Abstract
In recent years, the pollution of aquatic environments with pharmaceuticals, including non-steroidal anti-inflammatory drugs (NSAIDs), has increased. This research investigated the effects of 7- and 28-day exposure to ibuprofen on the midgut ultrastructure of the tardigrade Paramacrobiotus experimentalis. The conducted research will enrich the knowledge on the effect of ibuprofen with histological analyses. In addition, the effect of ibuprofen has not been studied on tardigrades so far. Specimens were incubated in three concentrations of this drug: 0.1 μg/L (concentration commonly found in surface waters worldwide), 16.8 μg/L (concentration found in the rivers of large cities), and 1 mg/L (experimental concentration). In addition, the lethal concentration 50 (LC50) after 24 h incubation in ibuprofen was determined. Ultrastructural analyses showed the presence of degenerated mitochondria and autophagic structures in midgut digestive cells after incubation in ibuprofen, which was confirmed by LysoTracker Red staining. TUNEL staining showed DNA fragmentation – a marker of cell apoptosis – in digestive cells treated with ibuprofen. Furthermore, dihydroethidium (DHE) revealed signals emitted by ROS+ positive cells in midgut digestive cells, indicating oxidative stress. Ultrastructural changes and the number of signals indicating damage in the cell were correlated with increases in concentration and time of exposure to the stressor. The lack of ultrastructural changes in regenerative cells supports the theory that digestive cells of the midgut are one of the first barriers protecting the body against stressors.
1. Introduction
With rapid economic development, including such branches as medicine and pharmacy, the consumption of drugs (especially over-the-counter) has increased in recent years. This issue is linked to the broader problem of inappropriate drug disposal and ineffective removal methods targeting specific groups of pollutants in wastewater treatment plants (Fent et al. Citation2006; Szymonik & Lach Citation2012; Marchlewicz et al. Citation2015). As a result, pharmaceuticals can enter the aquatic environment as metabolites or in unchanged form. Among medicines in aquatic environments are non-steroidal anti-inflammatory drugs (NSAIDs), antibiotics, hormonal drugs, drugs regulating lipid metabolism, antiepileptic medications, and β-blockers (Fent et al. Citation2006; Nikolaou et al. Citation2007; Szymonik & Lach Citation2012). NSAIDs such as ibuprofen, acetylsalicylic acid, diclofenac, and naproxen are reported to be among the main classes of molecules contaminating aquatic ecosystems worldwide. They are commonly used for their analgesic, antipyretic, and anti-inflammatory properties in human and veterinary therapy (Boxall et al. Citation2012; Parolini Citation2020). Many of these substances are characterised by low susceptibility to degradation and a tendency to bioaccumulate, which exposes the organisms to prolonged, sometimes lifelong exposure (Harshkova & Aksmann Citation2019). The accumulation of pharmaceuticals, including ibuprofen, in water bodies raises concerns about their ecological impact and potential disruption of aquatic ecosystems.
One of the most popular over-the-counter NSAIDs is ibuprofen. It is used to treat acute and chronic pain as well as rheumatic diseases (Karaźniewicz Citation2007; Bushra & Aslam Citation2010; Rainsford Citation2013). It inhibits two cyclooxygenase enzymes involved in the synthesis of prostaglandins. They are involved in invertebrate functioning, e.g., ion flux regulation, temperature control, reproduction, cell aggregation, and host-parasite interactions (Stanley-Samuelson Citation1987; Bushra & Aslam Citation2010; Batucan et al. Citation2022).
Tardigrades (water bears) are microscopic invertebrates known for their resistance to various factors, such as low and high temperatures, lack of water, and radiation. They owe it to the process of cryptobiosis and, in some cases, to diapause, which help them to survive periods of unfavorable environmental conditions (Nelson et al. Citation2015). In their living environment, they are exposed to many chemical factors.
Therefore, this research aimed to analyze and describe the effects of short- and long-term exposure to ibuprofen on the ultrastructure of the midgut of Paramacrobiotus experimentalis Kaczmarek et al. Citation2020 (Tardigrada, Eutardigrada). It should be emphasized that most studies on the effects of ibuprofen focus on vertebrates, moreover, there are no data in the literature on the effect of this pharmaceutical on tardigrades. Most studies also focus on survival and reproduction, in this work we will focus on the less described ultrastructure of cells treated with ibuprofen. The use of multiple methods is justified by the need to obtain a comprehensive and detailed understanding of the biological responses and effects of ibuprofen exposure on the specimens of Pam. experimentalis. Each biomarker targets specific aspects of cellular and subcellular changes, providing a multidimensional view of the potential impacts of ibuprofen. The midgut (that the study focuses on), is the region responsible for digestion, secretion, and absorption. In tardigrades, it is composed of two types of cells: digestive and regenerative cells. The midgut is the first barrier against various stressors taken with food (Rost-Roszkowska et al. Citation2008, Citation2018; Nelson et al. Citation2015). The expected results are changes in the structure of organelles in the cells of the animal’s body, activation of autophagy, which acts as a protective mechanism, triggering cell death processes and increasing oxidative stress. In conclusion, investigating the effects of ibuprofen on Pam. experimentalis serves an ecologically significant purpose by addressing the potential consequences of pharmaceutical contamination in aquatic ecosystems. By providing insights into the effect of ibuprofen on Pam. experimentalis, the study aids in understanding the broader ecological implications.
2. Material and methods
2.1. Material
Paramacrobiotus experimentalis (Tardigrada, Eutardigrada, Macrobiotidae) is a predatory, gonochoric species that lays ornamented eggs freely into the environment. The animal’s body is transparent/white (Kaczmarek et al. Citation2020). Specimens were collected in 2013 from moss samples in eastern Madagascar (Toamasina and Antananarivo Province). The specimens for our study were obtained from Professor Łukasz Kaczmarek (Adam Mickiewicz University in Poznań, Poland). Animals were cultured in plastic Petri dishes with a matted bottom in a mixture of Żywiec Zdrój mineral water and distilled water (1:3) at a temperature of 19°C in a 12 h light–12 h dark cycle. They were fed with rotifers (Lecane inermis) originating from the culture collection of Aquatic Ecosystems Team of The Institute of Environmental Sciences, Jagiellonian University. Algae (Chlorella sp. and Chlorococcum sp.) were also added to the culture. The experiments in this study did not require an approval by an ethical committee.
2.2. Methods
2.2.1. Determination of LC50 of ibuprofen
In toxicological studies, LC50 (lethal concentration 50) is the concentration of a substance at which 50% of the studied population will die after a certain period. Specimens were transferred to a 12-well plate (6 individuals per well). The wells contained 3 ml of gradually increasing concentrations of ibuprofen solution in a culture medium within the range of 100–950 mg/L. Incubation in ibuprofen solutions lasted for 24 hours at a temperature of 19°C. After that time, the numbers of living and dead specimens were checked. Data were evaluated using the probit analysis statistical method (Finney Citation1952), as the standard method to evaluate dose–response data.
2.2.2. Experiment
Specimens were divided into ten groups – four control groups and six experimental groups (). Each group consisted of 50 animals cultured in 12-well plates with a matted bottom. The control group PeC was cultured in a culture medium (see 2.1). The control groups PeC0, PeC7 and PeC28 were cultured in culture medium with the addition of 1 ml of ethanol per liter of medium (). This was because, in the experimental groups, ibuprofen was dissolved in 1 ml of ethanol before being added to the medium (ibuprofen is more soluble in ethanol than in water). Such selected control groups made it possible to avoid changes caused by the presence of ethanol in the culture medium and those caused by the aging of the animals. The experimental groups consisted of specimens bred in 12-well plates containing culture medium with the addition of ibuprofen. In our research, three concentrations of ibuprofen were used: two environmental concentrations of 0.1 μg/L (Aus der Beek et al. Citation2016) and 16.8 μg/L (Mrowiec Citation2015), and a higher concentration of 1 mg/L. For each concentration, the experiment was carried out in two exposure times − 7 days and 28 days ().
Table I. Control and experimental groups.
2.2.3. Light and transmission electron microscopy
Specimens of Pam. experimentalis () were fixed in 2.5% glutaraldehyde in a 0.1 M phosphate buffer (pH 7.4) for seven days at 4°C, washed three times in the same buffer at room temperature (RT, 30 min each) and postfixed in 2% osmium tetroxide in a 0.1 M phosphate buffer for 2 hours (RT). The remaining steps of the procedure were carried out at room temperature. Then the material was washed again in a phosphate buffer three times, 10 minutes each, and dehydrated in a graded series of ethanol (30, 50, 70, 90, 96 and 100% for 10 min, 2 × 10 min, 15 min, 15 min, 15 min and 4 × 15 min, respectively). After dehydration, the material was incubated in a solution of 100% ethanol and acetone (1:1) for 15 minutes, washed twice in acetone (10 min each), and then incubated in a solution of acetone and epoxy resin (1:1) for 1.5 hours in closed Eppendorf tubes. After that time, the Eppendorf tubes were opened and left overnight for the acetone to evaporate. The next day, specimens were embedded in epoxy resin (Epoxy Embedding Medium Kit, Sigma). Semi-thin (800 nm) sections were cut on a Leica UCT25 ultramicrotome, stained with 1% methylene blue in 1% borax and analysed with an Olympus B×60 light microscope. Ultra-thin sections (70 nm) were cut on a Leica EM UC7 ultramicrotome, mounted on copper grids, stained with uranyl acetate and lead citrate, and then analysed in the Hitachi H500 transmission electron microscope at 75 kV.
Table II. The number of adult specimens of Pam. experimentalis for each method.
2.2.4. Confocal microscopy
2.2.4.1. TUNEL – detection of DNA fragmentation during apoptosis
Non-fixed specimens () were incubated on ice in a 0.1% solution of freshly prepared sodium citrate for 2 minutes. After that, they were washed in TBS (3 × 5 min) and stained with a TUNEL reaction mixture (In situ Cell Death Detection Kit TMR red, Roche Applied Science) for 1 hour at 37°C in darkness. After that period, the material was washed in TBS (3 × 5 min), next stained with 1 µg/ml DAPI in TBS (15 min, RT) in darkness, again washed in TBS (3 × 5 min) and mounted in Vectashield medium on a microscopic slide. Specimens were analysed using an Olympus FluoView FV 1000 confocal microscope.
2.2.4.2. LysoTracker Red – detection of acidic organelles
Non-fixed specimens () were incubated for 15 min in the dark in 2.5 mM LysoTracker Red DND-99 (Molecular Probes, L 7528, Thermo Fisher Scientific, Waltham, MA, USA) in TBS (pH 7.4, RT). After that, the material was washed with TBS and stained with 1 µg/ml DAPI in TBS for 15 minutes. Next, the specimens were washed in TBS (3 × 5 min) and mounted in Vectashield medium on a microscopic slide. Specimens were analysed using an Olympus FluoView FV 1000 confocal microscope.
2.2.4.3. Dihydroethidium (DHE) analysing superoxide levels (differentiation of ROS+ and ROS− cells)
Non-fixed specimens () were incubated TBS with 0.0025% Triton X100 (RT). Next they were stained with 30 µM DHE prepared from the 30 mM stock solution of DHE in DMSO (15 min, RT, in darkness). After that, the material was washed in TBS (3 × 5 min) and stained with 1 µg/ml DAPI in TBS (15 min, RT) in darkness. Next, the specimens were washed in TBS (3 × 5 min) and mounted in Vectashield medium on a microscopic slide. Specimens were analysed using an Olympus FluoView FV 1000 confocal microscope.
3. Results
3.1. LC50 of ibuprofen
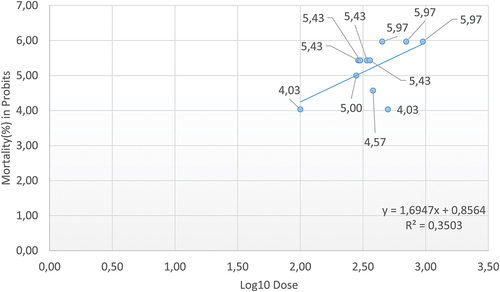
The LC50 of ibuprofen for Pam. experimentalis was computed based on probit analysis for 24 h exposure, and is 281.6 mg/L. The LC values are derived from the curve drawn using working probits and log doses (). The LC25, LC70, and LC95 values are collected in the table, as well as the 95% fiducial confidence interval (lower and upper limits), for all mentioned concentrations ().
Table III. Probit analysis of 95% confidence limits for effective concentrations of ibuprofen in Pam. experimentalis.
3.2. Ultrastructural changes in the midgut of Pam. experimentalis exposed to ibuprofen
3.2.1. Control groups (PeC, PeC0, PeC7, PeC28)
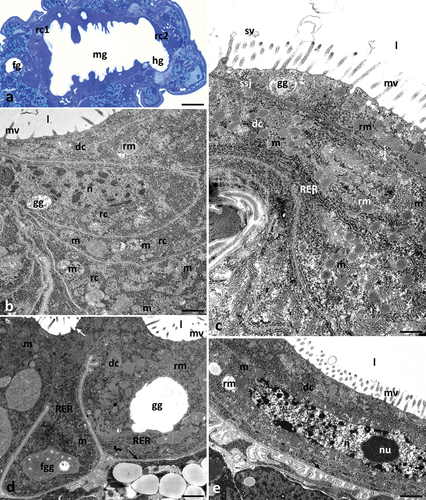
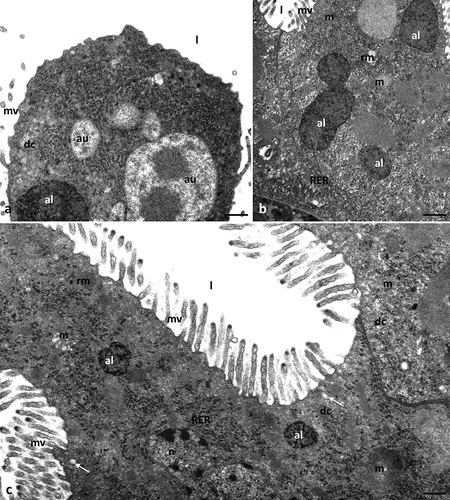
Ultrastructural analysis of the control groups cultured in the medium containing ethanol (PeC0, PeC7, PeC28) and without ethanol (PeC) showed no differences. Differences due to ageing were also not observed in the control groups (PeC0, PeC7, PeC28). Therefore, all control groups will be described together. The digestive system of tardigrades was composed of three parts: foregut, midgut and hindgut (). The midgut of Pam. experimentalis had a tube-like shape and spread along the entire length of the middle region of the body (). Its surface was strongly folded. The midgut epithelium was composed of two types of cells – digestive and regenerative. Regenerative cells were gathered at the ends of the midgut and formed two epithelial rings – anterior and posterior (). They had a crescent-like shape, and they lay on the thin non-cellular basal lamina. The apical parts of these cells did not reach the midgut lumen. The cytoplasm of these cells was poor in organelles. They had a large centrally located nucleus with a nucleolus, mitochondria with clearly visible cristae, short cisterns of rough endoplasmic reticulum and ribosomes (). Cubic-shaped digestive cells also lay on the thin non-cellular basal lamina (). The apical cell membrane of these cells formed microvilli and invaginations into the midgut lumen (). Secretory vesicles were observed near the apical membrane of the cells. Fusion of the vesicle membrane with the apical membrane of the digestive cell was observed, indicating merocrine secretion (). Moreover, small vesicles with an electron-lucent content were observed at the apical ends of the microvilli, indicating microapocrine secretion (). The nucleus of digestive cells was shifted near the basal membrane. Organelles and cell structures in digestive cells showed no regionalisation in distribution. Numerous mitochondria with distinct cristae, ribosomes, cisterns of rough endoplasmic reticulum, Golgi complexes, spheres of medium electron density reserve material and granules with a crystalline structure were present in the cell cytoplasm (). Smooth septate junctions and septate junctions were distinguished between adjacent digestive cells (). The basal membrane of these cells was slightly folded ().
Figure 2. Midgut of Pam. experimentalis in control groups – PeC, PeC0. PeC7, PeC28. (a) Localization of the midgut in animal body, LM, bar = 14.29 μm. (b) the ultrastructure of the midgut regenerative cells in the control group TEM, bar = 0.24 μm. (c-e) the ultrastructure of the midgut digestive cells in the control groups TEM (c) bar = 0.52 μm. (d) bar = 0.10 μm. (e) bar = 0.43 μm. Abbreviations: foregut (fg), midgut (mg), hindgut (hg), anterior epithelial rings (rc1), posterior epithelial rings (rc2), regenerative cells (rc), digestive cells (dc), mitochondrion (m), nucleus (n), nucleolus (nu), rough endoplasmic reticulum (RER), reserve material (rm), midgut lumen (l), microvilli (mv), gut granule (gg), forming gut granule (fgg), basal lamina (black arrow), merocrine secretion (white arrow), microapocrine secretion - secretory vesicle; (sv) septate junctions (sj), smooth septate junctions (ssj).
3.2.2. Experimental group Ib1a (concentration of ibuprofen 0.1 μg/L, 7 days)
Digestive cells had a cubic shape, and their apical membrane formed microvilli (). As in the control groups, the cytoplasm of regenerative cells was poor in organelles (). Compared to the control groups, no changes were observed in the ultrastructure of cell organelles in both types of cells ().
Figure 3. Midgut of Pam. experimentalis treated with ibuprofen for 7 days; concentration of ibuprofen 0.1 μg/L - Ib1a (a–b) and 16.8 μg/L - Ib1b (c–d), TEM. (a) the ultrastructure of the midgut digestive cells in the group Ib1a, bar = 0.20 μm. (b) the ultrastructure of the midgut regenerative cells in the group Ib1a, bar = 0.31 μm. (c;d) the ultrastructure of the midgut digestive cells in the group Ib1b, (c) bar = 0.36 μm, (d) bar = 0.32 μm. Abbreviations: digestive cell (dc), regenerative cell (rc), mitochondrion (m), nucleus (n), nucleolus (nu), rough endoplasmic reticulum (RER), reserve material (rm), gut granule (gg), microvilli (mv), midgut lumen (l), basal lamina (black arrow), merocrine secretion (white arrow).
3.2.3. Experimental group Ib1b (concentration of ibuprofen 16.8 μg/L, 7 days)
Digestive cells had a cubic shape. The apical cell membrane of digestive cells formed microvilli (). The ultrastructure of the digestive cells did not differ from the cells of the control group (). Also, no changes in the ultrastructure of regenerative cells were observed (not shown).
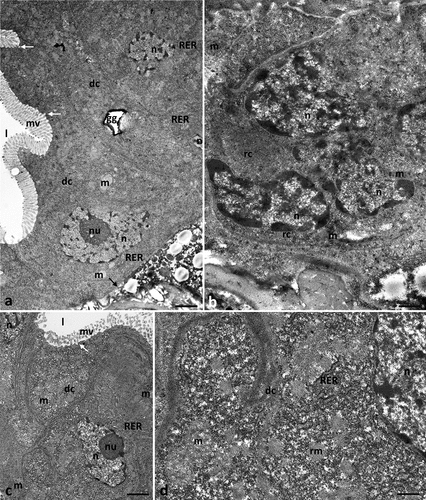
3.2.4. Experimental group Ib1c (concentration of ibuprofen 1 mg/L, 7 days)
The apical cell membrane of cubic digestive cells formed microvilli (). Few autophagic structures were observed in the cytoplasm of these cells (). Some mitochondria degenerated, losing cristae (). No changes were observed concerning the nucleus, nucleolus, or other cell organelles.
Figure 4. Midgut of Pam. experimentalis treated with ibuprofen for 7 days; concentration of ibuprofen 1 mg/L – Ib1c, TEM. (a–d) the ultrastructure of the midgut digestive cells in the group Ib1c, (a) bar = 0.29 μm, (b) bar = 0.32 μm, (c) bar = 0.39 μm, (d) bar = 0.33 μm. Abbreviations: autolysosome (al), autophagosome (au), digestive cell (dc), mitochondrion (m), rough endoplasmic reticulum (RER), reserve material (rm), gut granule (gg), microvilli (mv), midgut lumen (l), merocrine secretion (white arrow).
3.2.5. Experimental group Ib2a (concentration of ibuprofen 0.1 μg/L, 28 days)
The shape of regenerative and digestive cells did not differ from those of the control group. The apical cell membrane of digestive cells formed microvilli, which in some parts of the midgut were slightly shorter and less numerous than in the control group (). Crescent-shaped cells were poor in organelles. No changes were observed in their ultrastructure (). Numerous autophagosomes and autolysosomes were observed in the cytoplasm of the digestive cells (). Changes in the ultrastructure in the form of the loss of the characteristic cristae were observed in the mitochondria ().
Figure 5. Midgut of Pam. experimentalis treated with ibuprofen for 28 days; concentration of ibuprofen 0.1 μg/L – Ib2a, TEM. (a–b) the ultrastructure of the midgut digestive cells in the group Ib2a, (a) bar = 0.15 μm, (b) bar = 0.30 μm. c) the ultrastructure of the midgut regenerative cells in the group Ib2a, bar = 0.28 μm. Abbreviations: autolysosome (al), autophagosome (au), digestive cell (dc), regenerative cell (rc), mitochondrion (m), nucleus (n), rough endoplasmic reticulum (RER), microvilli (mv), midgut lumen (l), basal lamina (black arrow).
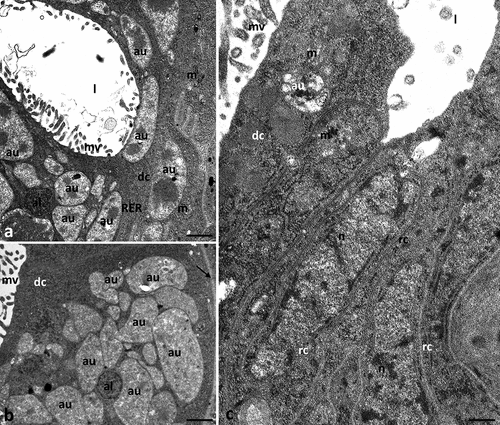
3.2.6. Experimental group Ib2b (concentration of ibuprofen 16.8 μg/L, 28 days)
Microvilli were formed on cubic-shaped digestive cells, but in some parts of the midgut they were shorter and less numerous (). In addition, the apical membrane of the digestive cells formed large evaginations directed to the midgut’s lumen, devoid of microvilli (). Numerous autophagic structures appeared in the cytoplasm of digestive cells (). Some mitochondria lost their cristae (). The remaining cell organelles showed no visible changes in their structure ().
Figure 6. Midgut of Pam. experimentalis treated with ibuprofen for 28 days; concentration of ibuprofen 16.8 μg/L – Ib2b, TEM. (a–c) the ultrastructure of the midgut digestive cells in the group Ib2b, (a) bar = 0.14 μm, (b) bar = 0.19 μm, (c) bar = 0.14 μm. Abbreviations: autophagosome (au), digestive cell (dc), mitochondrion (m), nucleus (n), nucleolus (nu), rough endoplasmic reticulum (RER), reserve material (rm), microvilli (mv), midgut lumen (l), merocrine secretion (white arrow).
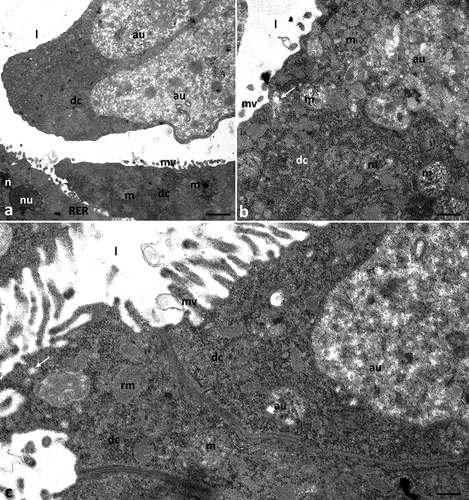
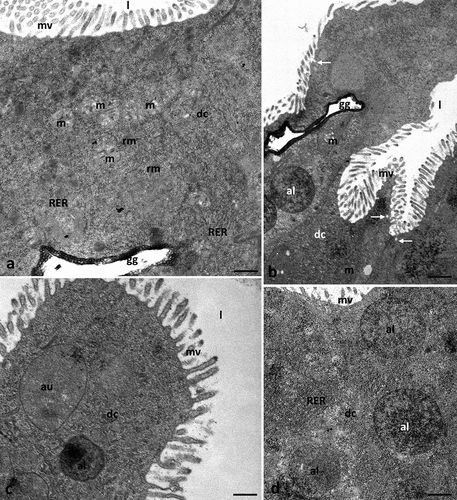
3.2.7. Experimental group Ib2c (concentration of ibuprofen 1 mg/L, 28 days)
The apical cell membrane of digestive cells formed microvilli () and large evaginations directed to the midgut’s lumen, which were devoid of microvilli (). Some mitochondria present in the cytoplasm degenerated, losing their cristae (). Numerous autophagic structures – autophagosomes and autolysosomes – were observed in the cytoplasm of the digestive cells ().
Figure 7. Midgut of Pam. experimentalis treated with ibuprofen for 28 days; concentration of ibuprofen 1 mg/L – Ib2c, TEM. (a–c) the ultrastructure of the midgut digestive cells in the group Ib2c, (a) bar = 0.29 μm, (b) bar = 0.32 μm, (c) bar = 0.21 μm. Abbreviations: autolysosome (al), autophagosome (au), digestive cell (dc), mitochondrion (m), nucleus (n), rough endoplasmic reticulum (RER), reserve material (rm), microvilli (mv), midgut lumen (l), merocrine secretion (white arrow).
3.3. Confocal microscopy analysis
3.3.1. ROS+/ROS− cells in the midgut of Pam. experimentalis exposed to ibuprofen
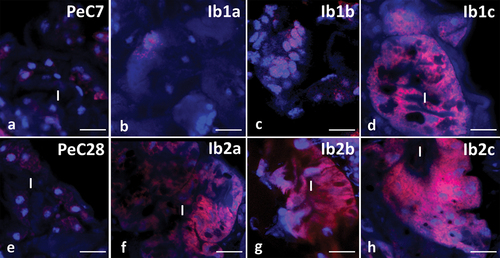
The use of dihydroethidium (DHE) revealed weak signals in the midgut cells of control groups (). Seven-day-long incubation in ibuprofen with concentrations of 0.1 μg/L and 16.8 μg/L showed that signals emitted by ROS+ positive cells were not stronger than in control groups (). However, after 7 days of treating tardigrades with ibuprofen (concentration 1 mg/L), signals emitted by ROS+ positive cells were visibly stronger (). A strong signal emitted by ROS+ cells was also observed in all study groups in which animals were treated with ibuprofen for 28 days ().
Figure 8. Midgut of Pam. experimentalis stained with Dihydroethidium (DHE) and DAPI, confocal microscopy. ROS-positive cells (red signals), nuclei (blue signals). (a;e) the midgut of Pam. experimentalis in control groups, (a) bar = 10.53 μm, (e) bar = 10 μm. (b) the midgut of Pam. experimentalis treated with ibuprofen for 7 days; concentration of ibuprofen 0.1 μg/L – Ib1a, bar = 9.38 μm. (c) the midgut of Pam. experimentalis treated with ibuprofen for 7 days; concentration of ibuprofen 16.8 μg/L – Ib1b, bar = 6.67 μm. (d) the midgut of Pam. experimentalis treated with ibuprofen for 7 days; concentration of ibuprofen 1 mg/L – Ib1c, bar = 8.89 μm. (f) the midgut of Pam. experimentalis treated with ibuprofen for 28 days; concentration of ibuprofen 0.1 μg/L – Ib2a, bar = 14.29 μm. (g) the midgut of Pam. experimentalis treated with ibuprofen for 28 days; concentration of ibuprofen 16.8 μg/L – Ib2b, bar = 11.11 μm. (h) the midgut of Pam. experimentalis treated with ibuprofen for 28 days; concentration of ibuprofen 1 mg/L – Ib2c, bar = 11.77 μm.
3.3.2. Apoptosis in the midgut of Pam. experimentalis exposed to ibuprofen
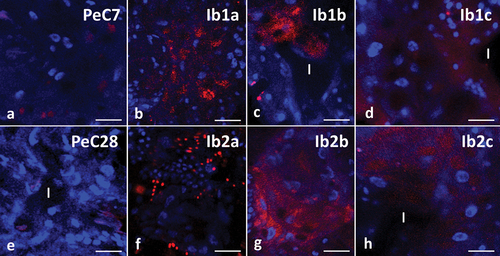
Qualitative analysis using TUNEL showed no nuclear DNA fragmentation, a marker of cell apoptosis, in any of the control groups (), similar to cells treated with three different concentrations of ibuprofen over 7 days (). The midgut cells of specimens treated with concentrations of 0.1 μg/L and 16.8 μg/L for 28 days showed stronger signals compared to the control groups (). The strongest signals were found in the midgut cells of the animals treated with a concentration of 1 mg/L for 28 days ().
Figure 9. Midgut of Pam. experimentalis stained with TUNEL assay and DAPI, confocal microscopy. Nuclei (blue signals), DNA fragmentation (red signals). (a;e) the midgut of Pam. experimentalis in control groups, (A) bar = 10 μm, (E) bar = 10.71 μm. (b) the midgut of Pam. experimentalis treated with ibuprofen for 7 days; concentration of ibuprofen 0.1 μg/L – Ib1a, bar = 11.77 μm. (c) the midgut of Pam. experimentalis treated with ibuprofen for 7 days; concentration of ibuprofen 16.8 μg/L – Ib1b, bar = 10.53 μm. (d) the midgut of Pam. experimentalis treated with ibuprofen for 7 days; concentration of ibuprofen 1 mg/L – Ib1c, bar = 8.33 μm. (f) the midgut of Pam. experimentalis treated with ibuprofen for 28 days; concentration of ibuprofen 0.1 μg/L – Ib2a, bar = 11.11 μm. (g) the midgut of Pam. experimentalis treated with ibuprofen for 28 days; concentration of ibuprofen 16.8 μg/L – Ib2b, bar = 8.33 μm. (h) the midgut of Pam. experimentalis treated with ibuprofen for 28 days; concentration of ibuprofen 1 mg/L – Ib2c, bar = 9.10 μm.
3.3.3. Autophagy in midgut of Pam. experimentalis exposed to ibuprofen
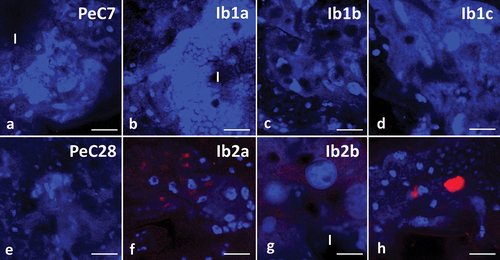
Using LysoTracker Red, it was possible to determine that the control groups showed few signals emitted by acid organelles – autolysosomes and lysosomes (). Similar results were also obtained in groups where specimens were incubated for 7 days with ibuprofen at concentrations of 0.1 μg/L and 16.8 μg/L (). However, in midgut cells of animals treated with ibuprofen at a concentration of 1 mg/L for 7 days, signals emitted by acid organelles were visibly stronger (). An incubation period of 28 days caused signals emitted by autolysosomes and lysosomes to be stronger regardless of the used concentration of ibuprofen ().
Figure 10. Midgut of Pam. experimentalis stained with LysoTracker Red and DAPI, confocal microscopy. Nuclei (blue signals), acidic organelles (red signals). (a;e) Autophagy in midgut of Pam. experimentalis in control groups, (a) bar = 12.93 μm, (e) bar = 10.53 μm. (b) Autophagy in midgut of Pam. experimentalis treated with ibuprofen for 7 days; concentration of ibuprofen 0.1 μg/L – Ib1a, bar = 14.29 μm. (c) Autophagy in midgut of Pam. experimentalis treated with ibuprofen for 7 days; concentration of ibuprofen 16.8 μg/L – Ib1b, bar = 11.11 μm. (d) Autophagy in midgut of Pam. experimentalis treated with ibuprofen for 7 days; concentration of ibuprofen 1 mg/L – Ib1c, bar = 10 μm. (f) Autophagy in midgut of Pam. experimentalis treated with ibuprofen for 28 days; concentration of ibuprofen 0.1 μg/L – Ib2a, bar = 8.33 μm. (g) Autophagy in midgut of Pam. experimentalis treated with ibuprofen for 28 days; concentration of ibuprofen 16.8 μg/L – Ib2b, bar = 12.5 μm. (h) Autophagy in midgut of Pam. experimentalis treated with ibuprofen for 28 days; concentration of ibuprofen 1 mg/L – Ib2c, bar = 10 μm.
4. Discussion
By focusing on various concentrations of ibuprofen and employing a diverse of methods, this research contributes to a comprehensive assessment of the risks associated with ibuprofen contamination. In research, three concentrations of ibuprofen were employed: two representative of environmental levels, specifically 0.1 μg/L (Aus der Beek et al. Citation2016) and 16.8 μg/L (Mrowiec Citation2015), alongside a higher concentration of 1 mg/L. The environmental concentrations were chosen to reflect real-world exposure scenarios, ensuring relevance to ecological contexts. By incorporating concentrations observed in the environment, aim to simulate conditions that organisms, including tardigrades, may encounter in their natural habitats. The inclusion of a higher concentration allows us to assess potential effects under more extreme exposure scenarios, providing insights into the biological responses of tardigrades across a range of ibuprofen concentrations. The study’s multi-faceted approach like LC50 determination, ultrastructural analysis, and confocal microscopy, provides a holistic view of the effects ranging from lethality to subcellular changes. These findings highlight the complexity of cellular responses to pharmaceutical contamination.
Harmful substances can enter a tardigrade’s body not only through the body wall but also with nourishments. Those stressors can cause irreversible alterations to the digestive cells (Rost-Roszkowska et al. Citation2008, Citation2018). The body wall and the digestive system form a barrier protecting the animal’s body against these stressors. The midgut is the most extensive part of the digestive system of tardigrades and is a place of digestion due to the lack of a cuticle (Nelson et al. Citation2015). Additionally, it is a region responsible for secretion and absorption (Rost-Roszkowska & Poprawa Citation2008; Rost-Roszkowska et al. Citation2011, Citation2019). It is formed by digestive cells as well as being detected in several species’ “crescent-like cells” that have a regenerative function. Their role is to differentiate into digestive cells. Regenerative cells form two distinct “epithelial rings” – anterior and posterior (Greven Citation1976; Rost-Roszkowska et al. Citation2011; Rost‐Roszkowska et al. Citation2013; Hyra et al. Citation2016; Citation2019).
The contamination of NSAIDs includes surface waters but also groundwater and rarely drinking water (Santos et al. Citation2010). It may result not only in consequences for aquatic organisms but also for the whole trophic chain (Parolini Citation2020). Ibuprofen has been detected worldwide in surface waters, groundwater, and tap water, with a maximum measured environmental concentration of 303 µg/L (Aus der Beek et al. Citation2016). The toxicity of ibuprofen towards water organisms has been investigated in aquatic invertebrates such as Daphnia magna (Heckmann et al. Citation2006, Citation2007; Hayashi et al. Citation2008; Gómez-Oliván et al. Citation2014), Hydra vulgaris (Pascoe et al. Citation2003), Planorbis carinatus (Pounds et al. Citation2008), Dreissena polymorpha (Parolini et al. Citation2011), and Corbicula fluminea (Aguirre-Martínez et al. Citation2015). The toxicity of ibuprofen has also been studied in vertebrates such as the fishes Rhamdia quelen (Mathias et al. Citation2018), Oryzias latipes (Flippin et al. Citation2007) as well as tadpoles of Bufo bufo (Aliko et al. Citation2021). Most of these studies investigated ibuprofen’s effects on mortality, growth, reproduction of animals, or expression of genes. There is no data on ultrastructural changes in invertebrate cells treated with ibuprofen.
The LC50 of the tested species for 24 h exposure is 282 mg/L. However, Pam. experimentalis is a gonochoric species with morphological differences between males and females, such as size differences (Kaczmarek et al. Citation2020). The experiment was conducted without dividing the individuals by sex. Recent research on the anhydrobiosis of this species has suggested that males may be less able to return to activity after rehydration than females (Nagwani et al. Citation2023). There was some evidence for the effect of sex on such resistance – a factor that should be analyzed in further research. Exposure to ibuprofen (200 mg/L) caused the death of all tested specimens of Daphnia magna after 24 hours (Du et al. Citation2016), and the 48-hour LC50 value for this species is reported to be 132.6 mg/L (Han et al. Citation2006). The LC50 test result for the green neon shrimp Neocaridina denticulata after 96-hour exposure to ibuprofen was 6.60 mg/L (Sung et al. Citation2014); in the freshwater keeled ramshorn snail Planorbis carinatus the 48- and 72-hour LC50 values were both 17.1 mg/L (Pounds et al. Citation2008); while the 72-hour LC50 value for the cnidarian Hydra attenuata was 22.3 mg/L (Quinn et al. Citation2008). For comparison, the 96-hour LC50 value of ibuprofen for African catfish Clarias gariepinus juveniles was determined to be 0.38 mg/L (Ogueji et al. Citation2018). However, the 96-hour LC50 value for the bluegill sunfish Lepomis macrochirus was 173 mg/L (Halling-Sørensen et al. Citation1998), and 142 mg/L for the fish Cirrhinus mrigala after 24-hour exposure (Saravanan et al. Citation2011). Studies conducted over the years have shown that ibuprofen toxicity varies from one species to another. Concentrations used in the experiment would most likely be of minor importance for survival. However, in a natural ecosystem, animals are exposed to ibuprofen concentrations for their whole lifespan. Other factors that need to be considered are the high biological activity of NSAIDs and the cumulative effects of drug mixtures (Parolini Citation2020).
Our study showed that ibuprofen causes changes at the ultrastructural level in the digestive cells of the midgut of Pam. experimentalis. After 7-day exposure to ibuprofen at concentrations of 0.1 μg/L and 16.8 μg/L, digestive cells showed no significant changes compared to the cells of animals from the control groups. However, exposure to this pharmaceutical for the same amount of time at a concentration of 1 mg/L showed the presence of autophagosomes in cells and changes in the ultrastructure of the mitochondria. Long-term, 28-day exposure at all three tested concentrations (0.1 μg/L, 16.8 μg/L, 1 mg/L) caused changes such as degenerated mitochondria and the presence of autophagosomes and autolysosomes in the cytoplasm of digestive cells. Evaginations directed to the midgut’s lumen, devoid of microvilli, are places where acidic organelles accumulate. This may indicate the possibility of later detachment of part of the cell with numerous autophagosomes and autolysosomes or the opening of autophagic structures into the midgut lumen to restore cell homeostasis. Autophagosomes are vesicles that containing cellular components, such as damaged organelles for degradation. Autolysosomes emerge as a consequence of the fusion between autophagosomes and lysosomes. This leads to the degradation material within autophagosomes by the enzymes present in lysosomes (Wang et al. Citation2022). The intensified autophagy observed in response to ibuprofen raises the possibility that the organism may be attempting to preserve the functionality of the tissue by removing damaged organelles. Regenerative cells showed no changes at the ultrastructural level at any concentrations tested, either in the short- or long-term incubation. The lack of changes in the crescent-like cells may be due to the lack of direct connection with the pharmaceutical (regenerative cells do not contact the midgut lumen). Ibuprofen caused changes in digestive cells by having direct contact with them, passing into the midgut along with the nourishments. This also confirms the role of digestive cells as the first barrier against stressors.
It is commonly known that mitochondria are one of the most important organelles ensuring the proper functioning of cells. Their crucial role in cell biology is to be the source of ATP, NADH, and GTP and participate in the biosynthesis of reactive oxygen species (Picard et al. Citation2013; Włodarczyk et al. Citation2019). Because of that, they are usually the first to respond to stressors. Changes in these organelles may disrupt the functioning of cells and cause irreversible damage (Rost-Roszkowska et al. Citation2021). Degeneration of mitochondria (loss of cristae) in Pam. experimentalis digestive cells was observed after 7-day incubation in ibuprofen (concentration 1 mg/L) and prolonged exposure time caused the same ultrastructural changes in all tested concentrations. No changes were observed in regenerative cells. Observed mitochondrial changes, have the potential to disrupt ATP production and, consequently, overall cell function. This may suggest a possible relationship between the observed ultrastructural changes and the animals response to ibuprofen. Also potentially affecting cellular functions, such as metabolism and growth. Treatment of storage cells of Hypsibius exemplaris for 7 days with paracetamol (at concentrations of 230 μg/L and 1 mg/L) caused mitochondria to degenerate, losing their cristae. The same study showed similar changes in these organelles after 28 days of incubation with ibuprofen, not only at concentrations of 230 μg/L and 1 mg/L but also at the much lower 0.2 μg/L (Wieczorkiewicz et al. Citation2024). As in the treatment with ibuprofen, specimens after long-term intubation in paracetamol showed changes at the ultrastructural level (degenerated mitochondria) with much lower concentrations that can be found in the environment. This indicates that prolonged exposure to this pharmaceutical can impact these animals. Similar changes were caused by other factors. Cadmium caused changes in mitochondria in germline cells of the earthworm Dendrobaena veneta (Siekierska & Urbańska-Jasik Citation2002) and both somatic and germline cells of Lithobius forficatus (Rost-Roszkowska et al. Citation2020a, Citation2020b, Citation2021, Citation2022; Poprawa et al. Citation2022), as well as combined cadmium and selenium in germline cells of the leech Erpobdella octoculata (Siekierska & Urbanska-Jasik Citation1998) and cadmium and copper in hemocytes of Steatoda grossa (Wilczek et al. Citation2018). Mitochondrial dysfunction may also be caused by nanodiamonds, as found in the gut epithelium in Acheta domesticus (Karpeta-Kaczmarek et al. Citation2016). The absence of cristae in the mitochondria of midgut epithelium cells of the freshwater shrimp Neocaridina davidi was also noted after treating the animals with insecticides (Ostróżka et al. Citation2022).
Confocal microscopy analysis showed a visible increase in DNA fragmentation, reactive oxygen species, and the presence of acidic organelles (autolysosomes, lysosomes) in midgut cells of specimens of Pam. experimentalis in most of the groups incubated in ibuprofen. Increasing concentrations of this drug caused the detection of more signals suggesting abnormalities in tardigrade cells. This in response to ibuprofen exposure suggests a multifaceted response of Pam. experimentalis to pharmaceutical stress. This complexity underscores the organisms ability to range of strategies as a response to stressors. They are observed even at lower concentrations after 28-day incubation. Increased intensity of autophagy in the cells of the studied tardigrade correlated with ibuprofen concentrations and length of incubation. Autophagy is an important process of growth regulation. Breakdown products are input to cellular metabolism. Energy generated in this way is used to build new proteins and membranes, which ultimately helps maintain the homeostasis of cells (Rabinowitz & White Citation2010). It is also a response to various types of stimuli, including nutrient deprivation, oxidative stress, chemical factors, or pathogens such as bacteria or microsporidia, helping to protect cells by eliminating damaged organelles or pathogens (Kelekar Citation2006; Glick et al. Citation2010; Rost-Roszkowska et al. Citation2011, Citation2013, Citation2018, Citation2020a, Citation2020b, Citation2022; Poprawa et al. Citation2022). During starvation-induced autophagy, reserve material accumulated in the cytoplasm of the digestive cells of animals can be exploited. This process can be caused by a lack of food in the environment, moulting, or hibernation (Hyra et al. Citation2016; Rost-Roszkowska et al. Citation2018). The 7-day treatment with a pharmaceutical concentration of 1 mg/L resulted in the appearance of acidic structures in cells responsible for removing damaged organelles, among others. Organelle damage is most likely related to the toxic effects of ibuprofen. Longer, 28-day incubation increased autophagy at all tested concentrations, which may indicate that even small amounts of this pharmaceutical, when exposure periods are long, can cause damage to organelles or proteins. The appearance of autophagic structures and, thus, the intensification of autophagy induced by pharmaceuticals have been described in storage cells of the tardigrade Hys. exemplaris incubated in paracetamol solution. In this species, the increase in autophagy was correlated with increased drug concentration and incubation time (Wieczorkiewicz et al. Citation2024). Autophagy in the digestive cells of Grevenius granulifer (formerly Isohypsibius granulifer granulifer – see Gąsiorek et al. Citation2019) infected with microsporidia was more intensive than in noninfected specimens; the purpose of this was to remove pathogens and damage organelles. This process has also been described in the cytoplasm of trophocytes of the same species as they are separated from the oocyte during late choriogenesis (Rost-Roszkowska et al. Citation2013). In this case, the intensification of autophagy directed the cell onto the path of apoptosis (Poprawa et al. Citation2015). In addition, a correlation between the intensity of autophagy and cell apoptosis or necrosis has been described in the midgut cells of tardigrades (Rost-Roszkowska et al. Citation2018). When too many autophagosomes, autolysosomes, and residual bodies appeared in the digestive cells, the cells began to die by apoptosis or necrosis (Rost-Roszkowska et al. Citation2018). Apoptosis of senescent cells may help maintain homeostasis in multicellular organisms when correlated with the proliferation of new cells (Hetts Citation1998). This process can be detected using markers such as TUNEL staining to detect DNA fragmentation. During apoptosis, the cell shrinks, and structural changes occur in organelles such as endoplasmic reticulum, Golgi complexes, and mitochondria. Then, “apoptotic bodies” are formed (Lee & Baehrecke Citation2001; Poprawa et al. Citation2015). This process can also be induced by many factors, such as cadmium, chromium, and nickel (Rana Citation2008). After the incubation of Pam. experimentalis with ibuprofen for 28 days, increased intensity of DNA fragmentation, a factor indicating that the cell has entered the path of apoptosis, was detected. The highest intensity of signals indicating DNA fragmentation was observed at a 1 mg/L concentration. Therefore, we propose that apoptosis is responsible for removing damaged cells to help maintain homeostasis in the animal organism. Cooperation of these processes was also described in the germarium Diatraea saccharalis (dos Santos et al. Citation2007), ovarian nurse cells of Drosophila virilis (Velentzas et al. Citation2007), and Adalia bipunctata (Mpakou et al. Citation2011) and in the midgut epithelium, salivary glands and gonads of Lithobius forficatus (Rost-Roszkowska et al. Citation2020a, Citation2020b; Poprawa et al. Citation2022).
Factors influencing apoptosis are reactive oxygen species (ROS), which are cytotoxic agents. They affect this process in pathological and physiological conditions (due to their antimicrobial properties). They have a destructive effect on both DNA and proteins (Simon et al. Citation2000; Zhang et al. Citation2020). Oxidative stress was observed in the midgut cells of Pam. experimentalis treated with ibuprofen for 28 days, regardless of the concentration of ibuprofen used, and after 7 days of incubation in this drug at a concentration of 1 mg/L. ROS levels in ibuprofen-treated cells introduces the potential for oxidative damage to cellular components this may contribute to the disruption of cell function and play a role in the observed ultrastructural changes and cellular responses. The significant accumulation of ROS induced by ibuprofen may have led to digestive cell death by apoptosis in this species. Increased oxidative damage to RNA and DNA has been described in coelomocytes and body wall tissue of the polychaete Glycera dibranchiata exposed to sulfide (Joyner-Matos et al. Citation2010). It has been shown that organophosphates and various classes of pyrethroids can also cause oxidative stress. The antimicrobial properties of ROS can be supported by their increased production in the midgut of Drosophila melanogaster upon bacterial challenge (Chaitanya et al. Citation2016). Studies on Neocaridina davidi shrimp have shown that long-term starvation increases the concentration of ROS in the intestine and liver while re-feeding reduces their concentration (Włodarczyk et al. Citation2019).
Conclusions
Our study showed that: (1) ultrastructural changes of digestive cells of Pam. experimentalis are positively correlated with the concentration and the time of exposure to the stressor; (2) the lack of ultrastructural changes in regenerative cells may be due to the lack of direct contact with the ibuprofen; (3) digestive cells are one of the first barriers against harmful substances; (4) mitochondria are the organelles most sensitive to the stressor; (5) with higher concentrations of ibuprofen and longer exposure time, in midgut cells activation and intensification of autophagy, DNA fragmentation and oxidative stress occur. (6) in future studies, a broader picture of the changes caused by ibuprofen in tardigrades may be obtained by flow cytometry, as well as studies on other organs such as gonads and storage bodies. By researching the effects of ibuprofen exposure on organisms like tardigrades, we provide insights into potential ecological risks of pharmaceuticals in the environment.
Analysis of the effect of ibuprofen on tardigrades requires further research. It seems particularly interesting to estimate the impact of this pharmaceutical on the ultrastructure and functioning of the gonads and to determine whether both sexes are equally sensitive to this stressor.
Author contributions
AM: Methodology, Validation, Investigation, Visualization, Writing - Original Draft, Writing - Review & Editing,
IP: Conceptualization, Supervision, Methodology, Investigation, Validation, Data Curation, Visualization, Writing - Original Draft, Writing - Review & Editing,
SS: Methodology, Visualization
EF: Methodology, Resources
Acknowledgments
We are very grateful to Dr Danuta Urbańska-Jasik, Dr Łukasz Chajec and MSc Kamil Janelt (University of Silesia in Katowice, Poland) for their technical assistance. We would like to thank Professor Agnieszka Babczyńska (University of Silesia in Katowice, Poland) for the substantive consultation. The authors are deeply indebted to Richard Ashcroft, biomedical editor (http://www.anglopolonia.com/home.html), for improving the English style.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All relevant data are within the paper.
References
- Aguirre-Martínez GV, DelValls AT, Martín-Díaz ML. 2015. Yes, caffeine, ibuprofen, carbamazepine, novobiocin and tamoxifen have an effect on Corbicula fluminea (Müller, 1774). Ecotoxicology and Environmental Safety 120:142–154. DOI: 10.1016/j.ecoenv.2015.05.036
- Aliko V, Korriku RS, Pagano M, Faggio C. 2021. Double-edged sword: Fluoxetine and ibuprofen as development jeopardizers and apoptosis’ inducers in common toad, Bufo bufo, tadpoles. Science of the Total Environment 776:145945. DOI: 10.1016/j.scitotenv.2021.145945
- Aus der Beek T, Weber FA, Bergmann A, Hickmann S, Ebert I, Hein A, Küster A. 2016. Pharmaceuticals in the environment—Global occurrences and perspectives. Environmental Toxicology and Chemistry 35(4):823–835. DOI: 10.1002/etc.3339
- Batucan NSP, Tremblay LA, Northcott GL, Matthaei CD. 2022. Medicating the environment? A critical review on the risks of carbamazepine, diclofenac and ibuprofen to aquatic organisms. Environmental Advances 7:100164. DOI: 10.1016/j.envadv.2021.100164
- Boxall ABA, Rudd MA, Brooks BW, Caldwell DJ, Choi K, Hickmann S, Innes E, Ostapyk K, Staveley JP, Verslycke T, Ankley GT, Beazley KF, Belanger SE, Berninger JP, Carriquiriborde P, Coors A, DeLeo PC, Dyer SD, Ericson JF, Gagné F, Giesy JP, Gouin T, Hallstrom L, Karlsson MV, Joakim Larsson DG, Lazorchak JM, Mastrocco F, McLaughlin A, McMaster ME, Meyerhoff RD, Moore R, Parrott JL, Snape JR, Murray-Smith R, Servos MR, Sibley PK, Straub JO, Szabo ND, Topp E, Tetreault GR, Trudeau VL, Van Der Kraak G. 2012. Pharmaceuticals and personal care products in the environment: What are the big questions? Environmental Health Perspectives 120(9):1221–1229. DOI: 10.1289/ehp.1104477
- Bushra R, Aslam N. 2010. An overview of clinical pharmacology of Ibuprofen. Oman Medical Journal 25(3):155. DOI: 10.5001/omj.2010.49
- Chaitanya RK, Shashank K, Sridevi P. 2016. Oxidative stress in invertebrate systems. Free Radicals and Diseases 19:51–68.
- dos Santos DC, Gregório EA, Moreli Silva de Moraes RL. 2007. Programmed cell death during early oogenesis in the Diatraea saccharalis germarium. Acta Microscopy 16:311–312.
- Du J, Mei CF, Ying GG, Xu MY. 2016. Toxicity thresholds for diclofenac, acetaminophen and ibuprofen in the water flea Daphnia magna. Bulletin of Environmental Contamination and Toxicology 97(1):84–90. DOI: 10.1007/s00128-016-1806-7
- Fent K, Weston AA, Caminada D. 2006. Ecotoxicology of human pharmaceuticals. Aquatic Toxicology (Amsterdam, Netherlands) 76(2):122–159. DOI: 10.1016/j.aquatox.2005.09.009
- Finney DJ. 1952. Probit Analysis. By D. J. Finney, M.A. Sc.D. [2nd ed. Pp. xiv + 318. Cambridge University Press, 1952. 35 s.]. Journal of the Institute of Actuaries 78(3):388–390. DOI: 10.1017/S0020268100052938
- Flippin JL, Huggett D, Foran CM. 2007. Changes in the timing of reproduction following chronic exposure to ibuprofen in Japanese medaka, Oryzias latipes. Aquatic Toxicology (Amsterdam, Netherlands) 81(1):73–78. DOI: 10.1016/j.aquatox.2006.11.002
- Gąsiorek P, Stec D, Morek W, Michalczyk Ł. 2019. Deceptive conservatism of claws: Distinct phyletic lineages concealed within Isohypsibioidea (Eutardigrada) revealed by molecular and morphological evidence. Contributions to Zoology 88(1):78–132. DOI: 10.1163/18759866-20191350
- Glick D, Barth S, Macleod KF. 2010. Autophagy: Cellular and molecular mechanisms. The Journal of Pathology 221(1):3–12. DOI: 10.1002/path.2697
- Gómez-Oliván LM, Galar-Martínez M, García-Medina S, Valdés-Alanís A, Islas-Flores H, Neri-Cruz N. 2014. Genotoxic response and oxidative stress induced by diclofenac, ibuprofen and naproxen in Daphnia magna. Drug and Chemical Toxicology 37(4):391–399. DOI: 10.3109/01480545.2013.870191
- Greven H. 1976. Some ultrastructural observations on the midgut epithelium of Isohypsibius augusti (Murray, 1907)(Eutardigrada). Cell and Tissue Research 166(3):339–351. DOI: 10.1007/BF00220130
- Halling-Sørensen BNNS, Nielsen SN, Lanzky PF, Ingerslev F, Lützhøft HH, Jørgensen SE. 1998. Occurrence, fate and effects of pharmaceutical substances in the environment-A review. Chemosphere 36(2):357–393. DOI: 10.1016/S0045-6535(97)00354-8
- Han GH, Hur HG, Kim SD. 2006. Ecotoxicological risk of pharmaceuticals from wastewater treatment plants in Korea: Occurrence and toxicity to Daphnia magna. Environmental Toxicology and Chemistry: An International Journal 25(1):265–271. DOI: 10.1897/05-193R.1
- Harshkova D, Aksmann A. 2019. Zanieczyszczenie środowiska niesteroidowymi lekami przeciwzapalnymi na przykładzie diklofenaku – przyczyny, skutki, bioindykacja. Kosmos 68(1):185–194. DOI: 10.36921/kos.2019_2487
- Hayashi Y, Heckmann LH, Callaghan A, Sibly RM. 2008. Reproduction recovery of the crustacean Daphnia magna after chronic exposure to ibuprofen. Ecotoxicology 17(4):246–251. DOI: 10.1007/s10646-008-0191-3
- Heckmann LH, Callaghan A, Hooper HL, Connon R, Hutchinson TH, Maund SJ, Sibly RM. 2007. Chronic toxicity of ibuprofen to Daphnia magna: Effects on life history traits and population dynamics. Toxicology Letters 172(3):137–145. DOI: 10.1016/j.toxlet.2007.06.001
- Heckmann LH, Connon R, Hutchinson TH, Maund SJ, Sibly RM, Callaghan A. 2006. Expression of target and reference genes in Daphnia magna exposed to ibuprofen. BMC Genomics 7(1):1–8. DOI: 10.1186/1471-2164-7-175
- Hetts SW. 1998. To die or not to die: An overview of apoptosis and its role in disease. Jama 279(4):300–307. DOI: 10.1001/jama.279.4.300
- Hyra M, Poprawa I, Włodarczyk A, Student S, Sonakowska L, Kszuk-Jendrysik M, Rost-Roszkowska MM. 2016. Ultrastructural changes in the midgut epithelium of Hypsibius dujardini (Doyère, 1840)(Tardigrada, Eutardigrada, Hypsibiidae) in relation to oogenesis. Zoological Journal of the Linnean Society 178(4):897–906. DOI: 10.1111/zoj.12467
- Joyner-Matos J, Predmore BL, Stein JR, Leeuwenburgh C, Julian D. 2010. Hydrogen sulfide induces oxidative damage to RNA and DNA in a sulfide-tolerant marine invertebrate. Physiological and Biochemical Zoology 83(2):356–365. DOI: 10.1086/597529
- Kaczmarek Ł, Roszkowska M, Poprawa I, Janelt K, Kmita H, Gawlak M, Fiałkowska E, Mioduchowska M. 2020. Integrative description of bisexual paramacrobiotus experimentalis sp. nov. (Macrobiotidae) from republic of Madagascar (Africa) with microbiome analysis. Molecular Phylogenetics and Evolution 145:106730. DOI: 10.1016/j.ympev.2019.106730
- Karaźniewicz M. 2007. Czterdzieści lat doświadczeń stosowania ibuprofenu w lecznictwie. Nowiny Lekarskie 76:195–197.
- Karpeta-Kaczmarek J, Augustyniak M, Rost-Roszkowska M. 2016. Ultrastructure of the gut epithelium in Acheta domesticus after long-term exposure to nanodiamonds supplied with food. Arthropod Structure & Development 45(3):253–264. DOI: 10.1016/j.asd.2016.02.002
- Kelekar A. 2006. Autophagy. Annals of the New York Academy of Sciences 1066(1):259–271. DOI: 10.1196/annals.1363.015
- Lee CY, Baehrecke EH. 2001. Steroid regulation of autophagic programmed cell death during development. Development 128(8):1443–1455. DOI: 10.1242/dev.128.8.1443
- Marchlewicz A, Guzik U, Wojcieszyńska D. 2015. Właściwości, występowanie i biodegradacja ibuprofenu w środowisku wodnym. Ochrona środowiska 37(1):65–70.
- Mathias FT, Fockink DH, Disner GR, Prodocimo V, Ribas JLC, Ramos LP, Cestari MM, de Assis HCS. 2018. Effects of low concentrations of ibuprofen on freshwater fish Rhamdia quelen. Environmental Toxicology and Pharmacology 59:105–113. DOI: 10.1016/j.etap.2018.03.008
- Mpakou VE, Velentzas AD, Velentzas PD, Margaritis LH, Stravopodis DJ, Papassideri IS. 2011. Programmed cell death of the ovarian nurse cells during oogenesis of the ladybird beetle Adalia bipunctata (Coleoptera: Coccinellidae). Development, Growth & Differentiation 53(6):804–815. DOI: 10.1111/j.1440-169X.2011.01288.x
- Mrowiec B. 2015. Farmaceutyki–zagrożenie środowiska wodnego. Edukacja Biologiczna i Środowiskowa 4:25–33.
- Nagwani AK, Melosik I, Kaczmarek L, Kmita H. 2023. Recovery from anhydrobiosis in the tardigrade Paramacrobiotus experimentalis: Better to be young than old and in a group than alone. bioRxiv 2023–2025 D oi: 10.1101/2023.05.22.541721.
- Nelson DR, Guidetti R, Rebecchi L. 2015. Phylum tardigrada. In: Thorp and Covich’s freshwater invertebrates. London, Elsevier: Academic Press. pp. 347–380.
- Nikolaou A, Meric S, Fatta D. 2007. Occurrence patterns of pharmaceuticals in water and wastewater environments. Analytical and Bioanalytical Chemistry 387(4):1225–1234. DOI: 10.1007/s00216-006-1035-8
- Ogueji EO, Nwani CD, Iheanacho SC, Mbah CE, Okeke CO, Yaji A. 2018. Acute toxicity effects of ibuprofen on behaviour and haematological parameters of African catfish Clarias gariepinus (Burchell, 1822). African Journal of Aquatic Science 43(3):293–303. DOI: 10.2989/16085914.2018.1465393
- Ostróżka A, Tiffert Z, Wilczek G, Rost-Roszkowska M. 2022. Can insecticide-free clean water regenerate the midgut epithelium of the freshwater shrimp after dimethoate treatment? Micron 155:103162. DOI: 10.1016/j.micron.2021.103162
- Parolini M. 2020. Toxicity of the non-steroidal anti-inflammatory drugs (NSAIDs) acetylsalicylic acid, paracetamol, diclofenac, ibuprofen and naproxen towards freshwater invertebrates: A review. Science of the Total Environment 740:140043. DOI: 10.1016/j.scitotenv.2020.140043
- Parolini M, Binelli A, Provini A. 2011. Chronic effects induced by ibuprofen on the freshwater bivalve Dreissena polymorpha. Ecotoxicology and Environmental Safety 74(6):1586–1594. DOI: 10.1016/j.ecoenv.2011.04.025
- Pascoe D, Karntanut W, Müller CT. 2003. Do pharmaceuticals affect freshwater invertebrates? A study with the cnidarian Hydra vulgaris. Chemosphere 51(6):521–528. DOI: 10.1016/S0045-6535(02)00860-3
- Picard M, Shirihai OS, Gentil BJ, Burelle Y. 2013. Mitochondrial morphology transitions and functions: Implications for retrograde signaling? American Journal of Physiology-Regulatory, Integrative & Comparative Physiology 304(6):R393–R406. DOI: 10.1152/ajpregu.00584.2012
- Poprawa I, Chajec Ł, Chachulska-Żymełka A, Wilczek G, Student S, Leśniewska M, Rost-Roszkowska M. 2022. Ovaries and testes of Lithobius forficatus (Myriapoda, Chilopoda) react differently to the presence of cadmium in the environment. Scientific Reports 12(1):6705. DOI: 10.1038/s41598-022-10664-4
- Poprawa I, Hyra M, Kszuk-Jendrysik M, Rost-Roszkowska MM. 2015. Ultrastructural changes and programmed cell death of trophocytes in the gonad of Isohypsibius granulifer granulifer Thulin, 1928 (Tardigrada, Eutardigrada, Isohypsibiidae). Micron 70:26–33. DOI: 10.1016/j.micron.2014.11.008
- Pounds N, Maclean S, Webley M, Pascoe D, Hutchinson T. 2008. Acute and chronic effects of ibuprofen in the mollusc Planorbis carinatus (Gastropoda: Planorbidae). Ecotoxicology and Environmental Safety 70(1):47–52. DOI: 10.1016/j.ecoenv.2007.07.003
- Quinn B, Gagné F, Blaise C. 2008. An investigation into the acute and chronic toxicity of eleven pharmaceuticals (and their solvents) found in wastewater effluent on the cnidarian. Hydra Attenuata Science of the Total Environment 389(2–3):306–314. DOI: 10.1016/j.scitotenv.2007.08.038
- Rabinowitz JD, White E. 2010. Autophagy and metabolism. Science 330(6009):1344–1348. DOI: 10.1126/science.1193497
- Rainsford KD. 2013. Ibuprofen: Pharmacology, therapeutics and side effects. Basel & Heidelberg: Springer Science & Business Media.
- Rana SVS. 2008. Metals and apoptosis: Recent developments. Journal of Trace Elements in Medicine and Biology 22(4):262–284. DOI: 10.1016/j.jtemb.2008.08.002
- Rost‐Roszkowska MM, Poprawa I, Kaczmarek Ł. 2013. Autophagy as the cell survival in response to a microsporidian infection of the midgut epithelium of Isohypsibius granulifer granulifer (Eutardigrada: Hypsibiidae). Acta Zoologica 94(3):273–279. DOI: 10.1111/j.1463-6395.2011.00552.x
- Rost-Roszkowska M, Janelt K, Poprawa I. 2019. Fine structure of the midgut epithelium of thulinius ruffoi (Tardigrada, Eutardigrada, Parachela) in relation to oogenesis and simplex stage. Arthropod Structure & Development 49:128–136. DOI: 10.1016/j.asd.2018.12.002
- Rost-Roszkowska MM, Janelt K, Poprawa I. 2018. The role of autophagy in the midgut epithelium of Parachela (Tardigrada). Zoomorphology 137(4):501–509. DOI: 10.1007/s00435-018-0407-x
- Rost-Roszkowska M, Poprawa I. 2008. Ultrastructure of the midgut epithelium in Dactylobiotus dispar (Tardigrada: Eutardigrada) during encystation. Zoologica Poloniae 53(1–4):19. DOI: 10.2478/v10049-008-0002-7
- Rost-Roszkowska M, Poprawa I, Chajec Ł, Chachulska-Żymełka A, Leśniewska M, Student S. 2020b. Effects of short-and long-term exposure to cadmium on salivary glands and fat body of soil centipede Lithobius forficatus (Myriapoda, Chilopoda): Histology and ultrastructure. Micron 137:102915. DOI: 10.1016/j.micron.2020.102915
- Rost-Roszkowska M, Poprawa I, Chajec Ł, Chachulska-Żymełka A, Wilczek G, Skowronek M, Student S, Leśniewska M. 2022. Hazards related to the presence of cadmium in food–Studies on the European soil centipede, Lithobius forficatus. Science of the Total Environment 845:157298. DOI: 10.1016/j.scitotenv.2022.157298
- Rost-Roszkowska M, Poprawa I, Chajec Ł, Chachulska-Żymełka A, Wilczek G, Wilczek P, Student S, Skowronek M, Nadgórska-Socha A, Leśniewska M. 2020a. Influence of soil contaminated with cadmium on cell death in the digestive epithelium of soil centipede Lithobius forficatus (Myriapoda, Chilopoda). The European Zoological Journal 87(1):242–262. DOI: 10.1080/24750263.2020.1757168
- Rost-Roszkowska M, Poprawa I, Chajec Ł, Chachulska-Żymełka A, Wilczek G, Wilczek P, Tarnawska M, Student S, Leśniewska M. 2021. Effects of cadmium on mitochondrial structure and function in different organs: Studies on the soil centipede Lithobius forficatus (Myriapoda, Chilopoda). The European Zoological Journal 88(1):632–648. DOI: 10.1080/24750263.2021.1912199
- Rost-Roszkowska MM, Poprawa I, Klag J, Migula P, Mesjasz-Przybyłowicz J, Przybyłowicz W. 2008. Degeneration of the midgut epithelium in Epilachna cf. nylanderi (Insecta, Coccinellidae): Apoptosis, autophagy, and necrosis. Canadian Journal of Zoology 86(10):1179–1188. DOI: 10.1139/Z08-096
- Rost-Roszkowska MM, Poprawa I, Wójtowicz M, Kaczmarek Ł. 2011. Ultrastructural changes of the midgut epithelium in Isohypsibius granulifer granulifer Thulin, 1928 (Tardigrada: Eutardigrada) during oogenesis. Protoplasma 248(2):405–414. DOI: 10.1007/s00709-010-0186-9
- Santos LH, Araújo AN, Fachini A, Pena A, Delerue-Matos C, Montenegro MCBSM. 2010. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. Journal of Hazardous Materials 175(1–3):45–95. DOI: 10.1016/j.jhazmat.2009.10.100
- Saravanan M, Karthika S, Malarvizhi A, Ramesh M. 2011. Ecotoxicological impacts of clofibric acid and diclofenac in common carp (Cyprinus carpio) fingerlings: Hematological, biochemical, ionoregulatory and enzymological responses. Journal of Hazardous Materials 195:188–194. DOI: 10.1016/j.jhazmat.2011.08.029
- Siekierska E, Urbanska-Jasik D. 1998. The effect of cadmium and selenium ions on the ovary structure in leech Herpobdella octooculata [L.]. Folia Morphologica 57(1).
- Siekierska E, Urbańska-Jasik D. 2002. Cadmium effect on the ovarian structure in earthworm Dendrobaena veneta (Rosa). Environmental Pollution 120(2):289–297. DOI: 10.1016/S0269-7491(02)00152-5
- Simon HU, Haj-Yehia A, Levi-Schaffer F. 2000. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5(5):415–418. DOI: 10.1023/A:1009616228304
- Stanley-Samuelson DW. 1987. Physiological roles of prostaglandins and other eicosanoids in invertebrates. The Biological Bulletin 173(1):92–109. DOI: 10.2307/1541865
- Sung HH, Chiu YW, Wang SY, Chen CM, Huang DJ. 2014. Acute toxicity of mixture of acetaminophen and ibuprofen to Green Neon Shrimp, Neocaridina denticulate. Environmental Toxicology and Pharmacology 38(1):8–13. DOI: 10.1016/j.etap.2014.04.014
- Szymonik A, Lach J. 2012. Zagrożenie środowiska wodnego obecnością środków farmaceutycznych. Inżynieria i Ochrona Środowiska 15:249–263.
- Velentzas AD, Nezis IP, Stravopodis DJ, Papassideri IS, Margaritis LH. 2007. Apoptosis and autophagy function cooperatively for the efficacious execution of programmed nurse cell death during Drosophila virilis oogenesis. Autophagy 3(2):130–132. DOI: 10.4161/auto.3582
- Wang Y, Que H, Rong Y. 2022. Autophagosomal components recycling on autolysosomes. Trends in Cell Biology 32:897–899.
- Wieczorkiewicz F, Sojka J, Poprawa I. 2024. Effect of paracetamol on the storage cells of Hypsibius exemplaris —ultrastructural analysis. Zoological Journal of the Linnean Society 200(1):258–268. DOI: 10.1093/zoolinnean/zlad051
- Wilczek G, Wiśniewska K, Kozina B, Wilczek P, Rost-Roszkowska M, Stalmach M, Skowronek M, Kaszuba F. 2018. Effects of food contaminated with cadmium and copper on hemocytes of steatoda grossa (Araneae: Theridiidae). Ecotoxicology and Environmental Safety 149:267–274. DOI: 10.1016/j.ecoenv.2017.12.007
- Włodarczyk A, Wilczek G, Wilczek P, Student S, Ostróżka A, Tarnawska M, Rost-Roszkowska M. 2019. Relationship between ROS production, MnSOD activation and periods of fasting and re-feeding in freshwater shrimp Neocaridina davidi (Crustacea, Malacostraca). PeerJ 7:e7399. DOI: 10.7717/peerj.7399
- Zhang T, Yan Z, Zheng X, Wang S, Fan J, Liu Z. 2020. Effects of acute ammonia toxicity on oxidative stress, DNA damage and apoptosis in digestive gland and gill of Asian clam (corbicula fluminea). Fish & Shellfish Immunology 99:514–525. DOI: 10.1016/j.fsi.2020.02.046