?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study presents primary observations of the natural diet of two endemic fishes, Delminichthys ghetaldii and Telestes dabar, along with an examination of their niche overlap. The feeding habits of these species were analyzed based on a dataset comprising 115 specimens collected from five karst poljes during the year 2018. Statistical analyses, including one-way ANOVA and Tukey’s HSD post-hoc testing, were employed to discern significant differences in body size and weight among the five populations of Delminichthys ghetaldii. Numeric indices such as numerical abundance, weight percentage of abundance, and frequency of occurrence, alongside a composite index (IRI - index of relative importance), were applied to elucidate the feeding strategy of these species. The diversity of diet and poljes utilization was assessed using Simpson’s diversity index, while predation selectivity was analyzed through the electivity index. Considerable overlap in the diet of Delminichthys ghetaldii was observed among the five poljes, as well as in the overlap between these two species. The findings of this study indicate that Delminichthys ghetaldii exhibits a zoophagous planktivorous diet, i.e. carnivorous, while Telestes dabar demonstrates a phytofagous planktivorous diet, i.e. herbivorous.
1. Introduction
The investigation of the dietary patterns and feeding behaviors of freshwater fish species remains a subject of continual scrutiny, as it forms the foundational basis for the formulation of effective fisheries management programs concerning fish capture and culture (Oronsaye & Nakpodia Citation2005). Analyzing the dietary preferences of organisms in their natural habitats contributes significantly to the comprehension of growth dynamics, population abundance, productivity, and geographical distribution (Fagade & Olaniyan Citation1972). Alterations in dietary preferences may be linked to variations in prey availability, energy requirements, ontogenetic growth phases, morphological constraints, or shifts in habitat utilization (Stephens et al. Citation2007). Accurate knowledge regarding the dietary proclivities of endemic and protected species is particularly indispensable for ensuring their appropriate management and conservation.
The Dinaric region stands recognized as a European biodiversity hotspot (Gaston & David Citation1994), celebrated for its elevated endemism, notably in aquatic (Bănărescu Citation2004) and subterranean fauna (Sket et al. Citation2004). Typically, these species exhibit restricted and localized distributions, inhabiting only a handful of specific locales. Minnows dwelling in karstic water streams are representatives of endemic ichthyofauna. Among them are the species of three genera Delminichthys, Phoxinellus, and Telestes, commonly referred to as “gaovice” in the region, which manifest a distinctive lifestyle. The life cycle of these endemic fishes is intricately linked to karstic habitats characterized by pronounced fluctuations in water level regimes. During autumn and spring floodings, these species emerge from subterranean karstic water streams to surface estaveles, retreating underground as water levels recede (Vuković & Ivanović Citation1971).
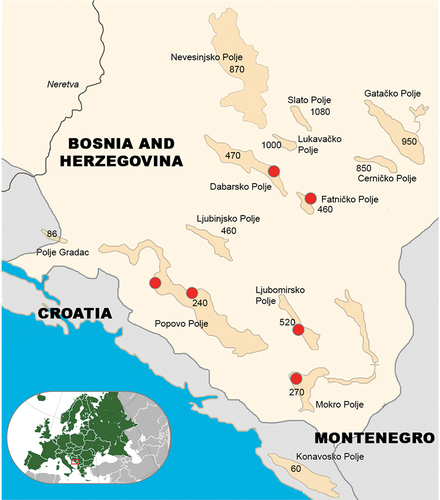
The species Delminichthys ghetaldii was initially documented as Phoxinellus ghetaldii in the caves of the Popovo Polje (Bosnia and Herzegovina) by Franz Steindachner in 1882. In the same year, he also described Phoxinellus pstrossii from the Trebišnjica River near Trebinje (Bosnia and Herzegovina), which flows through Popovo Polje. A subsequent revision of this genus by Bogutskaya and Zupančič (Citation2003) resulted in the division of ten Phoxinellus species into two groups, one of which further delineated into two subgroups. Freyhof et al. (Citation2006) identified Phoxinellus as a paraphyletic taxon based on nuclear and mtDNA sequences, highlighting three unrelated monophyletic units: Phoxinellus, Delminichthys, and Telestes. Following this taxonomic refinement, Zupančič and Bogutskaya (Citation2000) consolidated the two species (Phoxinellus pstrossi and Phoxinellus ghetaldii) under Delminichthys ghetaldii. The distribution of D. ghetaldii spans isolated poljes in Eastern Herzegovina, the microregion in Bosnia and Herzegovina that is a unique hydrogeological entity, bounded to the west by the valley of the lower course of the Neretva River, to the south by the coast of the Adriatic Sea, to the east by the massif of mountain Orjen and the northern boundary is the watershed’s divide towards the headwaters of the Neretva and Drina rivers (Milanović Citation1979). All five poljes: Dabarsko, Fatničko, Ljubomirsko, Popovo, and Mokro are traversed by the Trebišnjica River and that ends in the Konavosko polje in Croatia.
Similarly, the species Telestes metohiensis, was initially described as Paraphoxinus metohiensis from the Dabarsko Polje by Franz Steindachner in 1882. In addition to the already existing species in this area, Bogutskaya et al. (Citation2012), discovered two new species: Telestes dabar and Telestes miloradi. These species inhabit streams or shallow canals characterized by minimal current and clean water (Bogutskaya & Zupančič Citation2003), with subterranean waters serving as refuge during unfavorable conditions (Markotić Citation2013). T. metohiensis inhabits the Gatačko, Cerničko and Nevesinjsko poljes in Eastern Herzegovina, while T. miloradi species inhabits Ljuta River in Konavosko Polje in Croatia. T. dabar inhabits the Vrijeka and Opačica rivers in the Dabarsko Polje of Eastern Herzegovina (Bogutskaya et al. Citation2012).
The karst poljes of Eastern Herzegovina encompass the geographic expanse of the municipality of Trebinje. Recent macro-surface karst relief forms are shaped by neotectonic processes in the region, primarily influenced by corrosive and fluvial processes (Gnjato Citation1991). Morphologically, this area exhibits typical karst terrains, featuring highly developed forms of karst erosion, including corrosion forms, sinkholes, valleys and karst poljes. In addition to the karst poljes, the significance of larger karst valleys and numerous sinkholes is underscored.
The aim of this research is to scrutinize the dietary composition of sympatric and syntopic D. ghetaldii and T. dabar within poljes in Eastern Herzegovina. Prior to this investigation, both qualitative and quantitative data regarding the feeding habits of D. ghetaldii and T. dabar were absent. Consequently, this study constitutes the first report on their feeding behaviors, shedding light on the overlap in feeding niches during sympatric and syntopic occurrences.
2. Material and method
2.1. Study area
Popovo Polje, situated in Bosnia and Herzegovina, stands as one of the largest poljes, renowned for its distinctive karstic phenomena, including the Trebišnjica River, recognized as one of the world’s largest sinking rivers, and the Vjetrenica Cave system located in the valley’s west-southwest parts. Encompassing an area of 68.4 square kilometers, the polje is covered by fertile (alluvial) deposits, with dimensions stretching approximately 37 km in length and 1–3 km in width (Milanović Citation1979). Notably, the river bed at the study site comprises large rocks (25%), gravel (55%), and sand (30%) (AQEM Consortium Citation2002). The bed is about 2 m wide, with a maximum depth of 150 cm measured in April on the researched stretch. Vegetation, predominantly the macrophyte Ceratophillum demersum (Linnaeus Citation1753), covers 20–50% of the stream bed while the banks are flanked by shallots (Ranunculus spp., Linnaeus Citation1753) and agricultural lowland grain poljes. Water was clear, temperature 8.1◦C with minor deflections, conductivity 333 µS cm − 1, and pH value 8.07. Most of the fish were taken from the smaller cave at Dračevo (42°51'17.39' N 18°03'35.40'' E) and from the well Pokrivenik (42°51'59.99' N 17°57'59.99'' E) at Čvaljina. Fish fauna consisted mainly of Delminichthys ghetaldii and Phoxinus karsticus (Bianco & De Bonis Citation2015) and to a lesser extent of Squalius svalize (Heckel & Kner Citation1857). All common groups of macroinvertebrates’ bottom fauna communities were present, but Trichoptera and Nematoda were dominant.
Mokro Polje, situated south of Trebinje, spans an area of 18 km2 with an elevation ranging from 275 to 268 m. The river bed at the research site is characterized by large rocks (40%), gravel (35%), and sand (25%). The wellhead was approximately 5 m wide with a maximum depth of 100 cm measured in February on the researched stretch. The stream bed was 20–30% covered by vegetation predominantly consisting of the macrophyte Mentha aquatica (Linnaeus Citation1753). Surrounding banks feature grass (Carex spp., Linnaeus Citation1753) and adjacent agricultural lowland grain poljes and a nearby vineyard. The water was clear, temperature 7.83 ◦C, conductivity in the range of 290 µS cm − 1 and pH value 7.83. All the fish were taken from the flooded polje which was created from wellheads and springs in polje. The fish fauna in Mokro Polje is exclusively represented by Delminichthys ghetaldii, with macroinvertebrate communities, including Diptera, Trichoptera, and Oligochaeta, contributing to the overall ecosystem, with dipteran Chironomus thummi (Kieffer Citation1911) prevailing in abundance.
Ljubomirsko Polje is about 12.5 km2 in size. It is about 10 km long and between 500 and 1000 m wide. It also has numerous sinkholes, especially in the western part, as well as the stone blocks and caves, and dolomite cracks over which water flows during heavy rainfall. At the researched locality, the stream bed consists of large rocks (45%), gravel (45%), and sand (10%), as assessed during the study. The stream is about 4 m wide, with a maximum depth of 200 cm measured in April on the researched stretch. The stream bed was 30–60% covered with vegetation predominantly consisting of the macrophyte Berula erecta (Huds.) Coville Citation1893. The banks are flanked by willows (Salix spp. Linnaeus Citation1753). The water was clear, with a temperature of about 11◦C with minor deflections. Conductivity was 333 µS cm−1 and the pH value was 7.6. Most of the fish were taken from the smaller wadeable Ljubomirski Stream. Fish fauna is exclusively represented by D. ghetaldii, with macroinvertebrate communities featuring dominantly coleopteran Elmis aenea (Müller Citation1806) and snail Ancylus fluviatillis (Müller Citation1774).
Dabarsko and Fatničko poljes are geographically interconnected poljes in Eastern Herzegovina. They are located merely 3 km apart. The larger Dabarsko Polje lies NW to the slightly smaller Fatničko Polje. As the sinks cannot receive all the rain water, both poljes are usually flooded between November and May (Milanović Citation1979). Both poljes are flooded annually and are part of both Neretva and Trebišnjica river’s basins. Flood areas of Dabarsko and Fatničko poljes are 16,69 km2 and 7,27 km2 in surface, respectively. Various small streams and rivers appear in springs there, flow over the poljes and at then submerge into sinks on their other sides. In Fatničko Polje, the river bed consists of large rocks (15%), gravel (55%), and sand (30%), while in Dabarsko Polje at researched locality stream bed consists of large rocks (25%), gravel (35%), and sand (40%). The river bed in Fatnica stream is about 6 m wide, with a maximum depth of 70 cm measured in April while in the village of Dabar there is a small stream Vrijeka River which is about 3 m wide, with a maximum depth of 90 cm measured in June on the researched stretch. In Fatničko Polje, a dominant macrophyte was Veronica anagalis-aquatica (Linnaeus Citation1753), while in Vrijeka Stream bed was 20–50% covered with vegetation predominantly consisting of the macrophyte Mentha aquatica. The banks of Fatnica stream are flanked by grass (Poa spp., Linnaeus Citation1753), while in Vrijeka Stream are flanked by shallots (Ranunculus spp.) and agricultural lowland grain poljes. On both localities, the water was clear, with a temperature of 9◦C. Conductivity values in Fatničko and Dabarsko poljes were 254 µS cm−1 and 340 µS cm−1, respectively, and the pH values were 7.83 and 7.97, respectively. All the fish in Fatničko Polje were taken from the flooded polje during rainy season, while the fish in Dabarsko Polje were taken from the Vrijeka stream. Fish fauna consisted predominanty of D. ghetaldii in Fatnica and only few T. dabar. Conversly, in Dabarsko Polje T. dabar was a predominant species and D. ghetaldii was a less abundant. In both poljes, all common groups of macroinvertebrate communities were present, but in Fatničko Polje Chironomus thummi prevailed in abundance over other taxa, while in Dabarsko Polje Gammarus fossarum (Koch Citation1835) was dominant ().
Figure 1. Position of karst poljes (Popovo, Mokro, Dabarsko, Fatničko and Ljubomirsko) with their altitudes (in meters above the sea level), with the red circles denoting locations in them where the samples of Delminichthys ghetaldii and Telestes dabar originated from; in the bottom right corner, the position of Bosnia and Herzegovina within Europe is displayed.
2.2. Fish sampling and laboratory work
Sampling of individuals was attempted from 2016 to 2019, but due to uneven flooding regime, we were able to catch them in limited numbers only in 2018 from March to May, when all poljes were flooded (). Therefore, it is impossible to track the diet throughout all seasons. They were procured from daily catch of artisanal fishermen or standard, battery-powered, backpack electrofishing gear and taken to the laboratory. A total of 115 specimens (>50 cm) of D. ghetaldii and T. dabar were collected from five sampling sites. D. ghetaldii were sampled at Popovo (42° 96' 45'' N, 18°55′37″ E, 240 m asl in altitude; 7 indviduals − 2 males, 5 females), Mokro (42 39'' 35'''N, 18 20' 03''''E, 270 m asl in altitude, 6 individuals − 5 males, 1 female) and Ljubomirsko (42° 46' 3.54''N, 18° 21' 6.804″″ E; 520 m asl in altitude, 24 individuals − 10 males, 14 females) karst poljes. Both species were sampled at Fatničko (43° 1' 54.12''N, 18° 19′ 0.768″″ E; 460 m altitude; D.ghetaldii : 35 individuals-17 males, 18 females; T.dabar − 2 individuals-2 females individuals) and Dabarsko (44° 57‘ 15.3432’‘N, 15° 18’ 11.592”” E,470 m altitude, D.ghetaldii: 4 individuals- 3 males, 1 females; T.dabar 37 individuals-18 males, 19 females) poljes, traversed by the Trebišnjica River.
Table I. Data of sampling and the number of Delminichthys ghetaldii and Telestes dabar individuals.
Each specimen was measured for standard length (SL, in cm) and weight (W, in g). The gut content was removed and weighed and prey categories were determined to the lowest possible systematic category under an inverted microscope Model IB magnifications from 10 to 40 X.
2.3. Macroinvertebrates and plankton sampling
The study design incorporated a comparative analysis of both the diet composition and the environmental availability of prey. The prey categories under consideration included macroinvertebrates (such as insects, mollusks, arachnids) and plankton, which encompassed both phytoplankton (algae) and zooplankton (including Cladocerans, Copepods, and Rotifers). Notably, the sampling of available prey was conducted concurrently with the collection of data on fish diet, ensuring a synchronous evaluation of the dietary preferences of the fish species in relation to the abundance of environmental prey.
Benthic macroinvertebrates were collected by standard sampling with a benthological hand net (mesh size: 500 µm) from all available habitats (standard multihabitat sampling procedure). Three samples were taken at the beginning, middle and end of the transect at each studied locality (AQEM Consortium Citation2002).
Plankton organisms were sampled with a plankton EFE and GB nets, made of Monodur Nytal (Nylon), pore diameter from 20 µm, placed in five locations at each studied stretch at the same time when the fish were caught. At each site, three samples were taken at the beginning, middle and end of the studied locality if possible. All species from benthos and plankton samples were counted and identified to the family, species or genus level.
2.4. Data analysis
2.4.1. Prey importance and diet diversity
The assessment of prey significance and the structure of the diet involved an analysis that utilized the abundance (A) of individual prey categories, their frequency of occurrence (F), and the index of relative importance (IRI). The calculation of abundance was based on both the quantity and weight of the consumed prey categories:
Where N represents the number of individuals of each consumed prey type (i), and Nt represents the total number of consumed prey. W stands for the weight of each consumed prey type (i), and Wt represents the total weight of consumed prey. Another frequently utilized parameter in assessing prey significance is the frequency of occurrence,
which is determined by the proportion of stomachs containing a specific type of prey:
P represents the number of predators in which each prey category (i) occurred, and Pt represents the total number of predators.
Regarding the assessment of prey importance in the diet, while both abundance and frequency of occurrence are individual indicators, the index of relative importance (IRI) consolidates all three factors. It is calculated as follows:
AN represents the abundance based on the number of prey categories, AW represents the abundance based on the weight of the prey categories, and F represents the frequency of its occurrence (Pinkas et al. Citation1971; Pita et al. Citation2002).
Diet diversity (poljes) was evaluated using Simpson’s diversity index (AQEM Consortium Citation2002).
The value ranges from 0 to 1, indicating low to high diversity, respectively. Here, Ni represents the number of prey categories (benthos/plankton) i, and Nt is the total number of all prey categories (benthos/plankton).
2.4.2. Diet overlap among species and poljes
Diet overlap was evaluated using Pianka’s modification of the MacArthur and Lewin index (Pianka Citation1973), calculated as follows:
In this context, where j and k represent the subgroups for which the overlap is being estimated, AN signifies the proportion of the number of prey types of category i. O represents the resulting value ranging from 0 to 1, indicating low to high overlap.
2.4.3. Predation selectivity (electivity)
To assess predation selectivity, the electivity index, as described by Vanderploeg and Scavia (Citation1979), was utilized. This index is computed using Chesson’s coefficient (Wi) from Chesson’s work in 1978:
After obtaining the value of di, representing the proportion of a specific prey category (i) in the diet, and ei, representing the proportion of the same prey category (i) in the environment (benthos/plankton), the calculation of electivity is performed as follows:
The formula indicates that E, the electivity index, can range from −1 to + 1. A value of −1 suggests negative selection or that the prey is unavailable to the predator, whereas a value of +1 indicates positive selection. It’s noted that all calculations were carried out using MS Excel 2010.
2.4.4. Statistical analysis
The body size and weight underwent an Analysis of Variance (ANOVA) to ascertain their discriminatory capacity through the examination of the significance of variation among the five D. ghetaldii populations.
The Tukey HSD post-hoc test (Sokal & Rohlf Citation1981) served to determine between which particular populations there was a significance in variation for each of these two characters. The program Statistica for Windows (ver. 7) was used for this analysis.
3. Results
The size distribution of fish within the five populations of D. ghetaldii across the five karst poljes exhibited significant variability, as detailed in . Univariate testing using ANOVA revealed that body size and weight were significantly different among five D. ghetaldii populations (Fsize = 27.605, dfsize = 1,4, psize < 0.001; Fweight = 18.361, dfweight = 1,4, pweight < 0.001). Tukey’s HSD post-hoc testing for body size and weight revealed that fishes from Popovo Polje were similar to the ones from Mokro Polje, and that fishes from Ljubomirsko and Dabarsko poljes were also similar to each other, whereas those from Fatničko Polje were significantly different from all of them ().
Table II. Descriptive data of Delminichthys ghetaldii and Telestes dabar samples including length (mm) and weight (g) from the different poljes in Eastern Herzegovina.
Table III. Results of the post-hoc Tukey HSD testing between five populations of Delminichthys ghetaldii from poljes (PP, Popovo; MP, Mokro; DP, Dabarsko; FP, Fatničko; LP, Ljubomirsko) for body size (SL, above the diagonal) and weight (W, below the diagonal).
3.1. Benthos, plankton and diet samples
All identified benthos and plankton taxa, along with taxa identified as prey in diet samples, were consolidated into 18 prey categories (). This consolidation aimed to compute the proportion of consumed prey types for each species in all karst poljes across each prey category.
Table IV. The complete list of prey taxa found in the diet samples of Delminichthys ghetaldii and Telestes dabar was combined into 18 distinct prey categories.
The macroinvertebrate community observed in Popovo Polje exhibited Trichoptera as the most abundant group, accounting for 23% of the community. Nematoda followed closely, constituting 19%, while Chironomidae contributed 12%. Simuliidae represented 11.95%, Hydracarina 5.62%, Coleoptera 5.02%, Ephemeroptera 5%, Crustacea 2.30%, and Oligochaeta had a representation of 1.87% within the community. In the plankton sample, Rotatoria was the most substantial component at 7.92%, followed by Cladocera with a share of 5.02%, and Copepoda contributing 1.30% to the community.
In Mokro Polje, the macroinvertebrate community exhibited a predominant presence of Chironomidae, constituting a substantial 70.87% participation in the community. Oligochaeta followed with a share of 4%, while Trichoptera accounted for 3.7%, Tipulidae for 3.2%, and Simulidae for 2.2%. In the plankton sample, Cladocera took the lead with a participation rate of 6.83%, followed by Rotatoria at 5%, and Copepoda represented with 4.20%.
In Ljubomirsko Polje the sample is characterized by a rich benthic fauna, most likely because we sampled in the stream. The most abundant species were from the order Coleoptera with a share of 24.01% in community, classes Gastropoda with 15.0%, and Plecoptera by the share in the number of 10.00%. Other groups contributed slightly, as follows: Ephemeroptera 9.10%, Hydracarina with 8%, Trichoptera 7.0%, Chironomidae with 4% and Oligochaeta 3.47%. In the plankton sample, the most abundant were Cladocera with participation of 14.7%, Rotatoria with 3% and Copepoda with a share of 1.72% in community.
The macroinvertebrate community in Fatničko Polje closely resembles that of Mokro Polje, primarily characterized by the dominance of Chironomidae at 63.82%. Hydracarina follows with a participation rate of 7.09%, Simulidae with 5.67%, Coleoptera with 3.09%, Crustacea with 2.67%, Gastropoda with 2%, and finally, Oligochaeta with a participation rate of 1.5% within the community. In plankton samples, Rotatoria took the lead with a share of 8.11%, followed by Cladocera with 3.45%, and Copepoda with 2.60%, further contributing to the community’s composition.
In Dabarsko Polje, the macroinvertebrate community was sampled from the Vrijeka stream. The most dominant species was the crustacean Gammarus fossarum, constituting 35% of the community. Larvae of Coleoptera followed with a participation rate of 12%, while larvae of Ephemeroptera, primarily of Baetis rhodani (Pictet Citation1834), accounted for 6%. Larvae of Trichoptera represented 4.9%, Chironomidae 3%, Hydracarina 3%, Oligochaeta 3%, and Nematoda with a representation of 1.1%. In the plankton community, Cladocera exhibited the highest abundance at 24%, followed by Rotatoria with 5%, and Copepoda contributing 3% to the community. These proportions were utilized to calculate the electivity index for each polje sample.
3.2. Diet description according to number (AN) and weight (AW) abundance, frequency of occurrence (F), and index of relative importance (IRI)
The analysis of the importance of various prey categories in the diet of D. ghetaldii and T. dabar, along with the identification of the five most crucial prey items according to all indices for the population (), underscores notable distinctions. The diet of D. ghetaldii exhibits discernible variations among different isolated karst poljes, while the diet of T. dabar between the two isolated karst poljes remains relatively consistent. Notably, the diet disparity between these two species is particularly pronounced. Specifically, D. ghetaldii in Fatničko and Mokro karst poljes demonstrated a notable preference for Chironomidae, whereas fishes in Ljubomirsko and Dabarsko karst poljes exhibited a preference for zooplankton (Cladocerans) and smaller macroinvertebrates such as Hydracarina and smaller larvae of Coleoptera. Nematoda was found to be prevalent in the diet of D. ghetaldii in Popovo Polje, where it constituted the most abundant prey across various indices (AN, AW, F, and IRI) (). Chironomidae were ubiquitously present in the environment of all poljes, along with the consistent presence of Rotatoria and Cladocerans. Conversely, the diet of T. dabar exhibited a complete dominance of Bacillariophyta in both poljes.
Table V. The top five prey categories in the diet of Delminichthys ghetaldii across five karstic poljes (PP, Popovo; MP, Mokro; DP, Dabarsko; FP, Fatničko; LP, Ljubomirsko) and Telestes dabar in two karst poljes (DP, Dabarsko; FP, Fatničko) based on the abundance (AN), weight percentage of abundance (AW), frequency of occurrence (F) and index of relative importance (IRI). Only values for the first five prey categories for each karst polje are displayed.
3.3. Diversity
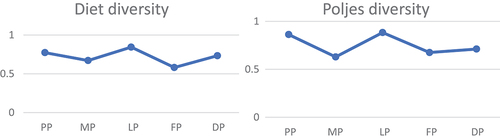
Regarding the diversities of prey categories in both diet and environment, the Simpson’s diversity index exhibited its lowest values in Fatničko and Mokro, while the highest values were recorded in Popovo and Ljubomirsko karst poljes (). All Simpson’s diversity index values fell within the range of 0.6 to 0.9, indicating a medium to high level of diversity.
3.4. Diet overlapping among poljes and among species
The analysis of diet overlap encompassed assessments between diet samples of D. ghetaldii from each of the karst poljes, as well as between D. ghetaldii and T. dabar in Fatničko and Ljubomirsko karst poljes. A “Pianka’s overlap index” value exceeding 0.6 is considered indicative of high overlap (). The lowest diet overlap was observed between Ljubomirsko and Mokro karst poljes, as well as between Fatničko and Dabarsko karst poljes. Conversely, the highest diet overlap occurred between Fatničko and Mokro karst poljes, as well as between Ljubomirsko and Dabarsko karst poljes. Notably, the diet overlap values between D. ghetaldii and T. dabar in Fatničko and Dabarsko karst poljes were quite low (with the values of the Pianka’s indices of 0.01 and 0.02, respectively).
Table VI. Diet overlaps of Delminichthys ghetaldii among five poljes (PP, Popovo; MP, Mokro; DP, Dabarsko; FP, Fatničko; LP, Ljubomirsko) high overlapping is in shaded (0–1, low–high).
3.5. Selectivity (electivity)
The Electivity index was calculated by comparing the diet of each species with the benthos and plankton samples in five poljes. Benthos and plankton were used to represent the proportion of prey categories in the environment based on AN. In each poljes, the composition of macrozoobenthos and plankton was compared with their occurrence in the diet (). The comparison focused on the most significant prey categories in the diet, namely Chironomidae, Trichoptera, Hydracarina, and Ephemeroptera from macrozoobenthos, as well as Rotatoria and Cladocera from zooplankton and Bacillariophyta from phytoplankton.
Table VII. The electivity of the most significant prey categories in the diet of Delminichthys ghetaldii across five karst poljes (PP, Popovo; MP, Mokro; DP, Dabarsko; FP, Fatničko; LP, Ljubomirsko) and Telestes dabar in two karst poljes (DP, Dabarsko; FP, Fatničko) is classified as positive (+, > 0.3), neutral (0, −0.3–0.3), or negative (−, <−0.3) selection.
Regardless of the significance of Chironomidae as prey in the diet, D. ghetaldii consumed them in proportions higher than in the environment (i.e., positive selection) only in Fatničko Polje. In contrast, in Ljubomirsko and Dabarsko karst poljes, they were consumed in a lower proportion than that occurring in the environment (i.e., negative selection). In Mokro and Popovo karst poljes, the selection was neutral. D. ghetaldii exhibited a preference for Cladocera in all karst poljes (i.e., positive selection). Trichoptera larvae were negatively selected in Ljubomirsko, Fatničko, and Dabarsko karst poljes and neutrally selected in Popovo and Mokro karst poljes (). Rotatoria were neutrally selected in all karst poljes. D. ghetaldii consumed Hydracarina in proportions lower than in the environment (i.e., negative selection) in Popovo and Fatničko karst poljes, while in Ljubomirsko and Dabarsko karst poljes, selection for this prey category was neutral. For Ephemeroptera larvae, selection was neutral in Ljubomirsko and Popovo karst poljes and negatively selected in Dabarsko Polje. In the diet of T. dabar, Chironomidae, Hydracarina, Ephemeroptera larvae, and Rotatoria were negatively selected in both poljes where they occurred, while Cladocera was negatively selected in Dabarsko and positively selected in Fatničko karst poljes. Bacillariophyta were the most important prey category in the diet of T. dabar, the most abundant in the environment and they were positively selected ().
4. Discussion
The uneven number of individuals in samples featuring this research, might be owing to specific karst terrain and uneving flooding of karst poljes. It seems that, in addition to changes in annual occurrence of floodings, the lasting of flooding period is decreases, so it is likely that the fishes are strongly limited in opportunity come to the surface where they feed and spawn. Delminichthys ghetaldii is categorized as a vulnerable species (VU) on the IUCN Red List, while Telestes dabar is not listed yet due to a lack of data on this newly discovered species of the highly limited distribution. Regardless, that one’s season cannot provide a complete picture of the diet, the obtained results are an opportunity to ascertain the basic feeding characteristics of those endemic species (Kaćanski et al. Citation1978).
The examination of fish dietary habits through stomach analyses serves as a crucial method in fish ecology for investigating trophic relationships within aquatic communities (Fagbenro et al. Citation2000). Currently, there is no literature data pertaining to the natural diet of D. ghetaldii and T. dabar, which would enable direct comparisons with the information we obtained. In the existing literature, there’s a lack of available data concerning the diet of other species within the Delminichthys genus. Regarding the Telestes genus’ diet, there exist different findings among various studies. Our research findings align with Vuković (Citation1985), who observed that T. souffia from the Drina River exhibits omnivorous behavior, with a higher consumption of plant materials. This observation categorizes the species into the zoophytophagous subgroup, a classification also supported for T. montenegrinus by Krivokapić (Citation1992). However, reffering to Marčić et al. (Citation2017) and Zanella et al. (Citation2009), the diet of T. karsticus and T. ukleva, respectively, was primarily composed of aquatic insects. Nevertheless, these studies suggest that these species cannot be strictly categorized as an insectivorous species because the other categories of aquatic invertebrates, fish, and algae were also found in their diet.
This study provides first insights into the feeding habits of these endemic species. It reveals similarity in consumption of prey categories within the D. ghetaldii samples and substantial differentiation in feeding niches between the two species in sympatry.
Feeding intensity and frequency are directly correlated with meal size and digestion time (Fange & Grove Citation1979). Larger specimens displayed a reduced feeding frequency compared to their younger counterparts, as was observed in various fish species (Martin Citation1970), which is associated with the energetic costs with age or size (Webb Citation1978). The size structure and weight differed among five D. ghetaldii populations. Namely, the individuals from Ljubomirsko and Dabarsko karst poljes are significantly smaller and lesser in weight than individuals in the other three poljes (). Those samples consumed smaller prey, namely plankton, as the dominant source of their diet, despite the presence of an sample supply of macroinvertebrates in the environment. The reason for this phenomenon is not entirely clear, but it is plausible that habitat type plays a role. Specifically, species from these two poljes were found in small streams, whereas the other three populations were captured in flooded poljes or directly from caves. There were no significant differences in body size and weight between the two T. dabar populations (), which reflected to their uniformity in feeding habits.
D. ghetaldii exhibits zoophagous feeding habits, i.e., a carnivorous diet, demonstrating a broad dietary spectrum incorporating Rotifers, Copepods, Cladocerans, Nematodes, Oligochaeta, Crustacea, Trichoptera, Coleoptera, Plecoptera, Diptera, and Ephemeroptera (). Given their size, it is reasonable to infer that they feed as predators, rather than ram or filtrating feeders, selectively capturing individual prey. Across the five karst poljes, Chironomidae larvae were predominant in the diet of D. ghetaldii in two of them (Mokro and Fatničko), while Cladocerans dominated in Ljubomirsko and Dabarsko karst poljes and Nematodes prevailed in the Popovo Polje (). Dulić et al. (Citation2011) documented a substantial proportion of rotifers in the diet of small fish, a trend that diminished in the diet of larger fish, consistent with our findings (). Phytoplankton, particularly Bacillariophyta, emerged as the predominant prey in the diet of T. dabar, followed by zooplankton (e.g., Cladocerans, Copepods, Rotifers), and small pelagic macroinvertebrates (e.g., larvae of Dipterans) in a considerably smaller proportion. These observations suggest that T. dabar feature a planktivorous, i.e., phytoplanktivorous filtration feeding. It is plausible that during feeding, a small number of macroinvertebrate individuals are incidentally ingested as they rise up in the water column.
To assess the importance of prey, three parameters—AN, AW, and F—along with the integrated IRI index, were employed. The prey number (AN) provides insight into the frequency of fish foraging for each prey category, elucidating the inclination of fish to target a specific type of prey. Conversely, the prey weight (AW) indicates the quantity of each food item consumed and its proportion relative to other food items. A single prey category may carry more weight than numerous smaller ones. Analysis of the feeding index (F) allows us to discern the particular prey types that minnows are likely to prioritize; thus, if F exceeds 50%, it implies that most fish in the group will actively pursue a certain type of prey. Finally, the IRI index integrates all three parameters to identify a select few crucial prey categories from the array of available options (Liao et al. Citation2001). The uniformity in the proportions of AN and AW in the diet of D. ghetaldii suggests a consistent foraging pattern for prey of similar size and weight, with some exceptions observed for larger and heavier prey categories that exhibited higher proportions by weight than by number, such as Trichoptera larvae, Hirudinea, and Coleoptera larvae. As indicated by the IRI, important prey items in the diet of D. ghetaldii encompassed larvae of Chironomidae, Trichoptera, and Ephemeroptera, along with Rotatoria, Cladocera, and Nematoda. In the diet of T. dabar, significant prey items identified by IRI included Bacillariophyta and Rotatoria (). The lowest values of Simpson’s diversity index, recorded for both the diet of D. ghetaldii and bottom fauna, were observed in Fatničko and Mokro karst poljes, while the highest values were documented in Popovo and Ljubomirsko karst poljes (). These results underscore the substantial influence of habitat characteristics and prey dynamics (i.e., availability) on the feeding habits of D. ghetaldii and T. dabar. The elevated diversity indices in Popovo and Ljubomirsko karst poljes are likely attributed to the more permanent and enduring river habitats in these locations. The diminished diversity indices in Fatničko and Mokro karst poljes can be attributed to the transient nature of the habitat, characterized by a flooded, shallow lake. In these poljes, the less favorable conditions, coupled with insufficient time for both plankton and benthos to proliferate in terms of individual numbers and/or size, limit on available food resources for fish. Consequently, fish in these poljes are constrained to smaller and/or less abundant food items, reflecting the reduced diversity in both the plankton and benthos communities. This, in turn, results in curtailed species diversity within the stomach contents of the fish.
The highest degree of diet overlap among D. ghetaldii populations was observed between individuals from Mokro and Fatničko karst poljes, with a slightly smaller overlap between those from Dabarsko and Ljubomirsko karst poljes (). In Fatničko and Dabarsko karst poljes, there was no significant diet overlap between D. ghetaldii and T. dabar, given the predominantly carnivorous nature of D. ghetaldii and the planktivorous preference of T. dabar.
The electivity index results (E) indicate that Chironomidae were positively selected by D.ghetaldii in Fatničko and neutrally selected in Mokro karst poljes, where they predominate in the diet due to their high abundance in the environment. Cladocera were positively selected in all karst Poljes, while Rotifers were neutrally selected. Notably, Bacillariophyta algae were positively selected by T. dabar in both Fatničko and Dabarsko karst poljes. However, zooplankton (Rotatoria and Cladocera) was negatively selected in Dabarsko Polje, whereas Cladocera were positively selected in Fatničko Polje. All benthic prey categories in the diet of T. dabar were negatively selected (), supporting their classification as phytoplanktivores.
In conclusion, this study reveals that when occur in sympatry, D. ghetaldii and T. dabar occupy distinct feeding niches, utilizing different prey categories in the two karst poljes. D. ghetaldii exhibits a zoophagous, carnivorous diet, while T. dabar is consistently planktivorous, primarily phytoplanktivorius in both karst poljes where they were sampled. That indicates that these two sympatric fish species don’t live in syntopy for their feeding niche. D. ghetaldii in Ljubomirsko and Dabarsko karst poljes, being smaller in size, consumed smaller prey items, while those in other karst poljes consumed larger prey. Regarding feeding diversity, it was highest in fishes from stream habitats in Ljubomirsko and Popovo karst poljes and lowest in fishes from temporary, short-lasting habitats in Fatničko and Mokro karst poljes.
Acknowledgments
The Laboratory of Hydropower Plant of Trebisnjica River granted the chemical parameters of water for the researched locations. Numerous local artisanal fishermen provided us specimens of both species that we used in this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- AQEM Consortium. 2002. Manual for the application of the AQEM method. A comprehensive method to assess european streams using benthic macroinvertebrates, developed for the purpose of the water framework directive, version 1.0. Duisburg, Germany: AQEM Consortium.
- Aubé CN. 1836. Iconographie et histoire naturelle des coléoptères d’Europe. Tome cinquième. Hydrocanthares. [Livraison 1]. Paris: Méquignon-Marvis Père et Fils. p. 415.
- Bănărescu PM. 2004. Distribution pattern of the aquatic fauna of the Balkan Peninsula. In: Griffiths H, Kryštufek B, Reed J, editors. Balkan biodiversity: Pattern and process in the European Hotspot. Dordrecht: Kluwer Academic Publishers. pp. 203–219. DOI: 10.1007/978-1-4020-2854-0_12.
- Bianco PG, De Bonis S. 2015. A taxonomic study on the genus Phoxinus (Acthinopterigy, Cyprinidae) from Italy and western Balkans with description of four new species: P. ketmaieri, P. karsticus, P. apollonicus and P. likai. Researches on Wildlife Conservation, IGF Publishing 4:1–17.
- Bogutskaya NG, Zupančič P. 2003. Phoxinellus pseudalepidotus, a new species from the Neretva basin (Teleostei: Cyprinidae) with an overview of the morphology of Phoxinellus species in Croatia and Bosnia and Herzegovina. Ichthyological Exploration of Freshwaters 14:369–383.
- Bogutskaya NG, Zupančič P, Bogut I, Naseka AM. 2012. Two new freshwater fish species of the genus Telestes (Actinopterygii, Cyprinidae) from karst poljes in Eastern Herzegovina and Dubrovnik littoral (Bosnia and Herzegovina and Croatia). ZooKeys 180:53–80. DOI: 10.3897/zookeys.180.2127.
- Burckhardt R. 1900. Beiträge zur Anatomie und Systematic der Laemargiden. Anatomischer Anzeiger 18:488–492. Carlin, 1934.
- Coville FV. 1893. 1893 Contributions from the United States National Herbarium. Vol. 4. Arlington, VA, U.S.A.: Contr. U.S. Natl. Herb. p. 115.
- Curtis J. 1834. XXXVII. Descriptions of some hitherto nondescript British species of May-flies of Anglers. London and Edinburgh Philosophical Magazine and Journal of Science 4(21):212–218. Douglas. DOI: 10.1080/14786443408648304.
- Douglas JW, Scott J. 1869. British Hemiptera: Additions and corrections. source: On-line Systematic Catalog of Plant Bugs (Insecta - Heteroptera - Miridae. Entomologist’s Monthly Magazine 5:259–268; 293–297.
- Draparnaud J-P-R. 1805. Histoire naturelle des mollusques terrestres et fluviatiles de la France. pp. 2.
- Dujardin F. 1841. Histoire naturelle des Zoophytes, Infusoires, comprenant la physiologie et la clasification de ces animaux et la manière de les étudier à l’aide du microscope. Paris: Librarie Encyclopédique de Roret. pp. i–xii, 1–684.
- Dulić Z, Stanković M, Rašković B, Spasić M, Ćirić M, Grubišić M, Marković Z. 2011. Role and significance of zooplankton in semi-intensive carp production. In Marković Z, editor. Vth International Conference “Aquaculture & Fishery”. Belgrade: University of Belgrade, Faculty of Agriculture. pp. 66–71.
- Ehrenberg CG. 1838. Die Infusionsthierchen als vollkommene Organismen. Ein Blick in das tiefere organische Leben der Natur. Leipzig: Verlag von Leopold Voss. pp. i–xviii, [1–4], 1[548]. 64 pls.
- Fagade SO, Olaniyan CI. 1972. The biology of the West African shad Ethmalosa fimbriata (Bowdich) in the Lagos Lagoon, Nigeria. Journal of Fish Biology 4(4):519–533. DOI: 10.1111/j.1095-8649.1972.tb05699.x.
- Fagbenro O, Adedire CO, Ayotunde EO, Faminu EO. 2000. Haematological profile, food composition and digestive enzyme assay in the gut of the African bony-tongue fish, Heterotis (Clupisudis) niloticus (Cuvier 1829) (Osteoglossidae). Tropical Zoology 13(1):1–9. DOI: 10.1080/03946975.2000.10531125.
- Fange R, Grove D. 1979. Digestion. In: Hoar WS, Randall DJ, Brett JR, editors. Fish physiology, bioenergetics and growth. Vol. 8. New York: Academic Press, Inc. pp. 161–260.
- Fischer S. 1851. Beitrage zur Kenntnis der in der Umgegend von St Petersburg sich findenden Cyclopiden. Bulletin de la Société Impériale des Naturalistes de Moscou 24:409–438.
- Freyhof J, Lieckfeldt D, Bogutskaya NG, Pitra C, Ludwig A. 2006. Phylogenetic position of the Dalmatian genus Phoxinellus and description of the newly proposed genus Delminichthys (Teleostei: Cyprinidae). Molecular Phylogenetics and Evolution 37(2):416–425. DOI: 10.1016/j.ympev.2005.07.024.
- Gaston KJ, David R. 1994. Hotspots across Europe. Biodiversity Letters 2(4):108–116. DOI: 10.2307/2999714.
- Geoffroy EL. 1762. Histoire abrégée des insectes qui se trouvent aux environs de Paris, dans laquelle ces animaux sont rangés suivant un ordre méthodique. Tome premier. Paris: Durand. p. xxviii + 523. 10 pl.
- Gnjato R. 1991. Istočna Hercegovina – regionalno geografski problemi razvoja. Banja Luka: Geografsko društvo Bosne i Hercegovine. p. 239.
- Heckel JJ, Kner R. 1857. Die Süsswasserfische der Österreichischen Monarchie, mit Rücksicht auf die angränzenden Länder. Leipzig: EASIN — Réseau européen d’information sur les espèces exotiques. pp. i–xii + 1–388. DOI: 10.5962/bhl.title.8197.
- Kaćanski D, Jerković L, Hafner D, Aganović M. 1978. On the nutrition of some species of fishes in the Buško Blato. Ichthyologia 10(1):67–75.
- Kieffer J-J. 1907-1911. Species des Hyménoptères d’Europe et d’Algérie. Paris: Tome X. pp. 1014.
- Koch CL. 1835. Deutschlands Insecten, herausgegeben von Dr. G. W. F. Panzer, fortgesetzt von Dr. G. A. W. Herrich‐Schäffer. Regensburg: Heft. Fr. Pustet. p. 134.
- Krivokapić M. 1992. Ishrana endemične podvrste jelšovke Leuciscus souffia montenegrinus (Vuković, 1963) iz rijeke Morače (Crna Gora, Jugoslavija). Glasilo republičkog zavoda zaštite prirode Prirodnjačkog muzeja Podgorica 25:93–103.
- Kützing FT. 1844. Die Kieselschaligen. Bacillarien oder Diatomeen. Nordhausen: World Register of Marine Species. p. 152. 30 pls.
- Liao H, Pierce CL, Larscheid JG. 2001. Empirical assessment of indices of prey importance in the diets of predacious fish. Transactions of the American Fisheries Society 130(4):583–591. DOI: 10.1577/1548-8659(2001)130<0583:EAOIOP>2.0.CO;2.
- Linnaeus C. 1753. Species Plantarum. Vol. 1. London: Holmiae, Impensis Laurentii Salvii. [L. Salvius, Stockholm.]. p. 392.
- Linnaeus C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. Holmiae [= Stockholm]: L. Salvii. p. 824.
- Marčić Z, Sučić I, Ćaleta M, Buj I, Mustafić P, Zanella D. 2017. Seasonal profile of the diet of the endemic dace Telestes karsticus Marčić & Mrakovčić 2011 (Cyprinidae, Leuciscinae). Journal of Applied Ichthyology 33(5):943–949. DOI: 10.1111/jai.13397.
- Markotić I. 2013. Biological and ecological characteristics of Mostar minnow, Phoxinellus pseudalepidotus Bogutskaya & Zupančič, 2003 (Teleostei: Cyprinidae) from Mostarsko blato, Bosnia and Herzegovina. Doctoral thesis, Department of Biology, Faculty of Science, University of Zagreb.
- Martin NV. 1970. Long-term effects of diet on the biology of the lake trout and the fishery in Lake Opeongo, Ontario. Journal of the Fisheries Research Board of Canada 27(1):125–146. DOI: 10.1139/f70-013.
- Milanović P. 1979. Hidrogeologija karsta i metode istraživanja, HET Institut za korištenje i zaštitu voda na kršu. Trebinje: HET. p. 302.
- Müller OF. 1774. Vermium terrestrium et fluviatilium, seu animalium infusorium, Helminthicorum, et testaceorum, non marinorum, succincta historia Vol. 2. Havniæ & Lipsiæ. (Heineck & Faber). pp. I–XXXVI, 1–214, 10.
- Müller OF. 1776. Zoologiæ Danicæ prodromus, seu animalium Daniæ et Norvegiæ indigenarum characteres, nomina, et synonyma imprimis popularium. Havniæ. (Hallager). pp. I-XXXII [= 1–32], 1–274.
- Müller PWJ. 1806. V. Macronychus, Krallenkäfer. Eine neueKäfergattung. mit der Beschreibung einer neuen Art vonHakenkäfer, Parnus Magazin für Insektenkunde 5:207–220.
- Oronsaye CG, Nakpodia FA. 2005. A comparative study of the food and feeding habits of Chrysichthys nigrodigitatus and Brycinus nurse in a tropical river. Pakistan Journal of Scientific and Industrial Research 48:118–121.
- Pianka ER. 1973. The structure of lizard communities. Annual Review of Ecology,evolution and Systematic 4(1):53–74. DOI: 10.1146/annurev.es.04.110173.000413.
- Pictet FJ. 1834. Recherches pour servir à l’histoire et l’anatomie des Phryganides. Vol. 235. Geneva. pp. [20 plates]. DOI: 10.5962/bhl.title.8547.
- Pinkas L, Oliphant M, Iverson LK. 1971. Food habits of Albacore, Bluefin Tuna and Bonito in California waters. Vol. 152. Sacramento, CA, USA: Department of Fish and Game, State of California.
- Pita C, Gamito S, Erzini K. 2002. Feeding habits of the gilthead seabream (Sparus aurata) from the Ria Formosa (southern Portugal) as compared to the back seabream (Spondyliosoma cantharus) and the annular seabream (Diplodus annularis). Journal of Applied Ichthyology 18(2):81–86. DOI: 10.1046/j.1439-0426.2002.00336.x.
- Savigny JC. 1826. Analyse d’un Mémoire sur les Lombrics par Cuvier. Mémoires de l’Académie des sciences de l’Institut de France 5:176–184.
- Scopoli JA. 1763. Entomologia carniolica exhibens insecta Carnioliae indigena et distributa in ordines, genera, species, varietates. Methodo Linnaeana. Vindobonae [=Vienna]: J. Trattner. p. xxxvi + 420.
- Sket B, Paragamian K, Trontelj P. 2004. A census of the obligate subterranean fauna of the Balkan Peninsula. In: Griffiths H, Kryštufek B, Reed J, editors. Balkan biodiversity: Pattern and process in the European hotspot. Dordrecht: Kluwer Academic Publishers. pp. 309–322. DOI: 10.1007/978-1-4020-2854-0_18.
- Sokal RR, Rohlf FJ. 1981. Biometry: The principles and practice of statistics in biological research. San Francisco. pp. 859. DOI: 10.2307/2529087.
- Stephens DW, Brown JS, Ydenberg RC. 2007. Foraging: Behaviour and ecology. Écoscience 15(1):138–139.
- Straus HE. 1820. Mémoire sur les Daphina, de la classe des Crustacés (Secondi Partie). Memoires du Muséum d’Histoire Naturelle 6:149–162.
- Vanderploeg HA, Scavia D. 1979. Calculation and use of selectivity coefficients of feeding: Zooplankton grazing. Ecological Modelling 7(2):135–149. DOI: 10.1016/0304-3800(79)90004-8.
- Vuković N. 1985. Ekološke i biosistematske karakteristike Leuciscus souffia Risso, 1826 iz gornjeg toka reke Drine. Doctoral dissertation, Faculty of Science, University of Sarajevo, Sarajevo. pp. 191.
- Vuković T, Ivanović B. 1971. Freshwater fishes of Yugoslavia. Sarajevo: Zemaljski Muzej BiH. pp. 268.
- Webb PW. 1978. Partitioning of energy into metabolism and growth. In: Gerking SD, editor. Ecology of freshwater fish production. Blackwell, Oxford. pp. 184–214. DOI: 10.1007/978-1-349-81461-9.
- Zanella D, Mihaljević Z, Mrakovčić M, Ćaleta M. 2009. Ecology and diet of the endemic Telestes ukliva (Cyprinidae) in the Cetina River system, Croatia. Cybium 33(2):97–105.
- Zupančič P, Bogutskaya N. 2000. Description of a new species, Phoxinellus dalmaticus (Cyprinidae: Leuciscinae), from the Cikola river in the Krka River system, Adriatic basin (Croatia). Natura Croatica 9:67–81.