Abstract
Plutonium zwierleini is a large plutoniumid centipede of great evolutionary interest, occurring with isolated populations along the western Mediterranean area, from Spain to Italy. Due to its rarity and the extreme paucity of available records, P. zwierleini is among the least known Mediterranean chilopods, and scarce information is currently available on its ecology and natural history. Based on an extensive sampling effort carried out in Sicily between 2022 and 2023, we here provide additional occurrence localities for the species across Sicily, and new insights into its ecology. Overall, 29 novel Sicilian records of P. zwierleini, scattered across 21 localities, were collected thus increasing its known Sicilian distribution area by 117%, and the number of localities by 110%. The species was found in a wide range of habitats such as open areas, woods, buildings, and caves, characterizing Plutonium zwierleini as a habitat generalist, whose fine ecological preferenda need to be further explored. Moreover, to explore the diet and behaviour of the species, some specimens were kept in captivity. The captive individuals fed mostly on dead or poorly mobile soft-bodied prey and inert food, without ever displaying predatory behaviour; this suggests that, contrarily to what is currently assumed, P. zwierleini might be a scavenger rather than a predator. The potential distribution of Plutonium zwierleini in Sicily was inferred based on georeferenced occurrence records and climatic variables. The implemented MaxEnt model forecasts the possible occurrence of P. zwierleini on the whole island, with the single exception of its south-easternmost part, possibly due to the local pattern of precipitation seasonality. We hope that the present work might pave the way for further surveys aimed at a better understanding of the ecology of Plutonium zwierleini and the collection of new data in the other regions inhabited by this secretive species.
Introduction
Rarity is a recurring attribute among species (Lennon et al. Citation2004; McCreadie & Adler Citation2008 and references therein), however its meaning can take different forms. The rarity of a species is often defined based on various conditions, not always simultaneously present (Rabinowitz Citation1981; Longton & Hedderson Citation2000). Collecting and analysing ecological (Sazima & Carvalho-Filho Citation2003; Faraone et al. Citation2021), distributional (McCreadie & Adler Citation2008; Vecchioni et al. Citation2022) and phylogenetic (Cai et al. Citation2007; Faraone et al. Citation2020) data on rare or elusive species usually require strong efforts, nevertheless it is a great opportunity to delve into various aspects of their natural history and provide a sound basis for conservation and management measures.
Within the chilopod order Scolopendromorpha, the relatively poorly known family Plutoniumidae currently includes two genera: the genus Theatops Newport, 1844, with six extant and one extinct species with highly disjointed distribution ranges scattered throughout the Holarctic (Shelley Citation1997; Di et al. Citation2010; Edgecombe et al. Citation2023), and the genus Plutonium Cavanna, Citation1881, with the only species Plutonium zwierleini Cavanna, Citation1881. Within the Plutoniumidae family, P. zwierleini has a highly distinctive feature: spiracles are present in every body segment from the second to the penultimate one, in contrast to the alternate segment distribution observed in all the other Scolopendromorpha species (Schileyko & Pavlinov Citation1997; Shelley Citation1997; Fusco Citation2005; Bonato et al. Citation2017). This feature, which can be observed only in P. zwierleini and in the distantly related Geophilomorpha within the Chilopoda, was previously considered a plesiomorphy of the genus, but it is now rather considered an autoapomorphic character of the species (Shelley Citation1997; Edgecombe et al. Citation2023). The species is heavily armed, equipped both with the strong and venomous forcipules typical of Chilopoda, and with blade-edged claws on its terminal non-ambulatory enlarged legs. Its peculiar morphology (i.e., blindness, shape of posterior legs, and tracheal system) seems to be adaptive for inhabiting deep fissures (Manton Citation1965). Such an endogeic habit could at least partly explain the paucity of currently available records for the species, which should thus be ascribable to its low detectability. In fact, P. zwierleini, despite being one of the largest and most striking European chilopods, and often considered potentially harmful to humans, is among the least known and most enigmatic Mediterranean centipede species, and it is an extremely rare taxon both due to the extreme paucity of available observations and its restricted and fragmented distribution (Bonato et al. Citation2017).
Plutonium zwierleini is to date known from relatively few and scattered observations from Italy and Spain: in the time frame comprised between its description (Cavanna Citation1881) and 2015, only 25 records had been collected, of which about 44% from the last two decades of the nineteenth century (Shelley Citation1997; Bonato et al. Citation2017). Later on, Bonato et al. (Citation2017) updated the knowledge and distribution of the species by adding 19 additional records, obtained through an accurate census in the museum collections and by Citizen Science approach. The data reported by Bonato et al. (Citation2017) allowed to identify four different distribution clusters: 1, Sicily (13 records, 10 localities) 2, Southern Italy, Tyrrhenian coasts, mostly on the Sorrento Peninsula (11 records, nine localities) 3, Sardinia (15 records, 14 localities) 4, Southern Iberian Peninsula, Peribaetic system (two records, two localities).
Due to the scarce and fragmentary available information, it is currently not possible to characterize the ecology and habitat preferences of Plutonium zwierleini, which has been found in both hypogean and epigean biotopes within variable contexts such as woods, cultivated fields, pastures, caves, urban settlements and inside buildings between sea level and 1200 m a.s.l (Bonato et al. Citation2017). Similarly, very little is known of other aspects of its natural history, such as its feeding behaviour. Scolopendromorpha are mostly active opportunistic foragers, which feed largely on other invertebrate and vertebrate animals (Guizze et al. Citation2016 and references therein) and, occasionally, on carrion and vegetal matter (Heymons Citation1901; Guizze et al. Citation2016). A single observation of predation in the wild is known for P. zwierleini and was documented in Sardinia, where a large adult individual was observed preying on a young cave salamander, Speleomantes supramontis (Lanza et al., 1986) (Sanna et al. Citation2018): similarly to documented cases in captive-reared Theatops sp. (UnicoCelula Citation2012; Furball677 Citation2016a, Citation2016b), the centipede used its terminal legs as functional forcipules to pierce the prey (Manton Citation1965; Shelley Citation1997; Lewis Citation2010).
The phylogenetic relationship between Plutonium zwierleini and other species within the Plutoniumidae family is not fully resolved yet. The few available morphological and molecular analyses of Plutoniumidae mostly retrieve Theatops as a paraphyletic group with the monotypic genus Plutonium nested within it (see Bonato et al. Citation2017; Edgecombe et al. Citation2023, and references therein) so that a taxonomical revision of the family is pending and a more comprehensive taxonomical and geographical coverage of the genetic diversity of plutoniumid species is often advocated (Bonato et al. Citation2017; Edgecombe et al. Citation2023). Moreover, since sequence data are currently available for a single P. zwierleini specimen (Bonato et al. Citation2017), no information is to date available on the intraspecific genetic diversity and the phylogeography of the species.
Given the high evolutionary interest of the species within the Chilopoda and of the scarce information currently available on its distribution, ecology, we aimed to address these main goals: (1) providing additional records of P. zwierleini across Sicily, the region where its type locality occurs, (2) predicting its distribution across geographic space using bioclimatic environmental data, and (3) offering some insights into the natural history of this secretive taxon, including habitat preferences and feeding behaviour.
Materials and methods
Sampling and distribution data
Sampling was conducted in Sicily in some of the localities where Plutonium zwierleini was reported to occur following Bonato et al. (Citation2017), and in new randomly selected sites. The selection was arbitrary, given that the habitat preferences of P. zwierleini have not to date been precisely delineated, with the species being observed in a diverse array of habitats, including both hypogeal and epigeal environments (Bonato et al. Citation2017). Throughout the fieldwork period spanning from December 2022 to June 2023, a total of 61 sampling sessions were conducted across 53 distinct locations (Figure S1). These sites were broadly categorized into open habitats, including pastures, garrigues, and grasslands (71.2%), as well as natural and artificial woods (28.8%). The average altitude of the sampled localities was 529.4 ± 414.9 (20–1570) m a.s.l. [mean ± SD (range)]. Latitude and longitude for each locality were determined with a geographical positioning system (GPS) receiver. In addition, at each sampled site we recorded local environmental characteristics and identified the occurring Scolopendromorpha taxa at the finer possible taxonomic level through visual inspection. Maps showing the sampling sites and P. zwierleini distribution were produced using QGIS software v. 3.30.2 (QGIS Development Team Citation2016, http://www.qgis.org).
Field sampling involved daytime active searches, by examining potential shelters under natural (e.g., rock crevices, logs, and vegetal detritus) and artificial structures (e.g., bituminous sheets and wooden boards). At each site, 1–4 researchers conducted surveys, dedicating 10 minutes to 3 hours, depending on the extension of the area and shelter density. At sites where Plutonium zwierleini was found, the number of findings/hours per researcher was calculated as a detection rate.
The body length of each collected specimen was measured with a digital calliper with a 0.05 mm precision from the anterior margin of the head to the tip of the ultimate tergite.
Five P. zwierleini voucher specimens were deposited in the collection of the Zoology Section “La Specola”, Natural History Museum, University of Florence (Italy) with the collection numbers (MZUF): 379–383 (). The other specimens are currently stored at the Dept. STEBICEF of the University of Palermo, under the responsibility of the author FPF.
Table I. Novel records of Plutonium zwierleini in Sicily. The acronyms reported in the reference column refer to author names. The detection rate, DR, was calculated as the number of findings/hours per researcher.
In addition to field surveys, we employed Citizen Science (see also Faraone et al. Citation2017; Haklay et al. Citation2021), leveraging collaborative contributions from users in a zoology-oriented Facebook group “Fauna Siciliana” (https://www.facebook.com/groups/faunasiciliana) and records shared by colleagues. Contributors supplied location data, providing information on additional sites where Plutonium zwierleini occurs in Sicily (). For each observation recorded through social networks, we meticulously checked the original files and pictures, the species identification, and the location provided by the users themselves. Therefore, new records were validated only when documented by voucher specimens and/or photographs taken in the field showing diagnostic characters of P. zwierleini. Only validated reports are here reported.
In order to estimate the extent of the distribution of Plutonium zwierleini in Sicily we used the Minimum Convex Polygon (MCP) method, as implemented in the QGIS software.
Species distribution model and spatial analysis
To predict the distribution of Plutonium zwierleini across Sicily using environmental data, we employed correlative species distribution models (SDMs). For this elusive species, we used georeferenced data for 19 out of the 21 localities listed in , since only toponyms without reference to coordinates are known in the literature (see Bonato et al. Citation2017 and references therein). Among all available methods (Valavi et al. Citation2021), we utilized the Maximum Entropy Modeling (Maxent) algorithm, chosen for its demonstrated suitability as the minimum performance threshold is achieved with as few as 15 observations (Støa et al. Citation2019). We applied the “max_spec_sens” threshold (Hijmans Citation2023), representing the point at which the combined sensitivity (true positive rate) and specificity (true negative rate) are maximized. Bioclimatic data was collected from the WorldClim database, encompassing all the available 19 variables (Fick & Hijmans Citation2017).
Although subterranean habitats differ from aboveground areas, previous research has shown that each of these variables can significantly influence belowground conditions (Mammola & Leroy Citation2018). We initially considered all the 19 variables, however, as many were highly correlated (Figure S2), a variance inflation analysis (VIF) (Marquardt Citation1970) was conducted using the “vif” function of the “usdm” package (Naimi Citation2017) to select a set of non-correlated variables. This process, ultimately, led us to analyse isothermality [ratio of the mean diurnal range (Bio 2) to the annual temperature range (Bio 7) multiplied by 100], temperature seasonality, mean temperature of driest quarter, precipitation seasonality, precipitation of warmest quarter, and precipitation of coldest quarter (Table S1). Seasonality variables provide a measure of change over the course of the year, with larger values indicating a greater variability in the selected variable. Pseudo-absence data were generated using 1000 random points across the region of interest. Then, we evaluated which climatic variables significantly affect the distribution of our studied species. Since we did not find spatial autocorrelation in our dataset (Moran’s test for distance-based autocorrelation p = 0.49), we performed a general linear model with “glm” setting observations of presence/absence as dependent (outcome) variable and climate variables as independent (fixed) effects. We selected “binomial” as family distribution and conducted model diagnostic in “DHARMa” (Hartig Citation2022). All analyses have been performed in R v. 4.1.3 (R Core Team Citation2022).
Behavioural observations
Three live Plutonium zwierleini specimens, two individuals (44- and 102-mm body length) from the locality #4 and one individual (53-mm body length) from the locality #18 (), were kept in captivity between February and August 2023 with the aim of observing their behaviour. The larger specimen was housed in a 30 cm long × 25 cm wide plastic box, whereas the smaller ones were housed individually in 20 × 15 cm similar enclosures. The lids of the boxes were finely perforated with 2–3 mm holes to allow air exchange. The bottom was filled with a layer of about 2 cm of loam, and a rock slab fragment was added as a shelter. Each box was kept at room temperature (18–28°C) at the Dipartimento di Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche of the University of Palermo, with a natural daylight cycle, and misted with water once or twice a week to avoid dehydration. Various types of food were supplied once per week overnight inside the enclosures for 12 hours and the feeding response of the centipedes was recorded. Several different prey items, consisting of both alive and freshly dead small soil invertebrates and inert food (meat and fruits), were proposed. Three different responses have been registered: 1) Rejection (R), when the food item was totally ignored or avoided and found intact and in the same position after 12 hours, 2) Tasting (T), when the food item was briefly touched with the maxillae and mouth parts, and then ignored and/or found poorly damaged after 12 hours, and 3) Consumption (C), when the food item was entirely or almost entirely eaten.
In addition, we kept note of the observations previously collected on a 91 mm body length Plutonium zwierleini specimen from Sicily kept in captivity in 2019 by the authors AB and GS.
Results
Sampling and distribution
We here provide 29 novel Sicilian records of Plutonium zwierleini spanning from 2002 to 2023 and scattered across 21 localities ().
Ten Plutonium zwierleini specimens were found in six out of the 52 sampled localities (11.5%, see and ). Five additional specimens were collected in 2022 through non-targeted hypogean sampling sessions using pitfall traps as detailed in Nicolosi et al. (Citation2023), placed from 1 to 26 m from cave entrances (#11, #13, #15, #16; see also ). Eight of the localities where we directly found P. zwierleini are new for the species (), whereas locality #10 is close (~1.4 km) to the “Ficuzza” village (Province of Palermo), already mentioned by Attems (Citation1930). Although they fall within the same municipality, localities #20 and #21 were considered as different sites since they are located 4.7 kilometres apart, characterized by different habitats, and separated by a large dam reservoir. In every location where a targeted sampling session revealed the presence of P. zwierleini, its coexistence with other Scolopendromorpha was observed. Scolopendra cingulata Latreille, 1829, S. oraniensis Lucas, 1846, and Cryptopidae were syntopically found with P. zwierleini in localities #19, #20, #21; S. cingulata and S. oraniensis in #4; S. cingulata and Cryptopidae in #18; S. cingulata in #17. Furthermore, the presence of S. cingulata, S. oraniensis, and Cryptopidae was also verified in the non-targeted site #15.
Figure 1. Occurrence localities of Plutonium zwierleini in Sicily. Black circles indicate novel sites where the centipede was directly observed by the authors; Black triangles report data from citizen science and personal communications; White diamonds represent bibliographic records of the species obtained from Bonato et al. (Citation2017). See for more information on the novel occurrence localities of the species.
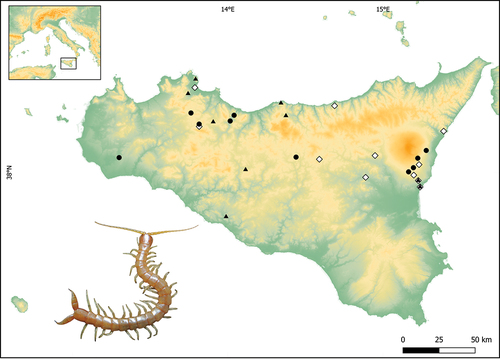
Two published Plutonium zwierleini localities, “Monte Gorna” and “Monte Pagano” (see Bonato et al. Citation2017), were surveyed without success. More than one observation was collected in localities #4 (three specimens during our 2023 fieldwork), #10 (two specimens in 2023) and #19 (two specimens in 2023, see and ). The detection rate of the species in the localities where it was found ranged from 0.17 to 0.50 records/hour per person (see for further details).
Figure 2. Four of the Plutonium zwierleini individuals collected in the field and photographed in a controlled environment. (a) 44 mm total length from Castelvetrano (Trapani province, locality #4); (b) 63 mm total length from Piana degli Albanesi (Palermo province, locality #19); (c) 102 mm total length from Castelvetrano (Trapani province, locality #4); (d) 114 mm total length from Castelvetrano (Trapani province, locality #4).

Ten further observations of Plutonium zwierleini were collected through the Citizen Science approach (). Six of the localities were new, whereas #6 and #8 confirmed the occurrence of the species in “Gravina di Catania” and #1 and #“7 in “Catania”, i.e., two sites already mentioned by Bonato et al. (Citation2017).
These new records expand the extent of occurrence (based on the MCP method) of Plutonium zwierleini in Sicily by approximately 117%, from 5990 ca. square kilometres, calculated by merging all the bibliographic localities (Shelley Citation1997; Bonato et al. Citation2017 and references therein), to 12991 ca. square kilometres, roughly 51% of the island surface.
The novel Plutonium zwierleini localities range from about 30 (#7) to 1164 m a.s.l. (#15) [412 ± 294 (30–1164) m a.s.l.]. As in other cases described in literature (Bonato et al. Citation2017), the habitats exhibit quite a variability, and can be both epigeal and hypogean (see records #11, #13, #15, and #16, ). Nevertheless, four out of the six localities derived by targeted samplings shared similar features, which are open areas such as pastures and uncultivated land with scattered rocks (). All the Citizen Science observations originated from human settlements and highly anthropized areas. Five centipedes were in fact found inside houses in rural and urban context (). In particular, specimens from localities #2 and #12 emerged from the drain pipes of a sink and a shower indoor, respectively, while at locality #14 P. zwierleini was found in an outdoor utility sink in a backyard.
Figure 3. Four of the Plutonium zwierleini habitat in Sicily. (a) Near Ficuzza, Monreale (Palermo province, locality #18); (b) Piana degli Albanesi (Palermo province, locality #19); (c) Caccamo (Palermo province, locality #20); (d) Alimena (Palermo province, locality #17).

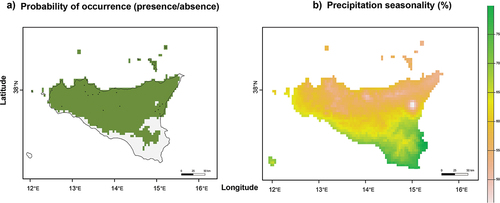
We found that the highest probability of occurrence of our studied species, inferred from our continuous predictions of habitat suitability (Figure S3) transformed into binary predictions, includes the whole surface of Sicily except a large section of its south-eastern part (), as also supported by presence data shown in . Of all the analysed climate variables, the general linear model pointed out that only precipitation seasonality plays a significant (p < 0.03) and negative (−0.32 ± 0.15 SE) role in predicting the occurrence of P. zwierleini () and shows the higher contribution value in the Maxent model (Table S2). As such, the likelihood of occurrence is greatest in areas where precipitation variations across the year are limited. Surprisingly, no temperature variables significantly affect the likelihood of occurrence.
Behavioural observations
The three captive Plutonium zwierleini individuals showed highly secretive habits. Most of the time was spent under the shelters. The activity took place almost exclusively at night and consisted of exploring the enclosures, feeding, and self-cleaning. Scarce and occasional superficial digging activity was observed. As observed in other Chilopoda (Lewis Citation1981), grooming behaviour is frequently performed by means of the maxillary complex, especially on the antennae and along the sides of the body trunk and each leg, lingering on each segment. It was also observed that all specimens drank from the water droplets on the sides of the enclosure after misting. Although our three captive P. zwierleini fed regularly, no increase in body length was detected in the two smallest individuals during the captivity period (respectively 140 and 199 days), while a growth of only 4 mm (3.9% of the whole length) was recorded in 199 days in the largest centipede. No moulting episodes or evidence were documented in a time interval of 199 days, likely due to the slow growth rate (see Brunhuber Citation1970) or, alternatively, to the consumption of the exuviae, a phenomenon already known for Chilopoda (Lewis Citation1981; McMonigle Citation2014). When alarmed, all specimens raised the last tergite upright and spread the terminal legs wide, displaying their pincer-like extremity. If stimulated, they did not hesitate to bite the forceps or the gloves, like other Scolopendromorpha (Lewis Citation1981), to pierce with the last two legs, and to emit an unpleasant smell, similar but less intense to that of the Julidae (Diplopoda).
Overall, 16 food types have been proposed (). No live items were accepted for all prey categories, except Formicidae larvae, which were readily accepted both alive and dead from all the Plutonium zwierleini specimens, and slugs, which were eaten sporadically (Table S3). Most preys were accepted only when dead, especially soft-bodied or poorly sclerotized invertebrates. Caterpillars (Lepidoptera) whereas woodlice (Oniscidae) were totally ignored by all centipedes. Among the inert foods, only the raw chicken meat was readily accepted and consumed by all specimens, while raw beef and fruits (banana) were only briefly tasted, with no evidence of actual feeding. A specimen from site #4 kept in captivity in 2019 also accepted live Lumbricidae (G. Signorello, unpublished data). Tactile contact with fast-moving prey (mainly arthropods) caused an immediate escape reaction in all individuals, even several hours after the introduction of live meals into the plastic boxes.
Figure 5. Foraging in captive Plutonium zwierleini. (a) Staphylinidae larvae; (b) Lumbricidae; (c) Scolopendra cingulata; (d) Banana; (e) Gryllidae; (f) Raw chicken meat.

Although observed in the field in a Sardinian population (Sanna et al. Citation2018), the use of the terminal legs during predation and feeding was never observed in our Plutonium zwierleini individuals, as well as in other captive Sicilian and peninsular Italian specimens (L. Cavigioli, J. Wojtkow, L. D’Aniello, pers. comm.).
Meal duration was highly variable, apparently based on food type and size. Some food items were tasted for a few seconds whereas, in other cases, very large meals (scarabaeid larvae, chicken meat) were consumed by the largest specimen, without interruption, up to 2 hours and 45 minutes. After each meal, the centipedes generally remained inactive under the shelter from three to six days.
Discussions
Distribution and ecology
The results from this study show that the distribution of Plutonium zwierleini in Sicily is significantly broader than previously documented. The new data increase the Sicilian extent of occurrence of the species. To date, there is still no past and present evidence of the presence of P. zwierleini from north-eastern and south-eastern Sicily (cf. Bonato et al. Citation2017), i.e., from the Peloritani mountains and Hyblean area; however, the latter area has only been marginally investigated in the frame of our fieldwork ( and and S1). Accordingly, a low probability of presence in south-eastern Sicily is also supported by the low values based on the maximum entropy of the climatic niche (). The species distribution modelling aligns with our novel observations and is consistent with records obtained from the literature spanning from 1881 to 2017 () (Bonato et al. Citation2017 and references therein).
Our results confirm that Plutonium zwierleini can be found in a wide range of biotopes, from natural sites, as oak woods, and caves, to extremely anthropized ones, such as buildings and urban areas. In anthropized areas, the repeated finding of this species inside or near drain pipes appears particularly interesting, but it is difficult to understand whether these circumstances are due to an effective use of hydraulic systems as biotopes or shelters, as it happens in other myriapods (Acosta Citation2003; Short & Huynh Citation2013), or if these animals simply get trapped there.
The syntopy of Plutonium zwierleini with other scolopendromorphs was observed in all sites, especially with medium to large-sized species (Scolopendra spp.), which were always abundant. This might indicate the absence of competitive exclusion between these large centipedes, perhaps in the context of particularly favourable biotopes for Scolopendromorpha.
With regard to bioclimatic variables, the wide extent of areas with high suitability values () indicates a wide spectrum of climatic conditions where the species could potentially be present (). Specifically, we found that the presence of Plutonium zwierleini seems to be limited by the seasonality of rainfall. These conditions could increase the risk of flooding, soaking or, conversely, excessive soil drying, serving as potential disruptive factors for chilopods (Uetz et al. Citation1979; Lewis Citation1981; Ivask et al. Citation2019; Menta & Remelli Citation2020).
The comprehensive data derived from our fieldwork corroborate that Plutonium zwierleini is a species characterized by a relatively low detectability, as it was identified in 11.5% of the sampled localities. This centipede shows a low detection rate when compared with the other large chilopod present in Sicily, the Mediterranean banded centipede, Scolopendra cingulata, which was recorded in 79.2% of the sampled areas. The outcome of the species distribution models, based on bioclimatic variables, does not align with the hypothesis of a patchy or localized distribution of P. zwierleini in the Sicilian territory (). Therefore, two alternative, not mutually exclusive, hypotheses emerge warranting future investigation with appropriate methodologies: (1) P. zwierleini exhibits low-density populations. Low local abundance is recognized as a factor that negatively influences the detectability of a species within a study area (Royle & Nichols Citation2003; Walsh et al. Citation2018). Indeed, low-density likely contributes to low detectability across various taxa, including predatory endogeic chilopods (Peretti et al. Citation2022); (2) P. zwierleini adopts predominately endogeic habits. Subterranean chilopods, as well as other cryptozoans (e.g., Kopecký & Tuf Citation2013), may have specific patterns of epigean activity, often constrained to limited time frames coinciding with periods of optimal external conditions (Lewis Citation1981; Tuf et al. Citation2006). Although, our sampling effort does not allow us to reveal a clear seasonal pattern of epigeal activity, we observed a reduced occurrence of the species during the warmest months, possibly due to increased aridity and temperature of the superficial soil layers (see Lewis Citation1981). Alternatively, they may surface in response to unpredictable events such as heavy rain and flooding, altering soil conditions (Lewis Citation1981). Therefore, it is possible that P. zwierleini primarily resides in deep soil layers thus rendering its occurrence under stones and in epigean habitats and subsequent detection rare or occasional.
Behaviour
A substantial part of the life history information on cryptozoic fauna is drawn from observations in captivity (Monge-Nájera et al. Citation1993; Punzo Citation2005; Fremlin & Taylor Citation2022). This reliance on captive observations stems from the elusive nature of cryptozoans and the inherent challenges associated with observing their behaviours in situ. This also extends to other Chilopoda taxa (Lewis Citation1981; McMonigle Citation2014; Guizze et al. Citation2016), where a significant portion of field records is based on opportunistic and isolated observations (Lewis Citation1981; Molinari et al. Citation2005; Noronha et al. Citation2015; Bonato et al. Citation2021).
Our data show distinctive feeding habits in captive Plutonium zwierleini individuals, setting them apart from other chilopod species examined under identical conditions. While the latter generally exhibit predatory behaviour using a combination of sit-and-wait and active foraging strategies (Lewis Citation1981; Lewis et al. Citation2010; McMonigle Citation2014; Guizze et al. Citation2016), our P. zwierleini specimens exhibited avoidance of live prey, except for soft-bodied and low-mobility preys such as ant larvae and slugs, and mostly consumed dead preys and inert food. The fast response observed in P. zwierleini upon detecting and consuming Formicidae larvae suggests that this resource might fall within its natural trophic spectrum, which aligns with expectations based on its relatively frequent occurrence under stones. Throughout the study period, sporadic instances of vegetable tasting, notably banana, were also observed (Table S3 and ), which is consistent with observations in several centipede species, where fruits and vegetables are briefly consumed, especially in prolonged absence of other resources (Lewis Citation1981; McMonigle Citation2014).
Although scavenging is widely reported in many chilopods (Wang Citation1945; Lewis Citation1981; Lewis et al. Citation2010), also under captivity condition (Lewis Citation1981; McMonigle Citation2014), it is generally considered as an optional habit that occurs in active opportunistic predators. Quite unexpectedly, our controlled environment observations suggest that Plutonium zwierleini predominantly exhibits a necrophagous-like behaviour. Conversely, the only feeding record of P. zwierleini in the field (Sanna et al. Citation2018) described a predatory behaviour on a living vertebrate, using terminal legs as forceps (as observed in Theatops spp., see above). This behaviour was never observed in our captive specimens, supporting what was previously suggested by Lewis (Citation2010) on the doubtful predatory functional use of the terminal legs.
However, despite what was inferred from our observations, we cannot rule out that the stress induced by the captivity conditions might have affected the feeding behaviour of our centipedes.
Conclusions
Despite its large size and evolutionary interest, Plutonium zwierleini is one of the least known taxa among the Mediterranean Scolopendromorpha. The present study allows to get a better insight on the distribution and ecology of the species, but also opens some challenging questions.
The dearth of information currently available about the natural history of this centipede is traditionally ascribed to its rarity and the consequent exceptional paucity of records throughout its wide west-Mediterranean distribution area, ranging from the southern Iberian Peninsula to peninsular Italy. Current study proves that, at least in Sicily, the species is less rare than expected and that dedicated surveys allow to collect valuable information and samples in a reasonable time span. Novel data collected in the frame of this survey increased its known geographic distribution range on the island by 117%, and the number of localities by 110% compared to those available in the literature (cf. Bonato et al. Citation2017 and references therein). Collected occurrence data confirmed that this centipede is a generalist species, whose fine ecological preferenda should, however, be explored with dedicated studies regarding microhabitat features. Furthermore, its frequent syntopy with other large centipede species suggests that exploring the ecological and behavioral aspects of multi-species Scolopendromorpha coexistence is a worthwhile area for further, in-depth investigations.
The diet composition of wild P. zwierleini inhabiting different habitats (e.g., caves vs. pasture lands) should be desirably investigated, possibly through NGS (cf. Bortolin et al. Citation2018; Bonato et al. Citation2021). Nevertheless, observations carried out on captive specimens allowed us to get some insights on the feeding behaviour of the species, suggesting that P. zwierleini might be a scavenger rather than a predatory species. This is somewhat at odds with the few observations available in nature for this species (Sanna et al. Citation2018) and those available in captivity for the closely related genus Theatops (UnicoCelula Citation2012; Furball677 Citation2016a, Citation2016b), although we cannot rule out an effect of the stress induced by their restrained conditions.
Eventually, molecular analyses including Plutonium zwierleini samples from the entire distribution range of the species are needed to shed light on the taxonomy of the Plutoniumidae, clarifying the relationships between the genera Plutonium and Theatops, and to account for the phylogeography of the species and the possible presence of cryptic species currently lumped under this binomen. We hope that the present work might pave the way for such studies and encourage the collection of new distributional and ecological data on Plutonium populations in the other regions inhabited by this charming and enigmatic species.
Supplemental Material
Download MS Word (1.8 MB)Acknowledgments
We are grateful to Doriana Calabretta, Katia Cespa, Simone Costa, Antonino Dentici, Paolo Galasso, Alessandro Marletta, Nicola Pavone, Michele Spoto, Giusi Surdo, Manfredi Paonita, and Roberto Viviano for kindly providing details of their field observations. We also thank Lucio Bonato for his precious advice, Luca Cavigioli, Luigi D’Aniello, and Jennifer Wojtkov for the valuable information on their captive specimens.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used for this work are available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2024.2324118
Additional information
Funding
References
- Acosta CA. 2003. The house centipede (Scutigera coleoptrata; Chilopoda): controversy and contradiction. Journal of the Kentucky Academy of Science 64(1):1–5.
- Attems C. 1930. Myriapoda. 2. Scolopendromorpha. Das Tierreich 54. Berlin: Walter de Gruyter & Co., Berlin-Leipzig. pp. 308.
- Bonato L, Orlando M, Zapparoli M, Fusco G, Bortolin F. 2017. New insights into Plutonium, one of the largest and least known European centipedes (Chilopoda): Distribution, evolution and morphology. Zoological Journal of the Linnean Society 180(4):887–909. DOI: 10.1093/zoolinnean/zlw026.
- Bonato L, Peretti E, Sandionigi A, Bortolin F. 2021. The diet of major predators of forest soils: A first analysis on syntopic species of Chilopoda through DNA metabarcoding. Soil Biology and Biochemistry 158:108264. DOI: 10.1016/j.soilbio.2021.108264.
- Bortolin F, Fusco G, Bonato L. 2018. Comparative analysis of diet in syntopic geophilomorph species (Chilopoda, Geophilomorpha) using a DNA-based approach. Soil Biology and Biochemistry 127:223–229. DOI: 10.1016/j.soilbio.2018.09.021.
- Brunhuber BS. 1970. Egg laying, maternal care and development of young in the scolopendromorph centipede, Cormocephalus anceps anceps Porat. Zoological Journal of the Linnean Society 49(3):225–234. DOI: 10.1111/j.1096-3642.1970.tb00738.x.
- Cai HX, Che J, Pang JF, Zhao EM, Zhang YP. 2007. Paraphyly of Chinese Amolops (Anura, Ranidae) and phylogenetic position of the rare Chinese frog, Amolops tormotus. Zootaxa 1531(1):49–55. DOI: 10.11646/zootaxa.1531.1.4.
- Cavanna G. 1881. Nuovo genere (Plutonium) e nuova specie (P. zwierleini) di Scolopendridi. Bullettino della Società Entomologica Italiana 13:169–178.
- Di Z, Cao Z, Wu Y, Yin S, Edgecombe GD, Li W. 2010. Discovery of the centipede family Plutoniumidae (Chilopoda) in Asia: A new species of Theatops from China, and the taxonomic value of spiracle distributions in Scolopendromorpha. Zootaxa 2667(1):51–63. DOI: 10.11646/zootaxa.2667.1.4.
- Edgecombe GD, Strange SE, Popovici G, West T, Vahtera V. 2023. An Eocene fossil plutoniumid centipede: A new species of Theatops from Baltic amber (Chilopoda: Scolopendromorpha). Journal of Systematic Palaeontology 21:1, 2228796. DOI: 10.1080/14772019.2023.2228796.
- Faraone FP, Giacalone G, Canale DE, D’Angelo S, Favaccio G, Garozzo V, Giancontieri GL, Isgrò C, Melfi R, Morello B, Navarria F, Russo G, Tinnirello V, Torre A, Torre D, Torre G, Urso G, Vinci P, Zizzo MG, Marrone F. 2017. Tracking the invasion of the red swamp crayfish Procambarus clarkii (Girard, 1852) (Decapoda Cambaridae) in Sicily: A “Citizen science” approach. Biogeographia - The Journal of Integrative Biogeography 32(1):25–29. DOI: 10.21426/B632135512.
- Faraone FP, Melfi R, Di Nicola MR, Giacalone G, Lo Valvo M. 2020. The genetic identity of the only Italian population of the genus Macroprotodon Guichenot, 1850 on the island of Lampedusa, Sicily. Vertebrate Zoology 70(2):235–240. DOI: 10.26049/VZ70-2-2020-09.
- Faraone FP, Russotto S, Giacalone G, Lo Valvo M, Belardi I, Mori E. 2021. Food habits of the Javelin sand boa Eryx jaculus (Linnaeus, 1758; Serpentes, Erycidae) in Sicily, Italy. Journal of Herpetology 55(4):452–458. DOI: 10.1670/20-047.
- Fick SE, Hijmans RJ. 2017. WorldClim 2: New 1 km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37(12):4302–4315. DOI: 10.1002/joc.5086.
- Fremlin M, Taylor S. 2022. Further observations on the carnivorous slug Testacella haliotidea Draparnaud, 1801. Essex Naturalist (New Series) 39:66–73.
- Furball677. 2016a. Centipede Eats Other Centipede 2. Available: https://www.youtube.com/watch?v=DOqHFyXwnRc&pp=ygUIVGhlYXRvcHM%3D. Accessed Nov 2023.
- Furball677. 2016b. Forceps centipede kills red centipede. Available: https://www.youtube.com/watch?v=-hwNkRwrFio&list=PL2pa26E5Bn1eifTLZJx9dYmDpHvMYXdlM&index=5. Accessed Nov 2023.
- Fusco G. 2005. Trunk segment numbers and sequential segmentation in myriapods. Evolution & Development 7(6):608–617. DOI: 10.1111/j.1525-142X.2005.05064.x.
- Guizze SPG, Knysak I, Katia C, Barbaro KC, Manoela Karam-Gemael M, Chagas Jr A. 2016. Predatory behavior of three centipede species of the order Scolopendromorpha (Arthropoda: Myriapoda: Chilopoda). Zoologia 33(6):1–7. DOI: 10.1590/S1984-4689zool-20160026.
- Haklay MM, Dörler D, Heigl F, Manzoni M, Hecker S, Vohland K. 2021. What is citizen science? The challenges of definition. In: Vohland K, Land-Zandstra A, Ceccaroni L, Lemmens R, Perelló J, Ponti M, Samson R, Wagenknecht K, editors. The science of citizen science. Amsterdam: Springer. pp. 13–33. DOI:10.1007/978-3-030-58278-4.
- Hartig F. 2022. Dharma: Residual diagnostics for (Hierarchical multi-level/Mixed) regression models. R package version 0.4.6. Available: http://florianhartig.github.io/DHARMa/. Accessed Dec 2023.
- Heymons R. 1901. Die Entwicklungsgeschichte der Scolopender. Zoologica Stuttgart 13:1–244.
- Hijmans RJ 2023. Predicts: Spatial prediction tools. R package version 0.1-11. Available: https://CRAN.R-project.org/package=predicts. Accessed Dec 2023.
- Ivask M, Kuu A, Meriste M, Kutti S, Raamets J. 2019. Chilopoda and Diplopoda of semi-natural flooded meadows in Matsalu. Pedobiologia 74(9):24–33. DOI: 10.1016/j.pedobi.2019.02.002.
- Kopecký O, Tuf IH. 2013. Podzemní populace pavouka plachetnatky čtyřzubé (Oreonetides quadridentatus (Wunderlich, 1972)). Západočeské entomologické listy 4:106–109.
- Lennon JJ, Koleff PJ, Green Wood JJD, Gaston KJ. 2004. Contributions of rarity and commonness to patterns of species richness. Ecology Letters 7(2):81–87. DOI: 10.1046/j.1461-0248.2004.00548.x.
- Lewis JGE. 1981. The biology of centipedes. Cambridge: Cambridge University Press. pp. 474.
- Lewis JGE. 2010. On the function of the ultimate legs of Cryptops and Theatops (Chilopoda, Scolopendromorpha). International Journal of Myriapodology 3(1):145–151. DOI: 10.1163/187525410X12578602960542.
- Lewis JGE, Daszak P, Jones CG, Cottingham JD, Wenman E, Maljkovic A. 2010. Field observations on three scolopendrid centipedes from Mauritius and Rodriguez (Indian Ocean) (Chilopoda: Scolopendromorpha). International Journal of Myriapodology 3(1):123–137. DOI: 10.1163/187525410X12578602960425.
- Longton RE, Hedderson TA. 2000. What are rare species and why conserve them? Lindbergia 25(2):53–61. DOI: 10.2307/20150038.
- Mammola S, Leroy B. 2018. Applying species distribution models to caves and other subterranean habitats. Holarctic Ecology 41(7):1194–1208. DOI: 10.1111/ecog.03464.
- Manton SM. 1965. The evolution of arthropodan locomotory mechanisms. Part 8. Functional requirements and body design in Chilopoda. Journal of the Linnean Society of London 46(306–07):251–484. DOI: 10.1111/j.1096-3642.1965.tb00500.x.
- Marquardt DW. 1970. Generalized inverses, ridge regression, biased linear estimation and nonlinear estimation. Technometrics 12(3):591–612. DOI: 10.2307/1267205.
- McCreadie J, Adler PH. 2008. Spatial distribution of rare species in lotic habitats. Insect Conservation and Diversity 1(3):127–134. DOI: 10.1111/j.1752-4598.2008.00017.x.
- McMonigle O. 2014. Centipedes in captivity - The reproductive biology and husbandry of chilopoda. Greenville: Coachwhip Publications.
- Menta C, Remelli S. 2020. Soil health and arthropods: From complex system to worthwhile investigation. Insects 11(3):1–23. DOI: 10.3390/insects11010054.
- Molinari J, Gutiérrez EE, De Ascençao AA, Arends A, Márquez RJ. 2005. Predation by Giant Centipedes, Scolopendra gigantea, on three species of bats in a Venezuelan cave. Caribbean Journal of Science 41(2):340–344.
- Monge-Nájera J, Barrientos Z, Aguilar F. 1993. Behavior of Epiperipatus biolleyi (Onychophora: Peripatidae) under laboratory conditions. Revista de biología tropical 41(3):689–696.
- Naimi B 2017. Package “usdm”. Uncertainty analysis for species distribution models. R-Cran, 18. Available: https://cran.rproject.org/web/packages/usdm/usdm.pdf.
- Nicolosi G, Giachino PM, Magrini P, Sabella G, Isaia M. 2023. Distribution and bioclimatic suitability of Duvalius hartigi, subterranean beetle from the lava caves of Mount Etna (Coleoptera: Carabidae, Trechinae). Fragmenta Entomologica 55(1):37–44. DOI: 10.13133/2284-4880/1510.
- Noronha JC, Battirola LD, Chagas-Júnior A, Miranda RM, Carpanedo RS, Rodrigues DJ. 2015. Predation of bat (Molossus molossus: Molossidae) by the centipede Scolopendra viridicornis (Scolopendridae) in Southern Amazonia. Acta Amazonica 45(3):333–336. DOI: 10.1590/1809-4392201404083.
- Peretti E, Cecchin C, Fusco G, Gregnanin L, Kos I, Bonato L. 2022. Shedding light on species boundaries in small endogeic animals through an integrative approach: Species delimitation in the centipede Clinopodes carinthiacus (Chilopoda: Geophilidae) in the south-eastern alps. Zoological Journal of the Linnean Society 196(2):902–903. DOI: 10.1093/zoolinnean/zlac008.
- Punzo F. 2005. The ability of Aphonopelma steindachneri (Ausserer) (Araneae: Theraphosidae) to detect and respond to chemosensory cues associated with a predator, Hogna carolinensis (Araneae: Lycosidae). Bulletin of the British Arachnological Society 13(5):169–171.
- QGIS Development Team. 2016. QGIS geographic information system. Open Source Geospatial Foundation Project. Available: http://www.qgis.org/
- R Core Team. 2022. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Available: https://www.R-project.org/
- Rabinowitz D. 1981. Seven forms of rarity. In: Synge H, editor The biological aspects of rare plant conservation. New York: Wiley. pp. 205–217.
- Royle JA, Nichols JD. 2003. Estimating abundance from repeated presence-absence data or point counts. Ecology 84(3):777–790. DOI: 10.1890/0012-9658(2003)084[0777:EAFRPA]2.0.CO;2.
- Sanna L, Bonato L, Marcia P, Zapparoli M. 2018. First record of predation by Plutonium zwierleini Cavanna, 1881 (Chilopoda Scolopendromorpha) on Speleomantes supramontis (Lanza, Nascetti & Bullini, 1986) (Amphibia Plethodontidae) in Sardinia, Italy. Riassunti 79°Congresso Nazionale Unione Zoologica Italiana, Lecce 25-28 settembre 2018. pp. 125. Available: http://www.uzionlus.it/documenti/Riassunti-UZI-2018.pdf.
- Sazima I, Carvalho-Filho A. 2003. Natural history of the elusive blenny Lupinoblennius paivai (Perciformes: Blenniidae) in coastal streams of southeast Brazil. Ichthyological Exploration of Freshwaters 14(2):175–184.
- Schileyko AA, Pavlinov IJ. 1997. A cladistic analysis of the order Scolopendromorpha (Chilopoda). Entomologica Scandinavica 51(Suppl.):33–40.
- Shelley RM. 1997. The holarctic centipede subfamily Plutoniuminae (Chilopoda: Scolopendromorpha: Cryptopidae) (Nomen correctum ex subfamily Plutoniinae Bollman 1893). Brimleyana 24:51–113.
- Short M, Huynh C. 2013. Four new species of Unixenus Jones, 1944 (Diplopoda, Penicillata, Polyxenida) from Australia. Zookeys 278:75–90. DOI: 10.3897/zookeys.278.4765.
- Støa B, Halvorsen R, Stokland JN, Gusarov VI. 2019. How much is enough? Influence of number of presence observations on the performance of species distribution models. Sommerfeltia 39(1):1–28. DOI: 10.2478/som-2019-0001.
- Tuf IH, Tufová J, Jeřábková E, Dedek P. 2006. Diurnal epigeic activity of myriapods (Chilopoda, Diplopoda). Norwegian Journal of Entomology 53:335–344.
- Uetz GW, van der Laan KL, Summers GF, Gibson PAK, Getz LL. 1979. The effects of flooding on floodplain arthropod distribution, abundance and community structure. The American Midland Naturalist 101:286–299. DOI: 10.2307/2424594.
- UnicoCelula. 2012. Theatops sp. feeding with terminal legs. Available: https://www.youtube.com/watch?v=i1sgW_fj2T4. Accessed Nov 2023.
- Valavi R, Guillera-Arroita G, Lahoz-Monfort JJ, Elith J. 2021. Predictive performance of presence-only species distribution models: A benchmark study with reproducible code. Ecological Monographs 92(1):e01486. DOI: 10.1002/ecm.1486.
- Vecchioni L, Faraone FP, Stoch F, Arculeo M, Marrone F. 2022. Diversity and distribution of the inland water decapods of Sicily (Crustacea, Malacostraca). Diversity 14(4):246. DOI: 10.3390/d14040246.
- Walsh JR, Pedersen EJ, Vander Zanden MJ. 2018. Detecting species at low densities: A new theoretical framework and an empirical test on an invasive zooplankton. Ecosphere 9(11):e02475. DOI: 10.1002/ecs2.2475.
- Wang YM. 1945. A preliminary report on Chilopoda at Ishan, Kwangsi, and Meitan, Kweichow. Journal of the New York Entomological Society 53:63–67.