Abstract
This study provides the first record of Lasioglossum subfasciatum (Imhoff, 1832) (Hymenoptera, Apoidea, Halictidae) in the Sila National Park (Calabria, Italy), a Biosphere Reserve included in the UNESCO program. The species has been classified as endangered (EN) on the IUCN Red List, throughout Europe. Three specimens were found during a monitoring activity, in May 2022. Here, we provide a description of the species, the environmental characteristics of the site where it was found and a chronological map of its European distribution.
1. Introduction
Halictidae is one of the largest families within the Apoidea, consisting of the subfamilies Rophitinae, Nomiinae, Nomioidinae, and Halictinae (Michener Citation2000; Danforth et al. Citation2008; Michez et al. Citation2019). The Halictinae is the largest and most diverse, with 2400 species distributed worldwide including 300 in Europe (Michez et al. Citation2019). Despite the ecological importance of sweat bees as pollinators, information on their distribution in Italy is fragmentary. Bibliographic data report species collected mainly in Piemonte, Friuli-Venezia Giulia, Emilia-Romagna, Tuscany, Umbria, Molise, Sicily, and Sardinia (Generani et al. Citation2001; Quaranta et al. Citation2004; Pagliano Citation2011; Forbicioni et al. Citation2019; Turrisi et al. Citation2021). Moreover, Flaminio et al. (Citation2021) contributed further data through a citizen science project. In southern Italy, some species have recently been documented in Sicily (Nobile & Turrisi Citation2015; Bella et al. Citation2020; Barletti et al. Citation2023). However, there is currently a lack of information on their distribution in Calabria, except for a few isolated observations (Nobile & Turrisi Citation2015).
The aim of this scientific note is to report for the first time the presence of Lasioglossum subfasciatum (Imhoff, 1832) in Sila National Park (Calabria, Italy). This species is currently considered as “threatened” by the International Union for Conservation of Nature (IUCN) and classified as “endangered” (EN) in Europe (Nieto et al. Citation2014).
2. Material and methods
The sampling of L. subfasciatum was performed in the Sila National Park (Macchia Sacra, Calabria, Italy; 39°18ʹ39.0ʹʹN 16°25ʹ31.2ʹʹE; 1686 m asl; ), an UNESCO Biosphere Reserve since 2014. Specimens were found during a monitoring activity, conducted monthly from May to August 2022, set up in response to the ministerial directive (DM 232/2020, Ministero dell’Ambiente e della Tutela del Territorio e del Mare), which mandates Italian national parks to implement measures for monitoring pollinator decline and preserving biodiversity. Three specimens of L. subfasciatum were observed during a visual census of a 250 m Pollard walk transect on the 25th of May. A single specimen was hand-collected, then killed in a cold chamber, identified, and preserved in the Laboratory of Morphofunctional Entomology at the University of Calabria. Digital images of the species were obtained using the Zeiss Microscope Stemi SV 11 Apo Stereoscope with the C-P8 Optikam PRO 8 Digital Camera Optika and ProView acquisition software.
Figure 1. (a) Map of Calabria (Italy) showing the area where Lasioglossum subfasciatum was observed. The yellow line shows the area of the Sila National Park. The red dot indicates the location of Macchia Sacra (39°18ʹ39.0ʹʹN 16°25ʹ31.2ʹʹE, 1686 m). Detail shows a satellite image (from Google Earth) on the right. Scale bar: 150 m. (b) Images of the collection site captured in May.
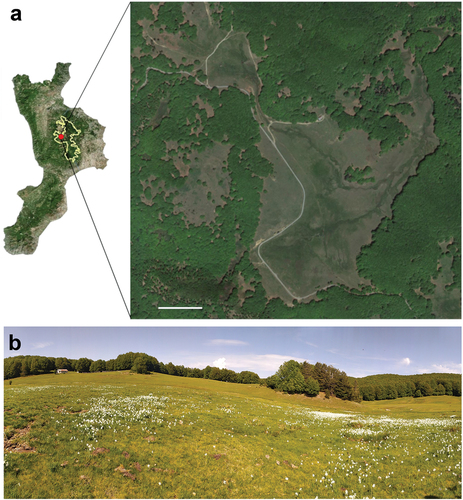
The site of the specimen collection was a mountain pastureland surrounded by beech forests (). The habitat is characterised by a mosaic of Sphagnum wetlands surrounded by mesic/dry pastures on raised areas (Hypericum calabricae-Asphodeletum macrocarpi) (Pellegrino et al. Citation2005; Mazzei & Brandmayr Citation2017). In May, the mean daily temperature at the site was 11.5°C, with a range of 0.4°C to 25.1°C (Arpacal Citation2024). The cumulative rainfall was estimated to be 37.8 mm. In summer, the average temperature is close to 18°C, with maximum reaching up to 28.9°C. Fluctuations in temperature and rainfall have a strong effect on the vegetation, causing rapid changes in flowering during the spring and summer seasons, with the genera Narcissus Linnaeus dominating in May (), Viola Linnaeus and Asphodelus Linnaeus in June and Cirsium Miller 1754 in July and August.
3. Results and discussion
Lasioglossum Curtis 1833 is a widespread genus and comprises more than 1,800 species exhibiting a wide ecological range with some species being eurytopic, others stenotopic (Collado et al. Citation2019; Venn et al. Citation2023). All species belonging to this genus are ground-nesting, except for a small number of species that can nest in rotting wood (Antoine & Forrest Citation2021). They exhibit different behaviours, including solitary, social, or cleptoparasitic (Michener Citation2000; Danforth et al. Citation2003). Their size ranges from 4 to 15 mm. The cuticle is typically dark without any yellow markings, and the antennal insertion is relatively high on the head. The female of Lasioglossum can be distinguished from other genera by the presence of a long furrow in the middle of the apical fringe of tergite 5 and the position of the tergite hair bands, which are located near the base of the tergite (Michez et al. Citation2019)
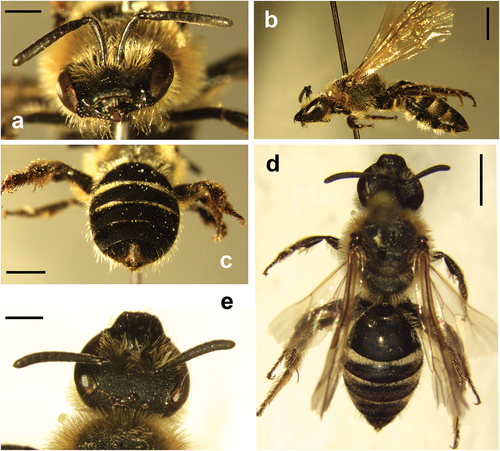
The specimen of L. subfasciatum was a female (10.91 mm in length; ), collected while actively foraging on Polygala alpestris ssp. meridionalis Arrigoni. Its head exhibits faint metallic reflections, which are also visible on the densely punctuated scutum covered in rusty hairs in fresh specimens (). The propodeum lacks carina, and the basal area is nearly crescent-shaped and rugose. The first tergum appears dull; punctures are sparse on the disc and denser on the margin, with smooth interspaces. Subsequent tergites are densely punctuated and opaque, with the second and third terga displaying a distinct proximal band of hairs. The male is distinguished from female by its ivory-yellow metabasitarsus, the sterna 2–4 adorned with long, dense hairs, and its genitalia characterised by long gonostyli slightly expanded at the apex.
Figure 2. Lasioglossum subfasciatum Imhoff, 1832; female. (a) Head, frontal view. (b) Habitus, lateral view. (c) Metasoma showing tergites. (d) Habitus, dorsal view. (e) Head, dorsal view. Scale bar: 1 mm (a, e), 2 mm (b, c, d).
The available information on L. subfasciatum is limited. Based on the bibliographic data, this species is distributed throughout Europe () (Ghisbain et al. Citation2023), from Spain to Greece, and from Italy to Poland, with many records in Switzerland and Germany (Ebmer Citation1988). Belgium is the north-western limit of its distribution in Europe. It also extends to the central Balkan peninsula in Serbia (Mudri-Stojnić et al. Citation2021) and is also found in Turkey and Iran (Warncke Citation1975; Pesenko Citation2000). However, the species has been reported to have disappeared in Belgium since the end of the 19th century (Pauly Citation2007). In Italy (), it has been recorded in Val d’Aosta (Steinmann Citation2002), Trentino-Alto Adige (Bonelli Citation1971), Emilia Romagna (Quaranta et al. Citation2004), Calabria [Aspromonte National Park; M. Cornalba Citation2017 in (Quaranta et al. Citation2018)] and Sicily (Pagliano Citation1988). The current area of occupancy (AOO) is probably less than 500 km2. This stenotic species inhabits montane meadows and has also been found even at altitudes of up to 2350 m asl in Greece (Grace Citation2010). It forages on a variety of flower species belonging to different families. The following host floral species are indicated in the literature: Acer pseudoplatanus L., 1753 (Aceraceae); Anthriscus sylvestris Hoffm., 1814 (Apiaceae); Centaurea scabiosa L., 1753, Taraxacum officinale Weber 1780 (Asteraceae); Brassica napus L., 1753, Cardamine pratensis L., 1753; (Brassicaceae); Knautia arvensis L., 1753 (Dipsacaceae); Ligustrum vulgare L., 1753 (Oleaceae); Potentilla verna L., 1753, Fragaria vesca L., 1753 (Rosaceae); Salix alba L., 1753 (Salicaceae); Valeriana officinalis L., 1753 (Valerianaceae); Tussilago L., 1753 (Asteraceae), and Gentiana cruciate L., 1753 (Gentianaceae) [(Quaranta et al. Citation2018) citing (Ebmer Citation1988; Westrich Citation1989; Grace Citation2010)]. In the past, the species may have inhabited extensive pastureland, which has since disappeared from much of Europe (Quaranta et al. Citation2018). Currently, the nesting biology and behaviour of the species are unknown, but it is presumed to be a solitary species, such as many other short-tongued halictids (Pesenko Citation2000).
Figure 3. Map of Europe showing 1242 records of Lasioglossum subfasciatum. The data are from the project PULSE (unpublished data, validated by S. Flaminio).
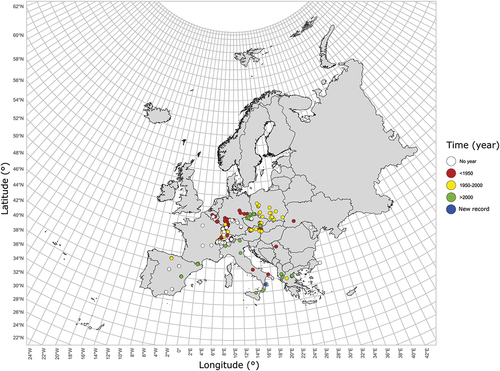
The species is currently classified as endangered (EN) based on criteria B2ab (iii) due to its small AOO, severe population fragmentation, continuing decline in the extent of occurrence (EOO), and reduction in the number of mature individuals (Nieto et al. Citation2014; Quaranta et al. Citation2018). The reasons for the decline in population and suitable habitat area of this species are unknown, but it is suspected that the disappearance of once extensive pastureland caused by agricultural intensification may have played a role (Quaranta et al. Citation2018). This species is listed in some National Red List or Red Data Book: Italy [Endangered; (Quaranta et al. Citation2018)], Belgium [Regionally Extinct; (Drossart et al. Citation2019)], Germany [Endangered; (Westrich et al. Citation2011)] and Switzerland [Vulnerable; (Amiet Citation1994)].
Actually, there are no targeted conservation efforts for this species in Italy. To ensure its conservation, it is important to protect and maintain its habitats. Our observation provides evidence of the importance of parks in conserving habitats and their associated species and adds new information to the scarce knowledge on the distribution of Apoidea in Calabria. Further research should be conducted to improve ecological understanding of the species and to identify threats for better protection and conservation.
Compliance with ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Acknowledgments
The authors are grateful to Dr Carmen Gangale, who provided the identification of the Polygala species and vegetation nomenclature.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Amiet F. 1994. Liste rouge des abeilles menacées de Suisse. In: Duelli P, editor. Rote Liste der gefährdeten Tierarten der Schweiz. Berne: Office fédéral de l’environnement, des forêts et du paysage. Berne, 38–44.
- Antoine CM, Forrest JRK. 2021. Nesting habitat of ground‐nesting bees: A review. Ecological Entomology 46(2):143–159. DOI: 10.1111/een.12986.
- Arpacal. 2024. Centro Funzionale Multirischi Arpacal. Available: https://www.cfd.calabria.it/.
- Barletti BR, Asensio A, Polidori C, Quaranta M, De la Rua P. 2023. Rediscovering the eusocial sweat bee Lasioglossum marginatum (Hymenoptera: Halictidae) in Sicily through DNA barcoding. Journal of Apicultural Research 62(2):263–265. DOI: 10.1080/00218839.2021.1896215.
- Bella S, Catania R, Nobile V, Mazzeo G. 2020. New or little-known bees from Sicily (Hymenoptera: Apoidea). Fragmenta Entomologica 52(1):113–117. DOI: 10.4081/fe.2020.418.
- Bonelli P. 1971. Imenotteri Aculeati della Regione Trentino-Alto Adige. Studi Trentini Di Scienze Naturali 43(B):208–235.
- Collado MÁ, Sol D, Bartomeus I, Duncan R. 2019. Bees use anthropogenic habitats despite strong natural habitat preferences. Diversity and Distributions 25(6):924–935. DOI: 10.1111/ddi.12899.
- Danforth BN, Conway L, Ji S. 2003. Phylogeny of eusocial Lasioglossum reveals multiple losses of eusociality within a primitively eusocial clade of bees (Hymenoptera: Halictidae). Systematic Biology 52(1):23–36. DOI: 10.1080/10635150390132687.
- Danforth BN, Eardley C, Packer L, Walker K, Pauly A, Randrianambinintsoa FJ. 2008. Phylogeny of Halictidae with an emphasis on endemic African Halictinae. Apidologie 39(1):86–101. DOI: 10.1051/apido:2008002.
- Drossart M, Rasmont P, Vanormelingen P, Dufrêne M, Folschweiller M, Pauly A, Vereecken N, Vray S, Zambra E, D’Haeseleer J. 2019. Belgian red list of bees. Mons, Belgium: Presse universitaire de l’Université de Mons. Available: http://www.atlashymenoptera.net.
- Ebmer AW. 1988. Kritische Liste der nicht-parasitischen Halictidae Österreichs mit Berücksichtigung aller mitteleuropäischen Arten (Insecta: Hymenoptera: Apoidea: Halictidae). Linzer Biologische Beiträge 20(2):527.
- Flaminio S, Ranalli R, Zavatta L, Galloni M, Bortolotti L. 2021. Beewatching: A project for monitoring bees through photos. Insects 12(9):841. DOI: 10.3390/insects12090841.
- Forbicioni L, Filippi L, Strumia F. 2019. Contributo alla conoscenza degli Imenotteri Apoidei dell’Isola d’Elba. Bollettino del Museo regionale di Scienze naturali di Torino 35(1–2):103–120.
- Generani M, Pagliano G, Scaramozzino P, Strumia F. 2001. Gli Imenotteri delle isole di Capraia, Giglio, Gorgona, Pianosa e Montecristo (Arcipelago Toscano). Frustula Entomologica 24(37):51–74.
- Ghisbain G, Rosa P, Bogusch P, Flaminio S, LE Divelec R, Dorchin A, Kasparek M, Kuhlmann M, Litman J, Mignot M. 2023. The new annotated checklist of the wild bees of Europe (Hymenoptera: Anthophila). Zootaxa 5327(1):1–147. DOI: 10.11646/zootaxa.5327.1.1.
- Grace A. 2010. Introductory biogeography to bees of the Eastern Mediterranean and Near East. UK: Bexhill Museum Sussex.
- Mazzei A, Brandmayr P. 2017. Agonum tulliae sp. n. From the Sila National Park (Calabria, southern Italy) (Coleoptera: Carabidae: Platynini). Journal of Entomological and Acarological Research 49(1). DOI: 10.4081/jear.2017.6677.
- Michener CD. 2000. The bees of the world. Vol. 1. JHU Press. Baltimore and London 913. ISBN 0-8018-6133-0.
- Michez D, Rasmont P, Terzo M, Vereecken N. 2019. Bees of Europe. Paris, France: Nap Éditions Paris, France ISBN : 9782913688346.
- Mudri-Stojnić S, Andrić A, Markov-Ristić Z, Đukić A, Vujić A. 2021. Contribution to the knowledge of the bee fauna (Hymenoptera, Apoidea, Anthophila) in Serbia. ZooKeys 1053:43. DOI: 10.3897/zookeys.1053.67288.
- Nieto A, Roberts SPM, Kemp J, Rasmont P, Kuhlmann M, Criado MG, Biesmeijer JC, Bogusch P, Dathe HH, De La Rúa P, De Meulemeester T, Dehon M, Dewulf A, Javier Ortiz-Sánchez F, Lhomme P, Pauly A, Potts SG, Praz C, Quaranta M, Michez D. 2014. European red list of bees. DOI: 10.2779/77003.
- Nobile V, Turrisi GF. 2015. New or little known Halictidae from Italy (Hymenoptera, Apoidea). Bollettino della Società Entomologica Italiana 147(1):39–42. DOI: 10.4081/BollettinoSEI.2015.39.
- Pagliano G. 1988. Catalogo degli Imenotteri italiani I. Halictidae. Bollettino Del Museo Civico Di Storia Naturale Di Venezia 38:85–128.
- Pagliano G. 2011. Ricerche imenotterologiche nell’isola di Lampedusa con descrizione di una nuova specie di Mutillide (Hymenoptera, Mutillidae). Fragmenta Entomologica 43(2):215–224. DOI: 10.4081/fe.2011.47.
- Pauly A. 2007. La collection Gerhardy, un témoignage de la richesse faunistique des environs de Malonne à la fin du XIXème siècle (Hymenoptera: Apoidea Halictidae). Notes Fauniques de Gembloux 60(3):133–139.
- Pellegrino G, Caimi D, Noce ME, Musacchio A. 2005. Effects of local density and flower colour polymorphism on pollination and reproduction in the rewardless orchid Dactylorhiza sambucina (L.) So? Plant Systematics and Evolution 251(2–4):119–129. DOI: 10.1007/s00606-004-0248-6.
- Pesenko IA. 2000. Bees of the family Halictidae (excluding Sphecodes) of Poland: Taxonomy, ecology, bionomics. Bydgoszcz Press, Bydgoszcz, Poland, Bydgoszcz ISBN: 83-7096-339-0.
- Quaranta M, Ambroselli S, Barro P, Bella S, Carini A, Celli G, Combal CP, Comoli R, Felicioli A, Floris I. 2004. Wild bees in agroecosystems and semi-natural landscapes. 1997–2000 collection period in Italy. Bulletin of Insectology 57:11–61.
- Quaranta MNAIN, Cornalba M, Biella P, Comba M, Battistoni A, Rondinini C, Teofili C. 2018. Red list IUCN of the Italian threatened bees [Lista Rossa IUCN delle api italiane minacciate]. Roma, Italy: Comitato Italiano IUCN e Ministero Dell’Ambiente e Della Tutela Del Territorio e Del Mare.
- Steinmann E. 2002. Die Wildbienen (Apidae, Hymenoptera) einiger inneralpiner Trockentäler. Jahresbericht Der Naturforschenden Gesellschaft Graubünden 111:5–26.
- Turrisi GF, Bella S, Catania R, La Greca P, Nobile V, D’urso V. 2021. Bee diversity in fragmented areas of Volcano Etna (Sicily, Italy) at different degrees of anthropic disturbance (Hymenoptera: Apoidea, Anthophila). Journal of Entomological and Acarological Research 53(1). DOI: 10.4081/jear.2021.10326.
- Venn S, Teerikangas J, Paukkunen J. 2023. Bees and pollination in grassland habitats in Helsinki (Finland) are diverse but dominated by polylectic species. Basic and Applied Ecology 69:1–12. DOI: 10.1016/j.baae.2023.03.003.
- Warncke K. 1975. Beitrag zur Systematik und Verbreitung der Furchenbienen in der Turkei (Hymenoptera, Apoidea, Halictus). Polskie Pismo Entomologiczne 45:81–128.
- Westrich P. 1989. Die Wildbienen Baden-Württembergs. Stuttgart: Eugen Ulmer Gmbh.
- Westrich P, Frommer U, Mandery K, Riemann H, Ruhnke H, Saure C, Voith J. 2011. Rote Liste und Gesamtartenliste der Bienen (Hymenoptera, Apidae) Deutschlands. Fassung, Stand Februar 3:373–416.
