Abstract
In this paper we provide integrative description (morphological and genetic) of the new eutardigrade species Mesobiotus mandalori sp. nov. from central Poland, found during research on the vertical distribution of Tardigrada in the Czerniejewskie Forests. Mesobiotus mandalori sp. nov. belongs to the harmsworthi group, but it differs from the members of this group in some morphometric characters of the bucco-pharyngeal apparatus, claws and/or eggs. Morphologically, Meb. mandalori sp. nov. is most similar to Meb. pseudocoronatus, but it differs from it by a larger bare egg diameter and smaller number of egg processes on egg circumference. Morphometrical data were supported by results of comparative genetic analysis of four molecular markers: 18S rRNA, 28S rRNA, ITS2 and COI. According to COI the new species is most similar to Meb. skorackii with the genetic distance of 21.1%.https://zoobank.org/urn:lsid:zoobank.org:act:0222E328-CF9E-4D04-8F8C-69C80515FEE2
Introduction
Tardigrada is a phylum of microscopic multicellular animals widely distributed in most aquatic and terrestrial ecosystems throughout the world (Nelson et al. Citation2015). Currently, approximately 1400 species and subspecies of tardigrades have been described (Degma & Guidetti Citation2009–2024). However, it is very likely that the actual number of taxa is much higher (Bartels et al. Citation2016).
The tardigrade fauna of Poland has been studied since the early of twentieth century (Jakubski Citation1915), and so far, 114 species have been reported from this area. However, most of the species were identified many years ago and the current state of knowledge on Polish tardigrades is far from complete (see discussion below).
During research on the vertical distribution of Tardigrada on trees in the complex of Czerniejewskie Forests in central Poland (30 km E of Poznań, near Kostrzyn Wielkopolski), the results of which will soon be published in a separate paper, a new species of the genus Mesobiotus (Vecchi et al. Citation2016b) was found in a few of collected samples.
Currently, in the genus Mesobiotus, 75 species (76 with newly described one) have been described which makes it the second richest genus in the family Macrobiotidae (Degma & Guidetti Citation2009–2024). What is more, this richness is still growing very fast: only since the beginning of 2020, nine new species from all around the world have been described (Degma & Guidetti Citation2009–2024). The genus Mesobiotus is morphologically divided into two main species groups that differ in egg shell morphology: i) the Meb. harmsworthi group, characterized by eggs with conical, or hemispherical, reticulated processes; ii) the Meb. furciger group which is characterized by branched egg processes (see e.g. Degma & Guidetti Citation2009–2024; Kaczmarek et al. Citation2020a).
In this paper we provide description of the new Mesobiotus species (harmsworthi group) prepared by means of an integrative taxonomy approach combining morphological, morphometric and genetic data.
Material and methods
Study area, sampling and specimens extraction
Samples were collected in the Czerniejewskie Forests near Wagowo in the Wielkopolska Province (52°26′N, 17°20′E). The Czerniejewskie Forests are a small complex of managed forests with an area of approx. 15 km2. The character of the forests is mixed: mostly pines (Pinus silvestris) with a great admixture of deciduous trees, such as the Quercus robur, Q. petraea, Betula pendula, Populus nigra and Populus tremula, and is representative of the typical mixed coniferous forests of west and central Poland.
Moss samples were collected in autumn from an entire tree trunk of Q. robur (samples were taken from the base of the tree to the crown, at intervals of 50 cm) height of a trunk of examined tree reached 10 m. Then, samples were packed into paper envelopes and labelled with the number of the tree and a numerical designation of the height. Later, they were delivered to the laboratory at the Faculty of Biology, Adam Mickiewicz University in Poznań, where they were slowly air-dried.
All samples were examined according to standard methods (Stec et al. Citation2016) with minor modifications for ecological studies. A cut-off piece of a sample (no bigger than 0.25 g) was weighed and placed in a beaker filled with 200 ml of H2O for eight hours. Afterwards, the moss was vigorously stirred within the beaker with the use of a glass rod. The supernatant (containing tardigrades, their eggs, and other animals inhabiting the moss alongside moss particles) was transferred into a 250 ml cylinder for 30 minutes, to allow all the particles to fall to the bottom of the cylinder. Then, the top 120 ml of water was discarded and the remaining 80 ml was stirred and poured onto 10 cm Ø glass Petri dishes. Tardigrades and their eggs were extracted using an Olympus SZ61 stereomicroscope (Olympus Corporation, Shinjuku–ku, Japan). All the remaining sediments were placed on four layers of paper towel, where they were left for five days for drying. The dried material was weighed and packed into envelopes.
A total of 50 animals and 22 eggs of the new species were extracted from the samples CF.A 2.20 (CF.A - Czerniejewskie Forests Autumn) at a height of 9.5 m (40 animals and 22 eggs), CF.A 2.19 at a height of 9.0 m (four specimens) and CF.A 2.18 at a height of 8.5 m (six specimens) from tree (52°25′29.46″N, 17°21′16.22″E). Of these, 39 specimens and 20 eggs were fixed on microscope slides in Hoyer’s medium, one specimen and two eggs were prepared for Scanning Electron Microscopy (SEM) analysis, and the remaining 10 individuals were used for DNA extraction and sequencing. Animals used for SEM and molecular analyses were not included in the type series.
Microscopy and imaging
Fixed tardigrades and their eggs were examined under Phase Contrast Microscopy (PCM) (Olympus BX41 associated with an Olympus SC50 digital camera, Olympus Corporation, Shinjuku–ku, Japan). Specimens (animals and eggs) for SEM imagining were prepared according to the protocol described in Roszkowska et al. (Citation2018) and examined under a high vacuum in a Hitachi S3000N Scanning Electron Microscope (Olympus Corporation, Shinjuku-ku, Japan).
All the figures were assembled in Corel Photo-Paint X6. For deep structures that could not be fully focused in a single photograph, a series of 2–8 images were taken every approximately 0.5 µm and then manually assembled into a single deep-focus image in Corel Photo-Paint X6.
Morphometry and morphological nomenclature
The sample size for morphometrics was chosen according to Stec et al. (Citation2016). All measurements are given in micrometres (µm). All structures were measured only if their orientation was appropriate. The pt index used is the ratio of the length of a structure to the length of the buccal tube, expressed as a percentage (Pilato Citation1981). Buccal apparatus type and claws were classified according to both Pilato and Binda (Citation2010) and Vecchi et al. (Citation2016b), while terminology used to describe the oral cavity armature (OCA) follows Pilato (Citation1972) with modifications described in Michalczyk and Kaczmarek (Citation2003). Body length was measured from the anterior extremity and the posterior end of the body, excluding the IV pair of legs. Measurements of the buccal apparatus, claws and eggs were based on recommendations by Kaczmarek and Michalczyk (Citation2017). The macroplacoid length sequence is presented according to Kaczmarek et al. (Citation2014). The egg-shell morphology follows Kaczmarek et al. (Citation2020a). Nomenclature of the cuticular bars on legs follow Kiosya et al. (Citation2021).
All morphometric data were processed with the usage of the template “Parachela” ver. 1.8, available from the Tardigrada Register (Michalczyk & Kaczmarek Citation2013) updated with the Thorpe’s normalization of the data (as in Massa et al. Citation2021 according to Bartels et al. Citation2011) (SM.1).
Tardigrade taxonomy follows Bertolani et al. (Citation2014) and Vecchi et al. (Citation2016b), while genera abbreviations follow Perry et al. (Citation2019).
Comparative genetic analysis
Before genomic DNA extraction, each specimen was identified in vivo using light microscopy. The DNA extraction was made from specimens using the Chelex®100 resin (Bio-Rad) extraction method (Casquet et al. Citation2012). Additionally, we applied modification in order to obtain tardigrade exoskeletons (Stec et al. Citation2020). The exoskeletons were removed from the obtained extract and were examined under stereomicroscope. Then, exoskeletons were sent back to the Department of Animal Taxonomy and Ecology, Faculty of Biology, Adam Mickiewicz University in Poznań.
We sequenced four molecular markers, which differ in effective mutation rates: three nuclear fragments, i.e., 18S rRNA, 28S rRNA and ITS2 as well as one mitochondrial fragment, i.e., COI gene sequences. All applied primers have been listed in . The 18S rRNA, 28S rRNA and COI gene fragments were amplified according to the protocols described in Kaczmarek et al. Citation2020b. In turn, the ITS2 sequences were amplified according to the protocol provided by Stec et al. Citation2018. Exonuclease I (20 U/μl, Thermo Scientific, Vilnius, Lithuania) and alkaline phosphatase FastAP (1 U/μl, Thermo Scientific, Vilnius, Lithuania) were applied to clean the PCR products. Sequencing in both directions was performed using the BigDyeTM terminator cycle sequencing method and ABI Prism 3130×l genetic analyser (Life Technologies).
Table I. Primers with their original references used for sequencing of three molecular markers of Mesobiotus mandalori sp. nov.
All obtained sequences have been deposited in GenBank (for the accession numbers please see subsection “DNA sequences”).
Obtained nuclear and mitochondrial DNA molecular markers were checked for quality and consensus sequences were created in BioEdit v. 7.2.5 (Hall Citation1999). Then, the Basic Local Alignment Search Tool (the BLAST; Altschul et al. (Citation1990)) search at NCBI was used to verify the homology of the obtained nuclear sequences with molecular markers deposited in the GenBank database. The COI haplotypes were generated using DnaSP v5.10.01 program (Librado & Rozas Citation2009) and were translated into amino acid sequences using the EMBOSS- TRANSEQ application (Rice et al. Citation2000; Goujon et al. Citation2010) to check for indels, pseudogenes and internal stop codons.
Genetic comparisons between nuclear sequences obtained by us and molecular markers available in GenBank of the genus Mesobiotus were used to supplement phenotypic description of the new species. Single sequence of molecular markers representing each Mesobiotus species were downloaded from the database. All sequences obtained in our study, and sequences downloaded from GenBank database as originated from Mesobiotus, were aligned with ClustalW Multiple Alignment tool (Thompson et al. Citation1994), implemented in BioEdit v. 7.2.5, using default settings. Overall, aligned sequences were trimmed to 707 bp (20 species), 708 bp (14 species), 264 bp (14 species) and 568 bp (19 species) for 18S rRNA, 28S rRNA, ITS-2 and COI molecular markers, respectively. Downloaded nucleotide sequences which were too short after genetic comparisons or represented different fragments of DNA markers were not applied and as a result not all species belonging to the genus Mesobiotus were represented in all data sets. Mega X (Kumar et al. Citation2018) was applied to calculate the uncorrected genetic distances (p-distance) for each DNA fragment. Genetic distances were computed between species of the Mesobiotus species and the GenBank accession numbers of applied sequences provided in the Supplementary Materials (SM.2-SM.5).
Species identification
Identification of the new species was carried out according to the key to the Mesobiotus species and other original papers (Pilato et al. Citation2010; Tumanov Citation2018, Citation2020; Kaczmarek et al. Citation2020a; Stec Citation2021).
Results
Taxonomic account of the new species
Phylum: Tardigrada Spallanzani Citation1776
Class: Eutardigrada Richters Citation1926
Order: Parachela (Schuster et al. Citation1980)
Superfamily: Macrobiotoidea Thulin Citation1928 (in Marley et al. Citation2011)
Family: Macrobiotidae Thulin Citation1928
Genus: Mesobiotus Vecchi et al. Citation2016a
Mesobiotus mandalori sp. nov. (, )
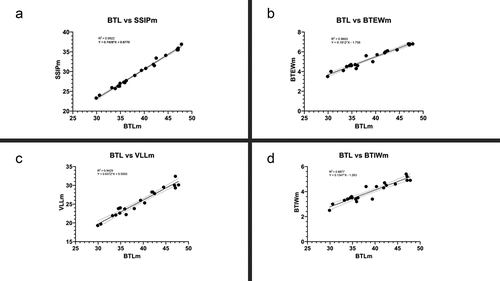
Table II. Linear regression of morphometric traits in relation to buccal tube length in Mesobiotus mandalori sp. nov. SSIPm represents Stylet support insertion point (µm), BTEWm represents Buccal Tube External width (µm), BTIWm represents Buccal Tube Internal Width (µm), VLLm represents Ventral Lamina Length (µm), M1m represents Macroplacoid 1 length (µm), M2m represents Macroplacoid 2 length (µm), M3m represents Macroplacoid 3 length (µm), Mim represents Microplacoid length (µm), MRm represents Macroplacoid Row length (µm) and PRm represents Placoid Row length (µm). The simple linear regression is performed using Graphpad Prisn 8.0.
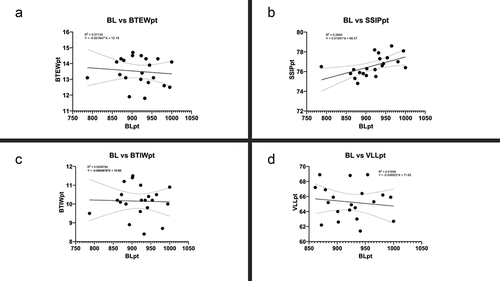
Table III. Linear regression of morphometric traits in relation to buccal tube length in Mesobiotus mandalori sp. nov. SSIPpt represents Stylet support insertion point (pt value), BTEWpt represents Buccal Tube External width (pt value), BTIWpt represents Buccal Tube Internal Width (pt value), VLLpt represents Ventral Laptina Length (pt value), PT1pt represents Macroplacoid 1 length (pt value), PT2pt represents Macroplacoid 2 length (pt value), PT3pt represents Macroplacoid 3 length (pt value), Mipt represents Microplacoid length (pt value), MRpt represents Macroplacoid Row length (pt value) and PRpt represents Placoid Row length (pt value). The simple linear regression is performed using Graphpad Prisn 8.0.
Only a small number of the new species character lengths were found to be non-isometric with regard to the buccal tube length following Thorpe’s normalization (SM.1). The range of pt indexes (min–max) for these characters did not differ significantly between the ranges estimated on the non-normalized data and the Thorpe’s normalized data (SM.1). Furthermore, results for simple linear regression analysis performed for measurements and pt value using GraphPad Prism 8.0 are presented in as well as (SSIP represents Stylet support insertion point, BTEW represents Buccal Tube External width, BTIW represents Buccal Tube Internal Width, VLL represents Ventral Lamina Length, M1 represents Macroplacoid 1 length, M2 represents Macroplacoid 2 length, M3 represents Macroplacoid 3 length, Mi represents Microplacoid length, MR represents Macroplacoid Row length, PR represents Placoid Row length m represents measured value in µm and pt represents pt value). For measured value all of the characters showed significance with p value < 0.0001, however, only SSIPpt showed significance with p value < 0.05.
Etymology
The name mandalori alludes to the fictional planet of Mandalore (home world of Mandalorians), from the Star Wars Universe. The first ever visual depiction of Mandalore showed this world as being covered by dense forests, and its inhabitants living in villages hidden in the tree canopies. This reflects the type locality of the new species, i.e. moss on trees in deep forest.
Material examined
In total 72 specimens (animals and eggs) were examined. The 39 animals and 20 eggs were mounted on microscope slides in Hoyer’s medium, one animal and two eggs were fixed on SEM stubs and 10 animals were processed for DNA sequencing.
Type depositories
The holotype (CF.A.2.20.9) with 50 paratypes, both animals and eggs (slides: CF.A.2.20./*, where the asterisk can be substituted by any of the following numbers: 2–14, –, 16–19, 21–27, 32, 34, 36, 43–45, 48, 50, 51, 54–55 and: CF.A.2.20.2/– part of the sample 2.20 taken for ecological studies) are deposited in the Department of Animal Taxonomy and Ecology, Institute of Environmental Biology, Adam Mickiewicz University, Poznań, Uniwersytetu Poznańskiego 6, 61–614 Poznań, Poland. 6 paratypes and two measured eggs (slides CF.A.2.20./*, where the asterisk can be substituted by any of the following numbers: 1, 15, 20, 41, 42, 57, 59) are deposited at the Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Sławkowska 17, 31–016 Kraków (Poland).
Description of the new species
Animals (all measurements and statistics in ). Live specimens white, but transparent after fixation in Hoyer’s medium (). Eyes present in all examined specimens (). Body cuticle smooth and without pores (). Granulation on legs I-IV present and clearly visible in PCM ().
Table IV. Measurements [in µm] and pt values of selected morphological structures of Mesobiotus. mandalori sp. nov. Table contains measurements [in µm] and pt values of selected morphological structures of the newly described species. “N” stands for the number of specimens/structures measured; “RANGE” refers to measurements taken for the smallest and the largest structure among all measured specimens; SD stands for standard deviation.
Figure 3. Mesobiotus mandalori sp. nov. — Phase-contrast microscope (PCM) and scanning electron microscope (SEM images of habitus morphology: (a) — PCM dorso-ventral projection (holotype); (b) — SEM dorsal projection (paratype). Scale bars in μm.
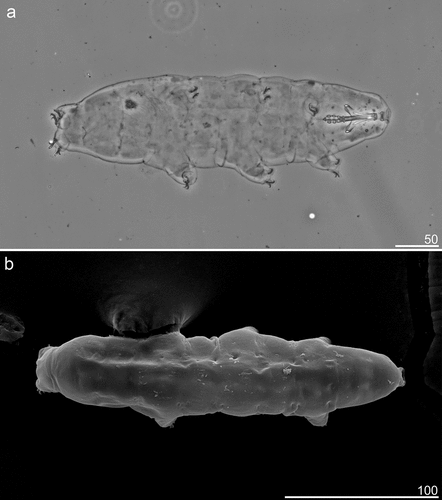
Figure 4. Mesobiotus mandalori sp. nov. —PCM images of claws I, III and IV: (a) — Claw I with smooth lunules (paratype); (b) —Claw III with smooth lunules (paratype); (c) — Claw IV with dented lunules (holotype). (d) — Lunules under claws IV with dented lunules (paratype). Filled flat arrowhead indicates lunules; filled indented arrowheads indicate the granulation; empty indented arrowheads indicate the indicates horseshoe structure connecting the anterior and the posterior claws; empty arrow indicates a single continuous cuticular bar under the claws; filled arrow indicates paired muscle attachments Scale bars in μm.
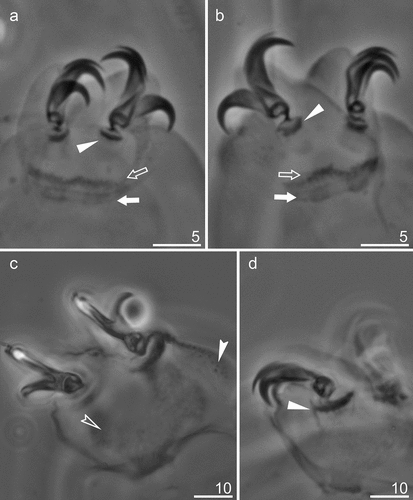
Claws of the Mesobiotus type with minute stalk, distinct distal part of the basal portion and short common tract (). Primary and secondary branches diverge at a point near half the claw height. All primary branches with accessory points (). The claws of the fourth pair of legs slightly longer than others (). Anterior (internal) and posterior (external) claws of legs IV similar in shape, however posterior claws slightly longer than anterior ones. Lunules under claws I-III smooth and slightly dentate on under claws IV (, filled unindented arrowheads; 4D, filled unindented arrowheads). Thin singular cuticular bars (, empty unindented arrowheads) and double muscle attachments under claws I-III present (, filled arrows). In addition, horse-shoe-shaped structures connecting anterior and posterior claws of IV pair of legs also present (, filled indented arrowhead).
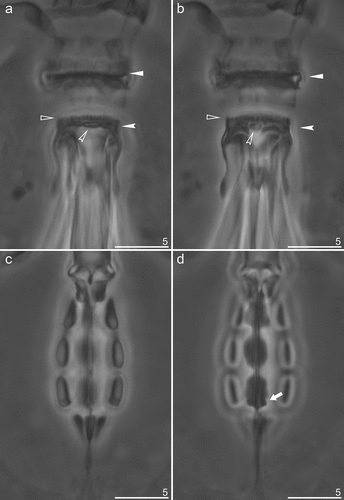
Bucco-pharyngeal apparatus of Mesobiotus type () with ventral lamina and 10 peribuccal lamellae (). Oral cavity armature (OCA) of the harmsworthi type, with three bands of teeth visible in PCM (). First (anterior) band of small teeth, in form of granules, clearly visible in PCM (, filled unindented arrowheads), a second (posterior) band of teeth in the form of a row composed of comma-shaped ridges, parallel to the main axis of the buccal tube (, empty unindented arrowheads); and finally the third band in the form of a system of three dorsal and three ventral transverse ridges (, indented arrowheads). Dorsal ridges similar in length and equally thick, however the lateral ones are thinning toward the external endings (). Lateral ventral ridges are curved, longer and thicker than the middle one, but thin toward the external endings, while the middle ventral ridge is shorter and roundish, and has the form of a singular tooth (). Pharyngeal bulb with apophyses, three rod-shaped macroplacoids and a large microplacoid located very closely to the last macroplacoid (). Macroplacoid length sequence 1 > 3 > 2. First and second macroplacoid without constrictions and protrusions. Third macroplacoid with shallow, distinct subterminal constriction, but without terminal protrusion (, filled arrow).
Figure 5. Mesobiotus mandalori sp. nov. —PCM images of the entire buccal apparatus: (a) — dorsal view of entire buccal apparatus (holotype); (b) — ventral view of entire buccal apparatus (holotype). Scale bars in μm.
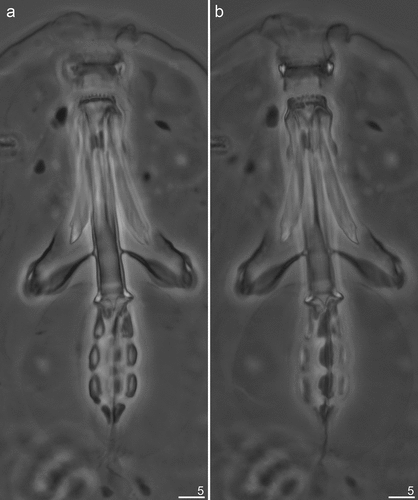
Figure 6. Mesobiotus mandalori sp. nov. —PCM images of oral cavity armature (OCA) and placoid morphology: (a) — dorsal view of OCA (paratype); (b) — ventral view of OCA (paratype); (c) — dorsal view of placoid morphology; (d) — ventral view of placoid morphology. Filled flat arrowheads indicate peribuccal lamellae; empty flat arrowheads indicate the first band of teeth; filled indented arrowheads indicate the second band of teeth; filled indented arrowheads indicate the third band of teeth, filled arrows indicate the middle ridge dorsal and ventral respectively; empty arrows indicate subterminal constrictions in the third macroplacoid. Scale bars in μm.
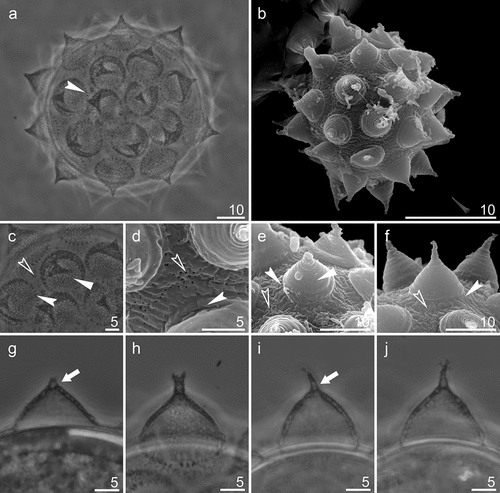
Eggs (all measurements and statistics in ). Spherical, white and laid freely. Egg chorion with processes in the shape of wide sharp cones () with thick, but flexible, shorter or longer endings (often broken) (). In some processes, its apical parts bifurcated or divided into even more short filaments (). In apical parts of some egg processes, small bubble-like internal structures are clearly visible in PCM (). Processes with two-layer walls () with a net of trabecular structures between both layers, forming reticular design, clearly visible in PCM (). In SEM, the surface of processes smooth or rarely with small pores on its bases (). Additionally, reticular design is sometimes visible under the smooth walls (). Egg processes bases surrounded by a crown of thickening (). Egg shell covered with small granules (in PCM), which in SEM are visible as small pores and wrinkles ().
Table V. Measurements [in µm] of selected morphological structures of eggs of Mesobiotus mandalori sp. nov. Table contains measurements [in µm] structures of eggs of newly described species. Where “N” stands for the number of specimens/structures measured; “RANGE” refers to measurements taken for the smallest and the largest structure among all measured specimens; SD stands for standard deviation.
Figure 7. Mesobiotus mandalori sp. nov. —PCM and SEM images of the eggs, egg surface, egg processes surface and midsections: (a) PCM image of the eggs; (b) SEM image of the egg; (c) PCM image of the egg surface; (d) SEM image of the egg surface; (e-f) SEM images of the egg processes surface; (g-j) PCM images of the egg processes midsections. Filled flat arrowhead indicates reticular design; filled indented arrowheads indicate crowns of strong thickenings around the process base; empty indented arrowheads indicate pores;filled arrow indicates bubble-like internal structures, Filled flat arrowheads indicate reticular design; filled arrows crowns of strong thickenings around the process base; empty arrows indicate pores. Scale bars in μm.
DNA sequences
We obtained good quality sequences for four applied molecular markers:
COI: GenBank: OP825090–91 (species voucher numbers: 2.18.1.2 and 2.18.1.3), 608–619 bp long;
18S rRNA: GenBank: OP829143–44 (species voucher numbers: 2.18.1.2 and 2.18.1.3), 1527–1543 bp long;
28S rRNA: GenBank: OP829056–57 (species voucher numbers: 2.18.1.2 and 2.18.1.3), 792–798 bp long;
ITS-2: GenBank: OP829058 (species voucher numbers: 2.18.1.2), 287 bp long.
Genetic distances
The ranges of uncorrected genetic p-distances between Meb. mandalori sp. nov. and other species of the genus Mesobiotus are as follows (for details see SM.2-SM.5):
COI: 21.1–27.8% (25.0% on average), with the most similar being Meb. skorackii Kaczmarek, Zawierucha, Buda, Stec, Gawlak, Michalczyk & Roszkowska, Citation2018 (GenBank: MW656257), and the least similar being Meb. furciger (Murray Citation1907) (GenBank: MW727960).
18S rRNA: 0.7–5.3% (3.2% on average), with the most similar being Meb. cf. barabanovi (GenBank: MN310392), and the least similar being Meb. dilimanensis Itang, Stec, Mapalo, Mirano-Bascos & Michalczyk, Citation2020 (GenBank: MN257048) and Meb. marmoreus Stec Citation2021 (GenBank: OL257858).
28S rRNA: 3.9–12.0% (8.3% on average), with the most similar being Meb. skorackii (GenBank: MW680636), and the least similar being Meb. marmoreus (GenBank: OL257870).
ITS-2: 22.7–27.2% (28.3% on average), with the most similar being Meb. occultatus Kaczmarek, Zawierucha, Buda, Stec, Gawlak, Michalczyk & Roszkowska, Citation2018 (GenBank: MH197155), and the least similar being Meb. marmoreus (GenBank: OL257863).
Differential diagnosis
Mesobiotus mandalori sp. nov. belongs to the harmsworthi group (according to Kaczmarek et al. Citation2011, Citation2018) with eggs with conical and reticulated processes with nonbranching apical parts and processes bases surrounded by the crown of dot-like structures or radial ridges. The new species is similar in those features to 19 taxa, however the new species differs from:
Mesobiotus binieki (Kaczmarek et al. Citation2011) (known only from the type locality in Bulgaria) by having shorter apical parts of the processes i.e. much shorter than conical basal parts (long spiniform apical parts, much longer than conical basal parts in Meb. binieki), smaller eggs (53.7–77.7 µm and 70.2–101.3 µm bare and full diameter, respectively, in the new species vs 85.1–94.5 µm and 108.7–114.7 µm bare and full diameter, respectively, in Meb. binieki), wider egg processes bases (12.6–19.0 µm in the new species vs 6.5–9.0 µm in Meb. binieki) and a smaller number of egg processes on egg circumference (10–12 in the new species vs 27–32 in Meb. binieki).
Mesobiotus coronatus (de Barros Citation1942) (known only from South America (Pilato et al. Citation2000; Kaczmarek et al. Citation2015)) by the absence of supplementary teeth in OCA, longer egg processes (9.8–16.3 µm in the new species vs 8.3–9.2 µm in Meb. coronatus) and wider egg processes bases (12.6–19.0 µm in the new species vs 8.2–10.4 µm in Meb. coronatus).
Mesobiotus diffusus (Binda & Pilato Citation1987) (known from Italy, Seychelles Tunisia (type locality) and New Zealand (Binda & Pilato Citation1995; Pilato & Binda Citation1996)) by the presence of eyes, absence of long flexible filaments on the top of egg processes, absence of the system of radial ridges on egg shell and the smaller number of egg processes on egg circumference (10–12 in the new species vs 15–23 in Meb. diffuses).
Mesobiotus dimentmani (Pilato et al. Citation2010) (known only from the type locality in Israel) by lower pt of the buccal tube external width (11.8–14.5 in the new species vs 19.3–23.2 in Meb. dimentmani), smaller eggs (bare egg diameter 53.7–77.7 µm in the new species vs 79.0–90.0 µm in Meb. dimentmani) and the smaller number of egg processes on egg circumference (10–12 in the new species vs 15–17 in Meb. dimentmani).
Mesobiotus helenae Tumanov & Pilato Citation2019 (known only from the type locality in New Zealand) by the presence of eyes, presence of granulation on legs, presence of indented lunules on IV pair of legs, having narrow apical parts of the processes i.e. much shorter than conical basal parts (long spiniform apical parts, much longer than conical basal parts in Meb. helenae), wider egg processes bases (12.6–19.0 µm in the new species vs 6.9–8.2 µm in Meb. helenae) and a smaller number of egg processes on egg circumference (10–12 in the new species vs approximately 22 in Meb. helenae).
Mesobiotus imperialis Stec Citation2021 (known only from the type locality in Vietnam) by, not divided ventro-median tooth in OCA, lower pt of buccal tube external width (11.8–14.5 in the new species vs 15.7–18.1 in Meb. imperialis), wider egg processes bases (12.6–19.0 µm in the new species vs 6.9–12.5 µm in Meb. imperialis) and a smaller number of egg processes on egg circumference (10–12 in the new species vs 15–18 in Meb. imperialis).
Mesobiotus insuetus (Pilato et al. Citation2014) (known only from the type locality in Italy) by the presence of eyes, presence of indented lunules on IV pair of legs, lower pt value of stylet supports insertion points (74.8–78.6 in the new species vs 79.0–79.4 in Meb. insuetus) and longer egg processes (9.8–16.3 µm in the new species vs 7.9–8.6 µm in Meb. insuetus).
Mesobiotus nikolaevae Tumanov Citation2018 (known only from the type locality in Croatia) by the presence of indented lunules on IV pair of legs, lower pt of external width of the buccal tube (11.8–14.7 in the new species vs 15.6–16.5 in Meb. nikolaevae), lower pt value of the length of the secondary branch of internal claws of III pair of legs (12.2–17.1 in the new species vs 17.2–22.0 in Meb. nikolaevae) and generally smaller number of egg processes on egg circumference (10–12 in the new species vs 12–14 in Meb. nikolaevae).
Mesobiotus occultatus Kaczmarek et al. Citation2018 (known only from the type locality on Svalbard, Norway) by the presence of indented lunules on IV pairs of legs, lower pt values for the length of secondary branches of internal claws of I and II pair of legs (I: 12.6–17.6 and II: 12.2–17.1 in the new species vs I: 18.2–20.9 and II: 17.7–21.2 in Meb. occultatus) and the smaller number of egg processes on egg circumference (10–12 in the new species vs 13–16 in Meb. occultatus).
Mesobiotus patiens (Pilato et al. Citation2000) (known from the Aeolian Islands and several other islands in the Tyrrhenian Sea, Italy (Pilato et al. Citation2000)) by the presence of eyes, presence of indented lunules on IV pairs of legs, lower pt of the buccal tube external width (11.8–14.5 in the new species vs 17.9–21.1 in Meb. patiens). The comparison is based on morphometric data provided by Pilato et al. (Citation2000) and Pilato and Lisi (Citation2009).
Mesobiotus perfidus (Pilato & Lisi Citation2009) (known only from the type locality on Seychelles Islands) by the presence of the first band of teeth in the OCA, presence of granulation on legs I–III and indented lunules on IV pairs of legs and having shorter apical parts of the processes i.e. much shorter than conical basal parts (long spiniform apical parts, longer than conical basal parts in Meb. perfidus).
Mesobiotus philippinicus Mapalo et al. Citation2016 (known from the type locality in the Philippines) by the non-divided ventro-median tooth in OCA, shallower subterminal-constriction on the third macroplacoid, higher pt value for ventral lamina length (61.4–68.9 in the new species vs 51.4–58.8 in Meb. philippinicus), generally shorter claws (see in the present paper and in Mapalo et al. (Citation2016)), having a surface of egg process without wrinkles (in SEM) and the smaller number of egg processes on egg circumference (10–12 in the new species vs 15–17 in Meb. philippinicus).
Mesobiotus pseudocoronatus (Pilato et al. Citation2006) (known only from the type locality on Seychelles Islands) by a larger bare egg diameter (53.7–77.7 µm in the new species vs approximately 50.1 µm in Meb. pseudocoronatus) and the smaller number of egg processes on egg circumference (10–12 in the new species vs approximately 14 in Meb. pseudocoronatus).
Mesobiotus pseudopatiens Kaczmarek & Roszkowska Citation2016 (known only from the type locality in Costa Rica) by the presence of eyes, presence of the first band of teeth, presence of granulation on legs I – III and indented lunules on IV pair of legs and shorter primary branches of internal claws of the third pair of legs (12.2–17.1 µm in the new species vs 17.4–21.1 µm in Meb. pseudopatiens).
Mesobiotus radiatus (Pilato et al. Citation1991) (known from Kenya and Tanzania (type locality) (Stec et al. Citation2018)) by the absence of granulation on the dorsal cuticle, the presence of eyes, non-divided ventro-median tooth in OCA, generally shorter claws (see in the present paper and in Stec et al. (Citation2018)), absence of bunches of thin, long, granulated and flexible filaments, and the absence of pores on the surface of apical parts of egg processes. The comparison is based on morphometric data provided by Pilato et al. (Citation1991) and Stec et al. (Citation2018).
Mesobiotus rigidus (Pilato & Lisi Citation2006) (known only from the type locality in New Zealand) by the presence of eyes, presence of granulation on legs I – III, having shorter apical parts of the processes i.e. much shorter than conical basal parts (long spiniform apical parts, similar in length to conical basal parts in Meb. rigidus).
Mesobiotus romani Roszkowska et al. Citation2018 by the presence of eyes, non-divided ventro-median tooth in OCA, different macroplacoid length sequence (1 > 3 > 2 in the new species vs 3 ≥ 1 > 2 in Meb. romani), having lower pt values of claws (see in the present paper and in Roszkowska et al. (Citation2018)), having shorter apical parts of the processes i.e. much shorter than conical basal parts (long spiniform apical parts, similar in length to conical basal parts in Meb. romani) and the smaller number of egg processes on egg circumference (10–12 in in the new species vs 16–17 in Meb. romani).
Mesobiotus simulans (Pilato et al. Citation2000) (known from Sardinia (type locality), a few other localities in Italy, and from Israel (Pilato et al. Citation2000, Citation2010) by having a lower pt of external width of the buccal tube (11.8–14.5 in the new species vs 17.5–29.1 in Meb. simulans), and having apical parts of the egg processes bifurcated or divided into even more short filaments. The comparison is based on the Meb. simulans description by Pilato et al. (Citation2000), and morphometric data provided by Pilato and Lisi (Citation2009).
Mesobiotus wuzhishanensis (Yin et al. Citation2011) by generally shorter claws (see in the present paper and in Yin et al. (Citation2011)), smaller eggs (53.7–77.7 µm and 70.2–101.3 µm bare and full diameter, respectively, in the new species vs approximately 85.8 µm and 114.1 µm bare and full diameter, respectively, in Meb. wuzhishanensis), smaller egg processes (9.8–16.3 µm in the new species vs approximately 18.5 µm in Meb. wuzhishanensis), wider egg processes bases (12.6–19.0 µm in the new species vs approximately 10.6 µm in Meb. wuzhishanensis) and a smaller number of egg processes on egg circumference (10–12 in the new species vs approximately 16 in Meb. wuzhishanensis).
Discussion
Tardigrades fauna of Poland consists of 114 species (115 including the newly-described species) (Dastych Citation1980, Citation1988; Kaczmarek & Michalczyk Citation2002, Citation2003, Citation2004, Citation2005; Kaczmarek et al. Citation2010, Citation2018, Citation2018b; Zawierucha Citation2011; Zawierucha et al. Citation2015; Nowak & Stec Citation2017, Citation2018; Stec et al. Citation2017; Gąsiorek & Degma Citation2018; Kayastha et al. Citation2021b), which makes around 7.8% of all known tardigrade species (Degma & Guidetti Citation2009–2024). At the same time, it is estimated that all of the known species of tardigrades in the world account for between one-half to one-fourth of the entire richness of this group (Bartels et al. Citation2016).
By contrast, the tardigrade fauna of Wielkopolska Province consists of only 30 known species (31 including the newly described species) (Kaczmarek & Michalczyk Citation2003, Citation2004, Citation2005; Kaczmarek et al. Citation2010; Nowak & Stec Citation2017; Stec et al. Citation2017; Kayastha et al. Citation2021b).
The genus Mesobiotus is a relatively new taxon that was excluded from the genus Macrobiotus in 2016 based on molecular and morphological data (Vecchi et al. Citation2016b) and initially consisted only of 55 species previously assigned to the genus Macrobiotus (Vecchi et al. Citation2016b; Degma & Guidetti Citation2009–2024). Four other species of this genus were described already in 2016 (including one described by Vecchi et al. (Citation2016b); Degma & Guidetti Citation2009–2024), followed by more. Up to end of 2022 17 new species of genus Mesobiotus were discovered (Degma & Guidetti Citation2009–2024; Kaczmarek et al. Citation2018; Tumanov Citation2018, Citation2020; Tumanov & Pilato Citation2019; Stec Citation2019, Citation2021, Citation2022). But studies on this genus did not end on description of new species. Recently the type species of the genus of Meb. harmsworthi (Murray Citation1907) was redescribed (Kaczmarek et al. Citation2018). Moreover, as was mentioned in the introduction, the genus Mesobiotus is morphologically divided into two main species groups Meb. harmsworthi group, and the Meb. furciger (see e.g. Kaczmarek et al. Citation2020a). It was recently shown that those two groups are not monophyletic and that their existence has no support in molecular phylogenetic data (Stec Citation2021; Stec et al. Citation2021; Short et al. Citation2022). As a result, they should be referred to as “morphogroups” only (Short et al. Citation2022) which are not taxonomic ranks. However, some tardigradologists began to wonder whether using this terminology is justified. Nevertheless, this term is often used in systematics papers, mostly because of its utility in describing newly found species (see e.g. Stec Citation2021; Short et al. Citation2022) and in this context it is used in this work.
As stated previously, to date 75 species have been described in Mesobiotus genus (Degma & Guidetti Citation2009–2024) (11 species in Meb. furciger group and 28 species in Meb. harmsworthi group), which makes around 5.1% of all known tardigrade species (Degma & Guidetti Citation2009–2024). Among all these species only Meb. harmsworthi (besides the newly described one) was reported from territory of Poland (Dastych Citation1980, Citation1988) (also found in Wielkopolska Province (Kaczmarek & Michalczyk Citation2003)). However, these records were published before the formal redescription of Meb. harmsworthi and the presence of this species in Poland is doubtful.
As mentioned above, the current state of knowledge of both the tardigrade fauna of Poland and the diversity of the Mesobiotus genus is far from complete, and faunistic research in these areas could still provide new discoveries.
Supplemental Material
Download MS Excel (12.2 KB)Supplemental Material
Download MS Excel (12.5 KB)Supplemental Material
Download MS Excel (13.5 KB)Supplemental Material
Download MS Excel (14.3 KB)Supplemental Material
Download MS Excel (114.8 KB)Acknowledgments
The authors wish to thank Cambridge Proofreading LLC (http://proofreading.org) for their linguistic assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2024.2341884
Additional information
Funding
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology 215(3):403–410. DOI: 10.1016/S0022-2836(05)80360-2.
- Bartels PJ, Apodaca JJ, Mora C, Nelson DR. 2016. A global biodiversity estimate of a poorly known taxon: Phylum Tardigrada. Zoological Journal of the Linnean Society 178(4):730–736. DOI: 10.1111/zoj.12441.
- Bartels PJ, Nelson DR, Exline RP. 2011. Allometry and the removal of body size effects in the morphometric analysis of tardigrades. Journal of Zoological Systematics and Evolutionary Research 49(s1):17–25. DOI: 10.1111/j.1439-0469.2010.00593.x.
- Bertolani R, Guidetti R, Marchioro T, Altiero T, Rebechci L, Cesari M. 2014. Phylogeny of Eutardigrada: New molecular data and their morphological support lead to the identification of new evolutionary lineages. Molecular Phylogenetics and Evolution 76:110–126. DOI: 10.1016/j.ympev.2014.03.006.
- Binda MG, Pilato G. 1987. Tardigrada dell’Africa. V. Notizie sui Tardigradi del Nord-Africa e descrizione della nuove specie Macrobiotus diffuses. Animalia 14(1/3):177–191.
- Binda MG, Pilato G. 1995. Remarks on tardigrades from the Seychelles, with a description of two new species. Tropical Zoology 8(1):1–6. DOI: 10.1080/03946975.1995.10539269.
- Casquet J, Thebaud C, Gillespie RG. 2012. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol‐stored spiders. Molecular Ecology Resources 12(1):136–141. DOI: 10.1111/j.1755-0998.2011.03073.x.
- Dastych H. 1980. Niesporczaki (Tardigrada) Tatrzańskiego Parku Narodowego. Monografie Fauny Polski PWN, Kraków 9:1–232.
- Dastych H. 1988. The Tardigrada of Poland. Monografie Fauny Polski PWN, Kraków 16:1–255.
- de Barros R. 1942. Tardigrados de Estado de Sao Paulo, Brasil. II. Gênero Macrobiotus. Revista Brasileira de Biologia 2:373–386.
- Degma P, Guidetti R. 2009–2024. Actual checklist of Tardigrada species (2009-2023, 42th Edition: 09-01-2023). DOI: 10.25431/11380_1178608.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3(5):294–299. Table 1.
- Gąsiorek P, Degma P. 2018. Three Echiniscidae species (Tardigrada: Heterotardigrada) new to the Polish fauna, with the description of a new gonochoristic Bryodelphax Thulin. Zootaxa 4410(1):77–96. DOI: 10.11646/zootaxa.4410.1.4.
- Goujon M, Mcwilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R. 2010. A new bioinformatics analysis tools framework at EMBL–EBI. Nucleic Acids Research 38:695–699. DOI: 10.1093/nar/gkq313.
- Hall TA. 1999. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41:95–98.
- Jakubski A. 1915. Opis fauny wrotków (Rotatoria) powiatu Sokalskiego z uwzględnieniem gromad brzuchorzęsków (Gartroprioga) i niesporczaków (Tardigrada). Wiadomości z Muzeum im. Dzieduszyckich, Lwów 1:1–166.
- Kaczmarek Ł, Bartylak T, Stec D, Kulp A, Kepel M, Kepel A, Roszkowska M. 2020a. Revisiting the genus Mesobiotus Vecchi et al. 2016 (Eutardigrada, Macrobiotidae) – remarks, updated dichotomous key and an integrative description of new species from Madagascar. Zoologischer Anzeiger 287:121–146. DOI: 10.1016/j.jcz.2020.05.003.
- Kaczmarek Ł, Cytan J, Zawierucha K, Diduszko D, Michalczyk Ł. 2014. Tardigrades from Peru (South America), with descriptions of three new species of Parachela. Zootaxa 3790(2):357–379. DOI: 10.11646/zootaxa.3790.2.5.
- Kaczmarek Ł, Gołdyn B, Czyz M, Michalczyk Ł. 2010. First records of Isohypsibius pushkini Tumanov, 2003 (Tardigrada, Eutardigrada, Hypsibiidae) from Poland. Biological Letters 47(2):81–85. DOI: 10.2478/v10120-009-0020-2.
- Kaczmarek Ł, Gołdyn B, Prokop ZM, Michalczyk Ł. 2011. New records of Tardigrada from Bulgaria with the description of Macrobiotus binieki sp. nov. (Eutardigrada: Macrobiotidae) and a key to the species of the harmsworthi group. Zootaxa 2781(1):29–39. DOI: 10.11646/zootaxa.2781.1.2.
- Kaczmarek Ł, Kosicki JZ, Roszkowska M. 2018. Tardigrada of Bory Tucholskie National Park, Zaborski Landscape Park, and their surroundings (Pomerania Province, Poland). Turkish Journal of Zoology 42:6–17. DOI: 10.3906/zoo-1705-44.
- Kaczmarek Ł, Michalczyk Ł. 2002. Calohypsibius schusteri Nelson et. McGlothlin, 1996 and Macrobiotus crenulatus Richters, 1904 – new species of water bears (Tardigrada) to the fauna of Poland. Przegląd Zoologiczny 46(1–2):67–69.
- Kaczmarek Ł, Michalczyk Ł. 2003. Niesporczaki (Tardigrada) Wielkopolskiego Parku Narodowego (środkowo-zachodnia Polska). Rocznik Naukowy Polskiego Towarzystwa Ochrony Przyrody Salamandra 7:55–64.
- Kaczmarek Ł, Michalczyk Ł. 2004. Diphascon (Diphascon) ilitsi (Schuster et Grigarick, 1965) gatunek niesporczaka (Tardigrada) nowy dla fauny Polski. Przegląd Zoologiczny 48(1–2):97–98.
- Kaczmarek Ł, Michalczyk Ł. 2005. Thulinius ruffoi Bertolani, 1981 rodzaj i gatunek niesporczaka (Tardigrada) nowy dla fauny Polski. Przegląd Zoologiczny 49(1–2):57–59.
- Kaczmarek Ł, Michalczyk Ł. 2017. The Macrobiotus hufelandi group (Tardigrada) revisited Zootaxa. Zootaxa 4363(1):101–123. DOI: 10.11646/zootaxa.4363.1.4.
- Kaczmarek Ł, Michalczyk Ł, McIness SJ. 2015. Annotated zoogeography of non-marine Tardigrada. Part II: South America. Zootaxa 3923(1):1–107. DOI: 10.11646/zootaxa.3923.1.1.
- Kaczmarek Ł, Roszkowska M. 2016. A new eutardigrade from Costa Rica with taxonomical and zoogeographical remarks on Costa Rican tardigrades. New Zealand Journal of Zoology 43(3):234–245. DOI: 10.1080/03014223.2016.1156553.
- Kaczmarek Ł, Roszkowska M, Poprawa I, Janelt K, Kmita H, Gawlak M, Fiałkowska E, Mioduchowska M. 2020b. Integrative description of bisexual Paramacrobiotus experimentalis sp. nov. (Macrobiotidae) from republic of Madagascar (Africa) with microbiome analysis. Molecular Phylogenetics and Evolution 145:106730. DOI: 10.1016/j.ympev.2019.106730.
- Kaczmarek Ł, Zawierucha K, Buda J, Stec D, Gawlak M, Michalczyk Ł, Roszkowska M, Rubal M. 2018. An integrative redescription of the nominal taxon for the Mesobiotus harmsworthi group (Tardigrada: Macrobiotidae) leads to descriptions of two new Mesobiotus species from Arctic. PLOS ONE 13(10):e0204756. DOI: 10.1371/journal.pone.0204756.
- Kayastha P, Roszkowska M, Mioduchowska M, Gawlak M, Kaczmarek Ł. 2021a. Integrative descriptions of two new tardigrade species along with the new record of Mesobiotus skorackii Kaczmarek et al. 2018 from Canada. Diversity 13(8):394 DOI: 10.3390/d13080394. (citated in SM2 – SM5).
- Kayastha P, Wiśniewska J, Kuzdrowska K, Kaczmarek Ł. 2021b. Aquatic tardigrades in Poland – a review. Limnological Review 21(3):147–154. DOI: 10.2478/limre-2021-0013.
- Kiosya Y, Pogwizd J, Matsko Y, Vecchi M, Stec D. 2021. Phylogenetic position of two Macrobiotus species with a revisional note on Macrobiotus sottilei Pilato, Kiosya, Lisi & Sabella, 2012 (Tardigrada: Eutardigrada: Macrobiotidae). Zootaxa 4933:113–135. DOI: 10.11646/zootaxa.4933.1.5.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K, Battistuzzi FU. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35(6):1547–1549. DOI: 10.1093/molbev/msy096.
- Librado P, Rozas J. 2009. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25(11):1451–1452. DOI: 10.1093/bioinformatics/btp187.
- Mapalo MA, Stec D, Mirano-Bascos D, Michalczyk Ł. 2016. Mesobiotus philippinicus sp. nov. the first limnoterrestrial tardigrade from the Philippines. Zootaxa 4126(3):411–426. DOI: 10.11646/zootaxa.4126.3.6.
- Mapalo M, Stec D, Mirano-Bascos D, Michalczyk L. 2017. An integrative description of a limnoterrestrial tardigrade from the Philippines, Mesobiotus insanis, new species (Eutardigrada: Macrobiotidae: Harmsworthi group). (citated in SM2 – SM5).
- Marley NJ, McInnes SJ, Sands CJ. 2011. Phylum Tardigrada: A re-evaluation of the Parachela. Zootaxa 2819(2819):51–64. DOI: 10.11646/zootaxa.2819.1.2.
- Massa E, Guidetti R, Cesari M, Rebecchi L, Jönsson KI. 2021. Tardigrades of Kristianstads Vattenrike Biosphere Reserve with description of four new species from Sweden. Scientific Reports 11(1):4861. DOI: 10.1038/s41598-021-83627-w.
- Michalczyk Ł, Kaczmarek Ł. 2003. A description of the new tardigrade Macrobiotus reinhardti (Eutardigrada: Macrobiotidae, harmsworthi group) with some remarks on the oral cavity armature within the genus Macrobiotus Schultze. Zootaxa 331(1):1–24. DOI: 10.11646/zootaxa.331.1.1.
- Michalczyk Ł, Kaczmarek Ł. 2013. The Tardigrada Register: A comprehensive online data repository for tardigrade taxonomy. Journal of Limnology 72(s1):175–181. DOI: 10.4081/jlimnol.2013.s1.e22.
- Mironov SV, Dabert J, Dabert M. 2012. A new feather mite species of the genus Proctophyllodes Robin, 1877 (Astigmata: Proctophyllodidae) from the long-tailed tit Aegithalos caudatus (Passeriformes: Aegithalidae)—morphological description with DNA barcode data. Zootaxa 3253(1):54–61 DOI: 10.11646/zootaxa.3253.1.2. Table 1.
- Murray J. 1907. XXV.—Arctic Tardigrada, collected by Wm. S. Bruce. Transactions of the Royal Society of Edinburgh 45(3):669–681. DOI: 10.1017/S0080456800011789.
- Nelson DR, Guidetti R, Rebecchi L. 2015. Phylum Tardigrada. In: Thor J, Covich A, editors Ecology and General Biology, Vol. 1. Freshwater Invertebrates. London: Academic Press. pp. 347–380.
- Nowak B, Stec D. 2017. The first record of Macrobiotus vladimiri Bertolani, Biserov, Rebecchi and Cesari, 2011 (Tardigrada: Eutardigrada: Macrobiotidae: hufelandi group) from Poland. Turkish Journal of Zoology 41(3):558–567. DOI: 10.3906/zoo-1609-22.
- Nowak B, Stec D. 2018. An integrative description of Macrobiotus hannae sp. nov. (Tardigrada: Eutardigrada: Macrobiotidae: Hufelandi group) from Poland. Turkish Journal of Zoology 42(3):269–286. DOI: 10.3906/zoo-1712-31.
- Perry E, Miller WR, Kaczmarek Ł. 2019. Recommended abbreviations for the names of genera of the phylum Tardigrada. Zootaxa 4608(1):145–154. DOI: 10.11646/zootaxa.4608.1.8.
- Pilato G. 1972. Structure, intraspecific variability and systematic value of the buccal armature of eutardigrades. Journal of Zoological Systematics and Evolutionary Research 10(1):65–78. DOI: 10.1111/j.1439-0469.1972.tb00785.x.
- Pilato G. 1981. Analisi di nuovi caratteri nello studio degli eutardigradi. Animalia 8:51–57.
- Pilato G, Binda MG. 1996. Two new species and new records of Macrobiotus (Eutardigrada) from New Zealand. New Zealand Journal of Zoology 23(4):375–379. DOI: 10.1080/03014223.1996.9518097.
- Pilato G, Binda MG. 2010. Definition of families, subfamilies, genera and subgenera of the Eutardigrada, and keys to their identification. Zootaxa 2404(1):1–52. DOI: 10.11646/zootaxa.2404.1.1.
- Pilato G, Binda MG, Catanzaro R. 1991. Remarks on some tardigrades of the African fauna with the description of three new species of Macrobiotus Schultze 1834. Tropical Zoology 4(2):167–178. DOI: 10.1080/03946975.1991.10539487.
- Pilato G, Binda MG, Lisi O. 2006. Three new species of eutardigrades from the Seychelles. New Zealand Journal of Zoology 33(1):39–48. DOI: 10.1080/03014223.2006.9518429.
- Pilato G, Binda MG, Napolitano A, Moncada E. 2000. The specific value of Macrobiotus coronatus DeBarros 1942, and description of two new species of the harmsworthi group (Eutardigrada). Bollettino delle sedute della Accademia Gioenia di Scienze Naturali 33:103–120.
- Pilato G, Lisi O. 2006. Macrobiotus rigidus sp. nov. new species of eutardigrade from New Zealand. Zootaxa 1109(1109):49–55. DOI: 10.11646/zootaxa.1109.1.5.
- Pilato G, Lisi O. 2009. Tardigrades from Seychelles Islands, with the description of three new species. Zootaxa 2124(1):1–20. DOI: 10.11646/zootaxa.2124.1.1.
- Pilato G, Lisi O, Binda MG. 2010. Tardigrades of Israel with description of four new species. Zootaxa 2665(2665):1–28. DOI: 10.11646/zootaxa.2665.1.1.
- Pilato G, Sabella G, Lisi O. 2014. Two new tardigrade species from Sicily. Zootaxa 3754(2):173–184. DOI: 10.11646/4299.
- Rice P, Longden I, Bleasby A. 2000. EMBOSS: The European molecular biology open software suite. Trends in Genetics 16(6):276–277. DOI: 10.1016/s0168-9525(00)02024-2.
- Richters F. 1926. Tardigrada. In: Kükenthal W, Krumbach T, editors Handbuch der Zoologie. Vol. 3. Berlin and Leipzig: Walter de Gruyter and Co. pp. 58–61.
- Roszkowska M, Stec D, Gawlak M, Kaczmarek Ł. 2018. An integrative description of a new tardigrade species Mesobiotus romani sp. nov. (Macrobiotidae: Harmsworthi group) from the Ecuadorian Pacific coast. Zootaxa 4450(5):550–564. DOI: 10.11646/zootaxa.4450.5.2.
- Sands CJ, SJ M, Marley NJ, Goodall-Copestake WP, Convey P, Linse K. 2008. Phylum Tardigrada: An “individual” approach. Cladistics 24(6):861–871. DOI: 10.1111/j.1096-0031.2008.00219.x. Table 1.
- Schuster RO, Nelson DR, Grigarick AA, Christenberry D. 1980. Systematic criteria of the Eutardigrada. Transactions of the American Microscopical Society 99(3):284–303. DOI: 10.2307/3226004.
- Short KA, Sands CJ, McInnes SJ, Pisani D, Stevens MI, Convey P. 2022. An ancient, Antarctic-specific species complex: Large divergences between multiple Antarctic lineages of the tardigrade genus Mesobiotus. Molecular Phylogenetics and Evolution 170(4):107429. DOI: 10.1016/j.ympev.2022.107429.
- Spallanzani L. 1776. Opuscoli di fisica animale, e vegetabile. Vol. 2. Il Tardigrado etc. Opusc. 4, sez, spec. Modena, Italy: Presso la Societa tipografica. pp. 222–253.
- Stec D. 2019. Mesobiotus datanlanicus sp. nov. a new tardigrade species (Macrobiotidae: Mesobiotus harmsworthi group) from Lâm Đồng Province in Vietnam. Zootaxa 4679(1):164–180. DOI: 10.11646/zootaxa.4679.1.10.
- Stec D. 2021. Integrative descriptions of two new Mesobiotus species (Tardigrada, Eutardigrada, Macrobiotidae) from Vietnam. Diversity 13(11):605. DOI: 10.3390/d13110605.
- Stec D. 2022. An integrative description of two new Mesobiotus species (Tardigrada: Eutardigrada: Macrobiotidae) with updated genus phylogeny. Zoological Studies 61:85. DOI: 10.6620/ZS.2022.61-85.
- Stec D, Gąsiorek P, Morek W, Kosztyła P, Zawierucha K, Michno K, Kaczmarek Ł, Prokop ZM, Michalczyk Ł. 2016. Estimating optimal sample size for tardigrade morphometry. Zoological Journal of the Linnean Society 178(4):776–784. DOI: 10.1111/zoj.12404.
- Stec D, Kristensen RM, Michalczyk Ł. 2020. An integrative description of Minibiotus ioculator sp. nov. From the Republic of South Africa with notes on Minibiotus pentannulatus Londoño et al. 2017 (Tardigrada: Macrobiotidae). Zoologischer Anzeiger 286:117–134. DOI: 10.1016/j.jcz.2020.03.007.
- Stec D, Roszkowska M, Kaczmarek Ł, Michalczyk Ł. 2018. An integrative description of a population of Mesobiotus radiatus (Pilato, Binda and Catanzaro, 1991) from Kenya. Turkish Journal of Zoology 42(5):523–540. DOI: 10.3906/zoo-1802-43.
- Stec D, Vecchi M, Calhim S, Michalczyk Ł. 2021. New multilocus phylogeny reorganises the family Macrobiotidae (Eutardigrada) and unveils complex morphological evolution of the Macrobiotus hufelandi group. Molecular Phylogenetics and Evolution 160:106987. DOI: 10.1016/j.ympev.2020.106987.
- Stec D, Zawierucha K, Michalczyk Ł. 2017. An integrative description of Ramazzottius subanomalus (Biserov, 1985) (Tardigrada) from Poland. Zootaxa 4300(3):403–420. DOI: 10.11646/zootaxa.4300.3.4.
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22:4673–4680. DOI: 10.1093/nar/22.22.4673.
- Thulin G. 1928. Über die Phylogenie und das System der Tardigraden. Hereditas 11:207–266. DOI: 10.1111/j.1601-5223.1928.tb02488.x.
- Tumanov DV. 2018. Mesobiotus nikolaevae sp. nov. (Eutardigrada: Macrobiotidae), a new species of Tardigrada from Croatia. Invertebrate Zoology 15(4):402–419. DOI: 10.15298/invertzool.15.4.08.
- Tumanov DV. 2020. Integrative description of mesobiotus anastasiae sp. nov. (Eutardigrada, Macrobiotoidea) and first record of lobohalacarus (Chelicerata, trombidiformes) from the Republic of South Africa. European Journal of Taxonomy 726:102–131. DOI: 10.5852/ejt.2020.726.1179.
- Tumanov DV, Pilato G. 2019. A new species of Eutardigrade (Macrobiotidae) from New Zealand. Zootaxa 4603(3):537–548. DOI: 10.11646/zootaxa.4603.3.6.
- Vecchi M, Cesari M, Bertolani R, Jönsoon IK, Guidetti R, Guidetti R. 2016a. Integrative systematic studies on tardigrades from Antarctica identify new genera and new species within Macrobiotoidea and Echiniscoidea. Invertebrate Systematics 30(4):303–322. DOI: 10.1071/IS15033.
- Vecchi M, Cesari M, Bertolani R, Jönsson KI, Rebecchi L, Guidetti R. 2016b. Corrigendum to: Integrative systematic studies on tardigrades from Antarctica identify new genera and new species within Macrobiotoidea and Echiniscoidea. Invertebrate Systematics 30(5):521–521. DOI: 10.1071/IS15033_CO.
- Yin H, Wang L, Li X. 2011. Two new species of genus Macrobiotus (Tardigrada: Macrobiotidae) from China. Proceedings of the Biological Society of Washington 124(3):165–178. DOI: 10.2988/11-05.1.
- Zawierucha K. 2011. Water bears (Tardigrada) of Okup Mały (Grabia Valley, Central Poland). Badania Fizjograficzne nad Polską Zachodnią, Seria C. Zoologia 52:47–52.
- Zawierucha K, Grzelak K, Kotwicki L, Kaczmarek Ł, Kristensen RM. 2015. First observation of the marine tardigrades Batillipes mirus and Batillipes noerrevangi (Arthrotardigrada, Batillipedidae) from a strongly brackish part of the Polish Baltic Sea coast. Marine Biology Research 11(8):859–868. DOI: 10.1080/17451000.2015.1024133.