ABSTRACT
OBJECTIVE: This research aims to compare the effects of sertraline and agomelatine on outpatients diagnosed with a “major depressive episode” in terms of sleep quality, sexual functioning, and metabolic parameters related to metabolic syndrome.
METHODS: This observational, open-labelled, 12-week follow-up study was carried out in the outpatient psychiatry clinic of a state research hospital. Included in the study were 60 outpatients admitted to the adult psychiatry clinic diagnosed with a “major depressive episode” who were subsequently prescribed agomelatine (25 mg/day) or sertraline (50 mg/day). Arizona Sexual Experience Scale (ASEX) and Pittsburgh Sleep Quality Index (PSQI) were performed during the 1st, 2nd, 4th, 8th and 12th weeks of treatment. The metabolic parameters; Body Mass Index, waist circumference, systolic and diastolic blood pressure, fasting blood glucose, liver enzymes (Alanine transaminase (ALT), Aspartate transaminase (AST) and lipid profiles – total cholesterol and total triglycerides, low density lipoprotein (LDL), high density lipoprotein and very low density lipoprotein – were assessed after the first interview (pre-treatment) and in the 12th week of treatment (post-treatment).
RESULTS: The PSQI scores of both the sertraline and agomelatine groups had declined significantly by the end of the follow-up, with the decline in PSQI scores in the agomelatine group being higher than the decline in the sertraline group. The decline in the ASEX scores of the sertraline group was not significant, while the score was significant in the agomelatine group at the end of the follow-up. However, the difference in the changes in the ASEX scores between the study groups was not significant. The unfavourable changes noted in metabolic parameters were: elevation of the mean LDL level in patients using sertraline, and an elevation in liver enzymes (AST and ALT) in the patients using agomelatine.
CONCLUSION: The favourable effects of agomelatine on sleep quality and the rapid onset of this effect may be beneficial in particular cases. Agomelatine may be an alternative drug for patients who complain of sexual side effects. Clinicians should evaluate the lipid profile of patients using sertraline, while liver function should be monitored in patients using agomelatine. Neither treatment led to unfavourable outcomes on most of the metabolic parameters.
Introduction
Agomelatine is a new antidepressant with a unique mechanism of action, and there have been several studies suggesting its efficacy and safety in the treatment of MDD [Citation1–3]. Agomelatine is a potent melatonin receptor-type 1a and 1b agonist and a serotonergic receptor 5-hydroxytryptamine receptor 2C (5-HT2c) antagonist.
Comparative studies of agomelatine with selective serotonin re-uptake inhibitors (SSRIs) or serotonin–noradrenaline re-uptake inhibitors (SNRIs) have demonstrated that agomelatine has a favourable tolerability profile [Citation4–6]. Discontinuations due to adverse effects have been found to be fewer in patients using agomelatine when compared to SSRI or SNRIs [Citation7,Citation8].
The favourable effects of agomelatine on sleep parameters have been reported in a number of previous studies. Corruble et al. [Citation1] found that agomelatine may bring additional long-term clinical benefits related to sleep-wake quality over escitalopram in depressive patients. In a study conducted by Quera-Salva et al. [Citation9], agomelatine was found to better preserve the number of sleep cycles, improve the morning condition and reduce daytime sleepiness when compared to escitalopram.
It would seem that sexual function is a favourable tolerability area for agomelatine. In an extensive review of data related to agomelatine and sexual dysfunction, it has been found that treatment with agomelatine is associated with a lower risk of sexual dysfunction, in contrast to the majority of first-line antidepressants. In that study, incidences of treatment-emergent sexual dysfunction with agomelatine (3%) were significantly lower than with a placebo (8.6%) and SSRIs (10.1%) [Citation10]. In another study, conducted by Montejo et al. [Citation11], it was found that agomelatine had a good sexual acceptability profile in both healthy men and women regarding such sexual parameters as “delayed orgasm/ejaculation,” “absence of orgasm/ejaculation,” and “total sexual impairment.”
Most antidepressants come with a risk of weight gain and metabolic disturbances, and there have been several studies to date indicating that the long-term use of SSRIs is also associated with weight gain [Citation12]. Some antidepressants that may promote weight gain may also impact upon serum lipid parameters [Citation13]. Any association between antidepressant use and incident Diabetes Mellitus remains inconclusive [Citation12]. The potential beneficial effects of melatonin on metabolic parameters have drawn attention in recent years. Melatonin levels are reduced in diseases associated with insulin resistance, such as metabolic syndrome (MS). As a chronobiotic and cytoprotective agent, melatonin retains a special place in the prevention and treatment of MS. Melatonin improves sleep efficiency and has antioxidant and anti-inflammatory properties, based partly on its role as a metabolic regulator [Citation14]. That said, the effects of melatonin agonists like agomelatine are needed to be better understood regarding their therapeutic value in MS [Citation15].
In a study comparing agomelatine and sertraline, it was claimed that agomelatine may offer some advantages over sertraline in the treatment of symptoms of depression and anxiety, as well as metabolic parameters in depressed patients with type 2 Diabetes Mellitus [Citation16].
This study aims to compare the effects of sertraline and agomelatine on sleep quality, sexual function and such metabolic parameters as body mass index (BMI), waist circumference (WC), systolic and diastolic blood pressure (SBP, DBP), fasting blood glucose (FBG), liver enzymes and lipid profile. Although several studies have identified the favourable effect of agomelatine on sleep and sexual function over other antidepressants, prospective studies are a few. On the other hand, the metabolic issues that relate to metabolic syndrome are less known for most antidepressants, particularly agomelatine.
Method
Study design and materials
This study is a part of a comprehensive project to assess the efficacy and tolerability profiles of agomelatine and sertraline, the first article of which was published in 2016 [Citation5] and included an efficacy and general tolerability profile. More specific issues related to drugs and their effect on sleep, sexuality, and metabolic parameters are covered in the present study.
This observational, open-labelled, 12-week follow-up study was carried out in the outpatient psychiatry clinic of a state research hospital in Istanbul, Turkey between February 2013 and March 2014. Before conducting this research, ethical committee approval was obtained from the institute (HNEAH-KAEK-2012/85). The study sample included outpatients admitted to the adult psychiatry clinic who were diagnosed as having a “major depressive episode” by a clinician based on the Diagnostic and Statistical Manual of Mental Disorders 4th edition, and who were prescribed agomelatine (25 mg/day) or sertraline (50 mg/day). These doses were considered equivalent doses of drugs [Citation17]. The other inclusion criteria for the outpatients were: new-onset depression and no recent use of psychotropic drugs (at least in the last month); and an absence of chronic physical disease, mental retardation, and comorbid psychiatric disease. Pregnant and puerperal patients and those with suicidal thoughts were excluded from this study. The patients who agreed to participate in the study all provided written and signed the informed consent before being included. The first clinician carried out a clinical follow-up and the second clinician, who was blind to information on the prescribed drug, made the assessments.
During the first interview, demographic and clinical data were obtained from the participants, and the Arizona Sexual Experience Scale (ASEX) [Citation18], Turkish version [Citation19] and Pittsburgh Sleep Quality Index (PSQI) [Citation20], Turkish version [Citation21] were applied. Further assessments of ASEX and PSQI were performed during the 1st, 2nd, 4th, 8th, and 12th weeks of treatment. The metabolic parameters of BMI (kg/m2), WC (cm), SBP and DBP (mmHg), FBG (mg/dL), liver enzymes (Alanine transaminase (ALT) (IU/L), Aspartate transaminase (AST) (IU/L) and lipid profiles (total cholesterol (TC) (mg/dL), total triglycerides (TT) (mg/dL), low density lipoprotein (LDL) (mg/dL), high density lipoprotein (HDL) (mg/dL), and very low density lipoprotein (VLDL) (mg/dL) were assessed both after the first interview (pre-treatment), and in the 12th week of treatment (post-treatment). Measurements of BMI, WC, and blood pressure were carried out by a nurse in the outpatient clinic. BMI was calculated as weight (kg)/height (m2). Blood pressure measurements were taken after the respondents had been seated at rest for 5 minutes. The metabolic parameters were analysed in the general biochemistry laboratory of the hospital.
During the follow-up period, six patients (four from the sertraline group and two from the agomelatine group) who did not attend the subsequent interviews and who did not respond to phone calls were excluded from the study, while three patients in the sertraline group were dropped due to adverse effects (two due to gastrointestinal problems like nausea and dyspepsia, and one due to insomnia). The study was terminated when the total number of patients that completed the study reached 30 in both groups.
Statistics
All statistical assessments were performed using the IBM SPSS Statistics version 20.0 program (IBM Co., Armonk, NY, United States), with rates and frequencies reported as raw numbers and percentages. Continuous variables were presented as a mean ± standard deviation. A chi-square test was used with a continuity correction in 2×2 tables to examine for possible differences in the categorical variables. A t-test for independent groups was used to evaluate differences in the continuous variables between independent groups, and a paired samples t-test was used to evaluate differences in the continuous variables before and after the treatment protocol. A Mann–Whitney U-test was also used to evaluate the differences in the continuous variables regarding the distribution of variables. After checking the preliminary assumptions, a repeated measures analysis of variance (with repeated contrasts) was carried out on six consecutive measurements of ASEX and PSQI, and “tests of between-subjects effects” was used to explore the main (group) differences. Effect sizes (η2 or r2) for statistically significant findings are calculated and presented in tables and figures. Statistical significance was accepted as p < .05.
Results
The initial doses were 25 mg/day for agomelatine and 50 mg/day for sertraline, while the mean doses at the end of follow-up period were 30.83 ± 10.75 for the agomelatine group and 59.16 ± 17.95 for the sertraline group, which were both close to the initial doses. A comparison of the demographic and clinical data of the patients using sertraline or agomelatine is presented in , from which it can be seen that no difference exists between the two groups in terms of age, sex, marital status, education level, occupation, and the number of past depressive episodes.
Table 1. Comparison of the demographical and clinical data of patients using sertraline or agomelatine.
The changes in the mean PSQI scores of the study groups in the follow-up period are presented in .
Figure 1. The changes in the mean PSQI scores of the study groups in the follow-up period. +The decline of PSQI scores in sertraline group throughout the follow-up period (effect size (η2) = 0.51). ++The decline of PSQI scores in agomelatine group throughout the follow-up period (effect size (η2) = 0.56). +++The difference in decline of PSQI scores between study groups (effect size (η2) = 0.87).
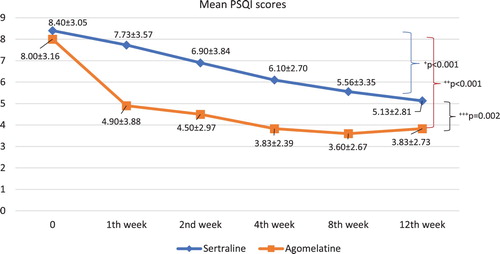
A comparison of the pre-treatment (time 0) and post-treatment (12th week) PSQI scores of the study groups shows that the PSQI scores of both the sertraline and agomelatine groups had declined significantly by the end of the follow-up (t-test; t = 5.527, df = 29, p < .001 and t = 6.054, df = 29, p < .001, respectively). The PSQI scores of the study groups throughout the follow-up period declined in the agomelatine group more than in the sertraline group (df = 1, F = 10.511, p = .002), and this difference in decline was particularly significant in the first week of treatment (df = 1, F = 6.137, p = .016).
The changes in the mean ASEX scores of the study groups in the follow-up period are shown in .
Figure 2. The changes in the mean ASEX scores of the study groups in the follow-up period. +The decline of ASEX scores in sertraline group throughout the follow-up period. ++The decline of ASEX scores in agomelatine group throughout the follow-up period (effect size (η2) = 0.20). +++The difference in decline of ASEX scores between study groups.
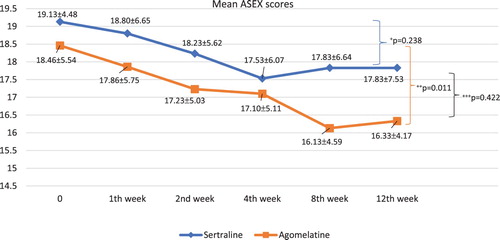
A comparison of the pre-treatment (time 0) and post-treatment (12th week) ASEX scores of the study groups shows that the ASEX scores of the sertraline group declined, but not to a significant degree (t = 1.205, df = 29, p = .238), while the ASEX scores of the agomelatine group had declined significantly by the end of the follow-up (t = 2.714, df = 29, p = .011). The difference in the changes of the ASEX scores of the two study groups throughout the follow-up period shows that the difference in the changes of the ASEX scores of the two study groups was not significant (df = 1, F = 0.655, p = .422), and this finding was true for all particular time periods.
A comparison of the pre-treatment (time 0) metabolic parameters with the post-treatment (12th week) metabolic parameters is shown in , from which it can be seen that only the mean LDL level changed significantly in the sertraline group and that the mean LDL level was higher at the end of the follow-up when compared to the baseline (p = .010). A small decline can be noted in the mean HDL level, although not statistically significant (p = .071). Liver enzymes were elevated significantly in the agomelatine group after treatment (p = .038 for AST and p = .002 for ALT), although in neither drug group did the elevation of the ALT and AST levels pass three times the upper normal limit in any patient. VLDL and TT levels were also decreased after treatment with agomelatine, but not to a significant level (p = .051, p = .067 respectively). No difference was identified in any other metabolic parameters from before and after treatment with agomelatine.
Table 2. Comparison of the pre-treatment (time 0) metabolic parameters with the post-treatment (12th week) metabolic parameters in patients using sertraline or agomelatine.
Discussion
The three parameters investigated in this study were sleep quality, sexual functioning, and metabolic parameters. In this single-centre, open-label, follow-up study agomelatine was found to be superior than sertraline in terms of improving sleep quality while sexual functioning did not differ between treatment arms. Also, minor metabolic changes were observed with sertraline elevating LDL and agomelatine AST and ALT levels.
Sleep quality
Sleep quality was found to improve significantly in both groups in this study, although the improvement in the agomelatine group was higher than in the sertraline group, and this difference was evident particularly in the first week of treatment. This finding is in line with previous studies, reflecting superior early improvement with agomelatine when compared to SSRIs [Citation1,Citation3,Citation9].
Quera-Salva et al. [Citation9] claim that the addition of a chronobiotic effect to the inhibition of 5-HT2c receptors may explain the early impact of agomelatine on sleep quality and alertness at awakening [Citation22]. In a study investigating the effects of psychotropic drugs on sleep-wake cycles, independent of diagnosis and symptom severity, antipsychotics were found to be related to longer sleep periods and duration, serotonin–norepinephrine re-uptake inhibitors to longer sleep periods, and agomelatine to earlier sleep onset [Citation23].
The favourable effect of agomelatine on sleep quality and the rapid onset of this effect may be useful particularly in situations requiring an urgent therapeutic effect, such as suicidal cases. Agomelatine may prove to be beneficial in patients that complain predominantly of sleep-awake problems.
Sexual functioning
In the agomelatine group, sexual function had improved by the end of the study, and while the sexual function scores were better at the end of this study in the sertraline group, they did not reach a significant level. The difference in the changes between the study groups was not significant at the end of this study. Although this does not reflect a superior effect of agomelatine over sertraline, this finding may reflect a mild favourable effect on sexual function in favour of agomelatine. Most studies have found that agomelatine has a better sexual tolerability than SSRIs or SNRIs [Citation24,Citation25] and has been shown to have a better sexual acceptability than to escitalopram [Citation11] and paroxetine [Citation10].
Agomelatine’s antagonistic action on the 5-HT2c receptors and the preclinically evidenced favourable effect of melatonergic agonism on sexuality may explain the better sexual acceptability of agomelatine compared to SSRIs [Citation10]. On the other hand, SSRIs and venlafaxine have been found to reduce dopaminergic transmission via the serotonin receptors in the mesolimbic area, which is associated primarily with inhibited sexual desire and orgasm [Citation26].
Clinicians should monitor patients for sexual side effects throughout the treatment period, and if such side effects develop, management options include waiting for spontaneous remission, decreasing medication doses, switching to alternative drugs, or adding an augmentation agent or antidote. Bupropion, mirtazapine, and buspirone have been studied as such augmentation agents/antidotes or substitution agents in the management of antidepressant-related sexual dysfunction [Citation27]. According to the results of the present study, agomelatine may be considered in such cases.
Metabolic parameters
The main unfavourable change in metabolic parameters in this study was the elevation of mean LDL levels in patients using sertraline, and an elevation in liver enzymes (AST and ALT) in patients using agomelatine.
In a large sample observational cohort study, it was found that the number of days of taking paroxetine and sertraline before the LDL test was related to higher LDL values, after accounting for age, sex, year of LDL testing, co-morbidity, depression, and lipid medication. The authors commented that the long-term use of paroxetine or sertraline might have a measurable adverse impact on cardiovascular risk in adults [Citation28]. In a prospective study investigating the relationship between SSRIs and metabolic syndrome abnormalities, the findings showed that a significant increase occurred in the parameters of weight, BMI, WC, fasting glucose, TC, LDL, and triglyceride after 16 weeks of treatment in the paroxetine group, and significant increases were seen in the levels of triglyceride in the citalopram and escitalopram groups. TC levels increased in the sertraline group, and there were significant reductions in the parameters of weight, TC, and triglyceride in the fluoxetine group [Citation29].
Mean HDL levels also declined in our study, but not to a significant degree. Considering the findings in the literature and in the present study, we recommend clinicians evaluate the lipid profile of patients using sertraline throughout treatment, and to use only with caution in patients with hyperlipidemia. Although not statistically significant, we observed a decline particularly in the TT and VLDL levels of the agomelatine group, although this finding needs to be corroborated by future studies into the potential of agomelatine in patients with hypertriglyceridemia.
The elevation of ALT levels above three times the upper normal limit indicates a clinically significant “drug-induced liver injury” [Citation30]. Fatigue and loss of appetite may be warning signs of liver injury, which may also overlap with symptoms of depression [Citation12]. The risk of liver toxicity seems to be more elevated among patients exposed to nefazodone, bupropion, agomelatine, and duloxetine [Citation30]. In a systematic review of agomelatine-induced liver injuries [Citation31], agomelatine was found to be associated with higher rates of liver injury than a placebo, escitalopram, paroxetine, fluoxetine, and sertraline, and a positive relationship was evidenced between agomelatine dose and liver injury. In a recent study of 896 patients with depression using agomelatine [Citation32], liver enzymes (AST, ALT, GGT, ALP) were found to be elevated after 12 weeks of treatment, although these elevations did not exceed three times the upper limits of the established norms.
In this study, the mean levels of ALT and AST demonstrated a mild but statistically significant elevation, although in neither drug group did the elevation of ALT levels reach above three times the upper normal limit in any patient. Liver function should be monitored in patients using agomelatine, particularly before treatment initiation and after increases in dose increments, and this is particularly important in patients with the pre-existing liver disease.
Considering the overall metabolic findings in this study, it can be said that neither the sertraline nor agomelatine treatments demonstrated an unfavourable effect on most of the metabolic parameters. Body mass index, WC, blood pressure, and FBG were the crucial metabolic parameters in a “metabolic syndrome” diagnosis, and no difference was noted in these parameters in either drug group after treatment, which indicates that these antidepressants are unlikely to cause metabolic syndrome. Accordingly, we could not confirm the findings of studies that reflect metabolic disturbances with SSRIs nor could we confirm the results of studies reflecting a superior effect of agomelatine to sertraline on crucial metabolic parameters. These findings should be evaluated considering the relatively short duration of this study when compared to a full course of depression treatment.
Conclusion
The favourable effect of agomelatine on sleep quality and the rapid onset of this effect may be particularly useful in cases requiring an urgent therapeutic effect. Although agomelatine did not demonstrate a superior effect over sertraline, a mildly favourable effect on sexual function in favour of agomelatine is observed, meaning that agomelatine may be a preferable alternative for patients complaining of sexual side effects. The LDL levels in patients using sertraline and the liver enzymes (AST and ALT) in patients using agomelatine were found to be elevated in this study. Clinicians should evaluate the lipid profile of patients using sertraline and use it only with caution in patients with hyperlipidemia, and liver function should be monitored in patients using agomelatine. Neither sertraline nor agomelatine had an unfavourable effect on most of the metabolic parameters related to metabolic syndrome.
The small number of patients, the open-label design, and the lack of randomization and placebo-control are the main limitations of this study. Shared method variance due to self-report scales, reporting bias, regression to the mean in consecutive measurements should also be kept in mind while considering the results of this study. Lastly, this study is conducted in outpatient setting and patients with comorbid psychiatric disorders, hospitalized patients, and patients with suicidal thoughts did not include to the study which may have led to study with a specific or moderate severity of depression. These are all limit the generalizability of the findings, and so further studies without these limitations are needed.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Corruble E, de Bodinat C, Belaïdi C, et al. Efficacy of agomelatine and escitalopram on depression, subjective sleep and emotional experiences in patients with major depressive disorder: a 24-wk randomized, controlled, double-blind trial. Int J Neuropsychopharmacol. 2013;16:2219–2234. doi: 10.1017/S1461145713000679
- Hale A, Corral RM, Mencacci C, et al. Superior antidepressant efficacy results of agomelatine versus fluoxetine in severe MDD patients: a randomized, double-blind study. Int Clin Psychopharmacol. 2010;25:305–314. doi: 10.1097/YIC.0b013e32833a86aa
- Kasper S, Hajak G, Wulff K, et al. Efficacy of the novel antidepressant agomelatine on the circadian rest-activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: a randomized, double-blind comparison with sertraline. J Clin Psychiatry. 2010;71:109–120. doi: 10.4088/JCP.09m05347blu
- Guaiana G, Gupta S, Chiodo D, et al. Agomelatine versus other antidepressive agents for major depression. Cochrane Database Syst Rev. 2013;17(12):CD008851.
- Akpınar E, Cerit C, Talas A, et al. Agomelatine versus sertraline: an observational, open-labeled and 12 weeks follow-up study on efficacy and tolerability. Clin Psychopharmacol Neurosci. 2016;14:351–356. doi: 10.9758/cpn.2016.14.4.351
- Kasper S, Corruble E, Hale A, et al. Antidepressant efficacy of agomelatine versus SSRI/SNRI: results from a pooled analysis of head-to-head studies without a placebo control. Int Clin Psychopharmacol. 2013;28:12–19. doi: 10.1097/YIC.0b013e328359768e
- Huang KL, Lu WC, Wang YY, et al. Comparison of agomelatine and selective serotonin reuptake inhibitors/serotonin-norepinephrine reuptake inhibitors in major depressive disorder: a meta-analysis of head-to-head randomized clinical trials. Aust N Z J Psychiatry. 2014;48:663–671. doi: 10.1177/0004867414525837
- Demyttenaere K, Corruble E, Hale A, et al. A pooled analysis of six month comparative efficacy and tolerability in four randomized clinical trials: agomelatine versus escitalopram, fluoxetine, and sertraline. CNS Spectr. 2013;18:163–170. doi: 10.1017/S1092852913000060
- Quera-Salva MA, Hajak G, Philip P, et al. Comparison of agomelatine and escitalopram on nighttime sleep and daytime condition and efficacy in major depressive disorder patients. Int Clin Psychopharmacol. 2011;26:252–262. doi: 10.1097/YIC.0b013e328349b117
- Montejo A, Majadas S, Rizvi SJ, et al. The effects of agomelatine on sexual function in depressed patients and healthy volunteers. Hum Psychopharmacol. 2011;26:537–542. doi: 10.1002/hup.1243
- Montejo AL, Deakin JF, Gaillard R, et al. Better sexual acceptability of agomelatine (25 and 50 mg) compared to escitalopram (20 mg) in healthy volunteers. A 9-week, placebo-controlled study using the PRSexDQ scale. J Psychopharmacol. 2015;29:1119–1128. doi: 10.1177/0269881115599385
- Carvalho AF, Sharma MS, Brunoni AR, et al. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom. 2016;85:270–288. doi: 10.1159/000447034
- McIntyre RS, Soczynska JK, Konarski JZ, et al. The effect of antidepressants on lipid homeostasis: a cardiac safety concern? Expert Opin Drug Saf. 2006;5:523–537. doi: 10.1517/14740338.5.4.523
- Cardinali DP, Vigo DE. Melatonin, mitochondria, and the metabolic syndrome. Cell Mol Life Sci. 2017;74:3941–3954. doi: 10.1007/s00018-017-2611-0
- Cardinali DP, Hardeland R. Inflammaging metabolic syndrome and melatonin: a call for treatment studies. Neuroendocrinology. 2017;104:382–397. doi: 10.1159/000446543
- Karaiskos D, Tzavellas E, Ilias I, et al. Agomelatine and sertraline for the treatment of depression in type 2 diabetes mellitus. Int J Clin Pract. 2013;67:257–260. doi: 10.1111/ijcp.12112
- Hayasaka Y, Purgato M, Magni LR, et al. Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord. 2015;180:179–184. doi: 10.1016/j.jad.2015.03.021
- McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26:25–40. doi: 10.1080/009262300278623
- Soykan A. The reliability and validity of Arizona Sexual Experiences Scale in Turkish ESRD patients undergoing hemodialysis. Int J Impot Res. 2004;16:531–534. doi: 10.1038/sj.ijir.3901249
- Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4
- Ağargün MY, Kara H, Anlar Ö. Pittsburgh Uyku Kalitesi İndeksi’nin Geçerliği Ve Güvenirliği. Turk Psikiyatr Derg. 1996;7:107–115.
- Quera Salva MA, Hartley S, Barbot F, et al. Circadian rhythms, melatonin and depression. Curr Pharm Des. 2011;17:1459–1470. doi: 10.2174/138161211796197188
- Robillard R, Oxley C, Hermens DF, et al. The relative contributions of psychiatric symptoms and psychotropic medications on the sleep-wake profile of young persons with anxiety, depression and bipolar disorders. Psychiatry Res. 2016;243:403–406. doi: 10.1016/j.psychres.2016.06.025
- Sapetti A. Agomelatine: an antidepressant without deterioration of sexual response. J Sex Marital Ther. 2012;38:190–197. doi: 10.1080/0092623X.2011.613095
- Reichenpfader U, Gartlehner G, Morgan LC, et al. Sexual dysfunction associated with second-generation antidepressants in patients with major depressive disorder: results from a systematic review with network meta-analysis. Drug Saf. 2014;37:19–31. doi: 10.1007/s40264-013-0129-4
- Bella AJ, Shamloul R. Psychotropics and sexual dysfunction. Cent European J Urol. 2013;66:466–471.
- Clayton AH, El Haddad S, Iluonakhamhe JP, et al. Sexual dysfunction associated with major depressive disorder and antidepressant treatment. Expert Opin Drug Saf. 2014;13:1361–1374. doi: 10.1517/14740338.2014.951324
- Wei F, Crain AL, Whitebird RR, et al. Effects of paroxetine and sertraline on low-density lipoprotein cholesterol: an observational cohort study. CNS Drugs. 2009;23:857–865. doi: 10.2165/11310840-000000000-00000
- Beyazyüz M, Albayrak Y, Eğilmez OB, et al. Relationship between SSRIs and metabolic syndrome abnormalities in patients with generalized anxiety disorder: a prospective study. Psychiatry Investig. 2013;10:148–154. doi: 10.4306/pi.2013.10.2.148
- Voican CS, Corruble E, Naveau S, et al. Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry. 2014;171:404–415. doi: 10.1176/appi.ajp.2013.13050709
- Freiesleben SD, Furczyk K. A systematic review of agomelatine-induced liver injury. J Mol Psychiatry. 2015;3:1484. doi: 10.1186/s40303-015-0011-7
- Medvedev VE. Agomelatine in the treatment of mild-to-moderate depression in patients with cardiovascular disease: results of the national multicenter observational study PULSE. Neuropsychiatr Dis Treat. 2017;13:1141–1151. doi: 10.2147/NDT.S129793
