Abstract
The flavone luteolin and the flavonol quercetin naturally occur in plants, providing some resistance against herbivores. However, despite suggesting the potential use of these compounds for plant protection, research on their relationship with aphids is limited. The aim of our study was to assess the effect of applying two flavonoids, quercetin and luteolin, at different concentrations, in vitro on the feeding behavior of the black bean aphid Aphis fabae Scopoli (Hemiptera: Aphididae). In most events associated with stylet activity, differences in probing behavior did not vary between the control gel and those with flavonoids. Higher concentrations of quercetin increased the number of penetrations, while lower concentrations of luteolin prolonged the first probing. The addition of flavonoids to the gels reduced passive ingestion. At a concentration of 0.1%, luteolin completely stopped salivation, and at 0.01%, it shortened the average duration of this activity. In events associated with active ingestion, differences in feeding behavior were observed between the control gel and those with quercetin at lower concentrations, affecting the time to the first stylet activity and the average time of active ingestion. Lower concentrations of luteolin prolonged the time to the first active ingestion, the duration of the first active ingestion, and the average time of this activity. Increased concentrations of quercetin and luteolin negatively affected aphid feeding behavior. Quercetin was better tolerated by A. fabae. A more noticeable effect was observed with an increase in luteolin concentration on the feeding behavior of A. fabae. These findings could be applied in biotechnological projects aiming to develop plants resistant to aphids and other herbivores.
Introduction
Present-day aphids have traditionally been controlled through the application of insecticides. However, chemical control poses numerous disadvantages for the health of organisms and environmental concerns (Casida & Durkin Citation2013; Köhler & Triebskorn Citation2013; Lamichhane et al. Citation2016). Many aphid species have developed resistance to several classes of insecticides (Bass et al. Citation2014; Foster et al. Citation2017). Due to the known harmful effects of chemical control, there is a growing trend in using alternative methods for aphid management. These methods include the use of antifeedants or attractants, causing the insect to either become disoriented or discouraged from feeding (Isman Citation2006; Zehnder et al. Citation2007; Pickett & Khan Citation2016; Rivera et al. Citation2020), especially in “push-pull” strategies (Frazier & Chyb Citation1995; Cook et al. Citation2007; Pickett et al. Citation2014). The behavior of aphids can be modified by chemical compounds, and the most potent chemicals often originate from natural sources, representing various groups of secondary plant compounds.
Flavonoids are ubiquitously present in plants and are known for their diverse beneficial biological effects, such as anti-inflammatory, antioxidant, antiviral, and anticancer effects (Middleton Citation1998; Pietta Citation2000; Simmonds Citation2001, Citation2003; Adlercreutz Citation2002; Jassim & Naji Citation2003). It has been demonstrated that flavonoids in plant tissues exhibit antifeedant, antibacterial, and antilarval effects, playing a crucial role in chemical defense against predators, competitors, and pathogens (Qi et al. Citation2008; Mitchell et al. Citation2016; Goławska et al. Citation2023; Paprocka et al. Citation2023). Due to the importance of flavonoids, breeding efforts, both conventional and involving genetic engineering, have been made to increase flavonoid levels in plants (Scarano et al. Citation2018; Marsafari et al. Citation2020). However, these modifications may impact various aspects of insect–plant interactions (Simmonds Citation2003). One such aspect involves phagostimulant and phagodeterrent effects. Goławska and Łukasik (Citation2012) suggested that the negative effects of flavonoids on the performance of aphids and possibly other insect herbivores could be a consequence of shortening or suppressing the feeding process. This supports the hypothesis that the insecticidal activity of flavonoids is associated with their influence on insect feeding behavior (Simmonds Citation2001). The observed effects are attributed to quercetin and luteolin.
Flavone luteolin and flavonol quercetin naturally occur in plants and play crucial roles, particularly during plant interactions with other organisms. These compounds are generally believed to be involved in plant–insect interactions, providing some resistance against herbivores (Calatayud et al. Citation1994; Simmonds Citation2001, Citation2003; Goławska & Łukasik Citation2012; Goławska et al. Citation2014b; Singh et al. Citation2021). They can act as repellents, antifeedants, or toxins (Isman Citation2006; Zehnder et al. Citation2007; Goławska & Łukasik Citation2012; Goławska et al. Citation2014b; Singh et al. Citation2021) and may induce oxidative stress within insect tissues (Łukasik et al. Citation2011). Quercetin has various effects on insects, particularly aphids (Hemiptera: Aphididae). An earlier study demonstrated that higher concentrations of quercetin added to a liquid diet increased developmental time, the pre-reproductive period and mortality, while decreasing fecundity and the intrinsic rate of natural increase of pea aphid Acyrthosiphon pisum Harris (Goławska et al. Citation2014b). Quercetin also exhibited deterrence activity against aphids: the woolly apple aphid (WAA), Eriosoma lanigerum (Hausmann) (Ateyyat et al. Citation2012), the mango aphid Toxoptera odinae (Van der Goot) (Xiao et al. Citation2019), and golden cap aphid Aphis craccivora Koch (Lattanzio et al. Citation2006). Exposure to quercetin reduced the colonization of winter wheat Triticum aestivum (Poaceae) by wingless females and larvae of bird cherry-oat aphid Rhopalosiphum padi L (Kozak et al. Citation2015). Researches by Chrzanowski et al. (Citation2011) suggested repellent activity of quercetin at concentration higher than 100 mM against larvae and females of the R. padi. Stec et al. (Citation2021) demonstrated that quercetin concentrations (0.1 and 0.5% ethanolic solutions) caused a slight reduction in phloem sap uptake during the first contact with the phloem sap for R. padi. It also shortened the time to reach phloem by green peach aphid Myzus persicae (Sulzer) but did not stimulate sap ingestion by A. pisum. Luteolin also has detrimental effect on insects feeding. It deters the feeding of the soybean aphid Aphis glycines. Exposure to luteolin demonstrated harmful effects on the bird cherry-oat aphid during the colonization of winter wheat seedlings (Kozak et al. Citation2015). Goławska and Łukasik (Citation2012) found that luteolin acted as antifeedants. When incorporated into artificial diets, luteolin disrupted the feeding behavior of pea aphid, A. pisum.
The precise mode of flavonoids’ insecticidal action and their influence on insect feeding behavior are not fully understood due to limited research. We hypothesized that the mode of action of these molecules involves modulation of sap palatability. Our study aimed to assess the effect of applying two flavonoids, quercetin and luteolin, in vitro on aphid stylet penetration activities in sucrose-agarose gels. We focused on A. fabae species, a polyphagous and efficient virus vector (Blackman & Eastop Citation2007). Under natural conditions in plant tissues, A. fabae may encounter luteolin and quercetin while probing (Lattanzio et al. Citation2006). Understanding the activity of these compounds in aphid probing holds potential for application in aphid control programs. We utilized the electrical penetration graph technique (EPG) to monitor the feeding behavior of black bean aphids exposed to the studied flavonoids in a sucrose-agarose gel experiments. EPG was chosen as the preferred technique because it provides continuous information on feeding/probing events, making it valuable for understanding aphid behavior (Sauvion et al. Citation2003; Goławska et al. Citation2020).
Materials and methods
Aphids
The black bean aphids (Order: Hemiptera; Suborder: Sternorrhyncha; Superfamily: Aphidoidea; Family: Aphididae; Genus: Aphis; Species: Aphis fabae Scopoli) (Węgierek & Wojciechowski Citation2004) used in this study were sourced from a wild population occurring on their winter host, guelder rose (Order: Dipsacales Dumort.; Family: Caprifoliaceae Juss.; Genus: Viburnum; Species: Viburnum opulus L.) (Mirek et al. Citation2020). We used fundatrigeniae, the most frequent morph of A. fabae among those infesting V. opulus. This morph remains on the host for the longest period and plays a pivotal role in the dynamic development of the population of heteroecious aphid species on the primary hosts (building of population) (Łukasik et al. Citation2023). Samples of aphids for the study were collected randomly during the third decade of May 2017 when the monitoring of population density was at its maximum. The viburnum trees were located in green areas around Siedlce, Poland (52°12'N, 22°17'E). The climate of the area is characterized by an annual mean temperature of 8.7°C, an annual mean relative air humidity of 79%, and a total rainfall of 526 mm (https://en.tutiempo.net). During the experimental period, the weather was typical for spring in eastern Poland.
Chemicals and gels
Luteolin and quercetin were purchased from Sigma-Aldrich, Poland (CN. 491703, CN. 117395, respectively). The impact of flavonoids on black bean aphid feeding behavior was investigated in vitro using sucrose-agarose gels. Sucrose, a basic sugar often used to attract aphids, influences their decision to feed or not to feed (Gabryś & Tjallingii Citation2002). Gels were prepared by incorporating 1.25% agarose (Sigma A-0169) into a 30% sucrose solution. Subsequently, one of the flavonoids was added to achieve concentrations of 0 (control), 0.1, 0.01, 0.001, and 0.0001%. After stirring the mixtures, they were heated in a water bath (75°C for 30 min) and then poured into plastic rings (10 mm high and 15 mm in diameter) covered with a stretched Parafilm M® membrane. Transparent gels formed after 1–2 min and were offered to aphids for probing.
Aphid probing and feeding behavior
Electrical Penetration Graphs (EPGs) (Tjallingii Citation1988) were employed to monitor the probing and feeding behavior of adult aphids exposed to flavonoids in a gel. The EPG allows the recording of various waveform patterns associated with aphid activities and stylet locations during penetration, typically within plant tissues (Sauvion & Rahbe Citation1999). EPG waveforms were recorded in a Faraday cage in the laboratory under conditions similar to those monitored in the green areas (approximately 21°C and 70% RH). Apterous adults were collected between 6 and 7 a.m. and then dorsally tethered on the abdomen with a gold wire (2 cm long, 20-μm in diameter) and water-based, conductive, silver paint (Demetron, L2027, Darmstadt, Germany). After a 2-h starvation period to recover from tethering, EPGs were initiated (between 9 and 10 a.m.) by carefully transferring the aphids to sucrose-agarose gel, individually placing them in the center of the membrane on the sucrose-agarose gels (one aphid per gel). A second electrode (a copper wire 9 cm long, 1 mm in diameter) was introduced into the gel. Aphids were connected to a Giga-4 EPG amplifier (Wageningen, Agricultural University, Entomology Department, The Netherlands) coupled to an IBM-compatible computer through a DAS 8 SCSI acquisition card (Keithley, USA). The EPGs were recorded under continuous laboratory lighting per day, and all EPG recordings were made for 10 aphids on 10 separate gels. EPG recordings began between 9 and 10 a.m., and aphid probing and feeding behavior were monitored for 2 h.
EPGs were acquired and analyzed with STYLET 2.2 software provided by W.F. Tjallingii. Waveforms were identified according to those found in sucrose-agarose gels (Sprawka & Goławska Citation2010; Goławska & Łukasik Citation2012; Goławska et al. Citation2014a, Citation2020), using an analogy to those defined and described for plants (Tjallingii Citation1988, Citation1990, Citation1994): pattern g-np (non-penetration), where the aphid’s stylet is outside the gel (analogous to the stylet being outside plant tissues); pattern g-C (stylet pathway phase), representing stylet activity in the gel (analogous to stylet activity in plant tissues, moving intercellularly through mesophyll or vascular tissues, and forming the salivary sheath); pattern g-E1 (sieve element phase) indicating salivation into the gel (analogous to salivation into the plant, where saliva is excreted into the phloem sieve tubes); and pattern g-G (xylem phase) indicating ingestion from the gel (analogous to active ingestion from the xylem). The time spent on each EPG waveform was measured and expressed per one insect.
Statistical analysis
The EPG parameters, including the duration of stylet activity in the gel, the duration of salivation into the gel, the duration of active ingestion from the gel, and the number of probes, were analyzed with the Kruskal–Wallis test, using the multiple comparisons test. The reported values represent the mean ± SE. p values < 0.05 were considered statistically significant.
To determine the effects of flavonoids on waveforms, a General Linear Model (GLM) with a Gaussian distribution was employed. In the analysis, response variables were the individual waveforms, and the compound, concentration, and interaction between these variables served
as predictor variables. All statistical analyses were performed using Statistica 12.0 (StatSoft Citation2014).
Results
Aphis fabae feeding behavior on gels with quercetin and luteolin
EPG recordings indicated that the addition of the flavonoids quercetin and luteolin to the sucrose-agarose gel affected the feeding behavior of A. fabae. The presence of g-C and g-E1 waveforms indicates that aphids probed the gel and excreted saliva into the gel in the control and in all flavonoid treatments. The absence of g-G waveform indicates that aphids did not actively ingest gels containing 0.1% luteolin. All EPG waveforms were observed on gels that contained ≤ 0.1% quercetin or ≤ 0.01% luteolin ().
Table I. Aphis fabae feeding behavior on a sucrose-agarose gel as affected by quercetin and luteolin and as indicated by EPG waveforms.
In A. fabae, stylet probing activities on gels with quercetin and luteolin occupied varied proportions of the 2-h experimental time. Generally, the total probing times were greatest for luteolin 0.1% and smallest for quercetin 0.1%. Aphids on luteolin 0.1% spent over 80% of their time on stylet probing activities and approximately 10% on non-probing (). For the studied concentrations, the proportion of the duration of salivation was similar for quercetin and varied for luteolin. On gels with luteolin, salivation occurred only in A. fabae on gels with 0.0001%, 0.001%, and 0.1% in 14%, 8%, and 3% of EPG-recorded aphids, respectively (). The proportion of the duration of ingestion varied and was the highest for quercetin 0.1% (44%) and lowest for luteolin 0.1% (0%) ().
Figure 1. Proportion of aphid activities on gels as affected by quercetin and luteolin during 2-hour EPG monitoring.
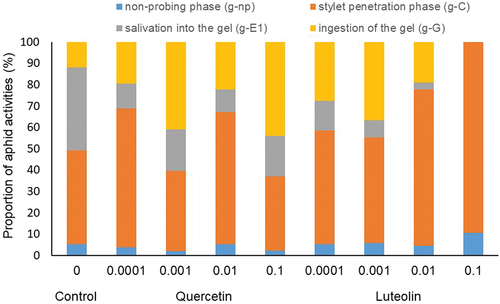
Concentrations of quercetin had significant effects on the number of g-C waveforms (Kruskal–Wallis test; H4,50 = 18.49; p = 0.001), time to the first g-C waveform (Kruskal–Wallis test; H4,50 = 12.98; p = 0.011), duration of the first g-C waveform (Kruskal–Wallis test; H4,50 = 15.54; p = 0.004) and average time of g-C (Kruskal–Wallis test; H4,50 = 16.45; p = 0.003) (). The number of penetrations was affected by treatments with quercetin at 0.01% and 0.1% (multiple comparison test; p = 0.001; 0.016, respectively). The number of penetrations on gels with quercetin at these concentrations was more than twice that of controls. The time to the first probing was shortened by quercetin at 0.1% (multiple comparison test; p = 0.049). On the gels with quercetin, aphids initiated their activity 11 times faster compared to the control. The duration of the first probe was prolonged over three times by quercetin at 0.0001% (multiple comparison test; p = 0.003) compared to the control and over seven times compared to quercetin at 0.1% (multiple comparison test; p = 0.020) ().
Table II. Aphis fabae stylet activity (g-C waveform) on a sucrose-agarose gel as affected by quercetin and luteolin.
Statistical differences were also observed for the average time of probing for quercetin at 0.0001% and for quercetin at 0.001% and 0.01% (multiple comparison test; p = 0.024; p = 0.002, respectively).
The concentrations of luteolin demonstrated significant effects on the time to the first g-C waveform (Kruskal–Wallis test; H4,50 = 25.34; p < 0.001). The timing to the first probing was extended twofold by luteolin at 0.001% (multiple comparison test; p = 0.030). The timing of the first probe differed varied for luteolin concentrations of 0.0001% and 0.1% (multiple comparison test; p = 0.005) as well as for luteolin at 0.001%, 0.01%, and 0.1% (multiple comparison test; p = 0.020, p < 0.001 respectively). On gels containing 0.1% luteolin, aphids initiated penetration the fastest, whereas on gels containing 0.001% luteolin, penetration was observed at the latest time (). Concentrations of luteolin also had significant effects on the duration of the first g-C waveform (Kruskal–Wallis test; H4,50 = 17.58; p = 0.002). Statistical differences were observed between the control and luteolin concentrations of 0.0001%, 0.001%, and 0.01% (multiple comparison test; p = 0.045, 0.003, 0.005, respectively). Compared to the control, approximately a fivefold longer first probe was observed on gels containing luteolin (). However, concentrations of luteolin did not have significant effects on the number of g-C waveforms (Kruskal–Wallis test; H4,50 = 2.86; p = 0.581) and average time of g-C (Kruskal–Wallis test; H4,50 = 3.71; p = 0.447) (). Concentrations of quercetin did not have significant effects on the time to the first g-E1 waveform (Kruskal–Wallis test; H4,50 = 8.07; p = 0.089), duration of the first g-E1 waveform (Kruskal–Wallis test; H4,50 = 5.78; p = 0.217), and average time of g-E1 (Kruskal–Wallis test; H4,50 = 9.12; p = 0.058) ().
Table III. Aphis fabae salivation (g-E1 waveform) into the sucrose-agarose gel as affected by quercetin and luteolin.
Concentrations of luteolin demonstrated significant effects on the time to the first g-E1 waveform (Kruskal–Wallis test; H4,50 = 28.71; p < 0.001), duration of the first g-E1 waveform (Kruskal–Wallis test; H4,50 = 27.42; p < 0.001), and average time of g-E1 (Kruskal–Wallis test; H4,50 = 34.39; p < 0.001) ().
The addition of luteolin to the gels resulted in the reduction or complete inhibition of aphid salivation (). At 0.1%, the flavonoid reduced the time to zero during which aphids salivated into the gels (). Aphids feeding on gels containing the lowest concentration of luteolin (0.0001%) exhibited this waveform earliest, while on gels with 0.01% luteolin, it appeared latest (multiple comparison test; p < 0.001 in all cases). The timing of the first salivation was shortened by luteolin at 0.001% and tended to decrease with higher concentrations of luteolin (multiple comparison test; p < 0.001). For aphids feeding on gels with the lowest concentration of luteolin (0.0001%), a longer time the first salivation was observed. A similar trend was observed for the average time of g-E1, which was generally reduced by luteolin (multiple comparison test; p < 0.001), decreasing as the concentration of luteolin increased ().
Concentrations of quercetin had significant effects on the time to the first g-G waveform (Kruskal–Wallis test; H4,50 = 19.56; p < 0.001), duration of the first g-G waveform (Kruskal–Wallis test; H4,50 = 10.07; p < 0.001), and average time of g-G (Kruskal–Wallis test; H4,50 = 13.78; p = 0.039) ().
Table IV. Aphis fabae active ingestion (g-G waveform) from the sucrose-agarose gel as affected by quercetin and luteolin.
Compared to the control group, aphids feeding on gels containing 0.0001% quercetin exhibited the first active ingestion more than six times later (multiple comparison test; p < 0.001), and for 0.001% luteolin, more than 10 times later (multiple comparison test; p = 0.007). The timing of the first g-G waveform was extended by 3.5 times with quercetin at 0.1% (multiple comparison test; p = 0.034). The average time of active ingestion also tended to be higher with the addition of quercetin, with statistical differences observed for quercetin at 0.001% (multiple comparison test; p = 0.015) and 0.1% (multiple comparison test; p = 0.041) (). The average time of g-G waveform on gels with quercetin was more than three times longer compared to the control. In other cases, no significant differences were noted.
The highest concentration of luteolin completely inhibited aphid active ingestion (g-G waveform). Luteolin at 0.1% reduced the total time that black bean aphids actively ingested the gels to zero, but the effect was not statistically significant (). This phase of aphid feeding generally increased at the lower concentrations of luteolin and was completely stopped by the highest concentration. As indicated by the g-G waveform, luteolin tended to delay the appearance of the first g-G waveform (Kruskal–Wallis test; H4,50 = 32.19; p < 0.001). Statistical differences were observed between the control and luteolin concentrations of 0.0001%, 0.001%, and 0.01% (multiple comparison test; p = 0.011, 0.008, 0.022 respectively). Compared to the control, an approximately 10 times later first active ingestion was observed on gels containing luteolin (). Statistical differences were also observed for the time to the first g-G waveform for luteolin at 0.1% and for luteolin at 0.0001%, 0.001%, and 0.01% (multiple comparison test; p < 0.001 in all cases). Luteolin, at lower concentrations, also tended to prolong active ingestion, as evidenced by the prolonged time of the first active ingestion (Kruskal–Wallis test; H4,50 = 27.82; p < 0.001) and total active ingestion (Kruskal–Wallis test; H4,50 = 27.20; p < 0.001) (). Compared to the control, on gels containing luteolin at 0.001%, four times longer first active ingestion was observed (multiple comparison test; p = 0.016) (). Statistical differences were observed for the duration of the first g-G waveform for luteolin at 0.1% and for luteolin at 0.0001%, 0.001%, and 0.01% (multiple comparison test; p = 0.002, p < 0.001, p = 0.014 respectively). Compared to the control, on gels containing luteolin at 0.001%, three times longer total active ingestion was observed (multiple comparison test; p = 0.021) (). Statistical differences were observed for the total time g-G waveform for luteolin at 0.1% and for luteolin at 0.0001% and 0.001% (multiple comparison test; p = 0.003, p < 0.001 respectively). The timing of the average active ingestion sought to be extended by luteolin at smaller concentrations but without statistical significance. The statistical differences were observed only for luteolin at 0.001% ().
Effect of quercetin and luteolin on aphis fabae penetration of sucrose-agarose gel
The statistical analysis revealed that the effect depended on the compounds and flavonoid concertation (). The tested compounds and their concentrations had a statistically significant impact on stylet activity on sucrose-agarose gel (). The analysis demonstrated the effect of the tested compounds and concentrations on the number of g-C waveforms (GLM; F7,72 = 7.97; p < 0.001), total time of g-C waveform (GLM; F7,72 = 12.76; p < 0.001), time to the first g-C waveform (GLM; F7,72 = 21.77; p < 0.001), and duration of the first g-C waveform (GLM; F7,72 = 5.97; p < 0.001). Significance for the duration of the first g-C waveform was affected by the tested compound. In the remaining cases, both the compound and concentration were found to be significant. Interactions between the tested factors were observed for the total time of the g-C waveform, time to the first g-C, and duration of the first g-C waveform ().
Table V. Statistical results of the GLM of A. fabae stylet activity (g-C waveform) on sucrose-agarose gel as affected by quercetin and luteolin.
Table VI. Statistical results of the GLM of A. fabae salivation (g-E1 waveform) on a sucrose-agarose gel as affected by quercetin and luteolin.
Table VII. Statistical results of the GLM of A. fabae active ingestion (g-G waveform) on a sucrose-agarose gel as affected by quercetin and luteolin.
The tested compounds and concentrations also had a statistically significant effect on A. fabae salivation (g-E1 waveform) on sucrose-agarose gel (). The analysis revealed an effect of the tested compounds and concentrations on the number of g-E1 waveforms (GLM; F7,72 = 8.10; p < 0.001), total time of g-E1 waveform (GLM; F7,72 = 8.77; p < 0.001), time to the first g-E1 waveform (GLM; F7,72 = 5.73; p < 0.001), and duration of the first g-E1 waveform (GLM; F7,72 = 3.74; p = 0.002). The analysis showed an effect of the tested compounds on the number of g-E1 and the total time of g-E1 waveforms, and an effect of the concentrations of the compounds on the total time of the g-E1 waveform, time to the first g-E1, and duration of the first g-E1 waveform (). There is no effect of the concentrations on the number of g-E1, and no effect of the compound on time to the first g-E1 and duration of the first g-E1 waveforms (). Interactions between the tested factors were found for the number of g-E1 waveforms, total time of g-E1 waveform, and time to the first g-E1 waveform ().
The studied compounds and concentrations also statistically affected A. fabae ingestion (g-G waveform) on sucrose-agarose gel (). The analysis demonstrated an effect of the tested compounds and concentrations on the number of g-G waveforms (GLM; F7,72 = 5.85; p < 0.001), total time of g-G waveform (GLM; F7,72 = 7.42; p < 0.001), time to the first g-G waveform (GLM; F7,72 = 7.74; p < 0.001), and duration of the first g-G waveform (GLM; F7,72 = 3.94; p = 0.001). For the number of g-G waveforms, total time of g-G, and time to the first g-G waveform, both compound and concentration were significant (). There is no effect of the compounds and concentrations on the duration of the first g-G waveform. Interactions between the tested factors were found for the total time of the g-G waveform, time to the first g-G, and duration of the first g-G waveform ().
Discussion
Flavonoids can modulate the feeding behavior of insects (Simmonds Citation2003; Goławska & Łukasik Citation2012; Goławska et al. Citation2014b). In the present study, we investigated the feeding response of A. fabae species to two flavonoids, quercetin and luteolin. Our aim was to explore the potential use of these compounds as chemical barriers that may prevent aphids from feeding. Plant traits, including plant chemistry, are next to the anatomical structure of the plant, the starting point for further breeding of cultivars resistant to pests (War et al. Citation2012; Reynolds et al. Citation2020). Wingless fundatrigeniae were used in the research. These morphs intensively exploit their hosts, leading to a decrease in the content of major nutritive compounds. Demonstrating the antifeeding influence of the investigated compounds on fundatrigeniae allows for the inhibition of aphid population development and, consequently, a reduction in the damage caused by them.
We demonstrated that quercetin and luteolin affect the feeding behavior of black bean aphids using the EPG method. This method can provide continuous information on feeding/probing events, detecting waveform aphid activities and stylet locations during penetration and feeding (Goławska & Łukasik Citation2012). Parameters such as total and average times of waveforms, duration, and frequency of events, number of probes, etc., are good indicators of probing/feeding aphids exposed to chemical or physical factors (Gabryś et al. Citation2015). Using the EPG method to monitor the feeding behavior of aphids exposed to chemicals in sucrose-agarose gels could help researches understand and overcome difficulties in using chemicals against insects (Goławska & Łukasik Citation2012; Goławska et al. Citation2014a, Citation2014b). The agarose-sucrose gels presumably mimic the tissues surrounding the sieve elements, whereas a Parafilm M® membrane containing sucrose syrup corresponds to sieve elements containing phloem sap (Urbańska et al. Citation1998). Sucrose is the basic stimulator of aphid feeding and is also an important source of metabolic energy necessary for the proper functioning of insect life processes. It is also the main transport sugar in plants. It is believed that sucrose stimulates the movement of aphid stylets in the intercellular spaces. By using gels, research can focus on the impact of various chemical compounds on the behavior of aphids, rather than solely on providing them with food. Previous works have demonstrated the use of the EPG technique to monitor the effects of individual compounds on probing and feeding behavior in vitro on sucrose-agarose gels (Goławska Citation2007; Sprawka & Goławska Citation2010; Sprawka et al. Citation2011; Goławska & Łukasik Citation2012; Sprawka et al. Citation2013; Goławska et al. Citation2014a, Citation2014b, Citation2020). Sucrose-agarose gels have also been employed for salivary protein detection and staining (Urbańska et al. Citation1998; Cooper et al. Citation2010).
In our study, EPG monitoring of aphid activities during probing sucrose-agarose gels with quercetin and luteolin showed variations in the feeding behavior of the studied aphids, depending on the flavonoid used and its applied concentration. Insect feeding can be affected at pre-ingestive, ingestive and postingestive phases (Frazier & Chyb Citation1995). The observed differences occurred before aphids started probing and both when aphid stylets were in sucrose-agarose gels and during salivation into the gel and ingestion from the gel.
In our study, waveforms generated by aphids were generally similar to those observed on plants and in liquid artificial diets (Tjallingii Citation1988; Sauvion et al. Citation2004). On gels containing ≤0.1% of quercetin and ≤ 0.01% of luteolin, we observed waveforms similar to those reported by Goławska et al. (Citation2020) on gels: g-np, g-C, g-E1, and g-G. A waveform analogous to passive ingestion on plants was not recorded on the sucrose-agarose gel. This is related to the fact that phloem sap is under pressure within the plants, and when the aphid pierces the phloem vessels, it can passively ingest phloem sap (Tjallingii Citation1990).
Aphids typically tend to start probing with no delay when no repellents are present at the surface (Powell et al. Citation1997). The first probe on gels with luteolin at 0.001% concentration was significantly delayed. No delay was observed for quercetin. On the contrary, at 0.1% concentration, the aphid made the first attempt faster. In other cases, there were no clear-cut differences in the time before the first probing. The value of the parameter describing the onset of probing on gels recorded by the EPG method could have been influenced by the conditions of the EPG experimental setup. Aphids were limited in freedom to move on the gels. They were attached to a golden wire. So, the tethered aphids were reluctant to probe, but if aphids had been free to move, they would probably go away. The aphids may have moved to the edge of the ring as well. During the first probes, aphids make decisions about the continuation or discontinuation of probes (Pettersson et al. Citation2017). For this reason, the duration of probing, the number and time of individual probes are good indicators that reflect the presence of factors that either stimulate or deter aphid probing during the pre-ingestive phase (Van Emden Citation2017; Wróblewska-Kurdyk et al. Citation2020). A. fabae were reluctant to penetrate the deeper gels with quercetin and luteolin. In A. fabae probing constituted 35% − 65% of all activities for gels with quercetin and 53% − 89% with luteolin. For the tested compounds, the number of penetrations into the gels was similar at all concentrations except for the gels with quercetin at 0.01% and 0.1%, where it was consistently three times longer than in the controls. Additionally, the duration of the first probe was elongated for 0.0001% quercetin and luteolin equal to or less than 0.01%. Deterrent factors present in the gels could have influenced it. Also, in the research of Goławska and Łukasik (Citation2012), the duration of the first probe tended to be prolonged by higher concentrations of luteolin. This phenomenon is observed for aphids on non- host plants or plants treated with deterrents (Prado & Tjallingii Citation1997; Souza et al. Citation2020).
Another observed activity on sucrose-agarose gels was the g-E1 waveform, indicating the salivation into the gel and analogous to excretion watery saliva on plants (Tjallingii Citation1990; Sauvion et al. Citation2004; Goławska et al. Citation2020). The proportion of E1 in A. fabae activities was a maximum of 20% of all activities on gels with quercetin, while it was a maximum of 14% and a minimum of 3% on gels with luteolin at 0.0001% and 0.01%, respectively. A. fabae exhibited this activity on all gels with quercetin and luteolin at concentrations ≥ 0.01%. Similar to the research by Goławska and Łukasik (Citation2012), in our study, on gels with luteolin at a concentration of 0.1%, aphids did not excrete saliva into the gel; higher concentrations of luteolin reduced or completely inhibited A. fabae salivation and also delayed the onset of salivation. In other cases, the time to the first, duration of the first, and average time of salivation did not differ significantly from the control, with one exception. A. fabae excreted saliva into the gel with luteolin at 0.01% for a shorter duration, which is challenging to interpret. Aphid saliva contains various enzymes associated with the detoxication of allelochemicals, such as peroxidases and oxidoreductases (Miles Citation1999; Van Bel & Will Citation2016). Paprocka et al. (Citation2023) hypothesized that quite often, aphids probably need to inactivate allelochemicals that occur in the plant leaf tissues, and because of it, aphid excrete of saliva.
Active ingestion from the gel, another observed activity of aphids on gels, lasted relatively long. As indicated by the g-G waveform, flavonoids tended to delay and prolong active ingestion. Aphid active ingestion was halted by a 0.1% concentration of luteolin. This waveform is analogous to the ingestion of xylem sap in plants (Goławska et al. Citation2020). Xylem is plant tissue that transports water and mineral salts (Kim et al. Citation2014). Aphids ingest xylem sap for osmoregulation, especially in cases of dehydration (Pompon et al. Citation2010; Pettersson et al. Citation2017). In our study, the time to the first g-G waveform was elongated on a gel containing quercetin in two cases (concentration 0.0001% and 0.001%). On gels containing luteolin, aphids started active ingestion from the gel later, with one exception. No active ingestion was observed on gels containing luteolin at a concentration of 0.1%. Average durations of g-G on gels containing 0.001% and 0.01% quercetin were longer than on control gels. Similarly, on gels containing 0.001% luteolin, average durations were longer. Also, the duration of the first g-G was longer on gels with 0.1% quercetin. It shows that flavonoids at these certain concentrations stimulated ingestion and did not reduce the ingestion. The proportions of g-G activity in total probing were relatively large. They ranged from 19% to 44% on gels with quercetin at 0.0001% and 0.1%, respectively, and it was 28%, 37%, and 19% on gels with luteolin at concentrations 0.0001, 0.001, and 0.01%. As indicated by the g-G waveform, the flavonoids tended to delay, prolong, or inhibit active ingestion. This finding aligns with earlier reports. The results obtained here confirm earlier reports by Goławska and Łukasik (Citation2012) and Goławska et al. (Citation2014b), showing that luteolin and quercetin prolonged the duration until the first g-G pattern was detected, the duration of the first g-G pattern, and the total time of g-G pattern for A. pisum.
In our study, observed differences and trends in aphid feeding indicated that the potency of behavioral effects increased with higher concentrations of quercetin and luteolin. Notably, the more observable effect of an increase in flavonoid concentration on the feeding of A. fabae occurred on gels with luteolin. This confirms the findings of others; for instance, Goławska et al. (Citation2014) demonstrated that a high concentration of quercetin added to artificial diets limited diet uptake by A. pisum. Detrimental effects of the flavone luteolin on the feeding behavior of the pea aphid have also been previously shown (Goławska & Łukasik Citation2012).
The results presented here confirm that quercetin and luteolin, act as feeding barriers for phytophagous insects (Chen et al. Citation2018; El Shafei & Abdelaziz Citation2020; Shi et al. Citation2020). Plants induced with herbivore resistance based on quercetin and luteolin affected feeding behavior, leading to negative effects on herbivores, such as diminished body growth and possibly feeding inhibition. Quercetin exhibited inhibitory effects on various lepidopteran species, including the cotton bollworm Helicoverpa armigera, the corn earworm Helicoverpa zea, and the tobacco budworm Heliothis virescens, and was lethal to the southern armyworm Spodoptera eridania larvae. Simmonds (Citation2001) demonstrated that quercetin inhibits the activity of mitochondrial ATPase and oxidoreductases, causing disturbances in the development of corn earworm larvae. Shi et al. (Citation2020) indicated that quercetin affected the food intake by the silk moth Bombyx mori Linnaeus (Lepidoptera: Bombycidae), inhibiting the expression of immune-related pathway genes in silkworms, potentially impacting growth and development. Similar results were found for the newborn cotton bollworm, where an increase in flavonoid concentration resulted in a remarkable increase in nymph mortality (Jadhav et al. Citation2012). Quercetin also exhibited deterrence activity against the mealworm beetle Tenebrio molitor larvae (Sosa et al. Citation2020). Goławska et al. (Citation2012) found that pea aphid daily fecundity was affected by luteolin, tricin, and chrysoeriol glycosides. Additionally, antifeedant activities of luteolin isolated from the herb Ajuga nipponensis were observed against larvae of the diamondback moth Plutella xylostella L. DBM (Huang et al. Citation2008). Luteolin and luteolin 49-glucuronide exhibited activity against second–instar larvae of the tobacco cutworm Spodoptera litura (Qi et al. Citation2008), and luteolin-7-O-glucoside from the cardoon Cynara cardunculus showed activity against fourth-instar larvae of the cotton leafworm Spodoptera littoralis (El Shafei & Abdelaziz Citation2020). Luteolin exhibited inhibitory effects on the Japanese beetle Popillia japonica (Coleoptera: Scarabaeidae) and lepidopteran species, including the beet armyworm Spodoptera exigua Hübner and the painted lady Vanessa cardui (Cipollini et al. Citation2008; O’Nill et al. Citation2010).
The utilization of antifeedants in pest-management programs holds significant potential as it addresses the need to protect specific crops while minimizing damage to non-target organisms. These substances function by reducing or preventing feeding, making plants unpalatable or toxic, thereby offering a novel approach to insect-pest and vector management. Despite the potential, there are relatively few studies exploring the use of flavonoids as antifeedants in insect control strategies. The current research findings recommend quercetin and luteolin as promising alternatives for insect antifeedants and deterrents. Both compounds have demonstrated the ability to prevent aphid feeding, offering a potential means to protect crop plants from insect infestation. However, these results also emphasize the need for further investigation to uncover the complexity of the physiological activity exerted by chemical compounds on insect. Future research endeavors should focus on unraveling the precise mode of activity and biological effects of quercetin and luteolin. A more comprehensive understanding of how these flavonoids interact with insect physiology will contribute to the development of effective and sustainable pest-management strategies.
Conclusions
In summary of our experimental findings, electrophysiological recordings (EPG) revealed that the feeding behaviors of black bean aphids on sucrose-agarose gels were significantly influenced by quercetin and luteolin. The investigated flavonoids effectively restricted aphid consumption, and at all tested concentrations, neither quercetin nor luteolin exhibited passive gel ingestion. We observed differences and trends in aphid feeding, with the strength of behavioral effects increasing with the applied concentrations of quercetin and luteolin. Notably, a more pronounced impact of increasing flavonoid concentration on A. fabae feeding behavior was observed in the case of luteolin. At the highest tested concentration (0.1%), luteolin demonstrated a noticeable effect, completely blocking both saliva secretion into the gel and active ingestion – an effect not observed with quercetin. This study demonstrated that the flavonoids, quercetin, and luteolin have a detrimental effect on black bean aphids. The antifeedant activity of these compounds on Aphis fabae includes an influence on the feeding behavior of aphids, consequently affecting their growth and development. Quercetin and luteolin, as distinctive chemical components, can be applied as insecticidal agents and incorporated into aphid control programs. Therefore, the use of quercetin and luteolin presents a promising and sustainable strategy for protecting plants from black bean aphids as insecticides in integrated pest management programs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Adlercreutz H. 2002. Phyto-oestrogens and cancer. The Lancet Oncology 3(6):364–373. DOI: 10.1016/S1470-2045(02)00777-5.
- Ateyyat M, Abu-Romman S, Abu-Darwish M, Ghabeish I. 2012. Impact of flavonoids against woolly apple aphid, Eriosoma lanigerum (Hausmann) and its sole parasitoid, Aphelinus mali (Hald.). The Journal of Agricultural Science 4(2):227–236. DOI: 10.5539/jas.v4n2p227.
- Bass C, Puinean AM, Zimmer CT, Denholm I, Field LM, Foster SP, Gutbrod O, Nauen R, Slater R, Williamson MS. 2014. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochemistry and Molecular Biology 51:41–51. DOI: 10.1016/j.ibmb.2014.05.003.
- Blackman RL, Eastop VF. 2007. Taxonomic issues. In: van Emden H, Harrington R, editors. Aphids as crop pests. Trowbridge, United Kingdom: Cromwell Publisher. pp. 1–29.
- Calatayud PA, Rahbé Y, Delobel B, Khuong-Huu F, Tertuliano M, Le Rü B. 1994. Influence of secondary compounds in the phloem sap of cassava on expression of antibiosis towards the mealybug Phenacoccus manihoti. Entomologia Experimentalis et Applicata 72:47–57. DOI: 10.1111/j.1570-7458.1994.tb01801.x.
- Casida JE, Durkin KA. 2013. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annual Review of Entomology 58(1):99–117. DOI: 10.1146/annurev-ento-120811-153645.
- Chen C, Han P, Yan W, Wang S, Shi X, Zhou X, Desneu N, Gao X. 2018. Uptake of quercetin reduces larval sensitivity to lambdacyhalothrin in Helicoverpa armigera. Journal of Pest Science 91:919–926. DOI: 10.1007/s10340-017-0933-1.
- Chrzanowski G, Czerniewicz P, Goławska S, Sytykiewicz H, Leszczyński B, Kozak A 2011. Repellent activity of flavonoid aglycones and catechol against Rhopalosiphum padi L. VIIth International Conference on Arthropods: Chemical, physiological, biotechnological and environmental aspects, 18-23 September. Poland, Białka Tatrzańska, pp. 79.
- Cipollini D, Stevenson R, Enright S, Eyles A, Bonello P. 2008. Phenolic metabolites in leaves of the invasive shrub, Lonicera maackii, and their potential phytotoxic and anti-herbivire effects. Journal of Chemical Ecology 34:144–152. DOI: 10.1007/s10886-008-9426-2.
- Cook SM, Khan ZR, Pickett JA. 2007. The use of push-pull strategies in integrated pest management. Annual Review of Entomology 52(1):375–400. DOI: 10.1146/annurev.ento.52.110405.091407.
- Cooper WR, Dillwith JW, Puterka GJ. 2010. Salivary proteins of Russian wheat aphid (Hemiptera: Aphididae). Environmental Entomology 39:223–231. DOI: 10.1603/EN09079.
- El Shafei SN, Abdelaziz S. 2020. Insects’ deterrent flavonoids from Cynara cardunculus for controlling cotton leafworm Spodoptera littoralis. Journal of Plant Protection and Pathology 11(6):315–320. DOI: 10.21608/jppp.2020.111730.
- Foster SP, Devine G, Devonshire AL. 2017. Insecticide resistance. In: van Emden H, Harrington R, editors. Aphids as crop pests. Wallingford, United Kingdom: CABI Publisher. pp. 426–447. DOI:10.14411/eje.2009.008.
- Frazier JL, Chyb S. 1995. Use of feeding inhibitors in insect control. In: Chapman R, de Boer G, editors. Regulatory mechanisms in insect feeding. New York, United States of America: Chapman & Hall Publisher. pp. 364–381.
- Gabryś B, Dancewicz K, Gliszczyńska A, Kordan B, Wawrzeńczyk C. 2015. Systemic deterrence of aphid probing and feeding by novel - Damascone analogues. Journal of Pest Science 88:507–516. DOI: 10.1007/s10340-014-0635-x.
- Gabryś B, Tjallingii WF. 2002. The role of sinigrin in host plant recognition by aphids during initial plant penetration. Entomologia Experimentalis et Applicata 104(1):89–93. DOI: 10.1046/j.1570-7458.2002.00994.x.
- Goławska S. 2007. Deterrence and toxicity of plant saponins for the pea aphid acyrthosiphon pisum Harris. Journal of Chemical Ecology 33(8):1598–1606. DOI: 10.1007/s10886-007-9333-y.
- Goławska S, Łukasik I. 2012. Antifeedant activity of luteolin and genistein against the pea aphid, Acyrthosiphon pisum. Journal of Pest Science 85(4):443–450. DOI: 10.1007/s10340-012-0452-z.
- Goławska S, Łukasik I, Chojnacki AA, Chrzanowski G. 2023. Flavonoids and phenolic acids content in cultivation and wild collection of European cranberry bush Viburnum opulus L. Molecules 28(5):2285. DOI: 10.3390/molecules28052285.
- Goławska S, Łukasik I, Kapusta I, Janda B. 2012. Do the contents of luteolin, tricin, and chrysoeriol glycosides in alfalfa (Medicago sativa L.) affect the behavior of pea aphid (Acyrthosiphon pisum)? Polish Journal of Environmental Studies 21(6):1613–1619.
- Goławska S, Łukasik I, Sprawka I, Sytykiewicz H, Chojnacki A. 2020. Comparison of probing/feeding behavior for diet analysis to control strategy: A case study on aphids. Allelopathy Journal 49(1):113–124. DOI: 10.26651/allelo.j/2020-49-1-1258.
- Goławska S, Sprawka I, Goławski A, Matok H. 2014a. Are agarose-sucrose gels useful for studying the probing and feeding behavior of aphids? Australian Journal of Crop Science 8(2):263–270.
- Goławska S, Sprawka I, Łukasik I, Goławsk A. 2014b. Are naringenin and quercetin useful chemicals in pest-management strategies? Journal of Pest Science 7:173–180. DOI: 10.1007/s10340-013-0535-5.
- Huang Z, Zhou FC, Xu D, Afzal M, Bashir MH, Ali S, Freed S. 2008. Antifeedant activities of secondary metabolites from Ajuga nipponensis against Plutella xylostella. Pakistan Journal of Botany 40(5):1983–1992.
- Isman M. 2006. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual Review of Entomology 51(1):45–66. DOI: 10.1146/annurev.ento.51.110104.151146.
- Jadhav DN, Mallikarjuna N, Rathore A, Pokle D. 2012. Effect of some flavonoids on survival and development of Helicoverpa armigera (Hübner) and Spodoptera litura (fab.) (Lepidoptera: Noctuidae). Asian Journal of Agricultural Sciences 4:298–307. Available: http://oar.icrisat.org/id/eprint/6082.
- Jassim SA, Naji MA. 2003. Novel antiviral agents: A medicinal plant perspective. Journal of Applied Microbiology 95(3):412–427. DOI: 10.1046/j.1365-2672.2003.02026.x.
- Kim HJ, Park J, Hwang I. 2014. Investigating water transport through the xylem network in vascular plants. Journal of Experimental Botany 65(7):1895–1904. DOI: 10.1093/jxb/eru075.
- Köhler HR, Triebskorn R. 2013. Wildlife ecotoxicology of pesticides: Can we track effects to the population level and beyond? Science 341:759–765. DOI: 10.1126/science.1237591.
- Kozak A, Chrzanowski G, Sempruch C, Klewek A, Chwedczuk M. 2015. Effect of selected flavonoids on the behavior of the bird cherry-oat aphid (Rhopalosiphum padi L.) during the colonization of winter wheat. Progress in Plant Protection 55:202–206. DOI: 10.14199/ppp-2015-033.
- Lamichhane JR, Dachbrodt-Saaydeh S, Kudsk P, Messean A. 2016. Toward a reduced reliance in conventional pesticides in European agriculture. Plant Disease 110:10–24. DOI: 10.1094/PDIS-05-15-0574-FE.
- Lattanzio V, Lattanzio VMT, Cardinali A. 2006. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochemistry: Advances in Research 661:23–67.
- Łukasik I, Goławska S, Sytykiewicz H. 2023. Differences in oxidative stress markers and antioxidant enzyme activities in black bean aphid morphs (Aphis fabae Scop.) Fed on the primary host Viburnum opulus L. Antioxidants 11:2476. DOI: 10.3390/antiox11122476.
- Łukasik I, Goławska S, Wójcicka A, Goławski A. 2011. Effect of host plants on antioxidant system of pea aphid Acyrthosiphon pisum. Bulletin of Insectology 64:153–158.
- Marsafari M, Samizadeh M, Rabiei B, Mehrabi A, Koffas M, Xu P. 2020. Biotechnological production of flavonoids: An update on plant metabolic engineering, microbial host selection, and genetically encoded biosensors. Biotechnology Journal 15:1900432. DOI: 10.1002/biot.201900432.
- Middleton EJR. 1998. Effect of plant flavonoids on immune and inflammatory cell function. Advances in Experimental Medicine and Biology 439:175–182. DOI: 10.1007/978-1-4615-5335-9_13.
- Miles P. 1999. Aphid saliva. Biological Reviews 74:41–85. DOI: 10.1111/j.1469-185X.1999.tb00181.x.
- Mirek Z, Piękoś-Mirkowa H, Zając A, Zając M. 2020. An annotated checklist. In: Mirek Z, Zając A, editors. Vascular plants of Poland. Kraków, Poland: Polish Academy of Sciences. pp. 322–341.
- Mitchell C, Brennan RM, Graham J, Karley AJ. 2016. Plant defense against herbivorous pests: Exploiting resistance and tolerance traits for sustainable crop protection. Fronties in Plant Science 7:1132. DOI: 10.3389/fpls.2016.01132.
- O’Nill BF, Zangerl AR, Dermody O, Bilgin DD, Casteel CL, Zavala JA, DeLucia EH, Berenbaum MR. 2010. Impact of elevated levels of atmospheric CO2 and herbivory on flavonoids of soybean (Glycine max Linnaeus). Journal of Chemical Ecology 36:35–45. DOI: 10.1007/s10886-009-9727-0.
- Paprocka M, Dancewicz K, Kordan B, Damszel M, Sergiel I, Biesaga M, Mroczek J, Arroyo Garcia RA, Gabryś B. 2023. Probing behavior of Aphis fabae and Myzus persicae on three species of grapevines with analysis of grapevine leaf anatomy and allelochemicals. The European Zoological Journal 90(1):83–100. DOI: 10.1080/24750263.2022.2162615.
- Pettersson J, Tjallingii WF, Hardie J. 2017. Host-plant selection and feeding. In: van Emden H, Harrington R, editors. Aphids as crop pests. Wallingford, United Kingdom: CABI Publisher. pp. 173–195.
- Pickett JA, Khan ZR. 2016. Plant volatile-mediated signalling and its application in agriculture: Successes and challenges. New Phytologist 212(4):856–870. DOI: 10.1111/nph.14274.
- Pickett JA, Woodcock CM, Midega CAO, Khan ZR. 2014. Push–pull farming systems. Current Opinion in Biotechnology 26:25–132. DOI: 10.1016/j.copbio.2013.12.006.
- Pietta PG. 2000. Flavonoids as antioxidants. Journal of Natural Products 63(7):1035–1042. DOI: 10.1021/np9904509.
- Pompon J, Quiring D, Giordanengo P, Pelletier Y. 2010. Role of xylem consumption on osmoregulation in Macrosiphum euphorbiae (Thomas). Journal of Insect Physiology 56(6):610–615. DOI: 10.1016/j.jinsphys.2009.12.009.
- Powell G, Hardie J, Pickett JA. 1997. Laboratory evaluation of antifeedant compounds for inhibiting settling by cereal aphids. Entomologia Experimentalis et Applicata 84(2):189–193. DOI: 10.1046/j.1570-7458.1997.00214.x.
- Prado E, Tjallingii WF. 1997. Effects of previous plant infestation on sieve element acceptance by two aphids. Entomologia Experimentalis et Applicata 82(2):189–200. DOI: 10.1046/j.1570-7458.1997.00130.x.
- Qi SH, Zhang S, Qian PY, Wang BG. 2008. Antifeedant, antibacterial, and antilarval compounds from the South China sea seagrass Enhalus acoroides. Botanica Marina 51(5):441–447. DOI: 10.1515/BOT.2008.054.
- Reynolds M, Chapman S, Crespo-Herrera L, Molero G, Mondal S, Pequeno DNL, Pinto F, Pinera-Chavez FJ, Poland J, Rivera-Amado C, Saint Pierre C, Sukumaran S. 2020. Breeder friendly phenotyping. Plant Science: An International Journal of Experimental Plant Biology 295:110396. DOI: 10.1016/j.plantsci.2019.110396.
- Rivera MJ, Martini X, Conover D, Mafra-Neto A, Carrillo D, Stelinski LL. 2020. Evaluation of semiochemical based push-pull strategy for population suppression of ambrosia beetle vectors of laurel wilt disease in avocado. Scientific Reports 10(1):2670. DOI: 10.1038/s41598-020-59569-0.
- Sauvion N, Charles H, Febvay G, Rahbe Y. 2003. Effects of jackbean lectin (ConA) on the feeding behavior and kinetics of intoxication of the pea aphid, Acyrthosiphon pisum. Entomologia Experimentalis et Applicata 110:31–44. DOI: 10.1111/j.0013-8703.2004.00117.x.
- Sauvion N, Nardon C, Febvay G, Gatehouse AMR, Rahbe Y. 2004. Binding of the insecticidal lectin concanavalin a in pea aphid, Acyrthosiphon pisum (Harris) and induced effects on the structure of midgut epithelial cell. Journal of Insect Physiology 50:1137–1150. DOI: 10.1016/j.jinsphys.2004.10.006.
- Sauvion N, Rahbe Y. 1999. Recording feeding behaviour of Hemiptera with the EPG method: A review. Annales de la Societe Entomologique de France 35:175–183.
- Scarano A, Chieppa M, Santino A. 2018. Looking at flavonoid biodiversity in horticultural crops: A colored mine with nutritional benefits. Plants 7(4):98. DOI: 10.3390/plants7040098.
- Shi G, Kang Z, Ren F, Zhou Y, Guo P, Bendena B. 2020. Effects of quercetin on the growth and expression of immune-pathway-related genes in silkworm (Lepidoptera: Bombycidae). Journal of Insect Science 20(6):23. DOI: 10.1093/jisesa/ieaa124.
- Simmonds MSJ. 2001. Importance of flavonoids in insect-plant interactions: Feeding and oviposition. Phytochemistry 56:245–252. DOI: 10.1016/S0031-9422(00)00453-2.
- Simmonds MSJ. 2003. Flavonoid-insect interactions: Recent advances in our knowledge. Phytochemistry 64:21–30. DOI: 10.1016/S0031-9422(03)00293-0.
- Singh S, Kaur I, Kariyat R. 2021. The multifunctional roles of polyphenols in plant-herbivore interactions. International Journal of Molecular Sciences 22(3):1442. DOI: 10.3390/ijms22031442.
- Sosa ME, Tonn CE, Guerreiro E, Giordano OS. 2020. Bioactividad de flavonoides sobre larvas de Tenebrio monitor. Revista de la Sociedad Entomologica Argentina 59:179–184. https://www.biotaxa.org/RSEA/article/view/32428
- Souza MF, Davis JA, Ranger C, Ranger C. 2020. Detailed characterization of Melanaphis sacchari (Hemiptera: Aphididae) feeding behavior on different host plants. Environmental Entomology 49(3):683–691. DOI: 10.1093/ee/nvaa036.
- Sprawka I, Goławska S. 2010. Effect of the lectin PHA on the feeding behavior of the grain aphid. Journal of Pest Science 83(2):149–155. DOI: 10.1007/s10340-009-0281-x.
- Sprawka I, Goławska S, Czerniewicz P, Sytykiewicz H. 2011. Insecticidal action of phytohemagglutinin (PHA) against the grain aphid, Sitobion avenae. Pesticide Biochemistry and Physiology 100(1):64–69. DOI: 10.1016/j.pestbp.2011.02.006.
- Sprawka I, Goławska S, Goławski A, Czerniewicz P. 2013. Toxic and deterrent effects of phytohemagglutinin on the grain aphid Sitobion avenae. Biologia (Pakistan) 63(3):525–532. DOI: 10.2478/s11756-013-0175-5.
- StatSoft Inc. 2014. STATISTICA (data analysis) software system, version 12. Available: https://www.statsoft.com.
- Stec K, Kordan B, Gabryś B. 2021. Quercetin and Rutin as modifiers of aphid probing behavior. Molecules 26(12):3622. DOI: 10.3390/molecules26123622.
- Tjallingii WF. 1988. Electrical recording of stylet penetration activities. In: Minks A, Harrewijn P, editors. Aphids: Their biology, natural enemies and control. Amsterdam, Netherlands: Elsevier Publisher. pp. 95–108.
- Tjallingii WF. 1990. Continuous recording of stylet penetration activities by aphids. In: Campbell R, Eikenbary R, editors. Aphid–plant genotype interactions. Amsterdam, Netherlands: Elsevier Publisher. pp. 88–89.
- Tjallingii WF. 1994. Sieve element acceptance by aphids. European Journal of Entomology 91:47–52.
- Urbańska A, Tjallingii WF, Dixon AFG, Leszczynski B. 1998. Phenol oxidising enzymes in the grain aphid’s saliva. Entomologia Experimentalis et Applicata 86(2):197–203. DOI: 10.1046/j.1570-7458.1998.00281.x.
- Van Bel AJE, Will T. 2016. Functional evaluation of proteins in watery and gel saliva of aphids. Frontiers in Plant Science 7:1840. DOI: 10.3389/fpls.2016.01840.
- Van Emden HF. 2017. Host-plant resistance. In: Van Emden H, Harrington R, editors. Aphids as crop pests. Wallingford (Oxfordshire, UK): CABI. pp. 515–532.
- War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC. 2012. Mechanisms of plant defense against insect herbivores. Plant Signaling & Behavior 7(10):1306–1320. DOI: 10.4161/psb.21663.
- Węgierek P, Wojciechowski W. 2004. Aphids Aphidoidea (Hemiptera Sternorrhyncha). In: Bogdanowicz W, Chudzicka E, Pilipiuk I, Skibińska E, editors. Polish fauna - Characteristics and list of species, Vol. I. Warsaw, Poland: Museum and Institute of Zoology PAN. pp. 234–271.
- Wróblewska-Kurdyk A, Dancewicz K, Gliszczyńska A, Gabryś B. 2020. New insight into the behavior modifying activity of two natural sesquiterpenoids farnesol and nerolidol towards Myzus persicae (Sulzer) (Homoptera: Aphididae). Bulletin of Entomological Research 110(2):249–258. DOI: 10.1017/S0007485319000609.
- Xiao L, Carrillo J, Siemann E, Ding J. 2019. Herbivore-specific induction of indirect and direct defensive responses in leaves and roots. AoB Plants 11(1):lz003. DOI: 10.1093/aobpla/plz003.
- Zehnder G, Gurr GM, Kuhne S, Wade MR, Wratten SD, Wyss E. 2007. Arthropod pest management in organic crops. Annual Review of Entomology 52(1):57–80. DOI: 10.1146/annurev.ento.52.110405.091337.
