Abstract
Nest reuse is often observed in large- and medium-sized birds, including hole-nesters, while in smaller passerines building open-cup nests it is rarely recorded. In this note we report a case of multiple nest reuse, observed in a residential garden in Poland. The nest was located on the wall of a farm building partly overgrown by Boston Ivy at the height of 2.3 m. The nest was occupied eight times between 2007 and 2023 by three bird species: Grey and Pied Wagtails and Common Blackbirds. The nest was built by Grey Wagtails in 2007, but in 2009 it was reconstructed considerably by blackbirds. The Blackbird cup with mud lining survived until 2023 and contained all other nests. We suggest that the most plausible reason for multiple nest reuse was the high quality of this nest site. However, we also found that the nest was used during warm, dry springs but avoided when weather conditions at the beginning of the breeding season were adverse. We hypothesize that cold and wet springs – thought to be associated with high costs of reproduction – may have influenced birds’ decision not to reuse the nest. To our knowledge, this is the longest case of nest reuse reported for passerines building open-cup nests.
Introduction
Nest building is an essential activity for successful reproduction in most birds. However, nest building is also energetically costly (Mainwaring & Hartley Citation2013). The costs include collection and transportation of nest materials (Withers Citation1977; Collias Citation1986), but also a delayed start of egg-laying (Barclay Citation1988; Cavitt et al. Citation1999; Hauber Citation2002; Antonov & Atanasova Citation2003; Safran Citation2006), reduced clutch size (Weeks Citation1978, but see Conrad & Robertson Citation1993), and lower provisioning rates (Moreno et al. Citation2010), indirectly affecting seasonal reproductive success (Hauber Citation2002; Safran Citation2006). Additional costs are also associated with the risk of nest predation during trips to collect nest material (Lima & Dill Citation1990). Because nest building is energetically costly and time-consuming, it is not surprising that some birds reuse old nests (Hansell Citation2000). This primarily refers to medium- to large-sized birds, like raptors, crows or storks, whose nests are big and their construction takes many days (Burnham et al. Citation2009; Jiménez-Franco et al. Citation2014). Likewise, secondary cavity-nesting species often reuse nests because available nest-sites are limited (Brawn & Balda Citation1988). In populations suffering from high brood parasitism, nest reuse may minimize the attention of brood parasites (Mérő et al. Citation2022).
Most birds, however, build a new nest for each breeding attempt, and nest reuse is a relatively rare phenomenon, especially amongst passerines (Hansell Citation2000; Winkler Citation2004). This suggests that there are some costs associated with nest reuse. They may include increased risk of infections of overwintering parasites, which may be especially important in colonial species (Hoogland & Sherman Citation1976; Mazgajski Citation2007). Nest reuse may also increase nest predation rates as some predators remember the nest location (Sonerud Citation1985; Sonerud & Fjeld Citation1987; Otterbeck et al. Citation2019). Contents of old nests may also produce odors that probably make old nests more vulnerable to predators than new ones (Winkler Citation2004). Furthermore, reused nests may be of poor quality, being unable to hold the nest content (Mazgajski Citation2007) or requiring higher incubation costs (Moreno et al. Citation2010).
Among species that facultatively reuse old nests, some reuse them in a new breeding season (between-season nest reuse; Cavitt et al. Citation1999; Hauber Citation2002; Antonov & Atanasova Citation2003; Koenig et al. Citation2021), while others exclusively within the same breeding season (within-season nest reuse; Antonov & Atanasova Citation2003; Hałupka & Klimczuk Citation2011; Zieliński Citation2012; Chmielewski Citation2019; Mérő et al. Citation2022). Because nests of open-cup nesting passerines are usually fragile, as a rule these birds reuse their nests only during one breeding season (Hansell Citation2000). Furthermore, they usually reuse the same nest at most twice, and cases of using the same nest three or more times are extremely rare (Mérő et al. Citation2022). Reused nests are usually re-occupied by the same pair (Wysocki Citation2004; Hałupka & Klimczuk Citation2011; Mérő et al. Citation2022) within the same season, or by the same species between seasons (Barclay Citation1988; Cavitt et al. Citation1999; Hauber Citation2002; Antonov & Atanasova Citation2003; Koenig et al. Citation2021). Reports of nest reuse by a different species are relatively rare (Bergin Citation1997; Wysocki Citation2004; Kwiecinski et al. Citation2024).
In this note we report one case of multiple nest reuse, involving three bird species, observed between 2007 and 2023.
Methods
Observations were carried out in a residential garden in the village of Sieniawka (50º46’38”N; 16º46'106''E; elevation 213 m) in SW Poland. The shape of the garden is rectangular (Supplementary material). There are two buildings in the garden, but most of its area of 0.22 ha is covered by vegetation: trees, bushes, lawns and many flowering plants. The garden is surrounded by other gardens, and a stream is running along its northern border. The owner of this garden (AW) has been attempting to locate all nests built in the garden and monitor them since 1990 (Wuczyński Citation2021; Wuczyński et al. Citation2021). Nest records regarding the breeding attempts presented in this note are archived in the Polish Nest Record Scheme (http://www.kgil.uwr.edu.pl/). The birds using the described nest were not banded.
The reused nest was located on a farm building partly overgrown by Boston Ivy Parthenocissus tricuspidata. It was situated on a wall of the building, facing east, at the height of 2.26 m from the ground (). The nest was supported from the bottom by a thick electrical cable running horizontally along the wall of the building, by strip of wood from the outside, and by ivy shoots ()). The nest site was protected by the roof of the house.
Figure 1. Overview of the location of the focal nest within the garden (a), and a close-up of the nest in 2015, 2017, 2020, and 2023 (b-e). Note the slight changes in the nest construction over the years. The 2023 photograph (e) shows the condition of the nest when it was 16 years old; two months after much of it fell down (see text). Arrow in section a shows the exact location of the nest on the wall of the farm building. For detailed location of the nest and the garden, the reader is referred to the Google earth file in the supplementary material (photos by Andrzej Wuczyński).

To find out if weather conditions might have influenced bird decisions whether to use the nest or not, we compared mean monthly temperatures (oC) and total monthly precipitation (mm) in April in years when the focal nest was (n = 8) and was not occupied (n = 9). Weather data were obtained from the regional meteorological station in Wrocław, about 40 km away. We used the weather data for April because all the birds started using (reconstructing) the nest already in April, even though some of them started egg-laying in May (see below for details).
Results
The nest was used eight times during 17 years (47.06%) of its observation (). It was occupied by three different bird species: once by the Common Blackbird Turdus merula (5.88% of all observation years), three times by the Grey Wagtail Motacilla cinerea (17.65%) and four times by the Pied Wagtail Motacilla alba (23.53%).
Figure 2. The years of nest occupation and details of the broods raised in the nest reused by three bird species marked with different colors, in the village of Sieniawka (Poland).
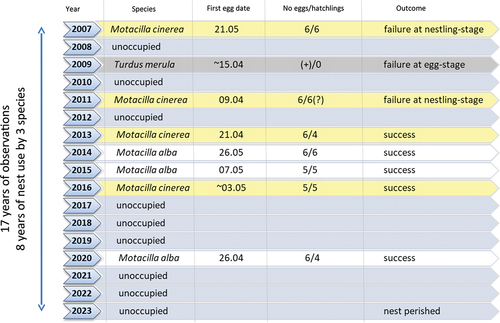
The nest was built in May 2007 by Grey Wagtails, but two years later it was reused by a pair of Common Blackbirds. Blackbirds reconstructed the nest considerably in early April 2009. Between 2011 and 2020 the nest was re-occupied six times by Pied and Grey Wagtails (details in ). The nest was somewhat modified (new nest material, including lining, was added) each time when it was occupied.
In all years only one clutch was laid in the nest. Most clutches (5 of 8) were successful. Interestingly, failures were recorded in the first three breeding attempts, while all later broods were successful. The eggs or nestlings disappeared simultaneously, suggesting a single episodes of predation. In 2011, one dead nestling of Grey Wagtail was found under the nest, with head injuries, whereas the other nestlings disappeared. In case of unsuccessful breeding attempts, the replacement clutches were laid nearby. On 7 August 2023 a part of the nest, including much of the mud lining, fell down after an intense rainfall.
The analysis of weather conditions in April revealed that in years when the nest was used, compared to years when it was not occupied, ambient temperatures were higher (monthly median 10.8°C vs 8.7°C, respectively), while precipitation lower (monthly medians equalled 20.5 vs 38 mm, respectively). The differences in precipitation between two categories of years (with the nest used/not used) were statistically significant (Mann-Whitney U-test: U = 12, p = 0.021, n1 = 8, n2 = 9), while differences in ambient temperatures were marginally significant (U = 16, p = 0.056).
Discussion
The nest was observed for 17 consecutive breeding seasons, and used eight times by three bird species. Such a long use of the same structure was probably due to the fact that the second nesting attempt was made by a pair of Common Blackbirds. Blackbird nests are solid cup-shaped structures made of grasses, leaves and other vegetation, bound together with mud (Cramp & Simmons Citation2004). The mud lining can survive for a long time. The blackbird nest formed a kind of “ledge”, and made this location much more attractive for Wagtails in the following years.
In our view, the most probable reason for multiple nest reuse was the high quality of this nest site. The nest was placed partly under the roof, protecting its content to some degree against precipitation and direct sunlight. The eastern exposure of the nest also protected it from the westerly winds that prevail in this area (Blazejczyk Citation2006). Its location on the wall probably made this nest-site difficult to reach for many predators. The presence of a stream, located fewer than 10 m from the nest, was certainly important for both species of Wagtails that usually breed close to watercourses (Cramp & Simmons Citation2004). The two Wagtail species often occupy man-made structures, and build their nests in various holes and on “ledges” (Cramp & Simmons Citation2004; Rodriguez & Rodriguez Citation2007). The cable running along the wall, together with some ivy shoots, formed a kind of platform suitable for nesting. Another possible reason for nest reuse may be the reproductive outcome in the preceding season. The breeding success in 2013–2016 could have contributed to the annual occupation of the nest in these years, not interrupted by years without breeding, as in earlier years. It has been hypothesized that the reuse of a nesting site may be associated with previous breeding success at the same site, although unambiguous confirmation of this relationship would require individually marked parental birds. This hypothesis is supported by some studies (Blancher & Robertson Citation1985; Styrsky Citation2005) but not others (Antonov & Atanasova Citation2003; Aguilar & Marini Citation2007). Furthermore, multiple reuse of the nest may be associated with saving energy, which is required during the nest-building process (Bergin Citation1997; Amat et al. Citation1999; Antonov & Atanasova Citation2003; Styrsky Citation2005).
The analysis of meteorological conditions in April, the month when birds started exploration of the nest site and took decision of its occupation, suggests that weather conditions might have affected bird decisions about reuse of the nest. We found that Aprils in the years when the nest was used were warmer and drier compared to years when the nest was not occupied. This suggests that birds avoided reusing the nest in adverse weather conditions. Although the roof of the building prevented rain from falling directly into the nest cup, precipitation did affect the nest condition. For example, in August 2023 the nest became soaked with water after an intense rainfall, which resulted in its damage and collapse. Furthermore, nests placed high above the ground are exposed to winds, which may significantly increase the rate of heat loss for reproducing birds (Eggers et al. Citation2006; Heenan & Seymour Citation2012). Therefore incubation costs in such nests may be considerably higher in colder breeding seasons compared to warmer ones. Overall, our analysis suggests that birds reused the nest in years with favourable weather conditions when incubation costs were probably relatively low, but avoided the site when weather at the beginning of the breeding season was adverse. Nest reuse may have been further encouraged by the breeding success in the previous season, as indeed nest losses were relatively low (37.5%) compared to other passerines building open-cup nests (Remeš et al. Citation2012). Therefore it is possible that the use of the focal nest as a nesting site in a given year was associated with a trade-off between the nest-site safety and its exposure to adverse weather conditions. Similar trade-offs have been described during the process of nest-site selection in a few bird species (Forstmeier & Weiss Citation2004; Eggers et al. Citation2006), but so far have not been discussed in the context of nest reuse.
In open-cup passerines nests are reused usually by the same species (Barclay Citation1988; Cavitt et al. Citation1999; Antonov & Atanasova Citation2003; Hałupka & Klimczuk Citation2011; Mérő et al. Citation2022) and only rarely by other species (Bergin Citation1997; Chmielewski Citation2019). It should be noted that various anecdotal reports of interspecific nest reuse may suggest that this phenomenon may only be rarely reported, but, in fact, it may be more common in nature. For example, in the same garden, breeding attempts of the House Sparrows Passer domesticus and the blackbirds were observed in nests built on last year’s nests of the Collared Dove Streptopelia decaocto, and a breeding attempt of the Black Redstart Phoenicurus ochruros was observed in a nest built on last year’s nest of the Barn Swallow Hirundo rustica (A. Wuczyński, unpublished obs.). Each time, however, it involved building of a new nest, based only on the nest of the previous species. Therefore, this does not fully correspond to the situation described in this report. The rarity of interspecific nest reuse in open-cup passerines is also confirmed by systematic studies of marked populations covering hundreds of broods. For example, Wysocki (Citation2004) found in Common Blackbirds that reused nests were usually occupied by the same breeding pair (91.4%), and much more rarely by other Blackbird pairs (5.7%), but only exceptionally by the Fieldfare Turdus pilaris (one case, 2.9%). For all of these reasons, we believe that multiple nest reuse by as many as three species, as we report here, indeed represents a rare case of nest reuse.
In summary, to our knowledge, the described case represents the longest reuse of the same nest among passerines building open-cup nests. Furthermore, the use of the same nest-cup by three different passerine species represents an unusual case. In addition, we have been able to show that nest reuse may be affected by weather conditions at the beginning of the breeding season. This factor has been neglected so far in the context of nest reuse. We hypothesize that the solid nest-cup constructed by the Blackbirds at the start of the observation period provided a durable platform that could be reused for many years, whereas the high quality of this nest-site made it attractive to three different bird species.
Supplemental Material
Download (2.9 KB)Acknowledgments
We would like to thank the three anonymous Reviewers for giving valuable comments which improved the manuscript. This study was supported by the Institute of Nature Conservation, Polish Academy of Sciences as a statutory activity, and also by the University of Wrocław, Faculty of Biological Sciences.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
A Google Earth file with location of the nest and the garden is available as part of the online article at: https://doi.org/10.1080/24750263.2024.2323570. File can be accessed using the application “Google Earth Pro”.
Additional information
Funding
References
- Aguilar TM, Marini MA. 2007. Nest and nest-site reuse within and between breeding seasons by three neotropical flycatchers (Tyrannidae). Brazilian Journal of Biology 67(3):537–540. DOI: 10.1590/S1519-69842007000300020.
- Amat JA, Fraga RM, Arroyo GM. 1999. Reuse of nesting scrapes by Kentish Plovers. The Condor 101(1):157–159. DOI: 10.2307/1370457.
- Antonov A, Atanasova D. 2003. Re-use of old nests versus the construction of new ones in the Magpie Pica pica in the city of Sofia (Bulgaria). Acta Ornithologica 38(1):1–4. DOI: 10.3161/068.038.0104.
- Barclay RMR. 1988. Variation in the costs, benefits, and frequency of nest reuse by Barn Swallows (Hirundo rustica). The Auk 105(1):53–60. DOI: 10.1093/auk/105.1.53.
- Bergin TM. 1997. Nest reuse by Western Kingbirds. Wilson Bulletin 109:737–741.
- Blancher PJ, Robertson RJ. 1985. Site consistency in kingbird breeding performance: Implications for site fidelity. Journal of Animal Ecology 53(3):1017–1027. DOI: 10.2307/4394.
- Blazejczyk K. 2006. Climate and bioclimate of Poland. In: Degórski M, editor. Natural and human environment of Poland. A geographical overview. Warszawa: Polish Academy of Sciences and Polish Geographical Society. pp. 31–48.
- Brawn JD, Balda RP. 1988. Population biology of cavity nesters in Northern Arizona: Do nest sites limit breeding densities? The Condor 90(1):61–71. DOI: 10.2307/1368434.
- Burnham KK, Burnham WA, Newton I. 2009. Gyrfalcon Falco rusticolus post-glacial colonization and extreme long-term use of nest-sites in Greenland. Ibis 151(3):514–522. DOI: 10.1111/j.1474-919X.2009.00939.x.
- Cavitt JF, Pearse AT, Miller TA. 1999. Brown Thrasher nest reuse: A time saving resource, protection from search-strategy predators, or cues for nest-site selection? The Condor 101(4):859–862. DOI: 10.2307/1370076.
- Chmielewski S. 2019. A case of interspecific nest use in thrushes (Turdidae). The Wilson Journal of Ornithology 131(2):419–422. DOI: 10.1676/18-177.
- Collias EN. 1986. Engineering aspects of nest building by birds. Endeavour 10:9–17. DOI: 10.1016/0160-9327(86)90044-X.
- Conrad KF, Robertson RJ. 1993. Clutch size in eastern phoebes (Sayornis phoebe). I. The cost of nest building. Canadian Journal of Zoology 71(5):1003–1007. DOI: 10.1139/z93-133.
- Cramp S, Simmons KEL. 2004. BWPi: Birds of the Western Palearctic interactive (DVD-ROM). Sheffield: BirdGuides Ltd.
- Eggers S, Griesser M, Hystrand M, Ekman J. 2006. Predation risk induces changes in nest-site selection and clutch size in the Siberian jay. Proceedings of the Royal Society B: Biological Sciences 273(1587):701–706. DOI: 10.1098/rspb.2005.3373.
- Forstmeier W, Weiss I. 2004. Adaptive plasticity in nest-site selection in response to changing predation risk. Oikos 104(3):487–499. DOI: 10.1111/j.0030-1299.1999.12698.x.
- Hałupka L, Klimczuk E. 2011. Nest reuse in the Eurasian Reed Warbler Acrocephalus scirpaceus. Ornis Polonica 52:69–71.
- Hansell MH. 2000. Bird nests and construction behaviour. Cambridge, U.K: Cambridge University Press.
- Hauber ME. 2002. Is reduced clutch size a cost of parental care in Eastern Phoebes (Sayornis phoebe)? Behavioral Ecology and Sociobiology 51(6):503–509. DOI: 10.1007/s00265-001-0450-2.
- Heenan CB, Seymour RS. 2012. The effect of wind on the rate of heat loss from avian cup–shaped nests. Public Library of Science ONE 7(2):e32252. DOI: 10.1371/journal.pone.0032252.
- Hoogland JL, Sherman PW. 1976. Advantages and disadvantages of Bank Swallow (Riparia riparia) coloniality. Ecological Monographs 46(1):33–58. DOI: 10.2307/1942393.
- Jiménez-Franco MV, Martínez JE, Calvo JF. 2014. Patterns of nest reuse in forest raptors and their effects on reproductive output. Journal of Zoology 292(1):64–70. DOI: 10.1111/jzo.12085.
- Koenig WD, Hallock EM, Weber DJ, Walters EL. 2021. Nest cavity reuse by the cooperatively breeding Acorn Woodpecker. Ornithology 138(2):ukaa088. DOI: 10.1093/ornithology/ukaa088.
- Kwiecinski Z, Podkowa P, Kosicki JZ. 2024. The reuse of Song Thrush (Turdus philomelos) nests by the Red-backed shrike (Lanius collurio) in an intensive agricultural landscape: A coincidence or a new solution? European Zoological Journal 91(1):75–80. DOI: 10.1080/24750263.2023.2294086.
- Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: A review and prospectus. Canadian Journal of Zoology 68(4):619–640. DOI: 10.1139/z90-092.
- Mainwaring MC, Hartley IR. 2013. The energetic costs of nest building in birds. Avian Biology Research 6(1):12–17. DOI: 10.3184/175815512X13528994072997.
- Mazgajski TD. 2007. Effect of old nest material on nest site selection and breeding parameters in secondary hole nesters — a review. Acta Ornithologica 42(1):1–14. DOI: 10.3161/068.042.0107.
- Mérő TO, Žuljević A, Kolykhanova O, Lengyel S. 2022. Reuse of nests in the Great Reed Warbler Acrocephalus arundinaceus: A behavior to save time and energy and to deter nest parasites? Ecology and Evolution 12(10):e9452. DOI: 10.1002/ece3.9452.
- Moreno J, Lobato E, González-Braojos S, Ruiz-de Castañeda R. 2010. Nest construction costs affect nestling growth: A field experiment in a cavity-nesting passerine. Acta Ornithologica 45(2):139–145. DOI: 10.3161/000164510X551291.
- Otterbeck A, Selås V, Tøttrup Nielsen J, Roualet E, Lindén A. 2019. The paradox of nest reuse: Early breeding benefits reproduction, but nest reuse increases nest predation risk. Oecologia 190:559–568. DOI: 10.1007/s00442-019-04436-7.
- Remeš V, Matysioková B, Cockburn A. 2012. Long-term and large-scale analyses of nest predation patterns in Australian songbirds and a global comparison of nest predation rates. Journal of Avian Biology 43(5):435–444. DOI: 10.1111/j.1600-048X.2012.05599.x.
- Rodriguez B, Rodriguez A. 2007. Breeding biology of Grey Wagtail Motacilla cinerea canariensis on Tenerife, Canary Islands. Acta Ornithologica 42(2):195–199. DOI: 10.3161/068.042.0203.
- Safran RJ. 2006. Nest-site selection in the barn swallow, Hirundo rustica: What predicts seasonal reproductive success? Canadian Journal of Zoology 84(11):1533–1539. DOI: 10.1139/z06-176.
- Sonerud GA. 1985. Nest hole shift in Tengmalm’s owl Aegolius funereus as defence against nest predation involving long-term memory in the predator. Journal of Animal Ecology 54(1):179–192. DOI: 10.2307/4629.
- Sonerud GA, Fjeld PE. 1987. Long-term memory in egg predators: An experiment with a Hooded Crow. Ornis Scandinavica 18(4):323–325. DOI: 10.2307/3676904.
- Styrsky JN. 2005. Influence of predation on nest-site reuse by an open-cup nesting neotropical passerine. The Condor 107(1):133–137. DOI: 10.1093/condor/107.1.133.
- Weeks HP. 1978. Clutch size variation in the Eastern Phoebe in Southern Indiana. The Auk 95:656–666.
- Winkler DW. 2004. Nests, eggs, and young: Breeding biology of birds. In: Podulka S., Rohrbaugh, RW Jr., Bonney, editors. Handbook of bird biology. New York: Cornell Lab of Ornithology. pp.8.1–.8.152.
- Withers PC. 1977. Energetic aspects of reproduction by the cliff swallow. The Auk 94:718–725. DOI: 10.2307/4085268.
- Wuczyński A. 2021. Hail-induced nest mortality and possible fright molting of a passerine bird during the pre-incubation period. The Wilson Journal of Ornithology 132(2):476–481. DOI: 10.1676/1559-4491-132.2.476.
- Wuczyński A, Hałupka L, Maroń A. 2021. First records of conspecific brood parasitism in two species of small passerines: lesser whitethroat and common linnet. Polish Journal of Ecology 69(2):124–133. DOI: 10.3161/15052249PJE2021.69.2.005.
- Wysocki D. 2004. Nest re-use by Blackbirds — the way for safe breeding? Acta Ornithologica 39(2):164–168. DOI: 10.3161/068.039.0202.
- Zieliński J. 2012. Nest reuse by Eurasian Blackcap Sylvia atricapilla. Ardea 100(1):98–100. DOI: 10.5253/078.100.0115.
