Sample Size Calculations in Clinical Research has its third edition appeared in 2017, written by Professors Shein-Chung Chow (Duke University), Jun Shao (University of Wisconsin-Madison), Hansheng Wang (Peking University) and Yuliya Lokhnygina (Duke University). These authors are not only famous statistical researchers in top-rated universities, but also well established in pharmaceutical industry because of their primary and fundamental research work in biopharmaceutical statistics.
Clinical research is a lengthy and costly process for providing accurate and reliable assessment of the efficacy and safety of pharmaceutical entities under investigation. To ensure the success of studies conducted at various phases of clinical development, sample size calculation plays a crucial role. It is usually conducted through a pre-study power analysis, with the purpose of selecting a sample size to achieve a desired statistical power for correct detection of a clinically meaningful difference of the pharmaceutical entity under study if such a difference truly exists.
The first two editions of the book, published, respectively, in 2003 and 2007, are well praised in the statistics community and pharmaceutical industry; for example, the following are reviews from well-known statistical journals:
‘... this is a useful, comprehensive compendium of almost every possible sample size formula. The strong organization and carefully defined formulae will aid any researcher designing a study.’ – Biometrics
‘This impressive book contains formulae for computing sample size in a wide range of settings. One-sample studies and two-sample comparisons for quantitative, binary, and time-to-event outcomes are covered comprehensively, with separate sample size formulae for testing equality, non-inferiority, and equivalence. Many less familiar topics are also covered ....’ – Journal of the Royal Statistical Society
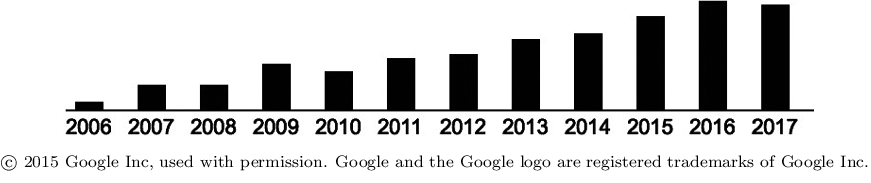
The following citation statistics provided by Google Scholar shows that the second edition of this book is highly cited with a steady increasing impact throughout the last decade.
Total citations: 743 (ended in September 2017)
The new third edition is updated throughout, including many new sections and five new chapters on emerging topics. Together with the 15 chapters in the second edition, the third edition has a total of 20 chapters providing a comprehensive and unified presentation of statistical concepts and practical applications, a balanced summary of current and emerging clinical issues, regulatory requirements, and recently developed statistical methodologies for sample size calculation in various phases of clinical research and development. Some interesting special features of the book are the following:
It compares the relative merits and disadvantages of statistical methods for sample size calculations.
It provides formulas and/or procedures for determination of sample size required not only for testing equality, but also for testing non-inferiority/superiority and equivalence (similarity) under a parallel-group design or a cross-over design with equal or unequal ratio of treatment allocations.
It presents real-world examples from areas such as cardiovascular medicine, the central nervous system, anti-infective medicine, oncology and women's health.
It provides sample size calculation for dose–response studies, microarray studies and Bayesian approaches. It explores emerging topics such as two-stage seamless adaptive designs, cluster randomised trial design, zero-inflated Poisson distribution, clinical trials with extremely low incidence rates and clinical trial simulation.
Chapter 1 is an introduction on regulatory requirement, basic concepts in hypothesis testing and the issue of sample size determination. Numerous updates have been included in this chapter of the third edition. Chapter 2 discusses some issues that need to be considered prior to sample size calculation. In practice, it is not uncommon to perform sample size calculation with inappropriate test statistics, study designs or even wrong hypotheses. The simplest problems of comparing means and proportions are treated in Chapters 3–5, where a new section on negative binomial regression is added to Chapter 5 of the third edition. For sample size calculation in goodness-of-fit tests and contingency tables considered in Chapter 6, new results on the use of strata are added in the new edition. Chapters 7 and 8 focus on the important area of time-to-event data. Chapter 9 considers comparing variabilities. A new section is added to Chapter 10 for sample size in bioequivalence testing. It is about the sample size requirement for analytical similarity in assessment of biosimilar products, a relatively new area in pharmaceutical development. Chapters 11–14 and 20 remain the same as those in the second edition, although many updates are made in these chapters. Five new chapters are in the third edition. They are Chapter 15 for cluster randomisation trial design, Chapter 16 for zero-inflated Poisson population, Chapter 17 for clinical trials with extremely low incidence rate, Chapter 18 for two-stage adaptive trial design and Chapter 19 for sample size based on clinical trial simulation. In the past decade, tremendous progress has been made in statistical methodology to utilise innovative design and analysis in pharmaceutical research to improve the chance of success in clinical development. This results in the five new chapters for sample size calculation in new developed methods.
Similar to the previous editions, the third edition concentrates on concepts and implementation of methodology rather than technical details. Because the details in mathematics and statistics are kept as fundamental as possible and concepts and implementation are illustrated through examples, this book is well suited for pharmaceutical and clinical scientists and biostatisticians as a tool book. We also believe that this new and expanded edition is a useful reference or textbook for scientists, researchers, biostatisticians, students and reviewers in academia, pharmaceutical industry and regulatory agencies.

