected in 1976 during a PIN expedition. Body fragmentarily preserved, head not preserved; fore and hind wings, hind tibia and tarsus preserved ().
Diagnosis
Abdomen apex lacks bilobed structures, no ovipositor. Abdomen apically with a lingulate cauda.
Specimen description
Poorly preserved specimen. Fore wing (), length – 1.08, width – 0,42. Distance from base of wing to end of pterostigma – 0.78. Pt short – 0.29 and wide – 0.09. Vein Rs, length – 0.34, departing in distal part of Pt at angle of 77°. Vein M not connected with main stem, with 2 branches. Common part length – 0.27, M1+2 – 0.25, M3+4 – 0.13. Bifurcation at angle of 63°. Veins CuA departing from main stem separately, length of CuA1 – 0.44, CuA2 – 0.15. Hind wing ()), length – 0.73, width – 0.22. Single transverse vein length – 0.32. Both pairs of wings with scaled texture. Hind tibia length – 0.25, 4× longer than tarsal segment II – 0.06, covered with short setae (); tarsal segment II strongly tapered apically with 2 pairs of setae on sides (). Apical five tergites of abdomen covered with small nodules. Cauda lingulate ().
Remarks
Abdomen apex lacks bilobed structures (present in Taimyraphis rasnitsyni, T. bekermigdisovae, Khatangaphis and Retinaphis), no ovipositor (present in Retinaphis). Abdomen apically with a lingulate cauda.
Although the specimen is not as well preserved as the holotype, its description extends the list of diagnostic characters to include details of fore- and hind wing venation. The description of hind legs includes their chaetotaxy. The shape of the cauda is re-examined.
Genus Retinaphis Kononova, Citation1975
Type species Retinaphis glandulosa Kononova, Citation1975, by original designation and monotypy.
Diagnosis
Antennae 6-segmented, half as long as body, rhinaria annular, antennal segment VI larger than segment III. Bases of cubital veins on fore wing running very close to each other. CuA1 not separated from main stem. Hind wing with single transverse vein. Abdomen apex with bilobed, doubled structures, ovipositor present.
Remarks
Unlike the holotype, the hind wings of PIN 3311/1108 are well preserved. On the hind wings, the transverse vein extends well beyond half the length of the wing. This feature should be considered diagnostic for the species. The ratio of the hind tibia to tarsus, that Kononova (Citation1975) used to distinguish Retinaphis from Tajmyraphis, cannot be used to distinguish these genera.
Retinaphis glandulosa Kononova, Citation1975
Figure 4. (a) Retinaphis glandulosa, ventral side of the body; (b) R. glandulosa, ventral side of the body, view under fluorescence microscope; (c) R. glandulosa, right antenna; (d) R. glandulosa, apical part of rostrum; (e) R. glandulosa, hind tarsus; (f) R. glandulosa, fore and hind wing; (g) R. glandulosa, apical part of abdomen, Ov – ovipositor.
Material
Neotype PIN 3311/1145a. The right bank of the Maimecha River 3 km upstream from its confluence with the Kheta River (a left tributary of the Khatanga River), Yantardakh Hill (Yantardakh). An almost completely preserved alate form (fore- and hind wings, right hind leg partly destroyed) ().
Diagnosis
As for the genus, because of monotypy.
Redescription
Head, length – 0.1, frons margin convex. Compound eyes large, diameter – 0.1, directed downward. Small tubercles with triommatidium located on postero-lateral margin of eye halfway along its height. Antennae short and quite thick, 6-segmented, 3 apical setae on last segment (on small terminal process), 2–4 more below (). Single setae each on remaining segments. Secondary rhinaria on all flagellar segments (3 annular rhinaria on each segment). Length of antennal segments: I – 0.02–0.03, II – 0.04, III – 0.06–0.08, IV – 0.03–0.04, V – 0.04–0.05, VI – 0.08–0.09. Rostrum long – 0.31, reaching half abdomen length. Last segment – 0.05 long, penultimate one – 0.03 long (). On last segment 4 pairs of setae. Rostrum pointed.
Thorax, length – 0.27. Mesosternum, length – 0.09, width – 0.13 wide (below arms), width of arms – 0.19. Front edge straight. Arms rather large, ending above transverse suture. Fore wing, length – 0.99, width – 0.37 (). Pterostigma short – 0.2 and wide – 0.09, with blunt end. Length from base of wing to end of pterostigma – 0.67. Vein Rs departing from Pt distally, in proximal part slightly curved at angle of 76°. Vein M not connected with main stem, with single branch at angle of 64°, common part of M – 0.35, M1+2 – 0.14, M3+4 – 0.22, ramification behind of base of Rs. Veins CuA departing from main stem close to each other. CuA1 twice as long as CuA2. Hind wing (), length – 0.69, width – 0.21, transverse vein, reaching far beyond half wing length. Legs short, covered with sparse, short setae. Trochanters clearly distinguished. Tarsi short, conical, first segment small (). One pair of setae on first tarsal segment, 2–3 pairs on second segment. Length of fore leg: trochanter – 0.02, femur – 0.11–0.15, tibia – 0.14–0.18, tarsus – 0.05–0.06 (segment I – 0.01–0.02, II – 0.03–0.05); middle leg: coxa – 0.04–0.05, trochanter – 0.03, femur – 0.09–0.13, tibia – 0.15–0.19, tarsus – 0.04–0.07 (segment I – 0.01–0.02, II – 0.03–0.05); hind leg: coxa – 0.05, trochanter – 0.03–0.04, femur – 0.1–0.16, tibia – 0.24, tarsus – 0.08 (segment I – 0.02, II – 0.06).
Abdomen, length – 0.26. Segmentation clearly preserved. Abdominal tergites with irregular small sclerites. Dorsal side roof-like with flat margin parts. Two paired structures at end of abdomen. Dorsal ones spade-shaped (as long as wide – 0.03), covered with scale-like cuticular structures. Cuticular structures endowed with stout, bristly setae subequal in length to plates. Second pair of projections located below and forming U-shaped structure, much slenderer, finger-like with apical parts bulbous, more strongly sclerotised. Ventral structures slightly shorter – 0.02 with distinct incision between them. Ovipositor at base, width – 0.05, length – 0.06 ().
Remarks
The new material is better preserved than the specimen that was primarily described by Kononova (Citation1975). It is also the only available representative of the species because the holotype has been seriously damaged due to storing amber in oil. The redescription includes detailed information on the number of antennal rhinaria and a description of the apical segments of the rostrum. Precise sizes, morphology and venation of wings are presented for the first time. Irregular sclerites on the abdomen, which may correspond to glandular cells or cuticular structures reported by Kononova, are commented on. A detailed description of paired processes at the abdomen termination is supplemented with precise measurements.
Aphids incertae sedis
Larva I type (Kononova, Citation1977).
Additional material
3311/798. Larva fully preserved but very indistinct, due to the properties of amber. Fom the right bank of the Maimecha River 3 km upstream from its confluence with the Kheta River (a left tributary of the Khatanga River), Yantardakh Hill (Yantardakh). An apterous aphid, whole body preserved.
Diagnosis
Antennae 5-segmented, with terminal process shorter than half length of basal part of segment, rhinarium circular.
Redescription
Body elongate, oval, length – 0.5, width – 0.2. Abdomen shorter than the rest of the body. Antennae 5-segmented (segment I – 0.01, II – 0.03–0.04, III – 0.02–0.04, IV – 0.02–0.03, V – 0.05–0.06). Segments II and III with single setae as long as segment length. Last segment with single bristle basally, apical part of terminal process with bundle of several stout bristles not as long as segment width. Eyes composed of 3 ommatidia. Rostrum reaching at least past half abdomen length, with blunt end (segment III – 0.02; IV – 0.03), covered with numerous setae from halfway along its length. Two longest setae as long as segment width at base. Legs massive, segment I with 5 stout setae (might be tibial setae?), segment II with 2 pairs of long setae (as long as the segment), 1 pair preapical capitate setae. Abdominal tergites strongly sclerotised, cuticula with fine furrows. Each abdominal segment with single, short and stout bristle on sides. Dorsum of last abdominal segment with 2 stout bristles.
Remarks
Kononova (Citation1977) described three types of aphid larvae (larva I type, larva II type and larva III type). They have no formal assignment to any genus described from Taimyr amber or to a specific family. The specimen described above may represent a sciotaxon (sensu Bengtson Citation1985) of the type I larva of Kononova (Citation1977).
The description of the larva includes the number of antennal segments and their chaetotaxy as well as the description of the apical part of rostrum. The structure and chaetotaxy of the abdomen are presented here for the first time.
Cladistic analysis of the morphological characters of Tajmyraphidoidea
The analysis includes all species of the superfamily Tajmyraphidoidea, scored for 31 morphological characters and 26 species, including the outgroup taxon. The parsimony analysis yielded the most parsimonious cladogram (), of 188 steps, with consistency index (CI) = 34, and retention index (RI) = 56. Weighting characters in the analysed taxa, based on different K values (3–8), produced stable results in analyses of morphological datasets, and the topology and length of the trees were identical.
Figure 5. Parsimonious tree of the Tajmyraphidoidea. Characters in red: homoplastic; characters in green: non-homoplastic; characters in white: reversion.
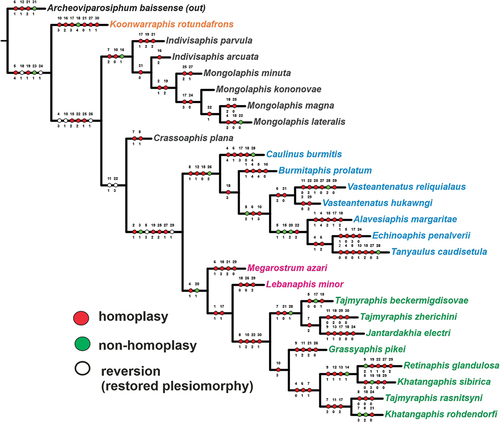
Figure 6. The diagnosed characters (character state: green − 0, blue − 1) of the parsimonious trees: (a) green branches (character 31 − 0) and (b) blue branches (character 23 − 1) support the clade of the Tajmyraphidoidea. Rose colour − character unknown.
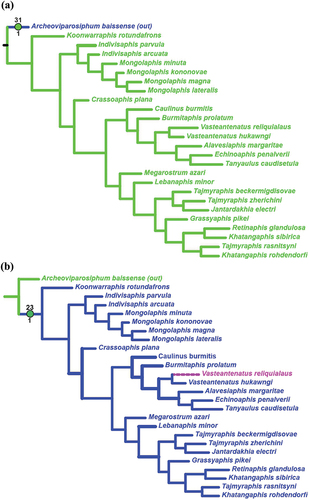
Figure 7. Parsimonious tree. The blue branches show character 25 − 1 and indicate the Mongolaphidinae.
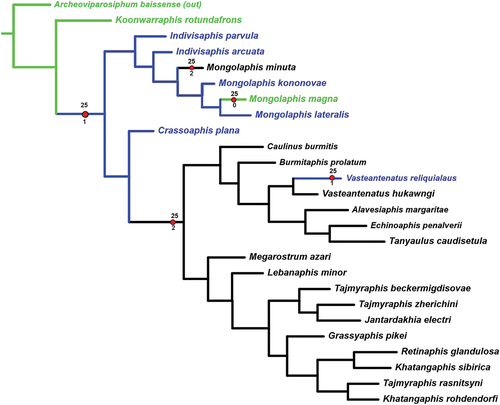
Figure 8. Parsimonious trees. (a) character 3 − 1 (blue) supports the Tajmyraphididae + Burmitaphididae; (b) character 26 − 2 (black) supports the Burmitaphididae; (c) character 20 − 1 (blue) supports Lebanaphididinae? + Tajmyraphidinae.
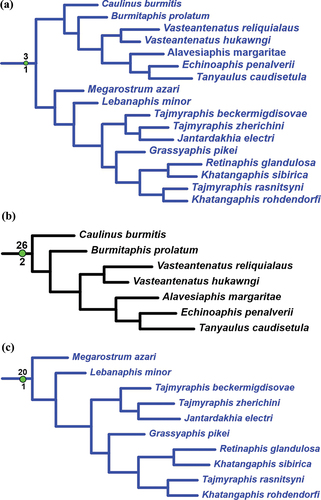
Cladistic relationships within the superfamily Tajmyraphidoidea are visualised in , and all recognized characters are listed in . The obtained clades were analysed using synapomorphies (green and white circles). Archeoviparasiphum baissense is involved as the outgroup taxon with one autapomorphy (31 − 1; porous siphons present) (). The sister branch embraces all considered genera of the superfamily Tajmyraphidoidea, whose monophyly is supported by one synapomorphy (23 − 1, vein M with one branch) and two reversions (18 − 1, CuA1 is 3× longer than CuA2, and 24 − 1, branching of the vein M behind the base of the Rs) ().
Table III. Synapomorphy (SA), putative synapomorphy (PSA), autapomorphy (AA), putative autapomorphy (PAA), reversion (restored plesiomorphy) (RM), and putative reversion (PRM) were established based on the parsimonious tree in . RM and apomorphy were recognised with diagnosed characters ().
The subordinate branches form an isolated branch of Koonwarraphis rotundafrons from Australia, with one autapomorphic character (18 − 4; CuA1 is less than twice as long as CuA2). It is sister to the large clade supported by three reversions (4 − 3, 10 − 1 and 26 − 1) and three homoplasies (15 − 2, 22 − 2 and 25 − 1). This clade diverges further in two, one subtending the Early Cretaceous genera Indivisaphis and Mongolaphis and supported by synapomorphy 16 − 1. The first genus appears paraphyletic and the second is monophyletic, supported by homoplasies 2 − 1 and 19 − 2.
The clade sister to the one above encompasses the Late Cretaceous fossils. It is confirmed with reversions 11 − 1 and 22 − 3 and consists of the isolated Crassoaphis () supported with homoplasies 7–1 and 8–1 and the large clade confirmed with synapomorphy 3 − 1 () and two reversions (5 − 3 and 29 − 1). This clade splits into two subequal branches. One of them is supported by the synapomorphy 26 − 2 and includes six genera assigned to Burmitaphididae (), all monotypical except Vasteantenatus which appears monophyletic (supported with homoplasies 6 − 2 and 21 − 0). Another branch is supported with the synapomorphy 20 − 1 and covers the remaining considered genera (). Of them, the monotypical Lebanaphis and Megarostrum currently form the subfamily Lebanaphidinae that is not monophyletic, and the other five that form a mass with species of both non-monotypical genera Tajmyraphis and Khatangaphis intermingled with other genera.
Discussion
Taimyr Peninsula fossil resins have been known for at least several centuries. However, it was only after two expeditions conducted by PIN researchers that the materials proved rich enough to conduct broader analyses of their origins, affinities of their faunas and abundant inclusions. Even preliminary examinations of the materials (Zherikhin & Sukatsheva Citation1973) indicated that the commonly used name “Taimyr amber” refers to variously aged resins collected at different sites. The differences are reflected in the taxonomic composition of the fauna (Zherikhin & Sukatsheva Citation1973). Rasnitsyn et al. (Citation2016) conducted a comparative analysis of arthropod assemblages found in Cretaceous fossil resins, including Taimyr amber. Arthropod-bearing Cretaceous resin sites were reviewed (Zhdanikha, Kresty: Begichevo Formation, upper Albian; Nizhnyaya Agapa: Dolgan Formation, Late Cenomanian; Romanikha, Ugolyak, Yantardakh: Kheta Formation, Santonian) and a list of the arthropods (identified at the family level) was provided. In total, 104 samples of Taimyr amber with aphid inclusions have been examined (). The results confirm the occurrence of aphids in the following locations ():
Kresty and Zhdanikha. Both sites in the Begichev Formation (upper Albian) are very poor in amber, and the percentage of fossil resin pieces containing arthropod inclusions is also very low (Zherikhin & Sukatsheva Citation1973; Zherikhin Citation1978). The oldest Taimyr aphids, Khatangaphis rohdendorfi, Tajmyraphididae and Nordaphis sukatchevae, have been recorded from there. The latter species is the oldest known representative of the extant family Drepanosiphidae (Heie & Wegierek Citation2011a).
Baikura is assigned to the upper Albian–lower Cenomanian Ognevka Formation. It is a rich insect-bearing site but aphids are poorly represented. Three inclusions yielded one newly described species, Baikuraphis abdominalis, of the family Aphididae and two others belonging to Ambaraphis baikurensis, Palaeoaphididae.
Romanikha, Ugolyak and Yantardakh are three sites in the Kheta Formation (Santonian). The largest and most well-studied insect-bearing site of the Kheta Formation is Yantardakh. The Romanikha and Ugolyak materials are very limited. Aphids are represented only by single inclusions/species: Khatangaphis sibirica, Tajmyraphididae; and Canadaphis ugolyaki, Canadaphididae. Yantardakh is the richest in aphid inclusions (93). Fifteen species have been described from that site: six species belong to the family Tajmyraphididae and some represent the extinct families Canadaphididae and Elektraphididae. The site also yielded three species representing the extant family Eriosomatidae and a single species each belonging to the extant families Drepanosiphidae and Aphididae. Outcrops of this formation revealed abundant inclusions of aphid larval stages (75).
Unfortunately, the analysis of Jantardakh’s fauna and flora and the entire Kheta Formation was hindered by an almost complete lack of palaeobotanical data on the Kheta Formation. In the absence of such data, the assessment of Taimyr’s forest amber climate was hardly possible (Perkovsky & Makarkin Citation2015; Sukhomlyn & Perkovsky Citation2023). A year ago we obtained a very important proxy. The geographically and temporally proximate Late Cretaceous deposits of the New Siberian Islands are dated Turonian–Coniacian, based on the fossil flora of Derevyannye Gory Formation of New Siberia Island (Herman et al. Citation2019), and late Coniacian [the lower age limit for the Derevyannye Gory Formation sedimentation is 88 ± 1.0 Ma based on U–Pb laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) dating of detrital zircons from Novaya Sibir Island (Kostyleva et al. Citation2022)]. The climate of the New Siberian Islands during the Turonian–Coniacian was very different from today. Here, in the high latitudes of the Arctic at a palaeolatitude of about 73–75° N, existed a rich forest vegetation in a much warmer and more humid climate than found there at present (Herman et al. Citation2019). Preliminary estimations of the palaeoclimate variables suggested a mean annual temperature (MAT) of 9.2°C, a warm month mean temperature (WMMT) of 17.2°С, and a cold month mean temperature (CMMT) of 1.1°С. The data indicate that, according to the Koppen classification of global climates (Köppen Citation1936; Peel et al. Citation2007), the plants of the New Siberian flora grew in a warm humid climate with a warm summer and a mild frost-free winter (Cfb climate). This corresponds well to the combination of temperate and cryophobic (sensu Archibald et al. Citation2023) elements present in the Santonian Taimyr amber fauna (our data).
The genus Khatangaphis is unique in having representatives of the same taxon (different species) in the oldest (Kresty) and the most recent ambers (Romanikha). No other similarities at the genus level could be traced.
Although Canadian amber is much younger than Taimyr amber (upper Campanian vs. upper Albian–Santonian, respectively), only a single species representing an extant family/subfamily Drepanosiphidae/Neophyllaphidinae (Richards Citation1966) has been reported from Canadian amber: Aniferella bostoni Richards, Citation1966. Other representatives of aphidofauna in Canadian amber belong to extinct families (Heie & Wegierek Citation2011a). Compared to the Canadian amber fauna, the aphid fauna in Taimyr amber is much more taxonomically diverse at higher taxonomic ranks. It contains representatives of five extinct families (Canadaphididae; Elektraphididae Steffan, 1968; Palaeoaphididae; Shaposhnikoviidae Kononova Citation1976; Tajmyraphididae) and three extant families (Aphididae, Eriosomatidae (= Pemphigidae), Drepanosiphidae).
Canadaphididae, Palaeoaphididae and Tajmyraphididae are known to have existed as early as the Early Cretaceous (Perkovsky & Wegierek Citation2017; Żyła & Wegierek Citation2020). The representatives of Drepanosiphidae, Eriosomatidae and Aphididae are new reports in the Upper Cretaceous inclusions. Due to unusual combinations of morphological characters, it is not possible to assign the reported species either to extant genera or even to extant subfamilies and tribes. Comparative analysis poses particularly serious problems when it comes to aphids within the family Tajmyraphididae. The representatives of that family (; Khatangaphis sibirica, Retinaphis glandulosa) were endowed with paired structures at the apex of the abdomen, which have no analogue in the extant forms (Kononova Citation1975) and are very difficult to interpret morphologically.
In extant aphids, the last abdominal tergite (IX) is transformed into an unpaired structure called the cauda (Kaszyca-Taszakowska et al. Citation2022). An anal aperture, which is located under the cauda, is protected from below by the last abdominal sternite, called the anal plate (representing abdominal sternite X). The structures called gonapophyses lie in front of the genital aperture and are believed to represent rudimentaries of ovipositor valves (Kaszyca-Taszakowska et al. Citation2022). The sternite anterior to the genital aperture is called the genital plate (representing abdominal segment VIII). In Drepanosiphidae and Hormaphididae, the plate is bilobed because of an indentation in the middle of the posterior margin. The structures observed at the end of the abdomen in Tajmyraphididae do not correspond to this description. Thanks to the examination of a well-preserved specimen, PIN 3311/1145 of Retinaphis glandulosa, where the ovipositor could be identified, it proved possible to regard them as homologous with structural elements of the apical part of the abdomen in extant aphids. A paired structure on the dorsum is homologous with the unpaired cauda in extant aphids. This is confirmed by its location as well as its cuticular microsculpture (short, pointed projections) and the occurrence there of long, dense setae (). A strongly curved sclerite lying below represents an anal plate. A well-developed ovipositor in Retinaphis glandulosa excludes the occurrence of gonapophyses; and the sternite in the form of a genital plate is not developed. The function of that dorsal structure (homologous with a cauda) is presently difficult to explain because in extant aphids the cauda is movable and used to remove the excess of honeydew. In Tajmyraphididae, nothing indicates the mobility of the structure and its paired character would significantly complicate fulfilling any functions similar to those in extant species. It seems possible that the structure was used only to keep honeydew drops away from the body (Kaszyca-Taszakowska et al. Citation2022) and protect the aphid from being covered with sugars.
Approximately 33% of the species from Yantardakh belong to modern families, and Baikuraphis is one of the two oldest representatives of the modern family. Thanks to the newly obtained data, we can assume that a significant part of the early evolutionary development of at least Aphididae and Drepanosiphidae took place in a moderately warm climate (and the climate of Baikura could have been cooler than that of Yantardach). Some adaptations that prevented Aphididae and advanced groups of Drepanosiphidae from succeeding in the tropics must have occurred (see Perkovsky & Wegierek Citation2018). The morphological features of Taimyraphidoidea (lack of trophobiotic organ) made symbiosis with ants unlikely and led to the extinction of that superfamily after the appearance of advanced groups of ants that entered symbiosis with hemipteres (Perkovsky & Wegierek Citation2018).
Taxonomic structure of Tajmyraphidoidea
Oviparosiphidae, one of the oldest known extinct families of aphids, is the closest to Tajmyraphidoidea and, for a long time, was considered a common ancestor of all extant Aphidoidea (Żyła et al. Citation2017) and, by inference, also of Taimyraphidoidea. These authors erroneously attest Oviparosiphidae is a polyphyletic group whilst their results imply that the family forms a paraphylum rather than a polyphylum being supported, together with Baissaphididae and Canadaphididae, by a number of apomorphies (Żyła et al. Citation2017, ); Tajmyraphididae were not included in their analysis). Archeoviparosiphum baissense Żyła, Homan and Franielczyk and Wegierek, Citation2015 is selected as the outgroup because of its particularly good preservation.
Our analysis () shows Taimyraphidoidea forms a monophylum with a sister pair of the lone, Early Cretaceous Koonwarraphis of Australia, and a clade of all other Tajmyraphidoidea. This would suggest a family-level distinction for Koonwarraphis. Yet we consider this premature because of our insufficient knowledge of that unique fossil, and we prefer to treat Koonwarraphis as an unplaced genus within the superfamily (Tajmyraphidoidea incertae sedis).
The clade sister to Koonwarraphis is split into two branches. One of them embraces two Early Cretaceous genera, Indivisaphis and Mongolaphis from Mongolia, that appear to represent a paraphylum (the first) and monophylum (the second). Another branch is split into the lone Early Cretaceous genus Crassoaphis from Mongolia and a larger monophylum of numerous Late Cretaceous genera. Żyła and Wegierek (Citation2020) referred to the above three Early Cretaceous genera as the subfamily Mongolaphidinae (within Tajmyraphididae), which now appears to be paraphyletic. To avoid paraphyly, we here exclude Crassoaphis from Mongolaphidinae.
The remaining large clade of the Late Cretaceous aphids forms a monophylum of two branches corresponding to Burmitaphididae and (Lebanaphidinae + Tajmyraphidinae) sensu Żyła and Wegierek (Citation2020), respectively. Our results falsify this construction, that is why we propose a re-arrangement. The sister relation of the current Mongolaphidinae and (Burmitaphididae + Lebanaphidinae + Tajmyraphidinae) suggests a full family rank for Mongolaphididae and equal rank for (Burmitaphididae + Lebanaphidinae + Tajmyraphidinae), hence the family Tajmyraphididae. Within the latter, Lebanaphidinae form a paraphylum and therefore are discarded as a suprageneric taxon, and Burmitaphididae are downgraded as a subfamily.
As a result, the system of Tajmyraphidoidea appears in the following form ():
Figure 9. Summarising the hypothetical tree with the proposal of the systematic relationships of the Tajmyraphidoidea. The five synapomorphies are distinguished for the respective clades.
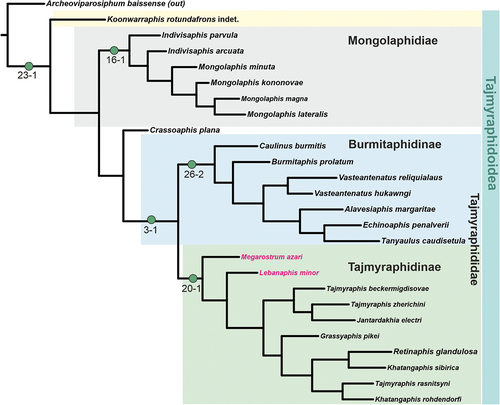
Superfamily Tajmyraphidoidea Kononova, Citation1975
Family Tajmyraphididae Kononova, Citation1975
Subfamily Tajmyraphidinae Kononova, Citation1975
Genus Tajmyraphis Kononova, Citation1975
Genus Grassyaphis Heie, Citation1996
Genus Jantardakhia Kononova, Citation1975
Genus Khatangaphis Kononova, Citation1975
Genus Lebanaphis Heie, Citation2000
Genus Megarostrum Heie, Citation2000
Genus Retinaphis Kononova, Citation1975
Subfamily Burmitaphidinae
Genus Burmitaphis Poinar and Brown 2005
Genus Alavesiaphis Penalver and Wegierek Citation2008
Genus Caulinus Poinar and Brown 2005
Genus Echinoaphis Wegierek, Cai and Huang 2019
Genus Tanyaulus Poinar, 2018
Genus Vasteantenatus Wegierek, Cai and Huang 2019
Genus incertae sedis
Genus Crassoaphis Żyła and Wegierek, Citation2020
Family Mongolaphididae
Genus Mongolaphis Żyła and Wegierek, Citation2020
Genus Indivisaphis Żyła and Wegierek, Citation2020
Genus incertae sedis
Genus Koonwarraphis Martin, Skidmore and Stilwell 2016
It is also worth mentioning is that for the present we set aside the question of monophyly of the genus-level taxa. Our results imply that some of those embracing more than one species (Tajmyraphis, Khatangaphis) appear non-monophyletic. However, to consider the monophyly of genera, more detailed knowledge is required of the morphology of fossils than it is currently available. That is why we prefer to rely on future research that may provide additional information about fossil aphids sufficient to launch a wider study.
Supplemental Material
Download MS Excel (30 KB)Acknowledgments
We are grateful to A. P. Rasnitsyn and I. D. Sukatsheva (Paleontological Institute of the Russian Academy of Sciences) for their constant attention to amber research and their invaluable contribution to the study of the entomofauna of Taimyr amber. The authors are indebted to the late E. A. Sidorchuk (PIN) for substantial help as well as for preparing and polishing the aphid specimens, and to Vyacheslav V. Martynov (Slavyansk) and Anatoly P. Vlaskin (SIZK) for finding aphid inclusions and for the primary treatment of the samples. Special thanks go to Dmitry Vorontsov, who prepared and took photographs of specimens of the genus Sidorchukaphis. We also thank David Morgado for improving the language; the reviewers for their valuable contributions, which led to the improvement of this manuscript; and Dr Łukasz Depa for consultations on the taxonomic diversity of extant aphids. Moreover, we are grateful to Prof. A. P. Rasnitsyn for essential comments and thorough manuscript proofreading.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2024.2310053.
Additional information
Funding
References
- Agnarsson I, Miller JA. 2008. Is ACCTRAN better than DELTRAN? Cladistics 24(2008):1032–1038. DOI: 10.1111/j.1096-0031.2008.00229.x.
- Archibald SB, Mathewes RW, Aase A. 2023. Eocene giant ants, 1145 Arctic intercontinental dispersal, and hyperthermals revisited: Discovery of fossil Titanomyrma (Hymenoptera: Formicidae: Formiciinae) in the cool uplands of British Columbia, Canada. The Canadian Entomologist 155. DOI: 10.4039/tce.2022.49.
- Bengtson S. 1985. Taxonomy of disarticulated fossils. Journal of Paleontology 59:1350–1358.
- Carpenter FM, Folsom JW, Essig EO, Kinsey AC, Brues CT, Boesel MW, Ewing HE. 1937. Insects and arachnids from Canadian amber. University of Toronto Students, Geology 1155 Series 40:7–62. DOI: 10.5281/zenodo.23722.
- Colombo WD, Gobbi FT, Perkovsky EE, Azevedo CO. 2021. Synopsis of the fossil Pristocerinae (Hymenoptera, Bethylidae), with description of two new genera and six species from Burmese, Taimyr, Baltic and Rovno ambers. 1160 Historical Biology 33(9):1736–1752. DOI: 10.1080/08912963.2020.1733551.
- Goloboff P, Farris J, Nixon K. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24(5):774–786. DOI: 10.1111/j.1096-0031.2008.00217.x.
- Heie OE. 1996. Palaeoaphididae and Tajmyraphididae in Cretaceous amber from Alberta, Canada (Hemiptera: Aphidinea). Annals of the Upper Silesian Museum in Bytom, Entomology 6-7:97–103.
- Heie OE, Azar D. 2000. Two new species of aphids found in Lebanese amber and a revision of the family Tajmyraphididae Kononova, 1975 (Hemiptera: Sternorrhyncha). Annals of the Entomological Society of America 93(6):1222–1225. DOI: 10.1603/0013-8746(2000)093[1222:TNSOAF]2.0.CO;2.
- Heie OE, Wegierek P. 2011a. A list of fossil aphids (Hemiptera, Sternorrhyncha, Aphidomorpha). Monographs of the Upper Silesian Museum 6:1–82.
- Heie OE, Wegierek P. 2011b. Table P-1 comparison of Aphidoidea classifications. In: Nieto NJM, Favret C, editors. Registers of family-group and Genus-group Taxa of Aphidoidea. León: Universidad de León. pp. 20.
- Herman AB, Kostyleva VV, Nikolskii PA, Basilyan AE, Kotel’nikov AE. 2019. New data on the Late Cretaceous flora of the New Siberia Island, New Siberian Islands. Stratigraphy and Geological Correlation 27:323–338. DOI: 10.1134/S0869593819030031.
- Kaszyca-Taszakowska N, Kanturski M, Depa Ł. 2022. Comparative studies of perianal structures in myrmecophilous aphids (Hemiptera, Aphididae). Insects 13:1160. DOI: 10.3390/insects13121160.
- Kononova EL. 1975. A new aphid family (Homoptera: Aphidoidea) from the Upper Cretaceous of the Tajmyr. Entomologicheskoe Obozrenie 54:795–807.
- Kononova EL. 1976. Extinct aphid families (Homoptera, Aphidinea) of the Late Cretaceous. Paleontological Journal 3:117–126.
- Kononova EL. 1977. New species of aphids (Homoptera, Aphidinea) from the Upper Cretaceous deposits of the Taimyr. Entomologicheskoe Obozrenie 56(3):588–600.
- Köppen W. 1936. Das geographische System der Klimate. In: mKöppen W, Geiger R, editors. Handbuch der Klimatologie. Berlin: Gebrüder Bornträger. pp. 1–44.
- Kostyleva VV, Moiseev AV, Shchepetova EV, Basilyan AE, Golionko BG, Nikolsky PA, Khissamutdinova AI, Malyshev NA, Verzhbitskiy VE, Obmetko VV, Borodulin AA. 2022. Results of the U/Pb dating of detrital zircons from Upper Cretaceous deposits in the Novaya Sibir Island (New Siberian Islands, Anjou Island Group). Lithology and Mineral Resources 57(3):218–233. DOI: 10.1134/S002449022203004X.
- Maddison WP, Maddison DR. 2023. Mesquite: A modular system for evolutionary analysis. Version 3.81. Available: http://mesquiteproject.org.
- Makarov KV, Perkovsky EE. 2020. Smallest and oldest false skin beetle: Paleobiphyllus ponomarenkoi gen. et sp. nov. (coleoptera: Cleroidea: Biphyllidae) from santonian taimyramber, northern Russia. Cretaceous Research 106:104238. DOI: 10.1016/j.cretres.2019.104238.
- Melnitsky SI, Ivanov VD. 2021. Two new species of the genus Archaeopolycentra (Trichoptera: Polycentropodidae) from Cretaceous Taimyr amber. Far Eastern Entomologist 444:1–7. DOI: 10.25221/fee.444.1.
- Nixon KC. 2004. ASADO, version 1.61 TNT-MrBayes Slaver (vl 5.30). Ithaca (NY): Published by the author. Accessed Aug 2023 17.
- Ogłaza B, Perkovsky EE, Wegierek P. 2022a. Khatangaphis sibirica Kononova, 1975 (Hemiptera: Sternorrhyncha:Tajmyraphididae) redescription. Palaeoentomology 5(1):066–070. DOI: 10.11646/palaeoentomology.5.1.7.
- Ogłaza B, Perkovsky EE, Wegierek P. 2022b. Canadaphis mordvilkoi, Kononova 1976 (Hemiptera: Sternorrhyncha: Canadaphididae) redescription and neotype designation. Zootaxa 5183(1):98–103. DOI: 10.11646/zootaxa.5183.1.11.
- Peel MC, Finlayson BL, McMahon TA. 2007. Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences 11(5):1633–1644. DOI: 10.5194/hess-11-1633-2007.
- Perkovsky EE, Makarkin VN. 2015. First confirmation of spongillaflies (Neuroptera: Sisyridae) from the Cretaceous. Cretaceous Research 56:363–371. DOI: 10.1016/j.cretres.2015.06.003.
- Perkovsky EE, Vasilenko DV. 2019. A summary of recent results in the study of Taimyr amber. Paleontological Journal 53(10):984–993. DOI: 10.1134/S0031030119100149.
- Perkovsky EE, Wegierek P. 2017. Oldest amber species of Palaeoaphididae (Hemiptera) from Baikura (Taimyr amber). Cretaceous Research 80:56–60. DOI: 10.1016/j.cretres.2017.08.013.
- Perkovsky EE, Wegierek P. 2018. Aphid–Buchnera–Ant symbiosis; or why are aphids rare in the tropics and very rare further south? Earth and Environmental Science Transactions of the Royal Society of Edinburgh 107:297–310. DOI: 10.1017/S1755691017000147.
- Rasnitsyn AP, Bashkuev AS, Kopylov DS, Lukashevich ED, Ponomarenko AG, Popov YA, Rasnitsyn DA, Ryzhkova OV, Sidorchuk EA, Sukatsheva ID, Vorontsov DD. 2016. Sequence and scale of changes in the terrestrial biota during the Cretaceous (based on materials from fossil resins). Cretaceous Research 61:234–255. DOI: 10.1016/j.cretres.2015.12.025.
- Richards WR. 1966. Systematics of fossil aphids from Canadian amber (Homoptera: Aphididae). The Canadian Entomologist 98:746–760. DOI: 10.4039/Ent98746-7.
- Sukhomlyn MM, Perkovsky EE. 2023. First carnivorous fungus from Santonian Taimyr amber. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 114:183–188. DOI: 10.1017/S1755691023000087.
- Zherikhin VV. 1978. Razvitie i smena melovykh i kainozoiskikh faunisticheskikh kompleksov (Trakheinyie i Khelicerovye). Trudy Paleontologicheskogo Instituta. Akademia Nauk SSSR, Moskva 165:1–198.
- Zherikhin VV, Sukatsheva ID. 1973. On the Cretaceous insectiferous “ambers” (retinits) in the North Siberia. In: Voprosy paleontologii nasekomykh (Doklady na 24-m Ezhegodnom chtenii pamyati N.A. Kholodkovskogo, 1971) [Problems of the Insect Palaeontology Lectures on the XXIV Annual Readings in Memory of N.A. Kholodkovsky]. Leningrad: Nauka. pp. 3–48.
- Żyła D, Homan A, Wegierek P, Hull JJ. 2017. Polyphyly of the extinct family Oviparosiphidae and its implications for inferring aphid evolution (Hemiptera, Sternorrhyncha). Public Library of Science ONE 12(4):e0174791. DOI: 10.1371/journal.pone.0174791.
- Żyła D, Wegierek P. 2020. Early stages of aphid evolution (Hemiptera, Sternorrhyncha, Aphidomorpha). Monographs of the Upper Silesian Museum 16:1–90. DOI: 10.5281/zenodo.4159532.
