Abstract
Within the family Calliphoridae, the genus Calliphora Robineau-Desvoidy, 1830, includes species of medical, veterinary and forensic relevance. This study reports for the first time the presence of Calliphora rohdendorfi (Grunin, 1966) in the Italian territory, namely in Calabrian Apennines (Southern Italy). The adults of the species were captured by bait bottle and yellow pan traps within a monitoring project of Diptera Brachycera involving Parco Nazionale dell’Aspromonte (Aspromonte National Park), Parco Nazionale della Sila (Sila National Park) and Parco Naturale Regionale delle Serre (Natural Regional Park of Serre), between 2018 and 2021. Calliphora rohdendorfi was identified on detailed morphological bases of both sexes. This was the first description of the general morphology of the female and its terminalia through digital photographs acquired by stereomicroscopy. The species was captured in pine and beech forests at an altitude between 1010 and 1820 m a.s.l. in shaded mountain areas. Prior to the finding in Southern Italy, this species showed an unusually disjointed distribution in Caucasus, Germany and Poland, thus the presence of C. rohdendorfi in Calabrian Apennines provides new and interesting data that require further investigation, because the species could play a role as flower visitor, pollinator and forensic indicator.
Introduction
The family Calliphoridae Brauer & Bergenstamm, 1889, commonly known as “blow flies” (Byrd & Castner Citation2001), includes about 1500 species (Pape et al. Citation2011) with a worldwide distribution (Byrd & Castner Citation2001). The number of species is going to be reevaluated due to the recent reordering of subfamilies (Yan et al. Citation2021). In most species, the adults exhibit typical metallic colors, such as green, blue, bronze, or black (Byrd & Castner Citation2001). This family includes species with medical, veterinary, forensic and economic importance. Most species are attracted by carrion and excrement and play an important ecological role in recycling of organic matter (Zumpt Citation1965; Rognes Citation1991; Byrd & Castner Citation2001; Scholl et al. Citation2009). In this family, numerous sarcosaprophagous species cause facultative myiasis, and others, in limited number, are agents of obligatory myiasis (Zumpt Citation1965; Rognes Citation1991; Hall & Farkas Citation2000; Scholl et al. Citation2009; Singh & Singh Citation2015; Pezzi et al. Citation2019, Citation2021, Citation2022). The sarcosaprophagous species of this family are currently used in forensic entomology to estimate the postmortem interval and are among the first insects colonizing human remains (Byrd & Castner Citation2001).
The cosmopolitan genus Calliphora Robineau-Desvoidy, 1830, includes about 100 species (https://animaldiversity.org; https://www.gbif.org), commonly associated with dead animal tissue and sometimes agents of facultative myiasis (Scholl et al. Citation2009). Within the species of the genus, Calliphora rohdendorfi (Grunin, 1966) has a complex systematic record. The species was described for the first time as Abago rohdendorfi Grunin, 1966 in the Caucasian region (Grunin Citation1966a, Citation1966b). In 1970, a different species was described under the name Calliphora rohdendorfi Grunin, 1970 (Grunin Citation1970a, Citation1970b), but when A. rohdendorfi was included in the genus Calliphora, the name Calliphora rohdendorfi Grunin, 1970 became a junior secondary homonym of Calliphora rohdendorfi (Grunin, 1966) and was therefore renamed as Calliphora grunini nom. nov. Schumann, 1992 (Schumann & Ozerov Citation1992).
After the first description in 1966, the species was found in other Caucasian sites (Grunin Citation1966a, Citation1966b, Citation1970a, Citation1970b; Schumann & Ozerov Citation1992; Rognes Citation2019), and recently in Germany (Adaschkiewitz & Gossner Citation2013; von Hoermann et al. Citation2022) and in Poland (Szpila Citation2015). Details about the distribution of C. rohdendorfi relevant to increase the knowledge about this species are described in a separate paragraph.
The present study records for the first time the presence of C. rohdendorfi in Italy, detected in Calabrian Apennines (Calabria, Southern Italy) in Parco Nazionale dell’Aspromonte (Aspromonte National Park), Parco Nazionale della Sila (Sila National Park) and Parco Naturale Regionale delle Serre (Natural Regional Park of Serre), between 2018 and 2021. The objectives of this study are to provide detailed morphological data on both sexes based on digital photographs acquired by stereomicroscopy and to report species distribution and habitat preferences in Southern Italy.
Status of distribution of Calliphora rohdendorfi
Calliphora rohdendorfi was described for the first time as Abago rohdendorfi Grunin, 1966, using as holotype one adult male found among preserved specimens collected in 1909, as reported, at the “foothills of Mt. Abago”, “Krasnodar Territory, Caucasian Reserve”. The locality “Mt. Abago” should correspond to “Gora Abago”, a mount within the Republic of Adygea included in the Caucasian Nature Biosphere Reserve. The names of the localities are based on the original article in Russian, published by Éntomologicheskoe Obozrenie (Grunin Citation1966a) and on its English translation, published by Entomological Review (Grunin Citation1966b). The holotype was later described as in poor state of preservation (Grunin Citation1970a, Citation1970b). Other 20 adults were later identified. Of these, 16 individuals, 11 males and 5 females, were collected between 1959 and 1969 (all in July except one male collected in May) in “Kavkazskiy reservation in the Adygey Autonomous Region”, identifiable with the Caucasian Nature Biosphere Reserve in the Republic of Adygea. Among the 11 males, two were found “near rotten meat and excrement” (Grunin Citation1970a, Citation1970b). In the “Teberda reservation in the Karachayevo-Cherkes Autonomous Region”, identifiable with the Teberda Nature Reserve in the Karachay-Cherkess Republic, two adult males were collected in September 1965, one of which was in an alpine meadow at about 2500 m a.s.l (Grunin Citation1970a, Citation1970b). In Armenia, two adult males were collected in 1969, one in “Ankavan” (probably identifiable with Hankavan, province of Kotayk) and in “Akhundov, Razdan District” (probably identifiable with Hrazdan, province of Kotayk). Again, all the names of localities are based on the original article in Russian, published by Éntomologicheskoe Obozrenie (Grunin Citation1970a) and on its English translation, published by Entomological Review (Grunin Citation1970b). The species was also detected in 1990 in “Tzej nahe Buron”, in the “nördlichen Ossetien (Kaukausus)”, probably identifiable with the Republic of North Ossetia-Alania. A total of 22 individuals (19 males and 3 females) were captured at 2200 m a.s.l. in July on flowers of Rubus sp. L. 1753 (Rosales: Rosaceae), also frequented by Calliphora vomitoria (Linnaeus, 1758), Calliphora loewi Enderlein, 1903, and Melinda caerulea (Meigen, 1826), now Melinda gentilis Robineau-Desvoidy, 1830 (Diptera: Calliphoridae) (Schumann & Ozerov Citation1992). The presence of C. rohdendorfi on flowers of Rubus sp. may support the role of this species as a pollinator. In the forests of Mount Teghenis, in the province of Kotayk (Armenia), a female was captured in July 2011, at about 2270 m a.s.l (Rognes Citation2019). According to a personal communication reported in Rognes (Citation2019), other individuals were captured in 2012 in Lagodekhi Nature Reserve, Kakheti district (Georgia).
In Central Europe, the species was found in Germany during an entomological fauna survey between 2008 and 2009, in Schwäbische Alb Biosphere Reserve (Baden-Württemberg), in Schorfheide-Chorin Biosphere Reserve (Brandenburg) and in Hainich National Park (Thuringia). A total of 23 adults (13 males and 10 females) were captured by window traps on spruce, beech, ash and pine trees, 16 of them at heights between 9.2 and 25 m (Adaschkiewitz & Gossner Citation2013).
The species was found in Poland between 2008 and 2012 in three deciduous forests (Szpila Citation2015). Two adult males were collected in the Borek reserve in August 2008, 7 males and 3 females in the Piwnicki Forest reserve in summer between August 2010 and June 2012, and other 7 males in the Płutowo reserve in June 2011. All three reserves are in Northern-Central Poland (Szpila Citation2015). In an experimental study performed in 2014 in the regions involved in the “Biodiversity Exploratories” programme in Germany, individuals of C. rohdendorfi resulted among the Calliphoridae visiting piglet carcasses between 2 and 4 days from the beginning of the experiment (von Hoermann et al. Citation2022).
The presence of C. rohdendorfi in Central Europe raises interesting questions. Recent studies on the family Calliphoridae carried out in Poland reveal that the first specimens of C. rohdehdorfi were identified in collections around 2008, about the same time when the species was detected in Germany (Szpila Citation2015).
Materials and methods
Within a monitoring project of Diptera Brachycera by traps (bait bottle, yellow pan and Malaise), samples were collected in four locations of the Calabrian Apennines: Parco Nazionale dell’Aspromonte (Aspromonte National Park) in 2018–2019, Parco Nazionale della Sila (Sila National Park) in 2020–2021, Parco Naturale Regionale delle Serre (Natural Regional Park of Serre) in 2020–2021, and a rural area within the campus of the University of Calabria (Rende, Cosenza) in 2019–2021 () (). For the three parks, five, six and five sites were, respectively, sampled, and for the rural area only one site (). To each site a code was assigned and the municipality (or locality), the geographic coordinates, the altitude, and the environmental and vegetation characteristics were recorded (). For each site, five bait bottle traps, one yellow pan trap and one Malaise trap were set. The bait bottle traps (volume 2 L) were set up as previously published (Hwang & Turner Citation2005). Inside the lower chamber, two plastic containers were placed, a 50-ml one with 25 g bovine liver and 20 ml saturated NaCl solution and another 100-ml one, with 50 g bovine liver and 40 ml of liquid protein bait (Dacus trap®, BioIberica, Barcelona, Spain). The distances among bait bottle traps were between 25 and 30 m. The traps were set on tree trunks or poles, attached by cable ties at 1.70–1.80 m height. The pan trap was made of 1-L yellow containers with holes on the sides to avoid collecting excess water. The trap was filled with 500 ml of saturated NaCl solution and 1 ml dish soap solution and set on a tree trunk different from those bearing the bait traps, or to a pole, attached by cable ties at the same height of the bait traps. The Malaise trap, of the traditional type (Townes Citation1972) was set on the ground. All three types of traps were examined, emptied of captured arthropods and reactivated every 15 days. The content of each trap was temporarily transferred to plastic containers with 80% ethanol, then washed in tapwater and stored in 80% ethanol until analysis. The taxa were identified by taxonomical keys. A preliminary separation of families and genera was performed (Rognes Citation1991; Oosterbroek Citation2006; Falk Citation2016) and the individuals of C. rohdendorfi were identified based on morphological characters of both sexes, as previously reported (Grunin Citation1966a, Citation1966b; Peris & Gonzáles-Mora Citation1989; Schumann & Ozerov Citation1992; Adaschkiewitz & Gossner Citation2013; Szpila Citation2015). For the general morphology of C. rohdendorfi, individuals were analyzed and photographed in toto by a stereomicroscope Nikon SMZ 800 (Nikon Instruments Europe, Amsterdam, The Netherlands), on which a Nikon Digital Sight DS-Fi1 camera (Nikon Instruments Europe) was mounted. Photographs were acquired with an image analysis software NIS-Elements Documentation (Nikon Instruments Europe). Terminalia immersed in distilled water were dissected by tweezers, fine scissors and pins, then analyzed and photographed by the previously mentioned equipment. The collected individuals of C. rohdendorfi were preserved in 80% ethanol at the Department of Biology, Ecology and Earth Sciences of the University of Calabria, and at the Department of Chemical, Pharmaceutical and Agricultural Sciences of the University of Ferrara. The terminology of morphological structures followed that of previously published studies (McAlpine et al. Citation1981; Rognes Citation1991; Szpila Citation2012; Tantawi et al. Citation2017). A total of 4 males and 4 females were examined for the detailed morphological study by photographs. About the ecological characteristics of sites, the genera and species of plants were identified according to local vegetation guides (Spampinato Citation2002; Spampinato et al. Citation2009; Pignatti et al. Citation2017a, Citation2017b, Citation2018).
Figure 1. Areas in the Calabrian Apennines sampled for Diptera Brachycera. Sites where individuals of Calliphora rohdendorfi were captured are indicated by white dots. Abbreviations: As, Parco Nazionale dell’Aspromonte (Aspromonte National Park); Re, municipality of Rende; Se, Parco Naturale Regionale delle Serre (Natural Regional Park of Serre); Si, Parco Nazionale della Sila (Sila National Park).
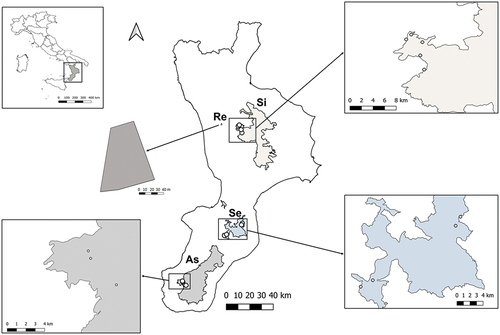
Table I. Areas, code sites, sampling sites, coordinates and their ecological characteristics. Abbreviations: As, Aspromonte National Park; Re, municipality of Rende; Se, Natural Regional Park of Serre; Si, Sila National Park.
Results and discussion
Morphology of both sexes based on stereomicroscopy
The morphological characters of both sexes on which the identification of the species was based are shown in . The adults are black with metallic blue reflections (). In the lower part of the head, yellow-reddish setae are clearly visible extending until the genae (). The palpi are yellow-brownish (), the basicosta is black () and the upper calypter has a dark border (). In the male, the fifth sternite has curved sides and a lobed posterior border (). The surstyli are longer than the cerci and tapering (). The ventrolateral processes of the aedeagus are notched (). The pregonite and the postgonite are slightly curved, but the first one is thicker and the postgonite, longer, is devoid of setae (). In the female, the ovipositor, shown for the first time by digital photographs acquired by stereomicroscopy (), is visible from the sixth tergite and sixth sternite down to the cerci in , respectively, in dorsal and ventral view. The sixth tergite has the shape of an upturned heart, with long setae distributed along the posterior border ().
Figure 2. General morphology of Calliphora rohdendorfi. (a) Habitus in lateral view of the adult female (scale bar: 2.5 mm). (b) Lateral view of the head of a female, showing the yellow-reddish setae in its lower part, extending until the genae (arrow) (scale bar: 500 µm) (c) Ventral view of the head of a female, with the proboscis removed to show the yellow-reddish setae (arrows) (scale bar: 500 µm). (d) Ventral view of the head of a female, with the proboscis removed to show the yellow-brownish palpi (scale bar: 500 µm). (e) Black left basicosta of a female (arrow) (scale bar: 1 mm). (f) Upper calypter of a male, with dark border (arrow) (scale bar: 1 mm). Abbreviations: lc, lower calypter; pa, palps; uc, upper calypter.
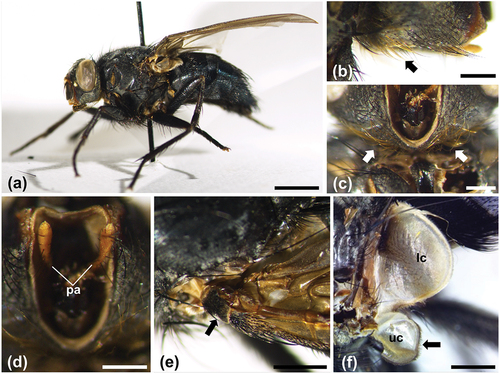
Figure 3. Calliphora rohdendorfi, male terminalia. (a) Dorsal side of the fifth sternite (scale bar: 500 µm). (b) Cerci and surstyli in lateral view (scale bar: 250 µm). (c) Cerci and surstyli in dorsal view (scale bar: 250 µm). (d) Aedeagus in lateral view (scale bar: 250 µm). (e) Pregonite and postgonite in lateral view (scale bar: 250 µm). Abbreviations: cs, cercus; prg, pregonite; psg, postgonite; su, surstylus.
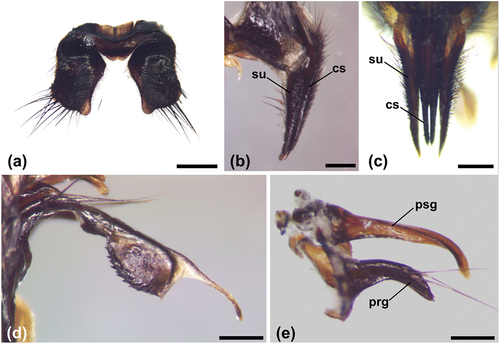
Figure 4. Calliphora rohdendorfi, ovipositor. (a) Ovipositor in dorsal view (scale bar: 500 µm). (b) Ovipositor in ventral view (scale bar: 500 µm). (c) Sixth tergite (scale bar: 500 µm). (d) Seventh tergite (scale bar: 500 µm). (e) Eighth tergite (scale bar: 250 µm). (f) Right sclerotized area of the eighth tergite (scale bar: 250 µm). (g) Clavate cerci and triangular epiproct (scale bar: 125 µm). (h) Sixth sternite (scale bar: 250 µm). (i) Seventh sternite (scale bar: 250 µm). (j) Eighth sternite with two terminal lobes (arrows) (scale bar: 250 µm). (k) Ovoidal hypoproct with long and short setae (scale bar: 125 µm). Abbreviations: ce, cerci; ep, epiproct; hy, hypoproct; sc-8, sclerotized area of the eighth tergite; st-6, sixth sternite, st-7, seventh sternite; st-8, eighth sternite; te-6, sixth tergite; te-7, seventh tergite. Other abbreviations as in .
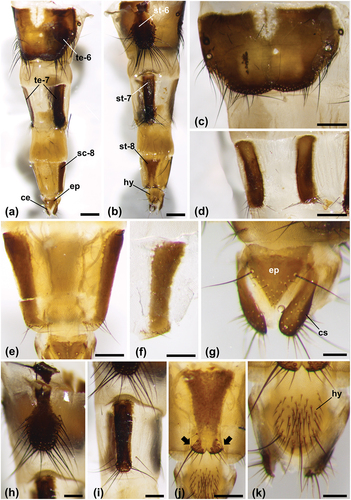
The seventh tergite is divided into two lateral sclerotized areas by a cuticular membrane. These areas are rectangular, slightly convex towards the center and with long setae in the posterior border (). As the seventh tergite, the eighth tergite is divided into two lateral sclerotized areas by a cuticular membrane. In this tergite, the areas are smaller and roughly rectangular, with shorter setae in the posterior border ().
The clavate cerci have setae of various length at their distal end (). The epiproct is triangular, with two long setae in the anterior part and several short setae in the posterior one (). The sixth sternite has a shovel shape with long setae on three sides (). The seventh sternite, rectangular, has long setae in the posterior part (). The eighth sternite has an inverted trapezoidal shape with two terminal lobes with setae (). The terminal hypoproct has an ovoidal shape with some long setae and many short ones (). These data are the first ones concerning the general morphology of the female and its terminalia obtained in this species by digital photographs acquired by stereomicroscopy. Previously, the female terminalia of C. rohdendorfi were documented only by line drawings (Schumann & Ozerov Citation1992).
Species distribution and habitat preferences in Southern Italy
A total of 156 individuals were captured, of which 126 females (81% of the total) and 30 males (19% of the total), in Aspromonte National Park, Sila National Park and Natural Regional Park of Serre () (). No individuals of C. rohdendorfi were ever captured by the Malaise traps. A total of 88 individuals were captured in Aspromonte National Park by bait bottle traps, of which 74 females (84%) and 14 males (16%) (). Calliphora rohdendorfi was collected from 1370 m (1As, pine forest) to 1650 m (4As, beech forest). Based on these data, in the Aspromonte National Park C. rohdendorfi has an altitude range of 280 m. The monitoring reveals the presence of this species from August to November in 2018 and from July to November in 2019. The site with the highest number of captured individuals is 3As (beech forest), with 42 individuals (83% females and 17% males). In site 1As (pine forest) there were 96% females and 4% males, and in site 4As (beech forest) there were 74% females and 26% males. No individuals of C. rohdendorfi were captured in 2As (pine forest) and in 5As (bog land).
Table II. Number of C. rohdendorfi individuals captured in the Aspromonte National Park between 2018 and 2019. Jul, July; Aug, August; Sep, September; Oct, October; Nov, November.
Table III. Number of C. rohdendorfi individuals captured in Sila National Park and in Natural Regional Park of Serre between 2020 and 2021. Jun, June; Dec, December. Other abbreviations as in .
In the Sila National Park, a total of 35 individuals were captured, of which 23 females (66%) and 12 males (34%) (). Calliphora rohdendorfi was collected from 1320 m (2Si, pine forest) to 1820 m (4Si, beech forest); therefore, the altitudinal range of the species in the Sila National Park is 500 m. In 2020, C. rohdendorfi was captured only by bait bottle traps (6 individuals, 5 females and 1 male), while in 2021 the species was captured by bait bottle traps (12 individuals, 8 females and 4 males) and yellow pan traps (17 individuals, 10 females and 7 males). No individuals of C. rohdendorfi were captured in the 5Si site (grazing land) and only one individual was captured in the bog land site 6Si. In this area, the species was present from June to December in 2020 and from July to November in 2021.
In the Natural Regional Park of Serre, a total of 33 individuals were captured by bait bottle traps, of which 29 females (88%) and 4 males (12%) (). Calliphora rohdendorfi was collected from 1010 m (3Se, beech forest) to 1189 m (4Se, beech forest); therefore, the altitudinal range of this species in the Natural Regional Park of Serre is about 200 m. The site with the highest number of captured individuals is 1Se (pine forest), with 26 individuals (85% females and 15% males). In sites 2Se (pine forest), 3Se (beech forest) and 4Se (beech forest), only females were captured, respectively, 3, 1 and 3. No individuals of C. rohdendorfi were captured in 5Se (grazing land). In this area, the species was present from August to December in 2020 and from September to November in 2021.
No individuals of C. rohdendorfi were captured by any type of trap in the rural area within the municipality of Rende.
This is the first report concerning the presence of Calliphora rohdendorfi (Grunin, 1966) (Diptera: Calliphoridae) in Italy. The species was detected in Calabria (Southern Italy) from 2018 to 2021 in three areas of the Calabrian Apennines (Aspromonte National Park, Sila National Park and Natural Regional Park of Serre) between June and December. The species was mainly found in pine and beech forests at an altitude between 1010 and 1820 m a.s.l., in shaded mountain areas. In all three areas, adults of both sexes of C. rohdendorfi were captured by bait bottle traps, thus the species was apparently attracted by bovine liver and protein bait. This is in agreement with previous data reporting that C. rohdendorfi is attracted by fruiting bodies of Phallus impudicus L., 1753 (Phallales: Phallaceae), whose odor resembles that of carrion, but also by pig liver in decomposition (Szpila Citation2015).
In Sila National Park, both sexes of C. rohdendorfi were captured by yellow pan traps, suggesting a possible role of the species as flower visitor and pollinator, as ascertained for several species of Calliphoridae (Cook et al. Citation2020, Citation2023). This hypothesis is also supported by the presence of C. rohdendorfi on flowers of Rubus sp. L. 1753 (Rosales: Rosaceae) (Schumann & Ozerov Citation1992).
The presence of C. rohdendorfi in Central Europe raises interesting questions. Based on the previous data on the species distribution, it is very likely that the presence of C. rohdendorfi in Central Europe is recent. However, it is difficult to explain the gap in the presence of the species between the Caucasian region and Central Europe (Szpila Citation2015), and after the detection of the species in Calabrian Apennines, the gap between Central Europe and Southern Italy.
Calliphora rohdendorfi is very similar to Calliphora subalpina (Ringdahl, 1931) in body habitus (Schumann & Ozerov Citation1992) and wing shape (Szpila et al. Citation2019). However, relevant differences between C. rohdendorfi and C. subalpina have been recently highlighted in genitalia of both sexes (Szpila Citation2015; Rognes Citation2019). These differences are supported by a direct comparison of the genitalia of both sexes of C. rohdendorfi, as reported in this study, with those previously reported for C. subalpina (Rognes Citation1991; Szpila Citation2012). Given the high morphological similarity of C. rohdendorfi with C. subalpina, it would be interesting to verify whether the specimens identified as C. subalpina in museum collections are specimens of C. rohdendorfi by morphological investigations and DNA barcoding, thus explaining the gaps and updating its distribution in Europe.
The presence of C. rohdendorfi in Calabria (Southern Italy), here reported for the first time, provides new interesting data about the disjointed distribution of this species, which should be more carefully investigated because of its possible role as flower visitor and pollinator, and also as forensic indicator.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adaschkiewitz W, Gossner MM. 2013. Einige Arten aus den Familien Anthomyiidae, Calliphoridae, Dolichopodidae, Drosophilidae, Muscidae und Phoridae (Diptera) neu für Deutschland. Studia Dipterologica 20:214–218.
- Byrd JH, Castner JL. 2001. Insects of forensic importance. In: Byrd JH, Castner JL, editors. Forensic entomology: The utility of arthropods in legal investigations. Boca Raton, FL: CRC Press LLC. pp. 43–79.
- Cook DF, Tufail MS, Voss SC, Deyl RA, Howse ET, Foley J, Norrish B, Delroy N, Shivananjappa SL. 2023. Blow flies (Diptera: Calliphoridae) ability to pollinate Hass avocado trees within paired tree enclosures. Journal of Applied Entomology 147(8):577–591. DOI: 10.1111/jen.13159.
- Cook DF, Voss SC, Finch JTD, Rader RC, Cook JM, Spurr CJ. 2020. The role of flies as pollinators of horticultural crops: An Australian case study with worldwide relevance. Insects 11(6):341. DOI: 10.3390/insects11060341.
- Falk S. 2016. Draft key to British Calliphoridae and Rhinophoridae. British Blowflies (Calliphoridae) and Woodlouse flies (Rhinophoridae). Available: http://www.stevenfalk.co.uk/files/21577/testkeytobritishblowflies132016.pdf.
- Grunin KJ. 1966a. New and little-known Calliphoridae (Diptera), mainly bloodsucking or subcutaneous parasites of birds. Éntomologicheskoe Obozrenie 45:897–903. (Article in Russian).
- Grunin KJ. 1966b. New and little-known Calliphoridae (Diptera), mainly bloodsucking or subcutaneous parasites of birds. Entomological Review 45:503–506. (English translation of Grunin 1966a published in Russian).
- Grunin KJ. 1970a. New species of Calliphoridae (Diptera) for the fauna of the USSR. Éntomologicheskoe Obozrenie 49:471–483. (Article in Russian).
- Grunin KJ. 1970b. Flies of the family Calliphoridae (Diptera) new to the USSR. Entomological Review 49:282–289. (English translation of Grunin 1970a published in Russian).
- Hall MJR, Farkas R. 2000. Traumatic myiasis of humans and animals. In: Papp L, Darvas B, editors. Contributions to a manual of Palaearctic Diptera (with special reference to flies of economic importance), volume 1, General and Applied Dipterology. Budapest, Hungary: Science Herald. pp. 751–768.
- Hwang C, Turner BD. 2005. Spatial and temporal variability of necrophagous Diptera from urban to rural areas. Medical and Veterinary Entomology 19(4):379–391. DOI: 10.1111/j.1365-2915.2005.00583.x.
- McAlpine JF, Peterson BV, Shewell GE, Teskey HJ, Vockeroth JR, Wood DM. 1981. Manual of Nearctic Diptera. Vol. 1. Ottawa, Canada: Research Branch Agriculture Canada. pp. 674.
- Oosterbroek P. 2006. The European families of Diptera – Identification, diagnosis, biology. Utrech, The Netherlands: KNNV Publishing. pp. 205.
- Pape T, Blagoderov V, Mostovski MB. 2011. Order Diptera Linnaeus, 1758. In: Zhang, Z.-Q. (Ed.). Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148(1):222–229. DOI: 10.11646/zootaxa.3148.1.42.
- Peris SV, Gonzáles-Mora D. 1989. About Calliphora and its allies (Diptera). Eos 65:165–201.
- Pezzi M, Bonacci T, Leis M, Mamolini E, Marchetti MG, Krčmar S, Chicca M, Del Zingaro CNF, Faucheux MJ, Scapoli C. 2019. Myiasis in domestic cats: a global review. Parasites & Vectors 12:372. DOI: 10.1186/s13071-019-3618-1.
- Pezzi M, Krčmar S, Mendicino F, Carlomagno F, Bonelli D, Scapoli C, Chicca M, Leis M, Bonacci T. 2022. Lucilia sericata (Diptera: Calliphoridae) as agent of myiasis in a goose in Italy and a review of myiasis by this species in birds. Insects 13(6):542. DOI: 10.3390/insects13060542.
- Pezzi M, Scapoli C, Chicca M, Leis M, Marchetti MG, Del Zingaro CNF, Vicentini CB, Mamolini E, Giangaspero A, Bonacci T. 2021. Cutaneous myiasis in cats and dogs: Cases, predisposing conditions and risk factors. Veterinary Medicine and Science 7(2):378–384. DOI: 10.1002/vms3.370.
- Pignatti S, Guarino R, La Rosa M. 2017a. Flora d’Italia e Flora Digitale, seconda edizione, volume primo. Milan, Italy: Edagricole, New Business Media srl.
- Pignatti S, Guarino R, La Rosa M. 2017b. Flora d’Italia e Flora Digitale, seconda edizione, volume secondo. Milan, Italy: Edagricole, New Business Media srl.
- Pignatti S, Guarino R, La Rosa M. 2018. Flora d’Italia e Flora Digitale, seconda edizione, volume terzo. Milan, Italy: Edagricole, New Business Media srl.
- Rognes K. 1991. Blowflies (Diptera, Calliphoridae) of Fennoscandia and Denmark. Fauna Entomologica Scandinavica 24:1–272.
- Rognes K. 2019. The Calliphoridae (Diptera) of Armenia. Zootaxa 4576(2):375–391. DOI: 10.11646/zootaxa.4576.2.11.
- Scholl PJ, Catts EP, Mullen GR. 2009. Myiasis (Muscoidea, Oestroidea). In: Medical and veterinary entomology. 2nd ed. Mullen GR, Durden LA, editors. San Diego, CA: Academic Press, Elsevier. pp. 309–338.
- Schumann H, Ozerov AL. 1992. Zum systematischen Status von Abago rohdendorfi Grunin, 1966 (Diptera, Calliphoridae). Deutsche Entomologische Zeitschrift 39(4–5):403–408. DOI: 10.1002/mmnd.19920390416.
- Singh A, Singh Z. 2015. Incidence of myiasis among humans—A review. Parasitology Research 114(9):3183–3199. DOI: 10.1007/s00436-015-4620-y.
- Spampinato G. 2002. Guida alla Flora dell’Aspromonte. Reggio Calabria, Italy: Laruffa Editore.
- Spampinato G, Cameriere P, Caridi D, Crisafulli A. 2009. Carta della biodiversità vegetale del Parco Nazionale dell’Aspromonte (Italia meridionale). Quaderni di Botanica Ambientale e Applicata 19:3–36.
- Szpila K. 2012. Key for identification of European and Mediterranean blowflies (Diptera, Calliphoridae) of medical and veterinary importance—adult flies. In: Forensic entomology, an introduction. 2nd ed. Gennard D, editor. Oxford, UK: Wiley-Blackwell. pp. 77–81.
- Szpila K. 2015. Calliphora rohdendorfi (Grunin, 1966) (Diptera: Calliphoridae) – New species to the Polish fauna. Dipteron 31:50–54.
- Szpila K, Żmuda A, Akbarzadeh K, Tofilski A. 2019. Wing measurement can be used to identify European blow flies (Diptera: Calliphoridae) of forensic importance. Forensic Science International 296:1–8. DOI: 10.1016/j.forsciint.2019.01.001.
- Tantawi TI, Whitworth TL, Sinclair BJ. 2017. Revision of the Nearctic Calliphora Robineau-Desvoidy (Diptera: Calliphoridae). Zootaxa 4226(3):301–347. DOI: 10.11646/zootaxa.4226.3.1.
- Townes H. 1972. A light-weight Malaise trap. Entomological News 83:239–247.
- von Hoermann C, Weithmann S, Sikorski J, Nevo O, Szpila K, Grzywacz A, Grunwald J-E, Reckel F, Overmann J, Steiger S, Ayasse M. 2022. Linking bacteria, volatiles and insects on carrion: The role of temporal and spatial factors regulating inter-kingdom communication via volatiles. Royal Society Open Science 9:220555. DOI: 10.1098/rsos.220555.
- Yan L, Pape T, Meusemann K, Kutty SN, Meier R, Bayless KM, Zhang D. 2021. Monophyletic blowflies revealed by phylogenomics. BMC Biology 19:230. DOI: 10.1186/s12915-021-01156-4.
- Zumpt F. 1965. Myiasis in man and animals in the old world. A textbook for physicians, veterinarians and zoologists. London, UK: Butterworth & Co.
