ABSTRACT
OBJECTIVE
Glial cell line-derived neurotrophic factor (GDNF), being a protective of dopaminergic neurons, is reported to modulate addictive behaviours and have a role as a negative regulator for biochemical and behavioural adaptations to drug abuse. We aimed to reveal impulsivity and serum GDNF levels in patients with heroin addiction and investigate their relationships in order to contribute to the understanding of behavioural aspects and biological mechanisms in heroin addiction via this study.
METHODS
This study was performed at the Department of Psychiatry of Ankara Numune Training and Research Hospital, Turkey. We recruited 129 heroin-dependent patients and 90 age, sex, and smoking-matched healthy controls with no major psychopathology. Barratt Impulsivity Scale-11, Hospital Anxiety and Depression Scale (HADS) and sociodemographic data form were applied to all participants. Laboratory analysis for serum GDNF levels was performed for each participant’s blood sample.
RESULTS
Total impulsivity scores and scores of Attentional Impulsivity, Motor Impulsivity, and Unplanned Impulsivity subscales were all higher in heroin addicts compared to the controls. Heroin addicts had also lower serum GDNF levels and lower GDNF levels were associated with high impulsivity and high HADS scores in heroin addicts.
CONCLUSION
Decrement in GDNF levels in heroin addiction seems as to be an important data which could be associated with impulsivity, anxiety, and depressive symptoms. GDNF could find a prominent place among the target molecules in the treatment of heroin addiction.
Introduction
Neurotrophic factors have an important role in the growth and differentiation of neurons during development and have been implicated in many forms of plasticity within the adult central nervous system.
The glial cell line-derived neurotrophic factor (GDNF) is one of the most potent neurotrophic factors influencing the dopaminergic system. It is reported that GDNF has distinct protective effects on the survival of midbrain dopaminergic neurons in both in vitro and in vivo studies [Citation1–4]. Preclinic studies demonstrated that GDNF was protective of dopaminergic neurons when administered in advance of degenerative toxins, and it also may have restorative effects on dying dopaminergic neurons [Citation5].
Neurotrophic factors and especially GDNF have raised considerable hope to be able to stop the progressive degeneration in neuropsychiatric diseases such as Parkinson Disease and Alzheimer Disease [Citation6]. Besides, animal studies indicate that GDNF modulates addictive behaviours and the role of GDNF as a negative regulator for biochemical and behavioural adaptations to drug abuse was reported [Citation7]. Opiate withdrawal was shown to regulate the expression levels of several neurotrophic factors in specific regions of adult brain [Citation8]. The rewarding effects and/or self-administration of cocaine and ethanol were found to be associated with up-regulation of GDNF levels in the ventral tegmental area (VTA) or striatum [Citation9,Citation10].
Messer et al. reported a functional interaction between GDNF and drugs of abuse at the level of the mesolimbic dopamine system and emphasized complex mechanisms that were likely to be induced in brain by chronic exposure to a drug of abuse [Citation11].
Garnicella et al. found that GDNF was highly effective in reducing heavy alcohol drinking and GDNF in VTA was more effective in inhibiting reacquisition of ethanol self-administration after the extinction period than in reducing ethanol self-administration before the period of abstinence [Citation12]. So, these findings were stated to suggest an important protective role for GDNF in abstinence and relapse processes.
In light of these data, we aimed to search on GDNF levels and heroin addiction which is a growing serious health problem in the world. We focused on GDNF because GDNF has so far more potent effects on the survival and protection of dopaminergic neurons compared to brain-derived neurotrophic factor (BDNF) and other neurotrophins [Citation3] and GDNF enhances the survival of midbrain dopamine neurons in vivo after being exposed to dopaminergic neurotoxins such as 6-hydroxydopamine in an obvious way [Citation1]. Additionally, it was shown that a single injection of GDNF into the midbrain could exert protective effects for at least a month [Citation2,Citation13].
Traits that are related to the extensive construct of disinhibition such as sensation seeking, impulsivity, and behavioural under control have been found to predict substance misuse disorders in adults and it is reported to be higher in substance-dependent patients [Citation14,Citation15].
Pompili et al. reported that substance-dependent patients have higher hopelessness, global psychopathology severity, impulsivity/aggression, and they are frequently depressed [Citation16].
As far as we know, there are no studies in the literature related to the association between GDNF levels, impulsivity, and clinical characteristics in heroin-dependent patients. Evidence-based data related to impulsive personality traits to the development and maintenance of substance misuse problems have been rising [Citation17–19].
We aimed to reveal impulsivity and serum GDNF levels in patients with heroin addiction and investigate their relationships in order to contribute the understanding of behavioural aspects and biological mechanisms in heroin addiction via this study.
Methods
Participants
This study was performed at the Department of Psychiatry of Ankara Numune Training and Research Hospital, Turkey. We recruited 129 heroin-dependent patients who admitted to our addiction clinic between January 2015 and May 2015; and 90 age, sex, and smoking-matched healthy controls with no major psychopathology.
Inclusion criteria for the heroin-dependent group were a diagnosis of heroin dependence according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, 18–65 years of age, and possessing at least five years of primary school education.
The exclusion criteria included anoxia or hypoxia during birth; the presence of a major medical disorder or neuropsychiatric symptoms secondary to a metabolic disorder or structural lesion and any comorbid psychiatric disorders in the heroin-dependent group.
We did a cross-sectional study to investigate the impulsivity and serum GDNF levels in heroin-dependent patients. The study received approval by the local ethics committee.
Every participant was examined by two trained psychiatrists after obtaining informed consent. Sociodemographic data form and the Barratt Impulsiveness Scale were applied to all participants.
Barratt Impulsivity Scale-11
The Barratt Impulsivity Scale (BIS) was developed by Barratt in 1959 and over time it has undergone many revisions. The latest form-11 was developed in 1995 [Citation20]. The scale consists of 30 items and 3 subscales: Attentional Impulsivity (AI), Motor impulsivity (MI), and Unplanned Impulsivity (UI). BIS evaluated four different sub-scores obtained; total points, UI, AI, and MI. High BIS scores are increased levels of the impulsivity index. Validity and reliability study in Turkey was performed by Güleç et al. in 2008 and they reported internal consistency rates (Cronbach’s alpha) as 0.82 for total score, 0.80, 0.70, and 0.64 for UI, MI, and AI subscales, respectively [Citation21]. In this study, the scoring key suggested by Patton et al. was used to evaluate the scale [Citation20].
Sociodemographic data form
Sociodemographic data were used in order to describe characteristics of patients and to determine differences among the groups. The form included age, sex, birth place, living place, education years, marital status, family type, occupation, monthly income, history of military service, history of prison, self and family history of physical and psychiatric diseases, first administration date, history of nicotine, alcohol, cannabis, cocaine and other illicit drugs used, detailed history of heroin usage (dosage, duration, dosage, etc.), and history of addiction treatment trials.
Blood samples and laboratory analysis
With informed consent of patients, 10 cm3 of peripheral venous blood samples was taken, placed in gel-containing tubes and centrifuged at 4000 rpm for 10 min to analyse the separated serum. Haemolysed and icteric serums were not used in this study.
Serum GDNF levels were evaluated with a commercial ELISA kit (BOSTER, Boster Biological Technology Co. Ltd, Fremont, CA, Code: EK0362, LOT: 851034314901). Its %CV value was <10, the measurement interval was 31.2–2000 pg/mL and sensitivity was <4 pg/mL.
Statistical analyse
All statistical analyses were performed with IBM SPSS ver.22.0. The Shapiro Wilk test was used as the normality test. Continuous variables were the Mann–Whitney U test when the data were not normally distributed. Categorical variables were compared using Pearson’s chi-squared test, the Fisher–Freeman–Halton test, and Fisher’s exact test. Correlations between variables were tested using Spearman correlation coefficients. A p-value <.05 was considered as significant.
Results
Overall, 129 heroin addicts and 90 healthy controls were enrolled. Demographic and clinical characteristics of two groups are contrasted in . There were no significant differences among the groups in age, gender, educational level, occupation, marital status, family construct, monthly income, and alcohol and nicotine consumption. Family history of addiction and history of prison rate were higher in the patient group (p = .024 and p = .000 respectively).
Table 1. Demographic characteristics of heroin addicts and controls.
Impulsivity
Total impulsivity scores and scores of AI, MI, and UI subscales were all significantly higher in heroin addicts as shown in .
Table 2. BIS scores in heroin addicts and controls.
GDNF
GDNF levels showed a significant statistical difference between heroin addicts and control groups (p = .002). Heroin addicts had lower levels of GDNF (mean ± SD:472.2 ± 272.7) compared to the control group (mean ± SD:583.1 ± 235.2).
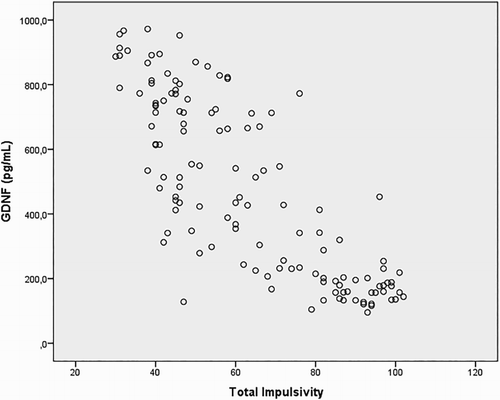
We tested correlations to analyse variables affecting GDNF levels in heroin addicts and have found that lower GDNF levels are associated with high impulsivity and high Hospital Anxiety and Depression Scale-Anxiety (HAD-A), Hospital Anxiety and Depression Scale-Depression (HAD-D) scores ().
There was an inverse correlation between serum GDNF levels and both daily dosage of used heroin (gram) and duration of addiction ().
Table 3. Variables affecting serum GDNF levels in heroin addicts.
Discussion
GDNF plays a crucial role in maintenance and survival of adult dopamine neurons [Citation22] and it has an important protective effect in abstinence and relapse processes of addiction.
We found that serum GDNF levels are significantly lower in heroin addicts compared to healthy controls and serum GDNF levels were inversely correlated with both daily dosage of used heroin and duration of addiction.
There is limited data about GDNF levels in heroin addicts in literature. It is found that a GDNF genotype was associated with a significantly increased risk for heroin dependence [Citation23].
Besides, GDNF has been the subject of research for many chronic and neurodegenerative diseases. It is reported that GDNF was significantly lower in patients with Major Depressive Disorder (MDD) and Mood Disorders (MD) compared to healthy controls [Citation24] and also patients with MD in a manic or depressive episode had lower GDNF levels compared to euthymic MD patients [Citation25].
GDNF was found to be associated with alcohol consumption [Citation26], addiction [Citation27], and Parkinson’s disease [Citation28].
Kotyuk et al. reported [Citation29] an association between a genetic variant of GDNF (rs3096140) and smoking behaviour.
In a recent study [Citation30], it was found that GDNF regulates dopamine transporter functioning in the striatum and reducing or deleting GDNF is associated with amphetamine-induced behaviour. These data suggest endogenous GDNF plays a crucial role in addiction and many other neuropsychiatric conditions.
Besides, GDNF genes were studied in patients with schizophrenia based on the neurodevelopmental ethio-pathogenesis of the disease and it is suggested that the GDNF gene may have an involvement in the genetic liability to Schizophrenia [Citation31].
GDNF was also found to affect synaptic and structural plasticity [Citation32]. Future studies may focus on the role of neurotrophic factors in drug-induced synaptic plasticity changes that potentially contribute to drug abuse and addiction.
We found an association between serum GDNF levels and both daily dosage of used heroin and duration of addiction but it is not clear that the lower levels of GDNF are a cause or result in heroin addiction.
In our study, total impulsivity scores and scores of AI, MI, and UI subscales were significantly higher in heroin addicts compared to healthy controls; and lower GDNF levels of heroin addicts were associated with increased impulsivity. We also found that lower levels of serum GDNF are associated with higher depression and anxiety scores.
It is reported that substance addicts are more impulsive [Citation33,Citation34], but the mechanism of this impulsivity is not clear. Our findings support the neurobiological aetiology of the impulsivity in heroin addicts.
Our study was a cross-sectional, case-control study and it cannot explain the cause-and-effect relationship between addiction and GDNF. Besides, significantly lower GDNF levels and its association with impulsivity, depression, and anxiety scores suggest a biological basis of heroin addiction and there are still many unknowns in this area.
It is reported that GDNF may facilitate or inhibit drug-taking behaviours in different pathways according to the drug type, addiction phase, and the brain site [Citation35]. Future studies should focus on effects of GDNF in terms of different phases of addiction with neuroimaging and advanced techniques.
Conclusions
One of the main regulator growth factor, GDNF levels are significantly lower in heroin addicts. There is a significant association between serum GDNF levels and impulsivity, anxiety, and depression rates.
Neurotrophic factors such as GDNF affecting the dopaminergic system seem to find a prominent place among the target molecules in the treatment of heroin addiction.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Rabia Nazik Yüksel http://orcid.org/0000-0003-1635-9176
Erol Göka http://orcid.org/0000-0001-7066-2817
References
- Beck KD, Valverde J, Alexi T, et al. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373:339–341. doi: 10.1038/373339a0
- Kearns CM, Gash DM. GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res. 1995;672:104–111. doi: 10.1016/0006-8993(94)01366-P
- Lin LF, Doherty DH, Lile JD, et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557
- Sauer H, Rosenblad C, Björklund A. Glial cell line-derived neurotrophic factor but not transforming growth factor beta 3 prevents delayed degeneration of nigral dopaminergic neurons following striatal 6-hydroxydopamine lesion. Proc Natl Acad Sci USA. 1995;92:8935–8939. doi: 10.1073/pnas.92.19.8935
- Aoi M, Tomita S, Ohmoto T. Single administration of GDNF into the striatum induced protection and repair of the nigrostriatal dopaminergic system in the intrastriatal 6-hydroxydopamine injection model of hemiparkinsonism. Restor Neurol Neurosci. 2000;17:31–38.
- Mickiewicz AL, Kordower JH. GDNF family ligands: a potential future for Parkinson’s disease therapy. CNS Neurol Disord Drug Targets. 2011;10:703–711. doi: 10.2174/187152711797247876
- Ron D, Janak PH. GDNF and addiction. Rev Neurosci. 2005;16:277–286. doi: 10.1515/REVNEURO.2005.16.4.277
- Numan S, Lane-Ladd SB, Zhang L, et al. Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J Neurosci. 1998;18:10700–10708.
- Barak S, Carnicella S, Yowell QV, et al. Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J Neurosci. 2011;31:9885–9894. doi: 10.1523/JNEUROSCI.1750-11.2011
- Green-Sadan T, Kuttner Y, Lublin-Tennenbaum T, et al. Glial cell line-derived neurotrophic factor-conjugated nanoparticles suppress acquisition of cocaine self-administration in rats. Exp Neurol. 2005;194:97–105. doi: 10.1016/j.expneurol.2005.01.020
- Messer CJ, Eisch AJ, Carlezon WA, et al. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26:247–257. doi: 10.1016/S0896-6273(00)81154-X
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43:35–43. doi: 10.1016/j.alcohol.2008.12.001
- Winkler C, Sauer H, Lee CS, et al. Short-term GDNF treatment provides long-term rescue of lesioned nigral dopaminergic neurons in a rat model of Parkinson’s disease. J Neurosci. 1996;16:7206–7215.
- Caspi A. The child is father of the man: personality continuities from childhood to adulthood. J Pers Soc Psychol. 2000;78:158–172. doi: 10.1037/0022-3514.78.1.158
- Fergusson DM, Boden JM, Horwood LJ. Recurrence of major depression in adolescence and early adulthood, and later mental health, educational and economic outcomes. Br J Psychiatry. 2007;191:335–342. doi: 10.1192/bjp.bp.107.036079
- Pompili M, Innamorati M, Lester D, et al. Substance abuse, temperament and suicide risk: evidence from a case-control study. J Addict Dis. 2009;28:13–20. doi: 10.1080/10550880802544757
- Dawe S, Gullo MJ, Loxton NJ. Reward drive and rash impulsiveness as dimensions of impulsivity: implications for substance misuse. Addict Behav. 2004;29:1389–1405. doi: 10.1016/j.addbeh.2004.06.004
- Nicola M D, Tedeschi D, De Risio L, et al. Co-occurrence of alcohol use disorder and behavioral addictions: relevance of impulsivity and craving. Drug Alcohol Depend. 2015;148:118–125. doi: 10.1016/j.drugalcdep.2014.12.028
- Moeller FG, Barratt ES, Dougherty DM, et al. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783
- Patton JH, Stanford MS. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1
- Güleç H, Tamam L, Güleç MY, et al. Psychometric properties of the Turkish version of the Barratt impulsiveness scale-11. Klinik Psikofarmakoloji Bülteni. 2008;18:251–258.
- Pascual A, Hidalgo-Figueroa M, Piruat JI, et al. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136
- Ma X-c, Chen C, Zhu F, et al. Association of the GDNF gene with depression and heroin dependence, but not schizophrenia, in a Chinese population. Psychiatry Res. 2013;210:1296–1298. doi: 10.1016/j.psychres.2013.08.025
- Takebayashi M, Hisaoka K, Nishida A, et al. Decreased levels of whole blood glial cell line-derived neurotrophic factor (GDNF) in remitted patients with mood disorders. Int J Neuropsychopharmacol. 2006;9:607–612. doi: 10.1017/S1461145705006085
- Barbosa IG, Huguet RB, Sousa LP, et al. Circulating levels of GDNF in bipolar disorder. Neurosci Lett. 2011;502:103–106. doi: 10.1016/j.neulet.2011.07.031
- Barak S, Ahmadiantehrani S, Kharazia V, et al. Positive autoregulation of GDNF levels in the ventral tegmental area mediates long-lasting inhibition of excessive alcohol consumption. Transl Psychiatry. 2011;1:e60. doi: 10.1038/tp.2011.57
- Carnicella S, Ron D. GDNF—a potential target to treat addiction. Pharmacol Ther. 2009;122:9–18. doi: 10.1016/j.pharmthera.2008.12.001
- Migliore M, Ortiz R, Dye S, et al. Neurotrophic and neuroprotective efficacy of intranasal GDNF in a rat model of Parkinson’s disease. Neuroscience. 2014;274:11–23. doi: 10.1016/j.neuroscience.2014.05.019
- Kotyuk E, Nemeth N, Ronai Z, et al. Association between smoking behaviour and genetic variants of glial cell line-derived neurotrophic factor. J Genet. 2016;95:811–818. doi: 10.1007/s12041-016-0701-7
- Kopra JJ, Panhelainen A, af Bjerkén S, et al. Dampened amphetamine-stimulated behavior and altered dopamine transporter function in the absence of brain GDNF. J Neurosci. 2017;37:1581–1590. doi: 10.1523/JNEUROSCI.1673-16.2016
- Michelato A, Bonvicini C, Ventriglia M, et al. 3′ UTR (AGG) n repeat of glial cell line-derived neurotrophic factor (GDNF) gene polymorphism in schizophrenia. Neurosci Lett. 2004;357:235–237. doi: 10.1016/j.neulet.2003.12.089
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078
- Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003
- Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007
- Ghitza UE, Zhai H, Wu P, et al. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010;35:157–171. doi: 10.1016/j.neubiorev.2009.11.009