Abstract
Species within the eutardigrade superfamily Macrobiotoidea are usually differentiated through their eggshell morphology. However, this trait can show very high intraspecific variability and, as consequence, species delimitation is challenging without molecular support. Although eggshell variability usually consists of small details, cases of species with very different egg morphotypes are known. Paramacrobiotus bifrons sp. nov. here described with an integrative approach, represents one of the species with extreme variability in eggshell morphology and it belongs to the areolatus group. The production by this gonochoric species of both areolatus-type and csotiensis-type eggs, the latter being rare in the group but most abundant in this species, was assessed by DNA barcoding, culturing, and inter-morphotype crosses. The reason underlying the production of two different eggshells resulted not related to tested culturing conditions, seasonality, or male’s influence. Confocal (with and without staining), Light, and Scanning Electron Microscopies analyses of the two egg morphotypes laid by P. bifrons sp. nov. allowed to redescribe the csotiensis-type egg, shed light on its composition (excluding chitin as a component), and to find shared details supporting the belonging of the two egg morphotypes to the same species.
http://www.zoobank.org/urn:lsid:zoobank.org:pub:E9519638-2CE9-414D-B96B-0F403845D4CA
http://www.zoobank.org/urn:lsid:zoobank.org:act:82DDACA5-F539-4A80-8418-8AF4A370B631
Introduction
The phylum Tardigrada includes small (length <1.2 mm and very often <0.5 mm) eight-legged micrometazoans closely related to arthropods, able to inhabit a large variety of terrestrial environments thanks to their unique anhydrobiotic ability (Guidetti et al. Citation2011; Nelson et al. Citation2019). From a taxonomic point of view, in the class Eutardigrada (representing the most common group of tardigrades in terrestrial environments) the available morphological characters useful for species discrimination are few. This is underlined by the widespread occurrence of cryptic and pseudocryptic species complexes or groups in the Phylum (see, e.g., the Milnesium tardigradum and granulatum groups, Michalczyk et al. Citation2012; the Echiniscus blumi-canadensis complex; Gąsiorek et al. Citation2017; the Mesobiotus harmsworthi group; Kaczmarek et al. Citation2018; the Ramazzottius oberhaeuseri complex; Stec et al. Citation2018; the Paramacrobiotus richtersi complex; Guidetti et al. Citation2019, the Macrobiotus persimilis-polonicus complex; Bertolani et al. Citation2023). Within these complexes, the detection of species is mainly based on DNA barcodes, karyology, and eggshell morphology, the latter representing one of the most used diagnostic characters at the species level in most eutardigrades laying freely deposited eggs (e.g., Bertolani & Rebecchi Citation1993; Bertolani et al. Citation1996; Guidetti et al. Citation2006, Citation2019; Mapalo et al. Citation2016; Kaczmarek et al. Citation2020; Stec et al. Citation2020a; Vecchi et al. Citation2023). In species laying ornamented eggs, the characterization of the eggshell morphology is indeed essential in species description and identification, especially when addressing taxa in the superfamily Macrobiotoidea (e.g., genera: Macrobiotus Schultze Citation1834, Mesobiotus; Vecchi et al. Citation2016, Minibiotus R. O.; Schuster et al. Citation1980, and Paramacrobiotus; Guidetti et al. Citation2009; Claxton Citation1998; Vecchi et al. Citation2016; Kaczmarek & Michalczyk Citation2017; Kaczmarek et al. Citation2017; Guidetti et al. Citation2019), and in the families Eohypsibiidae (e.g., Trygvadottir & Kristensen Citation2011; Hansen et al. Citation2017) and Ramazzottiidae (e.g., Stec et al. Citation2018; Guidetti et al. Citation2019). To further complicate the work of taxonomists, several species show different levels of intraspecific variability in eggshell morphology, particularly concerning eggshell processes shape (e.g., Dastych Citation2011; Mapalo et al. Citation2016, Citation2017; Stec et al. Citation2016, Citation2018; Zawierucha et al. Citation2016; Guidetti et al. Citation2019; Kihm et al. Citation2020). These situations are usually addressed as normal phenotypic variability within a species, therefore as a background noise in performing morphological species delimitation. However, in rare cases, this variability is so extreme that it can lead to taxonomic issues, especially dealing with low sample sizes (Stec et al. Citation2016). Therefore, alongside morphology, a molecular approach is crucial for tardigrade integrative taxonomy and phylogeny (Cesari et al. Citation2009, Citation2013; Bertolani et al. Citation2011; Guidetti et al. Citation2021).
Aside from taxonomy, few efforts have been made in investigating the causes of extreme differences in eggshell morphology that go beyond simple intraspecific genetic variability and could represent examples of genome- and/or environment-based phenotypic plasticity (e.g., Pennak Citation1953; Baumann Citation1966; Schuetz Citation1987; Hansen & Katholm Citation2002; Stec et al. Citation2016).
Within Macrobiotoidea, the genus Paramacrobiotus, described by Guidetti et al. (Citation2009), includes more than 40 species (Degma & Guidetti Citation2023; for a review of the genus, see Kayastha et al. Citation2023a). The genus has long been considered to include two main informal species groups, namely the richtersi complex and the areolatus group (see Guidetti et al. Citation2019; Stec et al. Citation2020b), only differing in the presence or absence of the microplacoid, respectively. Although each of these two species groups has a morphological support, the monophyly of the areolatus group is not supported by the most recent molecular phylogenetic analyses (see Guidetti et al. Citation2019; Stec et al. Citation2020b; Kayastha et al. Citation2023b). Within the areolatus group, species are mostly differentiated through morphological details of the eggshells, which yet show a general morphology of the areolatus-type (i.e., characterized by conical processes and the presence of areolae on the egg surface; Kaczmarek et al. Citation2017; Kayastha et al. Citation2023a). However, an exception to this typical egg morphology is represented, within the group, by the csotiensis-type egg, described as characterized by hemispherical processes covered with a hyaline layer (according to Iharos Citation1966a; Kaczmarek et al. Citation2017). The latter egg morphotype was, before the present study, only attributed to a single species, Paramacrobiotus csotiensis (Iharos Citation1966a), only known from Europe. Interestingly, there are many reports of csotiensis-type eggs found within the same moss fragment together with areolatus-type eggs, the latter belonging to Paramacrobiotus areolatus (Murray Citation1907) or Paramacrobiotus klymenki Pilato et al. Citation2012 (Binda & Pilato Citation1971; Binda Citation1980; Bertolani & Rebecchi Citation1988; Pilato et al. Citation2012). Despite the reports of this recurring coexistence, the major diversity between the two egg morphologies has always been interpreted as a noteworthy event of sympatry. The retrieval of the two egg morphotypes in five Italian moss samples allowed us to perform a specific study in order to further investigate this peculiar association. Tardigrades and eggs from these samples were analyzed with an integrated approach, including morphological observations at LM (Light Microscopy), SEM (Scanning Electron Microscopy), and CLSM (Confocal Laser Scanning Microscopy), molecular analyses, assessment of the reproductive strategies, and inter-morphotype crosses, allowing to find multiple lines of evidence that the two egg morphotypes, despite their overall morphological differences, belong to the same species.
Materials and methods
Analyzed material
For this study, five moss-on-rock samples containing tardigrades morphologically attributable to Paramacrobiotus of the areolatus group and eggs attributable to the areolatus and csotiensis morphotypes were collected in different Italian localities (): Sassi di Varana (C3886; Modena), Pompeano (C3891; Modena), Pontremoli (C4497; Massa-Carrara), Monte Sant’Angelo (C5062; Foggia), and Gombola (C5025; Modena) (). The moss from Gombola (firstly collected in June) was resampled in November to compare the ratio of abundance between the two egg morphotypes in different seasons. Animals and eggs have been extracted following the protocol described by Guidetti et al. (Citation2019).
Figure 1. Map of all localities in which csotiensis-type eggs were found, including samples collected for the present study (in red). For references, see .
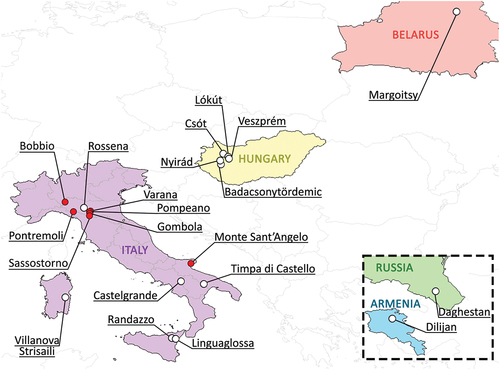
Table I. List of the sampled localities with sample code, coordinates, and altitudes. For each sample, performed analyses are indicated. LM = Light Microscopy; SEM = Scanning Electron Microscopy; CLSM = Confocal Laser Scanning Microscopy.
To investigate whether the two egg morphotypes belonged to the same species, each of the above-listed samples was used for one or more analyses, as indicated in .
In addition, reports of the association of csotiensis- and areolatus-type eggs from previous records were analyzed.
Proportion of the two egg morphotypes
The high number of extracted eggs of the two morphotypes allowed to estimate their abundance ratio, comparing the samples from Pontremoli (collected in November) and from Gombola (with two collections in June and in November). To calculate the 95% CI of the proportion of csotiensis-type eggs found in the three samples, we used the Clopper–Pearson method (Clopper & Pearson Citation1934) as implemented in the function “propCI” of the R package “prevalence v0.4.1” (Devleesschauwer et al. Citation2022). To test the null hypothesis that the proportion of the csotiensis-type eggs was the same in the tested samples, we performed a two-sided proportion test using the function “prop.test” of the R package “stats v4.3.1” (R Core Team Citation2023) with Yates’ continuity correction.
Karyotyping
Adult females with mature oocytes within the ovarium from Pontremoli and Gombola samples have been fixed in Carnoy fluid for 24 h, then stained on a slide with a drop of acetic lactic orcein, following the protocol by Rebecchi & Bertolani (Citation1994), to visualize chromosomes and assess the ploidy.
In order to reduce the time of the karyotyping procedure and allowing the recovery of the animals for further analyses, a new protocol to visualize the chromosomes within oocytes was tested: five females with mature oocytes within the ovarium from Gombola were stained in vivo with NucBlue [NucBlue™ Live ReadyProbes™ Reagent (Hoechst 33342); Thermo Fisher Scientific; 1:1 in distilled water] for 1 h, then mounted on temporary slide together with NucBlue water solution and observed at CLSM. Fluorescence signals were collected from stained specimens (light lenght excitation λex = 405 nm and emission signal collected λem = 450–470 nm) and images were acquired and post-elaborated either with Leica LAS X or Fiji V 1.53 u software (Schindelin et al. Citation2012). The animals were then recollected from the temporary slide and mounted on slides in Hoyer’s medium for further morphological analyses with LM.
Tardigrades culturing
Groups of tardigrades extracted from the Pontremoli and Gombola populations were cultured in two different conditions, i.e., in water or in moss, in order to always have available animals and eggs for the analyses and to monitor eventual changes in the ratio between the two laid egg morphotypes.
About 70 tardigrades randomly extracted from Pontremoli sample and 50 from Gombola sample were cultured in water as follows: animals were placed in bottom-scraped plastic Petri dishes (8 cm in diameter) with culturing water (50% distilled water and 50% “Monte Cimone” spring commercial water), at room temperature and natural light, and fed on algae (Chlorococcum sp.) and nematodes (Panagrellus pycnus Thorne Citation1938) ad libitum (protocol modified from Altiero & Rebecchi Citation2001). The cultures of both populations were monitored throughout 1 year.
Fifteen animals randomly extracted from the Pontremoli sample and 20 from the Gombola sample were cultured on moss as follows: animals were put on rinsed and well-hydrated small moss fragments collected from the original sample (i.e., gametophytes fragments with about 10 leaves, cleaned from animals and eggs) within small plastic capsules (1.5 cm in diameter), incubated in a humid chamber obtained with a glass Petri dish (10 cm in diameter) with few ml of water and its lid, at room temperature and natural light, and without providing any additional food source. The moss fragments were weekly water-sprayed and monthly replaced (protocol modified from Massa et al. Citation2023), and the cultures monitored for 5 months.
Life history traits acquisition and reproductive strategy assessment
Some life history traits (i.e., egg hatching time, age at sexual maturity, and number of eggs per clutch) were acquired from the water-cultured population from Gombola, by isolating 20 laid eggs, 10 per each morphotype. Hatchlings were labeled as “csotiensis-type” or “areolatus-type” referring to the emerging egg morphotype, individually cultured in small bottom-scraped plastic capsules (at room temperature, natural light, and fed with algae Chlorococcum sp. ad libitum). These were monitored until reaching sexual maturity, then observed in vivo to evaluate the kind of germ cells (oocytes or spermatozoa) present in the gonad, according to Rebecchi & Guidi (Citation1991). Finally, they were used to perform inter-morphotype crosses (see below, section “Inter-morphotype crosses”), and followed during their lifetime.
To investigate the reproductive strategy of the populations from Pontremoli and Gombola, respectively, 18 and 20 females with visible oocytes within the ovarium were isolated and individually cultured in water (with the same protocol reported above) up to 4 months, to obtain egg deposition.
Inter-morphotype crosses
Sexually mature animals from Gombola individually cultured in water since hatching and labeled as “csotiensis-type” or “areolatus-type” referring to the egg morphotype they emerged from (as reported above in the section “Reproductive strategy assessment and life history traits acquisition”) were used to perform inter-morphotype crosses, in order to test the interfertility between animals hatched from the two different egg morphotypes, as follows. First cross: a male hatched from the areolatus egg morphotype was paired with a female hatched from the csotiensis egg morphotype. Second cross: a male and a female both emerged from csotiensis-type eggs were paired, as a positive control. Third and fourth crosses: two mature males hatched from areolatus-type eggs were paired with two mature females whose emerging egg type could not be traced back. All four couples were fed daily and checked until eventual oviposition occurred. Laid eggs were removed and either mounted on slide in Hoyer’s medium or individually placed in new capsules, to be monitored until hatching.
Molecular analyses
Both egg morphotypes collected from the Gombola, Pontremoli, and Monte Sant’Angelo samples were incubated until hatching, and newborns were collected for molecular analyses, while the eggshells were mounted on slides in Hoyer’s medium as hologenophore voucher specimens (sensu Pleijel et al. Citation2008). One adult from Sassi di Varana was photographed prior to DNA extraction, obtaining a photographic hologenophore voucher specimen (following Cesari et al. Citation2013). Vouchers are listed in and are deposited in the Bertolani collection (University of Modena and Reggio Emilia, Italy). Genomic DNA was extracted from live specimens with Epicentre QuickExtract Kit (Lucigen) following the manufacturer’s protocol. When possible, cuticles were recovered and mounted on slides in Hoyer’s medium as additional hologenophore voucher specimens. Fragments of the cytochrome c oxidase subunit I (COI) and internal transcribed spacer 2 (ITS2) sequences were amplified using specific primers and PCR protocols, as listed in .
Table II. Sequence dataset used for molecular analysis of the COI and ITS2 markers, with GenBank accession numbers and codes of the vouchers obtained from the hologenophores or from the cuticles recollected after DNA extraction (a).
Table III. Primer pairs and associated PCR protocols used for the amplification of the ITS2 and COI markers.
Amplicons were gel purified using the Wizard Gel and PCR cleaning kit (Promega), and the sequencing reaction was performed through ABI Prism Big Dye Terminator v. 1.1 sequencing kit (Applied Biosystems), with the protocols described in Cesari et al. (Citation2022). Electropherograms were checked with the FinchTV 1.4.0 software (Geospiza Inc.) to identify and correct the presence of ambiguous bases. The COI sequences were translated into amino acids by using the invertebrate mitochondrial DNA genetic code in the program MEGA 11 (Tamura et al. Citation2021) to test for the presence of stop codons, and therefore of pseudogenes. The nucleotide sequences of the newly analyzed specimens have been submitted to GenBank; COI and ITS2 sequences of other P. areolatus group specimens obtained from GenBank were included in the analysis (for GenBank acc. n. see ). Nucleotide sequences were aligned with the MUSCLE algorithm (Edgar Citation2004), using default parameters implemented in MEGA11, and checked by visual inspection. P-distance matrixes have been computed by using MEGA 11 for all analyzed markers between all sequences obtained for the present study and available P. areolatus group sequences present on GenBank (), except for that of Paramacrobiotus lachowskae Stec et al. Citation2018, that was excluded from the analyses despite belonging (on a morphological basis) to the areolatus group, since it clusters within the richtersi complex (Kayastha et al. Citation2023b). Relationships among COI haplotypes or ITS2 sequences variability were estimated using haplotype/sequence parsimony networks, by applying the method described by Templeton et al. (Citation1992), as implemented in TCS 1.21 (Clement et al. Citation2000), and visualized using tcsBU (Santos et al. Citation2016). A 95% connection limit was employed as suggested by Hart and Sunday (Citation2007) as a useful general tool in species assignments and discovery. Species delimitation was also inferred on p-distance using the distance-based Assemble Species by Automatic Partitioning method (ASAP; Puillandre et al. Citation2021), available on the ASAP website (https://bioinfo.mnhn.fr/abi/public/asap/, accessed on 29/03/2023). A phylogenetic analysis was computed for both molecular markers in a maximum likelihood (ML) framework, using the program RAxML version 8.2.12 (Stamatakis Citation2014) as implemented in CIPRES, utilizing GTR+G as the evolutionary model. Bootstrap resampling with 1000 replicates was undertaken via the rapid bootstrap procedure of Stamatakis et al. (Citation2008) to assign support to branches in the ML tree.
Morphological analyses
Eggs belonging to the two morphotypes (i.e., areolatus and csotiensis) collected from Pontremoli and Gombola were individually incubated in culturing water at room temperature until hatching. Newborns (alongside their eggshells) and animals extracted from the Pontremoli, Gombola, and Monte Sant’Angelo samples were mounted on slides in Hoyer’s medium for LM observations. For all samples, LM observations and image acquisition were carried out both under phase contrast (PhC) and differential interference contrast (DIC). In addition, morphometric data collection was carried out (up to the maximum magnification: 100× oil objective) with a Leica DM RB microscope equipped with an AmScope MU1803 digital camera. Thirty animals and 12 areolatus-type eggs were measured following Kaczmarek & Michalczyk (Citation2017). Twelve csotiensis-type eggs were measured acquiring egg bare maximum diameter, egg bare minimum diameter, egg full diameter, and the height of three different suitable processes (i.e., entirely lying on the same focal plane) (Supp. Figure S1). Raw morphometric data were organized and analyzed using the template “Parachela” ver.1.8 by Michalczyk & Kaczmarek (Citation2013) available from the Tardigrada Register, also including the pt index (i.e., the percent ratio between the length of a structure and the length of the buccal tube), and with the addition of calculations for Thorpe’s Normalization by Massa et al. (Citation2021).
For the samples from Pontremoli, Gombola, and Pompeano, animals and eggs of each morphotype were prepared for scanning electron observations following the protocol by Camarda et al. (Citation2023) and observed through the Nova Nano SEM 450 available at the Centro Interdipartimentale Grandi Strumenti of the University of Modena and Reggio Emilia (CIGS-UNIMORE).
Animals and eggs of both morphotypes of the Gombola population were investigated by the CLSM Leica SP8 equipped with a white laser available at CIGS-UNIMORE to i. identify the eventual presence of chitin on the egg chorions; ii. characterize the structures of the two different egg morphotypes; iii. investigate the morphology of animal’s pharynx. The resulting images were post-elaborated (e.g., adjustment for contrast, intensity, signal-to-noise ratio, 3D reconstruction) either with Leica LAS X or Fiji V 1.53 u software (Schindelin et al. Citation2012). In particular, five animals and five eggs of each morphotype from Gombola were stained with calcofluor white to highlight chitin (CFW; Sigma; 1:50 in distilled water) for 1 h and then mounted on slides together with the CFW water solution. From stained animals and eggs, different fluorescence signals were collected and imaged as follows: for the CFW stained chitin signal, λex = 405 nm and λem = 425–470 nm; for the autofluorescence signal of unstained structures, λex = 488 nm and λem = 509–614 nm (following the protocol by Massa et al. Citation2024). Coverslips were bead-spaced (bead diameter <38 μm) to avoid the squeezing of animals or parafilm-spaced (parafilm thickness >100 µm) to avoid the squeezing of eggs, and Valap-sealed (McGreevy et al. Citation2018) to block solution evaporation.
Results
Previous and new reports of csotiensis-type eggs, together with their association with areolatus-type eggs, are reported in and . In most cases, the two egg morphotypes are found in the same moss samples; in a few cases, they were found in the same locality, but references did not give further indication if they were retrieved from the same moss fragment. The csotiensis-type eggs previously retrieved were all associated with the species Paramacrobiotus csotiensis. This species shows a Eurasian distribution and occurs most of the time associated with other species of the P. areolatus group (with which it shares the same adult morphology; ). In these cases, P. csotiensis coexisted with Paramacrobiotus areolatus (Murray Citation1907) and (only once) with Paramacrobiotus klymenki Pilato et al. Citation2012 (both last two species produce an areolatus-type egg).
Table IV. Summary of all previous records of csotiensis-type egg and its association with areolatus-type egg, with the addition of the samples analyzed in the present study. 1 The two egg morphotypes are reported to be found within the same moss sample; 2 the two egg morphotypes have been certainly found in same locality, but it is not possible to know whether they were also found within the same samples; * egg of Paramacrobiotus klymenki, to be considered of the areolatus type basing on the original description by Pilato et al. (Citation2012), and as also stated in Kaczmarek et al. (Citation2017).
Proportion of the two egg morphotypes
The abundance ratio between the two collected egg morphotypes in the samples of Pontremoli from November (150 eggs, of which 113 belonged to the csotiensis-type), Gombola from June (85 eggs, of which 64 belonged to the csotiensis-type), and Gombola from November (101 eggs, of which 76 belonged to the csotiensis-type) was skewed towards the csotiensis-type (about 3:1). The proportion tests did not find any significant difference between the estimated proportions of the csotiensis-type eggs in the three samples (X-squared = 0.00024, df = 2, p-value = 0.999). In detail, the proportion of the csotiensis-type eggs (mean [95% CI]) was as follows: Pontremoli (C4497) November, 75.4% [67.6%−82.0%]; Gombola (C5025) June, 75.3% [64.7%−84.0%]; Gombola (C5025) November, 75.2% [65.7%−83.3%].
The ~75% proportion of csotiensis-type eggs was also observed when the populations from those samples were cultured both in water and in moss.
Karyotyping
The populations from Pontremoli and Gombola showed a diploid karyotype, with six bivalent chromosomes within the oocytes (both with LM and CLSM; ), Supp. Figure S2m). The new protocol to visualize the chromosomes with CLSM proved to work and to be reliable.
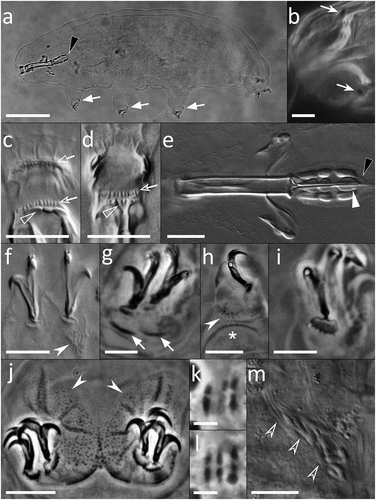
Figure 2. Paramacrobiotus bifrons sp. nov., animal morphology with LM. (a) holotype in toto; (b) eyespots visible in vivo; (c) buccal armature, dorsal view; (d) buccal armature, ventral view; (e) buccal-pharyngeal apparatus; (f) claws of the first pair of legs; (g) claws of the second pair of legs; (h) leg and claw of the third pair, internal side; (i) claws of the fourth pair of legs; (j) fourth pair of legs; (k-l) orcein-stained chromosomes, different planes of the same oocyte; (m) sperms within a sperm duct visible in vivo. Black arrowheads: filament departing from the third macroplacoid; white arrows: cuticular bars under claws; white indented arrows: eyespots; empty arrow: first band of teeth; empty indented arrows: second band of teeth; empty arrowheads: transversal crests; white arrowhead: constriction on the third macroplacoid; white indented arrowheads: granulation on legs; asterisk: pulvinus; empty indented arrowhead: sperms within a sperm duct. a, g–l: PhC; b–f, m: DIC. e–h, j: z-stacks. Scale bars: a = 50 µm; b, e–j, m = 10 µm; c–d = 5 µm; k–l = 2.5 µm.
Life history traits
Of the 20 individually cultured eggs from sample C5025 (Gombola), only five csotiensis-type and three areolatus-type eggs successfully hatched. Their life history data are reported in Supp. Table S1. The two egg morphotypes had similar hatching times (range 15–18 days for csotiensis-type eggs and 16–22 days for areolatus-type eggs). To reach sexual maturity (i.e., showing gametocytes in the gonad), newborns of both sexes from both egg morphotypes took 17–35 days. Once a female reached sexual maturity, no oviposition occurred unless a male, regardless the egg morphotype of origin, was present. The same female was never observed to lay two different egg morphotypes during her life. The number of laid eggs per clutch varied from two in the first oviposition (in females involved in the first and second inter-morphotype crosses) to up to 10 and 14 in adult females found in the sample (i.e., those involved in the third and fourth inter-morphotype crosses).
Reproductive strategy
Of the 18 individually cultured females with visible oocytes in the gonad (population from Pontremoli), two laid eggs after 2–3 days from the isolation (very probably they were already fertilized), while 16 of them never laid eggs. Two of these 16 females survived up to 121 and 137 days without laying eggs, always showing oocytes within their gonads. None of the 20 isolated females with visible oocytes in the gonad collected from the sample of Gombola laid eggs.
The in vivo inspection with LM on the individually cultured animals (deriving from the 20 isolated eggs used to collect life history traits) revealed the presence of males in both populations (Gombola and Pontremoli), which hatched from both egg morphotypes (Supp. Table S1).
Intermorphotype-crosses
The inter-morphotype crosses performed showed that individuals hatched from the two different egg morphotypes are interfertile. In the first cross (male from areolatus egg + female from csotiensis egg), the female laid two csotiensis-type eggs after 3 days post male introduction. After 8 days the couple was formed, both individuals were in simplex stage and the female already showed oocytes within the gonad, while the male still showed spermatozoa within the sperm ducts. Both csotiensis-type eggs laid by this couple hatched 13 and 15 days later. In the second cross (male + female both from csotiensis egg), an oviposition of three csotiensis-type eggs occurred after 4 days, and all eggs hatched within 15 days. In the third and fourth crosses (male from csotiensis egg + female from unknown egg), the females had been previously individually cultured for 79 days without laying eggs. After the pairs were formed, one female laid 10 csotiensis-type eggs after 6 days. Three of these eggs were mounted on slide for morphological observation, two never hatched, and five hatched within 22 days. The other female laid 14 csotiensis type-eggs after 4 days. Four of these eggs were mounted on slide for morphological observations, five never hatched, and six hatched within 20 days. Unfortunately, no females that were hatched from areolatus-type eggs survived in the individual culture condition, preventing to test the inter-morphotype cross with males hatched from csotiensis-type eggs.
Molecular characterization of the populations
The molecular analysis of the COI gene was carried out on a 660 bp dataset of 19 sequences, including the outgroup sequence of Macrobiotus kyoukenus Cesari et al. Citation2022, four sequences belonging to specimens of the areolatus group retrieved from GenBank, and 14 sequences obtained in the present study belonging to the populations from Pontremoli, Gombola, Sassi di Varana, and Monte Sant’Angelo (). The molecular analysis of the ITS2 fragment was carried out on a 500 bp dataset of 17 sequences, including the outgroup sequence of M. kyoukenus, four sequences belonging to specimens of the areolatus group retrieved from GenBank, and 12 sequences obtained in the present study belonging to the populations from Pontremoli, Gombola, and Monte Sant’Angelo ().
Concerning COI, all the populations analyzed for this study resulted quite similar, with p-distance values of 0.0–2.5% (Supp. Table S2). All the sequences obtained in the present study cluster together within the haplotype parsimony network, with no segregation based on the egg morphotype; the only exception is a newborn from an areolatus-type egg from Monte Sant’Angelo, which, however, has a p-distance of 2.5% from the other newborn from areolatus-type egg and of 2.4% from the newborn from csotiensis-type egg from the same sample. A population of Paramacrobiotus gr. areolatus from Castelbianco (Italy; Stec et al. Citation2020b) clusters within the haplotype network of the populations from Pontremoli, Gombola, Sassi di Varana, and Monte Sant’Angelo (p-distance values 1.5–3.0%; Supp. Table S2 and ). The best partition generated by ASAP (lower ASAP-score = 1.00; threshold p-distance: 7.40%) shows the presence of four groups of sequences, corresponding to four delimited species (). The first group is formed by all specimens of populations from Pontremoli, Gombola, Sassi di Varana, and Monte Sant’Angelo. The other three groups are made up of each individual haplotype pertaining to the other species of the areolatus group present in GenBank (including a specimen from the locus typicus of P. areolatus). The only exception is the P. gr. areolatus from Castelbianco (Italy; Stec et al. Citation2020b), which clusters with all specimens of the populations from Pontremoli, Gombola, Sassi di Varana, and Monte Sant’Angelo. The ML phylogenetic analysis for COI (Supp. Figure S3) shows results that are in agreement with what is described above.
Figure 3. Parsimony haplotype network obtained for the COI gene and parsimony sequence network for the ITS2 fragment of P. areolatus group. The circles are representing different haplotypes and are colored by populations following the legend. The connecting lines are representing single substitutions, while the white dots indicate the missing/ideal haplotypes/sequences. The diameter of the circles is proportional to the number of specimens presenting that specific haplotype/sequence, as indicated in the legend. nwb. = newborn. ASAP partitions for both markers are represented by background-colored boxes: orange background for COI (threshold distance 7.40%; lower ASAP score: 1.00) and grey background for ITS2 (threshold distance 17.89%; lower ASAP score: 1.50). The histograms generated by the ASAP software are showing the frequency distribution of each p-distance in comparison with all the considered P. areolatus group sequences. Red lines are indicating threshold distances.
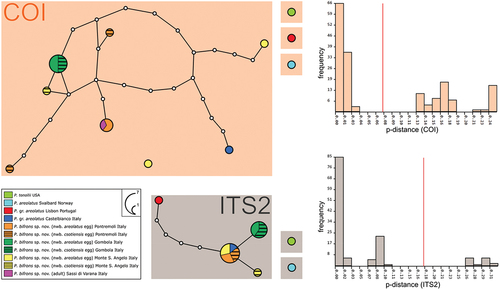
The results for the ITS2 analysis are in overall similar to those obtained for COI, but with a lower p-distance variability, ranging from 0.0% to 0.5% (, Supp. Table S3). Specifically, one sequence (shared by newborns from two csotiensis- and two areolatus-type eggs) was found in the Pontremoli sample, which is shared with P. gr. areolatus from Castelbianco (Italy; Stec et al. Citation2020b) and with two newborns from areolatus eggs type from Monte Sant’Angelo sample. This sequence is directly linked to that of a newborn from csotiensis-type egg from the Monte Sant’Angelo sample. A single sequence (shared by newborns from three csotiensis- and two areolatus-type eggs) was found in the Gombola sample (). Moreover, a population of P. gr. areolatus from Portugal (Stec et al. Citation2020b) clusters in the parsimony network of P. bifrons sp. nov., representing an individual sequence differing by five substitutions. The best partition generated by ASAP (lower ASAP-score = 1.50; threshold p-distance: 17.89% for ITS2) shows the presence of three groups of sequences, corresponding to three delimited species (). The first group corresponds to Paramacrobiotus tonollii (Ramazzotti Citation1956) from USA, the second corresponds to the sequence of P. areolatus (from locus typicus), and the third includes all sequences of the populations from Pontremoli, Gombola, and Monte Sant’Angelo, plus the two sequences retrieved from GenBank related to P. gr. areolatus from Portugal and Castelbianco (Italy; Stec et al. Citation2020b). The ML phylogenetic analysis for ITS2 (Supp. Figure S4) shows results that are in agreement with what is described above.
Taxonomic account
Due to peculiar characters of the animals and eggs retrieved from the analyzed populations, we describe the new species Paramacrobiotus bifrons sp. nov., characterized by the production of two markedly different egg morphotypes (i.e., csotiensis- and areolatus-type eggs).
Paramacrobiotus bifrons sp. nov.
ZooBank: urn:lsid:zoobank.org:act:82DDACA5-F539-4A80-8418-8AF4A370B631
Type locality
Moss on stone slabs on the roof of a small house, Gombola, Modena, Italy (C5025; 44°23’20”N 10°43’18”E, 454 m a.s.l.). Other localities in Italy: Sassi di Varana, Modena (C3886; 44°29’24”N 10°51’1”E, 369 m a.s.l.); Pompeano, Modena (C3891; 44°23’57”N 10°45’31”E, 642 m a.s.l.); Pontremoli, Massa-Carrara (C4497; 44°22’48”N 9°52’52”E, 263 m a.s.l.); Monte Sant’Angelo, Foggia (C5062; 41°43’12”N 15°56’01”E, 535 m a.s.l.).
Type repository
Holotype (C5025 s8a), 39 paratypes (C5025 s1-s6, s8-s10, s12), 20 csotiensis-type eggs (C5025 s13-s20), and 10 areolatus-type eggs (C5025 s23-s27, s31), at the Bertolani collection of the University of Modena and Reggio Emilia (Italy); 5 paratypes (C5025 s11), 4 csotiensis type-eggs (s22a,c,d,e), and 3 areolatus-type eggs (s22b, s33, s34) at the tardigrade collection of the Natural History Museum of Verona (Italy); 6 paratypes (C5025 s7), 2 csotiensis-type eggs (C5025 s21), and 2 areolatus-type eggs (C5025 s28–29) at the tardigrade collection of the Zoological Museum of the University of Copenhagen (Denmark). Vouchers deposited in the Bertolani collection of the University of Modena and Reggio Emilia, voucher codes in ).
Description
Animals length is 186–497 μm (morphometric data in , Supp. Table S4). Eyespots are present in alive specimens (), but usually fade after fixation. The body is whitish and the cuticle smooth, without pores (). A slight granulation (with LM) composed of spiny tubercules formed by grouped granules (with SEM) surrounds the legs (), ), appearing larger and thicker on legs IV (, ).
Figure 4. Paramacrobiotus bifrons sp. nov., animal morphology with SEM. (a) in toto; (b) mouth; (c) leg and claws of the first pair, internal side; (d) fourth pair of legs. White arrow: first band of teeth; empty arrowhead: ventral transversal crests; indented arrows: thickening at the base of the claw. Scale bars: a = 100 µm; b–d = 10 µm.
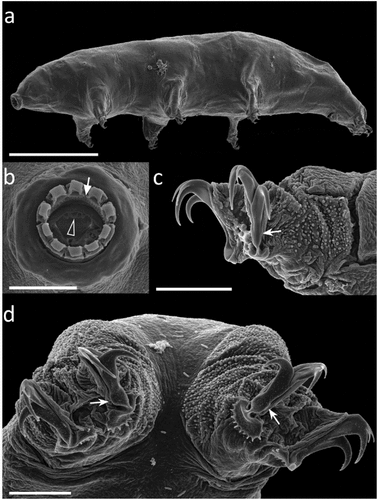
Table V. Paramacrobiotus bifrons sp. nov. List of selected characters measured in animals, including the number of measurements, the range for individual characters expressed in µm and pt, the mean and standard deviation expressed in µm and pt, the measurements of the holotype, and the values obtained from the application of Thorpe’s normalization (parameter b = slope of log Y versus log buccal tube length. Parameter a* = Y-intercept of Thorpe normalized traits versus buccal tube lenght; for the raw data, see supp. Table S4).
The buccal-pharyngeal apparatus is composed by an anteroventral mouth (, ), surrounded by 10 peribuccal lamellae (, ). The buccal armature is composed of an anterior band of small granular teeth, a posterior band of cusped teeth, three dorsal, long transversal crests () and three ventral transversal crests. The ventral median crest can be subdivided in two small teeth, while the two lateral crests are pointy and in shape of large teeth (, ). Within the pharyngeal bulb, large apophyses and three rod-shaped macroplacoids are present (the third with an evident terminal constriction), macroplacoids length sequence is 2 < 1 < 3, and the microplacoid absent (). The absence of the microplacoid is also confirmed with CLSM by the absence of autofluorescence signal and of the relative chitinous sac-like structure (see Massa et al. Citation2024) in the placoid line within the pharyngeal cage (Supp. Figure S2a-d). A filament (with a collagen-like nature, as suggested by their autofluorescence; see Massa et al. Citation2024) departs from each posterior extremity of the third macroplacoid (with LM and CLSM; , Supp. Figure S2a,c).
Claws are of the hufelandi type, with well-developed accessory points on the primary branch, and with a long and basally thickened common tract (), )). Claw bases with smooth lunules on legs I-III (), and clearly indented larger lunules on legs IV (), 4(d)). Two cuticular bars under the claws on legs I-III are present ()). A large and flat bulge (defined as a pulvinus) is present on the internal surface of legs I-III ().
Reproduction
The species is gonochoric (females and males were found; ) and diploid, with six bivalent chromosomes within the oocytes (, Supp. Figure S2m). The species produces two egg morphotypes, an areolatus-type egg (, , ) and a csotiensis-type egg (, ).
Figure 5. Paramacrobiotus bifrons sp. nov., areolatus-type egg with LM. (a) in toto; (b) egg processes; (c) fused egg processes; (d) areolae surrounding a process. White arrow: trabecular layer between the process walls. a–d: PhC, z-stacks. Scale bars: a–c = 10 µm; d = 5 µm.
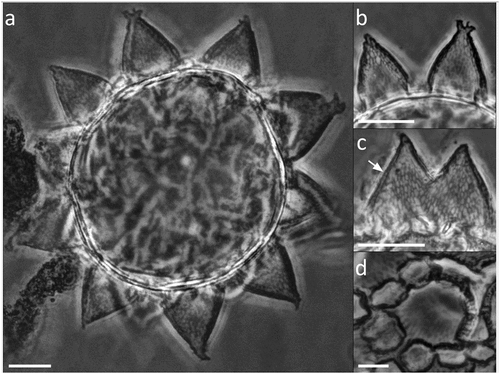
Figure 6. Paramacrobiotus bifrons sp. nov., areolatus-type egg with SEM. (a) in toto, red frame corresponding to (b); (b) basal portion of a process wall with small pores, closeup of (a); (c) two underdeveloped and two normal processes; (d) two fused processes; (e) process surrounded by areolae. White arrow: small pores on the basal portion of a process wall. Scale bars: a,c = 10 µm; b = 2 µm; d–e = 5 µm.
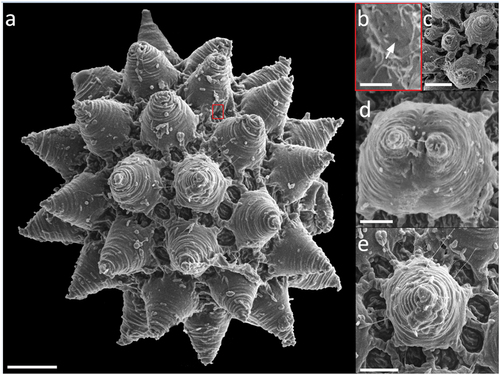
Figure 7. Paramacrobiotus bifrons sp. nov., csotiensis-type egg with LM. (a) in toto; (b) newborn with egg; (c) egg processes in shape of large, fused interconnected crests surrounding a group of areolae; (d) areolae; (e) trabecular layer between the process walls. White arrowheads: crest-shaped egg processes; white indented arrows: groups of areolae; white arrow: trabecular layer between the process walls. a–e: PhC; a–b, d: z-stacks. Scale bars: a,c = 10 µm; b = 25 µm; d = 5 µm; e = 2.5 µm.
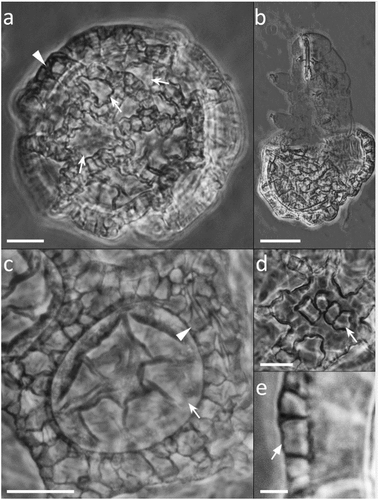
Figure 8. Paramacrobiotus bifrons sp. nov., csotiensis-type egg with SEM. (a–b) in toto; (c–d) basal portion of processes walls with small pores. White indented arrows: areolae; white arrows: small pores on the basal portion of processes walls. Scale bars: a–b = 10 µm; c–d = 2 µm.
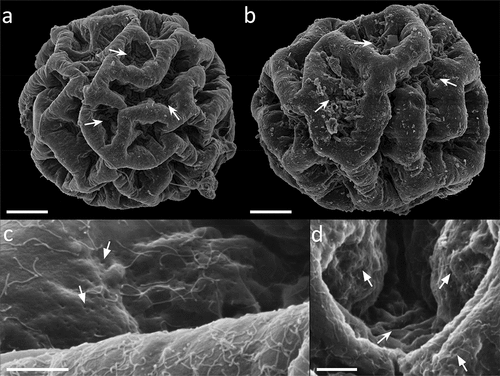
Figure 9. Paramacrobiotus bifrons sp. nov., csotiensis-type eggs aberrations with SEM (a–b) and LM (c–h). (a) in toto egg with small protrusions similar to underdeveloped areolatus-like processes, occurring where three or more crests fuse together; (b) small protrusion similar to areolatus-like process, closeup from (a); (c) in toto egg with aberrant processes appearing as hemispherical; (d) aberrant process appearing as hemispherical, with loose trabecular structures in its upper part, closeup of (c); (e–h) small protrusions similar to areolatus-like processes from various eggs. Arrowhead: csotiensis-type processes in the shape of crests; arrows: small protrusions similar to areolatus-like process; indented arrows: areolae. A-b: SEM; c–h: PhC, z-stacks. Scale bars: a, c = 10 µm; b, d–h = 2.5 µm.
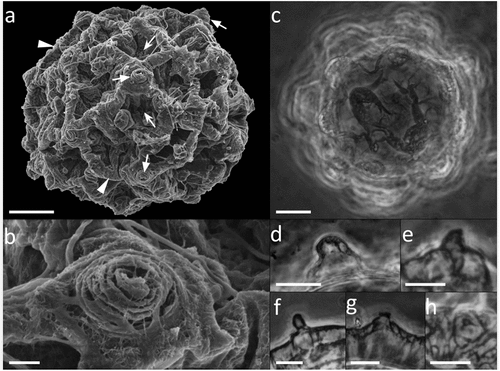
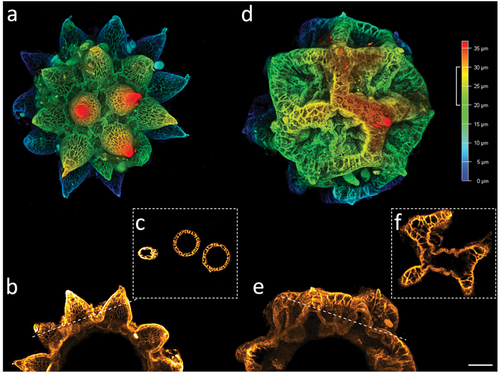
Figure 10. Paramacrobiotus bifrons sp. nov, areolatus- (a–c) and csotiensis- (d–f) type eggs with CLSM, unstained; (a,d) 3D reconstruction of a single hemisphere of the two egg morphotypes, color coded by depth as in the associated scale bar; (b,e) maximum projections of 10 µm sections of (a) and (d), respectively, corresponding to the depth indicated by the white squared bracket on the color-coded-by-depth scale bar; (c,f) cross sections obtained as indicated by the dashed lines in (b) and (e), respectively. b–c, e–f color coded by intensity of autofluorescence signal (from orange, lower, to white, higher). Scale bar: 10 µm.
Eggs
The areolatus-type egg is spherical, ornamented, and freely laid (morphometric data in , Supp. Table S4). Egg processes are elongated, conical, and point-tipped, occasionally bifurcated or branched (). The number of processes on egg circumference varies from 10 to 11. The trabecular layer between the process walls shows an alveolated/reticular pattern with LM and CLSM (), ). The surface of the processes is smooth or delicately wrinkled with SEM (). Occasionally, underdeveloped () or fused ()) processes occur. The proximal part of the external wall of the processes usually shows very small pores, only visible with SEM (). About 10 flat areoles surround each process ()); these are occasionally partly fused (), and have a smooth surface with LM () which appears delicately wrinkled with SEM (.
Table VI. Paramacrobiotus bifrons sp. nov. List of selected characters measured in csotiensis- and areolatus- type eggs, including the number of measurements, the range for individual characters, the mean and standard deviation (for the raw data see supp. Table S4).
The csotiensis-type egg is spherical or sub-ovoidal, ornamented, and freely laid (morphometric data in , Supp. Table S4). Egg processes are generally shaped as large, long, and interconnected crests (, )). An alveolated/reticulated trabecular structure is visible between the processes walls of the crests, similarly to what it occurs in the areolatus-type eggs (), )). The trabecular layer is larger and with looser trabeculae in the upper part of the crests, and thinner with denser trabeculae in the lateral walls (with LM and CLSM; )). The irregular areas delimited by the crests are composed of irregularly distributed areolae ()– ). The basal portion of the crests shows very small pores (only visible with SEM) ()). In some eggs, where three or more crests fuse together, small protrusions, seemingly underdeveloped areolatus-like processes, are present (), )). In a few other eggs, the processes do not appear as crests or cones, but as hemispherical processes with trabecular structures in their distal part ()).
Calcofluor white signal is absent in and on the egg chorion and processes in both egg morphotypes, suggesting a lack of chitin in the structures of the egg surface (Supp. Figure S2e–l).
Etymology
bifrons (Latin) means “two faced”, referred to the two egg morphotypes laid by the species.
Molecular characterization
Eight haplotypes for COI (from 13 specimens; p-distance 0.0%-3.0%, mean 1.1%), four sequences for ITS2 (from 14 specimens; p-distance 0.0%-1.6%, mean 0.2%) (GenBank acc. n. in ; mean p-distance in ; p-distance matrixes in Supp. Tables S2–3). The sequences of the species most similar to P. bifrons sp. nov. belong to Paramacrobiotus areolatus (Murray Citation1907): p-distance 16.0%–18.3% for COI and 8.5%–9.6% for ITS2.
Table VII. Mean genetic p-distances among the most similar sequences of P. areolatus group present in GenBank. and P. bifrons sp. nov. sequences and mean genetic p-distances inside P. bifrons sp. nov. sequences. Values below the diagonal show p-distances, while values above the diagonal indicate standard deviations.
Differential diagnosis
Paramacrobiotus bifrons sp. nov. differs from all other species of the genus Paramacrobiotus without the microplacoid within the pharynx (areolatus group) by producing two different egg morphotypes. The differential diagnosis was restricted to the more similar species of the P. areolatus group, the species of this group with smooth lunules on hind legs were not considered due to the clear difference from P. bifrons sp. nov., that has evident indented lunules on claws IV.
Specifically, P. bifrons sp. nov. differs from:
- Paramacrobiotus areolatus (Murray Citation1907) (based on Stec et al. Citation2020b redescription) by more evident teeth on lunules of claws IV, smaller pt of all claws (e.g., pt of primary branches 18.7–29.9 in the new species vs 29.2–37.5 in P. areolatus), less evident cuticular thickenings at the base of the claws, the presence of males (P. areolatus is most likely parthenogenetic; Stec et al. Citation2020b), shorter egg process with apex not elongated, smaller egg (bare diameter 51.5–66.4 µm in the new species vs 63.2–127.5 µm in P. areolatus; full diameter 75.9–100.0 µm in the new species vs 148.7–195.5 µm in P. areolatus), smaller process height (12.5–19.7 µm in the new species vs 26.8–46.5 µm in P. areolatus), smaller number of processes on the egg circumference (10–11 in the new species vs 12–15 in P. areolatus), COI p-distance values of 16.0%–18.3% and 8.5%–9.6% for ITS2.
- Paramacrobiotus csotiensis (Iharos Citation1966a) (based on the original description, on Pilato Citation1972, and on Kaczmarek et al. Citation2017, redescription) by an evident and wide granulation on all legs, first band of teeth clearly visible (both characters not reported in P. csotiensis by Iharos Citation1966a and not seen by Kaczmarek et al. Citation2017), overall well-built buccal armature (vs with delicate structures, specifically referring to the teeth of the posterior band, in P. csotiensis by Pilato Citation1972).
- Paramacrobiotus intii Kaczmarek et al. Citation2014 (based on the original description) by presence of the first band of granular teeth, cusped-shaped teeth of the second band (vs granular in P. intii), point-tipped egg processes (blunt egg processes in P. intii), smaller egg bare diameter (51.5–66.4 µm in the new species vs 75.3–113.5 µm in P. intii), smaller process base width (9.2–14.8 µm in the new species vs 22–34 µm in P. intii), generally lower process base/height (58–104% in the new species vs 100–160% in P. intii).
- Paramacrobiotus klymenki Pilato et al. Citation2012 (based on the original description and on Camarda et al. Citation2022) by the presence of eyespots, overall smaller claws (e.g., pt of posterior primary branch of claws IV: 21.8–31.2 in the new species vs 34.3–38.8 in P. klymenki from Camarda et al. Citation2022), a higher number and smaller areolae around each egg processes (according to Figure 7(e) of Pilato et al. Citation2012), narrower processes base width (9.2–14.8 μm in the new species vs 16.4–18.2 μm in P. klymenki from Pilato et al. Citation2012 and 16.7–19.3 µm from Camarda et al. Citation2022), lower process base/height ratio (58–104% in the new species vs 98–113% in P. klymenki from Pilato et al. Citation2012 and 125–141% from Camarda et al. Citation2022).
- Paramacrobiotus walteri (Biserov Citation1997/98) (based on the original description) by evidently thicker and shorter claws, accessory points distinctly separated from the claw branch (connected to the branch in P. walteri), smaller mean pt of primary branches of claws IV (25.8 and 27.2 in the new species vs 50.7 in P. walteri), lower difference in length between the primary and secondary claw branches of claws IV (clearly visible in Figures 2(f) and 4(f) from Biserov Citation1997/98), absence of small dots in the distal part of egg processes.
Discussion
The results of the present study, based on morphological, reproductive, molecular, and karyological data, prove that the animals hatched from the csotiensis- and the areolatus-type eggs belong to the same species, Paramacrobiotus bifrons sp. nov., answering a long-standing question emerged after the frequent finding, in different localities, of these two eggs within the same moss sample (Iharos Citation1966a; Binda & Pilato Citation1971; Binda Citation1980; Bertolani & Rebecchi Citation1988). As well as P. bifrons sp. nov., other eutardigrade species are known to lay different types of eggs, although this phenomenon remains rare. Murray (Citation1910) described the exclusively Antarctic species Mesobiotus polaris (Murray Citation1910) as characterized by the presence of eggs with different morphologies of the processes (i.e., hemispherical processes or processes terminating with sharp tips). Hansen & Katholm (Citation2002) reported the occurrence of two egg morphotypes in Bertolanius nebulosus (Dastych Citation1983), in this case identified as a summer egg (with conical processes) and a winter egg (with longer conical processes), pointing out seasonality as the main driver of such variation. Mesobiotus lusitanicus (Maucci & Durante Pasa Citation1984) is characterized by a typical egg morphology (with conical processes), a “transition” egg morphology, and an “anomalous” egg morphology, pointing out the great variability within the species, but possible causes are not reported. Similarly, Dactylobiotus ovimutans Kihm et al. Citation2020 can produce a range of different eggshell morphologies, varying from fully inflated conical processes to fully deflated, whose variability could not be related to environmental factors, since these types of eggs were laid under the same stable laboratory conditions. A high variability was also observed in the eggs of Ramazzottius subanomalus (Biserov Citation1985), this was related to intraspecific variability but also to genetic drivers related to incipient speciation (Stec et al. Citation2016). Paramacrobiotus bifrons sp. nov. also proved to lay both egg morphotypes in both culture facilities (i.e., in water and in moss), under constant culturing conditions. Despite protracted observations of the cultured populations and the sampling of the population from Gombola conducted in two different seasons, it was not possible to decipher the reason behind the production of two very different egg morphotypes. Our observations allowed to at least exclude some inducing factors, e.g., temperature and food source (as they were kept constant in both cultures), and other seasonal drivers, since the analyses of the samples from Gombola, collected in different seasons (i.e., June and November), revealed the same abundance ratio between the two egg morphotypes (3:1, skewed towards the csotiensis-type egg). Additionally, the two egg morphotypes showed similar hatching times in the same experimental conditions, therefore one of the two being a resistant egg (e.g., over-wintering/summering) seems unlikely. Either males or females originated from both egg morphotypes also, excluding a possible role of the offspring’s sex in determining eggshell morphology. The inter-morphotype crosses performed not only allowed to obtain partial (due to the lack of testing the fertility of the F1 generation) results in testing the biological species concept against the morphological evidence and helping in species delimitation, but also allowed to exclude the male parent’s influence in determining the eggshell morphology (the couple csotiensis female and areolatus male only laid csotiensis-type eggs). With the present data, inducing factors that cannot be excluded are a maternal effect or a marked phenotypic plasticity, whose drivers remain unacknowledged but that could be worth investigating in the future research also to clarify the ecological function of the eggshell morphology in tardigrade species laying freely deposited eggs.
Analyzing the cases of documented sympatry between the two egg types laid by morphologically indistinguishable tardigrades, it should be noticed that the areolatus-type egg was always attributed to the species P. areolatus. In the past, many species of the areolatus group have been probably misidentified as P. areolatus due to the lack of a detailed redescription and, especially, the absence of molecular data (Stec et al. Citation2020b). Therefore, it is possible that the areolatus-type eggs previously retrieved together with P. csotiensis do not belong to P. areolatus, but rather correspond to the areolatus-type egg laid by P. bifrons sp. nov. or a closely related species. This especially refers to at least the Italian populations from Reggio Emilia (Bertolani & Rebecchi Citation1988), Matera (Binda Citation1980), and Catania (Binda & Pilato Citation1971), for which we have the indication of the two egg morphotypes being found within the same moss sample (). On the other hand, the molecular species delimitation suggests that a population labeled as P. gr. areolatus from Castelbianco (Italy) (Stec et al. Citation2020b) belongs to the species P. bifrons sp. nov. having a similar COI (lower p-distance 1.5%). This population is gonochoric as P. bifrons sp. nov. but, interestingly, the cultured individuals of Castelbianco only laid areolatus-type eggs (Matteo Vecchi, personal observation). The population from Castelbianco has been subsequently labeled as Paramacrobiotus cf. klymenki in Figure 1 by Kayastha et al. (Citation2023b), probably on morphological bases since the unavailability of molecular data for P. klymenki. However, it should be considered that the population from Castelbianco presents eyespots (Daniel Stec, personal communication) as well as P. bifrons sp. nov., a character which is instead absent in P. klymenki; therefore, further investigations will be certainly needed to clarify the relationships among these populations.
The nuclear marker ITS2, though supporting the results obtained for COI, proved to have an overall lower variability both at the species level (confirming the pattern observed by Stec et al. Citation2020b for the areolatus group) and at population level (as also observed by Kayastha et al. Citation2023b in a species of the richtersi group, Paramacrobiotus gadabouti Kayastha et al. Citation2023b).
Concerning the reports of P. csotiensis in literature (, ), it is worth noting that the finding of eggs of the csotiensis and areolatus morphotypes within the same moss sample is frequent, at least in Italy (Binda & Pilato Citation1971; Binda Citation1980; Bertolani & Rebecchi Citation1988; present study). There are two reports in the Italian territory of P. csotiensis found alone corresponding to two localities in Sardinia island (Binda & Guglielmino Citation1982): one consists in the finding of only one egg, while the other should be considered as dubious record, because it relies on the finding of only one animal which, according to the species description, is impossible to be distinguished from other species of the areolatus group. Several other records (Iharos Citation1966b; Binda Citation1980; Biserov Citation1991) also report the finding of eggs of the csotiensis-type not associated with eggs of the areolatus-type, further supporting the hypothesis of P. csotiensis and P. bifrons sp. nov. being two different species.
Recently, a redescription of P. csotiensis from type material, including pictures of the csotiensis-type egg at LM for the first time, was provided (Kaczmarek et al. Citation2017); unfortunately, the scarcity and quality of available type material prevented to add further morphological details in respect with that provided by Iharos (Citation1966a): some morphological details of P. csotiensis, indeed, still remain unknown (e.g., the presence of the first band of teeth in the buccal armature and the morphology of lunules under claws IV). In our opinion, the present description of the csotiensis-type egg is not completely consistent with the visible morphological characters. Thanks to different microscopy techniques (i.e., LM, SEM, and CLSM), the present study allowed a thorough morphological observation of the eggs produced by P. bifrons sp. nov., providing a more accurate description of the csotiensis-type egg and also revealing several shared details between the two egg types, i.e., the proximal part of the external wall of the processes showing very small pores (see , ), the presence of a trabecular layer interposed between the process walls (see )), and the presence of areolae-like structures (see , ). The presence on some csotiensis-type eggs of small areolatus-like conical processes (), ) or unconnected crests forming hemispherical processes suggests the possible derivation of the csotiensis-type egg from an areolatus-type egg. In the genus Paramacrobiotus, the general morphology of areolatus-type egg is indeed conserved and widespread, while the csotiensis-type egg was, so far, only associated with a single, rare species (P. csotiensis). Furthermore, CLSM granted to observe the detailed morphology of the internal trabecular layer of egg process walls in the two egg morphotypes of this new species (), and it allowed to exclude the presence of chitin in the structures of both egg morphotypes (Supp. Figure S2e–l). This last finding is particularly interesting because it represents the first report on the nature of the egg surface, which appears unexpectedly different from the animal surface (Bussers & Jeuniaux Citation1973; Greven & Peters Citation1986; Kristensen & Neuhaus Citation1999; Greven et al. Citation2016; Massa et al. Citation2024).
Authors contribution
RG, MV, SB collected the samples. RG, SB designed methodology; SB analyzed the samples and performed the experiments; MC, SB, MV performed molecular analyses; SB, EM performed CLSM analyses. LR, RG organized the lab work and provided funds. All authors contributed critically to the drafts preparation and gave final approval for the manuscript publication.
Supplemental Material Table S4
Download MS Excel (85.4 KB)Supplemental Material Table S3
Download MS Excel (12.3 KB)Supplemental Material Table S2
Download MS Excel (13.4 KB)Supplemental Material Table S1
Download MS Excel (11.3 KB)Supplemental Material Figures S3 and S4
Download MS Word (1,001.1 KB)Supplemental Material Figures S1 and S2
Download MS Word (1.1 MB)Acknowledgments
We are thankful to Prof. Roberto Bertolani for starting the idea of this project, Dr Lucia Piemontese for collecting the moss sample from Monte Sant’Angelo, and Dr Daniel Stec for sharing the information about the population from Castelbianco.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2024.2317465
Additional information
Funding
References
- Altiero T, Rebecchi L. 2001. Rearing tardigrades: results and problems. Zoologischer Anzeiger 240(3–4):217–221. DOI: 10.1078/0044-5231-00028.
- Baumann H. 1966. Lebenslauf und Lebensweise von Hypsibius (H.) oberhaeuseri Doyère (Tardigrada). Veröffentlichen Überseemuseum Bremen 3:245–258.
- Bertolani R, Cesari M, Giovannini I, Rebecchi L, Guidetti R, Kaczmarek Ł, Pilato G. 2023. The Macrobiotus persimilis-polonicus complex (Eutardigrada, Macrobiotidae), another example of problematic species identification, with the description of four new species. Organisms Diversity & Evolution 23:329–368. DOI: 10.1007/s13127-022-00599-z.
- Bertolani R, Rebecchi L. 1988. The tardigrades of Emilia (Italy). I. Rossena. Bolletino di zoologia 55:367–371. DOI: 10.1080/11250008809386634.
- Bertolani R, Rebecchi L. 1993. A revision of the Macrobiotus hufelandi group (Tardigrada, Macrobiotidae), with some observations on the taxonomic characters of eutardigrades. Zoologica Scripta 22:127–152. DOI: 10.1111/j.1463-6409.1993.tb00347.x.
- Bertolani R, Rebecchi L, Claxton SK. 1996. Phylogenetic significance of eggshell variation in tardigrades. Zoological Journal of the Linnean Society 116(1–2):139–148. DOI: 10.1111/j.1096-3642.1996.tb02339.x.
- Bertolani R, Rebecchi L, Giovannini I, Cesari M. 2011. DNA barcoding and integrative taxonomy of Macrobiotus hufelandi CAS Schultze 1834, the first tardigrade species to be described, and some related species. Zootaxa 2997:19–36. DOI: 10.11646/zootaxa.2997.1.2.
- Binda MG. 1980. Tardigradi di Lucania. Animalia 7:79–91.
- Binda MG, Guglielmino A. 1982. Tardigradi muscicoli e dulcacquicoli di Sardegna. Animalia 9:199–221.
- Binda MG, Pilato G. 1971. Nuovo contributo alla conoscenza dei Tardigradi di Sicilia. Bollettino delle sedute dell’Accademia Gioenia di Scienze Naturali, Catania 10:896–909.
- Biserov VI. 1985. Hypsibius subanomalus n. sp. (Eutardigrada, Hypsibiidae) du district d’Astrakhan. Zoologičeskij žurnal 64:131–135.
- Biserov.1997/98. Tardigrades of the Caucasus with a taxonomic analysis of the genus Ramazzottius (Parachela: Hypsibiidae). Zoologischer Anzeiger 236:139–159.
- Biserov VI. 1991. An annotated list of Tardigrada from European Russia. Zoologische Jahrbücher System 118:193–216.
- Bussers JC, Jeuniaux C. 1973. Chitinous cuticle and systematic position of Tardigrada. Biochemical Systematics and Ecology 1:77–78. DOI: 10.1016/0305-1978(73)90040-9.
- Camarda D, Lisi O, Mifsud D. 2022. First faunistic report of limno-terrestrial tardigrades (Tardigrada Doyère, 1840) from the Maltese Islands. Check List 18(4):779–792. DOI: 10.15560/18.4.779.
- Camarda D, Massa E, Guidetti R, Lisi O. 2023. A new, simplified, drying protocol to prepare tardigrades for scanning electron microscopy. Microscopy Research and Technique 1–11. DOI: 10.1002/jemt.24460.
- Cesari M, Bertolani R, Rebecchi L, Guidetti R. 2009. DNA barcoding in Tardigrada: the first case study on Macrobiotus macrocalix Bertolani & Rebecchi 1993 (Eutardigrada, Macrobiotidae). Molecular Ecology Resources 9(3):699–706. DOI: 10.1111/j.1755-0998.2009.02538.x.
- Cesari M, Giovannini I, Altiero T, Guidetti R, Cornette R, Kikawada T, Rebecchi L. 2022. Resistance to extreme stresses by a newly discovered Japanese tardigrade species, Macrobiotus kyoukenus (Eutardigrada, Macrobiotidae). Insects 13:1–21. DOI: 10.3390/insects13070634.
- Cesari M, Guidetti R, Rebecchi L, Giovannini I, Bertolani R. 2013. A DNA barcoding approach in the study of tardigrades. Journal of Limnology 72:182–198. DOI: 10.4081/jlimnol.2013.s1.e23.
- Claxton SK. 1998. A revision of the genus Minibiotus (Tardigrada: Macrobiotidae) with descriptions of eleven new species from Australia. Records of the Australian Museum 50(2):125–160. DOI: 10.3853/j.0067-1975.50.1998.1276.
- Clement M, Posada D, Crandall K. 2000. TCS: A computer program to estimate gene genealogies. Molecular Ecology 9:1657–1660. DOI: 10.1046/j.1365-294x.2000.01020.x.
- Clopper CJ, Pearson ES. 1934. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26(4):404–413. DOI: 10.1093/biomet/26.4.404.
- Dastych H. 1983. Two new Eutardigrada species from west Spitsbergen and the Tatra MTS. Bulletin de la Société des Amis des Sciences et des Lettres de Poznan 23:195–200.
- Dastych H. 2011. Ramazzottius agannae sp. nov., a new tardigrade species from the nival zone of the Austrian Central Alps (Tardigrada). Entomologische Mitteilungen aus dem Zoologischen Museum Hamburg 15:237–253.
- Degma P, Guidetti R. 2023. Actual checklist of Tardigrada species. DOI: 10.25431/11380_1178608.
- Devleesschauwer B, Torgerson P, Charlier J, Levecke B, Praet N, Roelandt S, Smit S, Dorny P, Berkvens D, Speybroeck N (2022). prevalence: Tools for prevalence assessment studies. R Package Version 0.4.1. https://cran.r-project.org/package=prevalence
- Edgar RC. 2004. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32(5):1792–1797. DOI: 10.1093/nar/gkh340.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3:294–299.
- Gąsiorek P, Stec D, Morek W, Michalczyk Ł. 2017. An integrative redescription of Echiniscus testudo (Doyère, 1840), the nominal taxon for the class Heterotardigrada (Ecdysozoa: Panarthropoda: Tardigrada). Zoologischer Anzeiger 270:107–122. DOI: 10.1016/j.jcz.2017.09.006.
- Greven H, Kaya M, Baran T. 2016. The presence of α-chitin in Tardigrada with comments on chitin in the Ecdysozoa. Zoologischer Anzeiger 264:11–16. DOI: 10.1016/j.jcz.2016.06.003.
- Greven H, Peters W. 1986. Localization of chitin in the cuticle of Tardigrada using wheat germ agglutinin-gold conjugate as a specific electron-dense marker. Tissue & Cell 18:297–304. DOI: 10.1016/0040-8166(86)90037-6.
- Guidetti R, Altiero T, Hansen JG. 2006. A new species of freshwater tardigrades from Disko Island (Greenland) increases an unsolved paradox in tardigrade systematics. Hydrobiologia 558(1):69–79. DOI: 10.1007/s10750-005-1408-6.
- Guidetti R, Altiero T, Rebecchi L. 2011. On dormancy strategies in tardigrades. Journal of Insect Physiology 57(5):567–576. DOI: 10.1016/j.jinsphys.2011.03.003.
- Guidetti R, Cesari M, Bertolani R, Altiero T, Rebecchi L. 2019. High diversity in species, reproductive modes and distribution within the Paramacrobiotus richtersi complex (Eutardigrada, Macrobiotidae). Zoological Letters 5:1–28. DOI: 10.1186/s40851-018-0113-z.
- Guidetti R, Massa E, Bertolani R, Rebecchi L, Cesari M. 2019. Increasing knowledge of Antarctic biodiversity: New endemic taxa of tardigrades (Eutardigrada; Ramazzottiidae) and their evolutionary relationships. Systematics & Biodiversity 17(6):573–593. DOI: 10.1080/14772000.2019.1649737.
- Guidetti R, Schill RO, Bertolani R, Dandekar T, Wolf M. 2009. New molecular data for tardigrade phylogeny, with the erection of Paramacrobiotus gen. nov. Journal of Zoological Systematics & Evolutionary Research 47(4):315–321. DOI: 10.1111/j.1439-0469.2009.00526.x.
- Guidetti R, Schill RO, Giovannini I, Massa E, Goldoni SE, Ebel C, Förschler MI, Rebecchi L, Cesari M. 2021. When DNA sequence data and morphological results fit together: Phylogenetic position of Crenubiotus within Macrobiotoidea (Eutardigrada) with description of Crenubiotus ruhesteini sp. nov. Journal of Zoological Systematics & Evolutionary Research 59(3):576–587. DOI: 10.1111/jzs.12449.
- Hansen J, Katholm AK. 2002. A study of the genus Amphibolus from Disko Island with special attention on the life cycle of Amphibolus nebulosus (Eutardigrada, Eohypsibiidae). In: Hansen JG, editor Arctic biology field course, Qeqertarsuaq, 2002. Copenhagen: Zoological Museum of Denmark, University of Copenhagen. pp. 129–163.
- Hansen JG, Kristensen RM, Bertolani R, Guidetti R. 2017. Comparative analyses of bertolanius species (Eohypsibiidae; eutardigrada) with the description of Bertolanius birnae sp. nov. From northern polar regions. Polar biology 40:123–140.
- Hart MW, Sunday J. 2007. Things fall apart: Biological species form unconnected parsimony networks. Biology Letters 3(5):509–512. DOI: 10.1098/rsbl.2007.0307.
- Iharos G. 1966a. Neue Tardigraden-arten aus Ungarn. Acta Zoologica Academiae Scientiarum Hungaricae 12:111–122.
- Iharos G. 1966b. A Bakony-hegyseg Tardigrada-faunaja. III. Kulonlenyomat az Allattani Kozlemenyek 53:69–78.
- Kaczmarek Ł, Bartylak T, Stec D, Kulpa A, Kepel M, Kepel A, Roszkowska M. 2020. Revisiting the genus Mesobiotus Vecchi et al., 2016 (Eutardigrada, Macrobiotidae)–remarks, updated dichotomous key and an integrative description of new species from Madagascar. Zoologischer Anzeiger 287:121–146.
- Kaczmarek Ł, Cytan J, Zawierucha K, Diduszko D, Michalczyk Ł. 2014. Tardigrades from Peru (South America), with descriptions of three new species of Parachela. Zootaxa 3790(2):357–379. DOI: 10.11646/zootaxa.3790.2.5.
- Kaczmarek Ł, Gawlak M, Bartels PJ, Nelson DR, Roszkowska M. 2017. Revision of the genus Paramacrobiotus Guidetti et al., 2009 with the description of a new species, re-descriptions and a key. Annales Zoologici 67:627–656. DOI: 10.3161/00034541ANZ2017.67.4.001.
- Kaczmarek Ł, Michalczyk Ł. 2017. The Macrobiotus hufelandi group (Tardigrada) revisited. Zootaxa 4363(1):101–123. DOI: 10.11646/zootaxa.4363.1.4.
- Kaczmarek Ł, Zawierucha K, Buda J, Stec D, Gawlak M, Michalczyk Ł, Roszkowska M, Rubal M. 2018. An integrative redescription of the nominal taxon for the Mesobiotus harmsworthi group (Tardigrada: Macrobiotidae) leads to descriptions of two new Mesobiotus species from Arctic. Public Library of Science ONE 13(10):e0204756. DOI: 10.1371/journal.pone.0204756.
- Kayastha P, Mioduchowska M, Warguła J, Kaczmarek Ł. 2023a. A review on the genus Paramacrobiotus (Tardigrada) with a New Diagnostic Key. Diversity 15(9):977. DOI: 10.3390/d15090977.
- Kayastha P, Stec D, Sługocki Ł, Gawlak M, Mioduchowska M, Kaczmarek Ł. 2023b. Integrative taxonomy reveals new, widely distributed tardigrade species of the genus Paramacrobiotus (Eutardigrada: Macrobiotidae). Scientific Reports 13(1):2196. DOI: 10.1038/s41598-023-28714-w.
- Kihm JH, Kim S, McInnes SJ, Zawierucha K, Rho HS, Kang P, Park TYS. 2020. Integrative description of a new Dactylobiotus (Eutardigrada: Parachela) from Antarctica that reveals an intraspecific variation in tardigrade egg morphology. Scientific Reports 10:1–11. DOI: 10.1038/s41598-020-65573-1.
- Kristensen RM, Neuhaus B. 1999. Special issue on tardigrada - the ultrastructure of the tardigrade cuticle with special attention to marine species. Zoologischer Anzeiger 238:261–282.
- Mapalo MA, Stec D, Mirano-Bascos D, Michalczik Ł. 2017. An integrative description of a limnoterrestrial tardigrade from the Philippines, Mesobiotus insanis, new species (Eutardigrada: Macrobiotidae: Harmsworthi group). The Raffles Bulletin of Zoology 65:440–454.
- Mapalo MA, Stec D, Mirano-Bascos D, Michalczyk Ł. 2016. Mesobiotus philippinicus sp. nov., the first limnoterrestrial tardigrade from the Philippines. Zootaxa 4126(3):411–426. DOI: 10.11646/zootaxa.4126.3.6.
- Massa E, Guidetti R, Cesari M, Rebecchi L, Jönsson KI. 2021. Tardigrades of Kristianstads Vattenrike biosphere reserve with description of four new species from Sweden. Scientific Reports 11(1):4861. DOI: 10.1038/s41598-021-83627-w.
- Massa E, Rebecchi L, Guidetti R. 2023. Effects of synthetic acid rain and organic and inorganic acids on survival and CaCO3 piercing stylets in tardigrades. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology 339:578–589. DOI: 10.1002/jez.2701.
- Massa E, Rebecchi L, Guidetti R. 2024. Composition and structural organization of tardigrades feeding apparatus focusing on chitin and other autofluorescent molecules. Zoological Journal of the Linnean Society 200(1):186–199. DOI: 10.1093/zoolinnean/zlad028.
- Maucci W, Durante Pasa MV. 1984. Macrobiotus lusitanicus sp. nov. nuova specie di Eutardigrado del Portogallo Nord-Occidentale (Tardigrada, Macrobiotidae). Bollettino del Museo Civico di Storia Naturale di Verona 11:319–326.
- McGreevy KM, Heikes KL, Kult S, Tharp ME, Goldstein B. 2018. Fluorescent cell staining methods for living Hypsibius exemplaris embryos. Cold Spring Harbor Protocols 11:db–prot106021. DOI: 10.1101/pdb.prot106021.
- Michalczyk Ł, Kaczmarek Ł. 2013. The Tardigrada Register: a comprehensive online data repository for tardigrade taxonomy. Journal of Limnology 72:175–181. DOI: 10.4081/jlimnol.2013.s1.e22.
- Michalczyk Ł, Wełnicz W, Frohme M, Kaczmarek Ł. 2012. Redescriptions of three Milnesium Doyère, 1840 taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 3154(1):1–20. DOI: 10.11646/zootaxa.3154.1.1.
- Murray J. 1907. XXV.—Arctic Tardigrada, collected by wm. S. Bruce. Transactions of the Royal Society of Edinburgh 45:669–681. DOI: 10.1017/S0080456800011789.
- Murray J. 1910. Canadian Tardigrada. In: Shackleton, C.V.O. Report for the scientific investigations. Murray J, editors. British Antarctic Expedition 1907–1909 under the command sir, Vol. I. London: William Heinemann, pp. 158–178. C.V.O. Report for the scientific investigations.
- Nelson DR, Bartels PJ, Guil N. 2019. Tardigrade ecology. In: Schill R, editor Water bears: The biology of Tardigrades. zoological monographs. Vol. 2. Cham: Springer. pp. 163–210.
- Pennak RW. 1953. Fresh-water invertebrates of the United States. New York: Ronald Press.
- Pilato G. 1972. Structure, intraspecific variability and systematic value of the buccal armature of eutardigrades. Journal of Zoological Systematics & Evolutionary Research 10(1):65–78. DOI: 10.1111/j.1439-0469.1972.tb00785.x.
- Pilato G, Kiosya Y, Lisi O, Sabella G. 2012. New records of Eutardigrada from Belarus with the description of three new species. Zootaxa 3179(1):39–60. DOI: 10.11646/zootaxa.3179.1.2.
- Pleijel F, Jondelius U, Norlinder E, Nygren A, Oxelman B, Schander C, Sundberg P, Thollesson M. 2008. Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Molecular Phylogenetics and Evolution 48(1):369–371. DOI: 10.1016/j.ympev.2008.03.024.
- Prendini L, Weygoldt P, Wheeler WC. 2005. Systematics of the Damon variegatus group of African whip spiders (Chelicerata: Amblypygi): Evidence from behaviour, morphology and DNA. Organisms Diversity & Evolution 5:203–236. DOI: 10.1016/j.ode.2004.12.004.
- Puillandre N, Brouillet S, Achaz G. 2021. ASAP: Assemble species by automatic partitioning. Molecular Ecology Resources 21(2):609–620. DOI: 10.1111/1755-0998.13281.
- R Core Team. 2023. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available: https://www.R-project.org/.
- Ramazzotti G. 1956. Tre nuove specie di Tardigradi ed altre specie poco comuni. Atti della Società Italiana di Scienze Naturali 95:284–291.
- Rebecchi L, Bertolani R. 1994. Maturative pattern of ovary and testis in eutardigrades of freshwater and terrestrial habitats. Invertebrate Reproduction & Development 26:107–117. DOI: 10.1080/07924259.1994.9672407.
- Rebecchi L, Guidi A. 1991. First SEM studies on tardigrade spermatozoa. Invertebrate Reproduction & Development 19:151–156. DOI: 10.1080/07924259.1991.9672169.
- Santos AM, Cabezas MP, Tavares AI, Xavier R, Branco M. 2016. tcsBU: A tool to extend TCS network layout and visualization. Bioinformatics 32(4):627–628. DOI: 10.1093/bioinformatics/btv636.
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: An open-source platform for biological-image analysis. Nature Methods 9(7):676–682. DOI: 10.1038/nmeth.2019.
- Schuetz G. 1987. A one year study on the population dynamics of milnesium tardigradum doyère in the lichen Xanthoria parietina (L.). In: Bertolani R, editor Biology of tardigrades: Selected symposia and monographs, UZI. Modena: Mucchi. pp. 217–228.
- Schultze CAS. 1834. Macrobiotus hufelandii animal e Crustaceorum Classe Novum, Reviviscendi Post diuturnam asphixiam et Aridiatem Potens. Berlin: Apud Carolum Curths.
- Schuster RO, Nelson DR, Grigarick AA, Christenberry D. 1980. Systematic criteria of the Eutardigrada. Transactions of the American Microscopical Society 1980:284–303. DOI: 10.2307/3226004.
- Stamatakis A. 2014. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9):1312–1313. DOI: 10.1093/bioinformatics/btu033.
- Stamatakis A, Hoover P, Rougemont J, Renner S. 2008. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57(5):758–771. DOI: 10.1080/10635150802429642.
- Stec D, Kristensen RM, Michalczyk Ł. 2020a. An integrative description of Minibiotus ioculator sp. nov. From the Republic of South Africa with notes on Minibiotus pentannulatus Londoño et al., 2017 (Tardigrada: Macrobiotidae). Zoologischer Anzeiger 286:117–134. DOI: 10.1016/j.jcz.2020.03.007.
- Stec D, Krzywański Ł, Zawierucha K, Michalczyk Ł. 2020b. Untangling systematics of the Paramacrobiotus areolatus species complex by an integrative redescription of the nominal species for the group, with multilocus phylogeny and species delineation in the genus Paramacrobiotus. Zoological Journal of the Linnean Society 188(3):694–716. DOI: 10.1093/zoolinnean/zlz163.
- Stec D, Morek W, Gąsiorek P, Kaczmarek Ł, Michalczyk Ł. 2016. Determinants and taxonomic consequences of extreme egg shell variability in Ramazzottius subanomalus (Biserov, 1985) (Tardigrada). Zootaxa 4208:zootaxa–4208. DOI: 10.11646/zootaxa.4208.2.5.
- Stec D, Morek W, Gąsiorek P, Michalczyk Ł. 2018. Unmasking hidden species diversity within the Ramazzottius oberhaeuseri complex, with an integrative redescription of the nominal species for the family Ramazzottiidae (Tardigrada: Eutardigrada: Parachela). Systematics & Biodiversity 16(4):357–376. DOI: 10.1080/14772000.2018.1424267.
- Stec D, Roszkowska M, Kaczmarek Ł, Michalczyk Ł. 2018. Paramacrobiotus lachowskae, a new species of Tardigrada from Colombia (Eutardigrada: Parachela: Macrobiotidae). New Zealand Journal of Zoology 45(1):43–60. DOI: 10.1080/03014223.2017.1354896.
- Tamura K, Stecher G, Kumar S, Battistuzzi FU. 2021. MEGA11: Molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution 38(7):3022–3027. DOI: 10.1093/molbev/msab120.
- Templeton AR, Crandall KA, Sing CF. 1992. A cladistic analysis of phenotypic association with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics 132:619–633. DOI: 10.1093/genetics/132.2.619.
- Thorne G. 1938. Notes on free-living and plant-parasitic nematodes. Proceedings of the Helminthological Society of Washington 5:64–65.
- Trygvadottir BV, Kristensen RM. 2011. Eohypsibiidae (Eutardigrada, Tardigrada) from the Faroe Islands with the description of a new genus containing three new species. Zootaxa 2886(1):39–62.
- Vecchi M, Cesari M, Bertolani R, Jönsson KI, Rebecchi L, Guidetti R. 2016. Corrigendum to: Integrative systematic studies on tardigrades from Antarctica identify new genera and new species within Macrobiotoidea and Echiniscoidea. Invertebrate Systematics 30:521–521. DOI: 10.1071/IS15033_CO.
- Vecchi M, Tsvetkova A, Stec D, Ferrari C, Calhim S, Tumanov D. 2023. Expanding Acutuncus: Phylogenetics and morphological analyses reveal a considerably wider distribution for this tardigrade genus. Molecular Phylogenetics and Evolution 180:107707. DOI: 10.1016/j.ympev.2023.107707.
- Wełnicz W, Grohme MA, Kaczmarek Ł, Schill RO, Frohme M. 2011. ITS–2 and 18S rRNA data from Macrobiotus polonicus and Milnesium tardigradum (Eutardigrada, Tardigrada). Journal of Zoological Systematics & Evolutionary Research 49:34–39. DOI: 10.1111/j.1439-0469.2010.00595.x.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T, editors PCR protocols: A guide to methods and applications. Orlando: Academic. pp. 315–322.
- Zawierucha K, Kolicka M, Kaczmarek Ł. 2016. Re-description of the Arctic tardigrade Tenuibiotus voronkovi (Tumanov, 2007) (Eutardigrada; Macrobiotidea), with the first molecular data for the genus. Zootaxa 4196:zootaxa–4196. DOI: 10.11646/zootaxa.4196.4.2.
