ABSTRACT
Even though extensive research has been directed toward optimization of the strain, media, and the process, yet there is limited knowledge on the performance and viability of large-scale PHB production lines exploiting cyanobacteria. In this study, the scale-up challenges associated with photosynthetic PHB production are listed. The high PHB producing cyanobacterial mutant, MT_a24, a randomly mutated strain of Synechocystis sp. PCC 6714, has been tested in pilot-scale trials for photosynthetic PHB production under non-sterile conditions. The MT_a24 obtained PHB content of 0.356 g L−1 and 1.7 g L−1 of glycogen from CO2 after 10 days of cultivation using a self-limiting media and non-optimized cultivation parameters. The results obtained here suggest that in order to achieve high PHB productivity values of the lab and to overcome the existing scalability issues reassessment of the optimized parameters needs to be performed during the pilot-scale trials.
Introduction
Microalgae are an alternative sustainable renewable production system, which does not need arable land for cultivation [Citation1]. Poly (3-hydroxybutyrate) (PHB) is a widespread intracellular storage compound found in prokaryotic organisms [Citation2], which can be used for the production of biodegradable plastics. Cyanobacteria are well-known for their ability to produce PHB from sustainable resources CO2 and sunlight [Citation3]. The use of PHB derived photosynthetically is not economically feasible due to high production costs. The price of cyanobacterial PHB can be reduced to make the material more competitive in the market either by increasing the productivities or reducing the cultivation costs. This, in turn, can be achieved by smart engineering solutions and physiological adaptations of production strains [Citation1].
PHB biosynthesis in cyanobacteria has been enhanced using optimization of cultivation conditions and multi-stage cultivation process which involves nitrogen or phosphorus limitation and the addition of sugars or organic acids, approaches which did not exploit the photosynthetic potential of cyanobacteria [Citation4]. As an alternative way to increase productivity, cyanobacteria have been engineered, however, these attempts have shown little success. Recently, the optimization of the acetoacetyl-CoA reductase ribosome binding site in Synechocystis led to increase in (R)-3-hydroxybutyrate production of up to 1.84 g L−1 in 10 days from CO2 and the highest productivity of 263 mg L−1 d−1 was obtained [Citation5]. As a substitute approach, random mutagenesis has also been used to obtain superior cyanobacterial strains in terms of growth and productivities. The authors previously showed the cyanobacterial strain MT_a24, a UV-mutated strain of Synechocystis sp. PCC 6714 produces PHB of up to 37 ± 4 % dry cell weight (DCW) under nitrogen and phosphorus limitation showing the highest productivity of 134 mg L−1 d−1 [Citation6]. Under lab conditions, it was also shown that media optimization can be used to increase PHB content in MT_a24 to up to 1.16 g L−1 [Citation7]. Although extensive research has been directed toward optimization studies, yet there is scarce knowledge on the performance and viability of large-scale photosynthetic PHB production lines.
After an initial estimation of the costs to see whether production is economically justifiable, cultivation should be tested in a pilot plant. The pilot-plant gives results concerning “time-space-yield” relationships and economic factors, also aiding in the selection of the criteria for scale-up. So far there exist a few reports on pilot-scale cyanobacterial PHB production under unsterile conditions. Troschl et al [Citation8]. in 2018 reported the production of 125 mg L−1 PHB in a 200-litre tubular photobioreactor using Synechocystis sp. CCALA192.
For a successfully integrated scale-up, the utmost importance is the proper technology-transfer from the laboratory to industrial scale. The task includes the elucidation of crucial information to be transferred from R & D to pilot-production and for development of the existing process to the production in industrial-scale [Citation9].
The challenges associated with the scale-up of microalgae cultures have already been discussed by various authors [Citation10,Citation11]. However, how to transfer and translate the specifications from lab to industry for cyanobacterial production systems has not been yet described.
In this current study, the authors aim at filling the knowledge gap between the laboratory and the industrial application showing sustainable and renewable production of PHB from CO2. This work will provide the scale-up scenario and the preliminary steps toward industrial production of cyanobacterial PHB, in order to facilitate understanding and characterization of the large-scale production and the necessary steps toward commercial production.
Material and methods
Strain and inoculum preparation
The strain MT_a24, a high PHB producing strain of Synechocystis sp. PCC 6714 was generated by UV-mutagenesis using the method described by Kamravamanesh et al. in 2018. For inoculum preparation complete BG-11 media was supplemented with 10 mM HEPES buffer, pH 8.2 and 5 mM NaHCO3 was used as a carbon source prior to inoculation. The self-limiting media for PHB production was a modified BG-11 media containing 1000 mg L−1 NaNO3 and 60 mg L−1 K2HPO4 optimized previously and described by Kamravamanesh et al. in 2019.
Determination of growth, glycogen, and PHB
Biomass growth was determined spectrophotometrically at 750 nm using a UV-Vis spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA) at 6–8 hour intervals.
Glycogen and PHB quantification and determinations were performed using a protocol which was previously described by Kamravamanesh et al. in 2018.
Bioreactor cultivations
Lab-scale bioreactor experiments were carried out under sterile conditions and light/dark cycles of 16:8 in a 1.5 L jacketed glass reactor with a working volume of 1 L (Applikon B.V, the Netherlands) as previously described by Kamravamanesh et al. [Citation12]. The temperature was maintained at 28 °C and pH was measured with a pH-electrode (Mettler Toledo GmbH, Vienna, Austria) and was automatically maintained at 8.5 by the addition of 0.5 M HCl or 0.5 M NaOH. Agitation speed was at 300 rpm. Gas flow was controlled by mass flow controllers for air and CO2 (Brooks Instrument, Matfiels, USA). The reactor was bubbled with a mixture of sterile filtered air and 2 % CO2 at a flow rate of 0.02 vvm (20 mL min−1). The illumination was done using LED strips wrapped around the reactor vessel providing a light intensity of 50 µmol m−2 s−1 photons in PAR.
The Pilot-scale cultivations were performed in a glass-house in a 40-litre glass, the tubular, vertical airlift reactor system at ecoduna AG in Bruck an der Leitha, Austria under non-sterile conditions. The pH was controlled at 8.5 using CO2 sparging and the circulation was done using the airlift effect.
All fermentation parameters and variable pump set-points in the lab were controlled using the process information management system Lucullus online monitoring system 3.2 (Securecell AG, Schlieren, Switzerland).
Photobioreactor experiments were performed in duplicates and one is shown as an example here. Samples were taken in triplicates at 24-hour intervals and were analyzed for biomass concentration, glycogen, and PHB content.
Results and discussions
Technology-transfer for scale-up
The scientific and technical feasibility of photosynthetic PHB production in the laboratory has already been endorsed. The question lies, however, in whether the technology can be supported and further developed to overcome existing scalability challenges to facilitate economic viability [Citation13].
Scaling-up in cyanobacterial processes have commonly been done by a factor of 10, for instance from 100 mL shake flasks to a 1 L reactor and from 1 L to 10 L reactor [Citation10]. However, the subsequent scale-up can be complex while the quality of the raw material used, production mode, system or critical process parameters also the performing equipment is changed. There are certain guidelines, which need to be considered for a systematic scale-up.
The first step toward scale-up is the estimation of the production scale in order to be commercially and economically relevant and viable. The reactor volume required for the production of 10 tons of PHB per year, assuming the highest reported productivity for the phototrophic cyanobacterial PHB production value of 263 mg L−1 d−1 [Citation5] and annual operation time of 365 days is around 120 m3. The assumptions used here are completely optimistic and so far only one report, at lab-scale has achieved such titres [Citation5]. In case the volumetric productivity is half of the mentioned value the reactor size must be doubled, also for commercial production 10 tones product per annum is used for high-value products and for the commodity, production of more than 1000 tons annually is considered commercial scale [Citation10]. The assumptions also do not include the efficiency of downstream processing comprising of biomass harvesting, PHB recovery, and product loss during extraction and purification or the risk of contamination or equipment breakdown. Moreover, in many locations, seasonal climate changes do not allow 365 days of production [Citation10]. Furthermore, the water requirement for such cultivation should be considered, taking in to account the water loss from the pond/photobioreactor due to evaporation. Zittelli et al [Citation14]. in 2013 have reported that the cost of building photobioreactor facilities can vary from 10 to 5000 € m−2 depending mainly on the size and reactor type. Thus, to prevent future bankruptcy the step by step scale-up planning needs to be performed. In this context, it is of primary importance to validate the lab-scale quantitative approach first in a pilot-plant with the goal to mimic the final production and operation mode.
Technology-transfer specifications
Establishment of the most efficient and inexpensive process for the production of PHB in cyanobacteria is only achievable when the issues of mass cultivations which are listed below are acknowledged beforehand.
The cultivation system
The main objective of scale-up is to enlarge the production quantities with similar or higher productivity and product quality [Citation11]. The most widely used systems for large-scale photosynthetic production are the moderately controlled, sophisticated photobioreactor systems (PBRs) and the industrially applied open-raceway ponds. Horizontal closed PBR systems, have several drawbacks over open raceway systems (oxygen accumulation, cell damage by shear stress, over-heating and the difficulty to scale-up) [Citation15]. As a result of these limitations, the PBRs are expensive to design, build and to operate, and the production cost of the biomass in PBRs may be one order of magnitude higher than in open ponds [Citation15]. On the other hand, the limitation for using genetically modified strains and maintaining monocultures in open systems remains as the main disadvantage of race-way ponds. Irrespective of the cultivation system used, there are certain technical and biological problems which need to be explained for successful scale-up after the detailed quantitative analysis of the process is performed in the lab.
Light
Light availability is one of the most important aspects of the photosynthetic operation. The light intensity and duration directly affect the photosynthetic efficiency of the microalgae biomass and has an influence on the biochemical composition of the cells and productivity [Citation16].
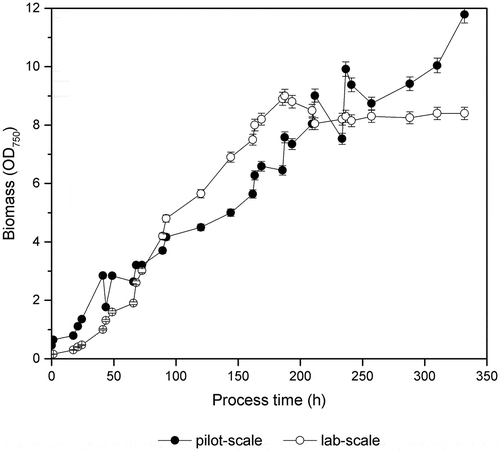
In all cultivation facilities, the growth of algae is limited by the shape and the dimensions of the cultivation vessel. Therefore, cultivation facilities have to be designed such that the light conversion efficiency is maximized, which means the use of dense cultures fully absorbing the delivered light [Citation17]. Unfortunately, the steep light gradient formed in these cultures results in overexposure of the upper layers of the culture and leads to low efficiency of light conversion [Citation17,Citation18]. To prevent prolonged light saturation, cultures have to be rapidly mixed [Citation18]. The photorespiration is a competing process to carboxylation, where the organic carbon is converted into CO2 without any metabolic gain [Citation17]. Under high or low irradiance, a high concentration of oxygen and reduced CO2 levels, the reaction equilibrium is shifted toward the photorespiration, as a result reducing the productivity [Citation17]. Therefore, in order to obtain optimum yield in microalgae mass cultivations, it is of utmost importance to minimize the effect of photorespiration. Furthermore, the influence of the day/night regime on the process and the productivity vary significantly from the dark/light cycles mimicked in the lab. In the growth of cyanobacterial mutant strain, MT_a24 [Citation7] on a self-limiting media in lab-scale and pilot-scale reactors is represented by optical density (OD) measurements at 750nm. Both cultivations show a very similar behavior until 180 hours of the process after that the flattening of growth for the process in lab-scale is observed. The stationary phase represented for the lab-scale process means the growth is seized as a result of nutrient starvation indicating the polymer synthesis phase. Although the cultivation conditions of outdoor culture are neither optimal nor controlled, the mutant MT_a24 obtains slightly higher OD750 for the pilot-scale cultures. However, fluctuation in OD750 is observed between the measurements performed in the morning and in the evening in pilot-scale cultures, which could be due to the changes in the irradiance spectrum of day/night regime. It has been known that even dense cultures of microalgae are exposed to large fluctuations in light intensity due to the daylight variations and the poor mixing [Citation17]. Therefore, it is of maximum importance to previously expose and adapt the cells to the day/night regime instead of routinely done dark/light cycles before starting the pilot-scale trials.
Figure 1. Represents the phototrophic growth of cyanobacterial strain MT_a24 on a self-limiting media under controlled defined conditions in one-litre photobioreactor and under non-sterile conditions of a 40-litre pilot-scale tubular photobioreactor. Data represent the mean ± SD from three measured samples of three independent runs of cultivation.
Temperature control
Maximum productivity can be obtained under optimal cultivation conditions. The authors previously showed the significance of temperature on growth and PHB productivity in the parent strain Synechocystis sp. PCC 6714 [Citation12].
Commonly, the outdoor cultures of microalgae are exposed to a range of environmental stresses. The most frequent combination is light and temperature fluctuations [Citation17]. The fluctuations in light intensity occur mainly in a range of 1–2 hours, the increase in temperature, however, is a slower process and occurs in a much longer period. This kind of de-synchronization, which affect photosynthesis and growth of outdoor algal cultures, results in a unique stress condition under which photoinhibition can be induced due to the sub-optimal temperature conditions at a relatively low light intensity [Citation17,Citation19]. While open ponds are limited by low temperatures in the morning, PBRs generally require cooling at midday [Citation15]. Shading, immersion in a water bath and water spraying are the most common solutions adopted to avoid overheating of outdoor PBRs [Citation15]. For that reason, preferably the microalgae production farm needs to be located in places with a moderate climate and minor temperature fluctuations during the night. Moreover, the cultures which are selected for outdoor cultivations need to be versatile or at least adapted to a wide temperature range.
Nutrient limitation
Environmental factors, particularly light, temperature, nutrient status, and salinity, not only affect photosynthetic efficiency and the productivity of microalgae but also influence the pattern, pathway, and activity of cellular metabolism and thus the dynamic cell composition [Citation20].
Carbon, nitrogen, and phosphorus are the three most important nutrients for autotrophic growth and their supply is key to microalgae biotechnology [Citation17]. Nitrogen and phosphorus are generally the essential constituents of cyanobacteria and play important role in cellular metabolic processes. Under nitrogen and phosphorus limitation, the photosynthetic efficiency falls and the focus is directed toward the accumulation of carbohydrates and fatty acids and in case of some cyanobacteria accumulation of PHB. Nitrate and ammonia are the most widely used nitrogen source in cyanobacterial cultivations. Use of ammonia makes the process control more challenging because the release of H+ ions leads to drastic pH changes, however, the advantage is the lower cost [Citation20]. The authors have previously shown that the nitrogen source used for growth can influence PHB productivity, in the case of Synechocystis sp. PCC 6714 [Citation12] the nitrate use was favorable to the final PHB content.
represents the glycogen and PHB accumulation for strain MT_a24 using a self-limiting media [Citation7] both in one-litre indoor and 40-litre outdoor pilot-scale reactor. The glycogen content in the outdoor culture () showed significant oscillations until the end of the process and reached a value of about 55 % (DCW) corresponding to 1.7 g L−1 after 330 hours of the process. As expected, the glycogen accumulation for the indoor one-litre reactor showed a constant increase and attained a maximum value of 49.5 % (DCW) corresponding to 1.4 g L−1 after 260 hours of the process and later started to reduce significantly.
Figure 2. Photosynthetic cultivation of MT_a24 using a self-limiting media both in a one-litre lab-scale reactor and a 40-litre pilot-scale photobioreactor (a) glycogen accumulation and (b) PHB content. Data represent the mean ± SD from three measured samples of three independent runs of cultivation.
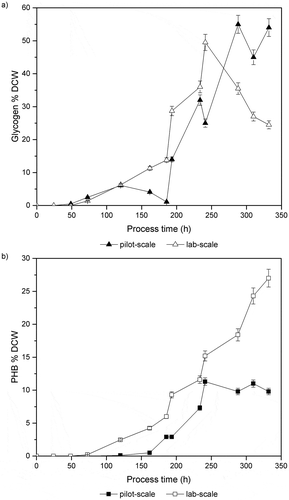
Also, the highest PHB content () in the outdoor culture observed was a value of 11.3 % (DCW) corresponding to 0.365 g L−1 in contrast to a maximum of 27 % (DCW) PHB (782 mg L−1) which was obtained for the lab-scale indoor process. While the authors have previously shown the significance of the dark/light cycle on the photosynthetic PHB productivity for the Synechocystis sp. PCC 6714 [Citation12] the PHB content for MT_a24 observed here in the lab-scale under dark/light cycles is lower than previously observed for this strain.
This diversity in the obtained results from outdoor and indoor cultivations could not be easily explained, however, one hypothesis could be the transition between dark/light cycles in the lab leads to a different carbohydrate metabolism than the naturally occurring day/night regime. The other difficulty could be the uncontrolled temperature of the pilot-reactor and the difference in the pH-control system. While PHB being the intracellular carbon reserve material can remobilize and be utilized as an energy source when there is no extracellular carbon source available. However, the main inspection could be the fact that the media has been optimized for the process parameters used in the lab and not the pilot.
This phenomenon indicates the complexity of the scale-up operation and hence presents the need for reassessment of all achievable cultivation parameters once moving from the lab to pilot studies. One of the most critical parameters being the media and the quality of water used for large-scale production. Further, the cost estimation for the media used for microalgae biomass production in a 100 tones process is about 3000 $ tonnes−1 [Citation11]. This implies that making an economically relevant process for the production of cyanobacterial commodity products such as PHB is only possible when alternative cheap sources such as agro-industrial wastewaters with high nitrogen and phosphorus content are applied. It has been shown that a 70–110 tones/(ha annum) microalgae facility using wastewater can result in a saving of 48,400–74,800 $/(ha annum) for nitrogen removal and 4,575–7,625 $/(ha annum) for phosphorus removal [Citation11]. Media plays as an important factor in cyanobacterial production systems and the productivities which are reported in the lab using the sterile and defined media may significantly differ from the values obtained using alternative resources.
Environmental stress
Controlled PBR systems can prevent fluctuations and variations in the culture. Nevertheless, the difficulty to operate and scale-up and the high production cost of the PBR systems is the main limitation associated with their commercial employment. On the other hand, the use of open raceway ponds due to changes in environmental conditions mainly pH, temperature shifts and contaminants may result in sudden culture failure.
The pH-static control in large-scale processes is mainly done via the CO2 sparging, during the photosynthesis the OH¯ ions accumulate in the system increase the pH leading to overshoot in CO2 concentration in the culture and during the respiration, the pH drops leading to little or no CO2 in the system, mainly happening at night when no CO2 is needed. Some microalgae become susceptible to invasion by other competing species when growing under slight pH stress [Citation21]. It is noteworthy that the sub-optimal environmental conditions may not only reduce the overall biomass or PHB productivity in cyanobacteria but also the potential instability which may lead to contamination and culture collapse. Essentially, the production of PHB in most cyanobacteria occur under nutrient limitation leading to culture instability and therefore the risk of contamination is increased during the PHB production phase. The sterilization of large surface PBRs is a difficult and expensive task and cultivation under unsterile conditions can be challenging for most lab strains. For some extremophile species, the pH control, extreme salinity or high temperature can be used to maintain the cultures clean during mass cultivations.
Large-scale cyanobacterial processes will surely contribute to the development of a sustainable industry for biomass and PHB production. Many wild-types and genetically modified species of cyanobacteria show potential for large-scale production, however, there is a lack of information on commercial trials. In laboratory-scale, the cyanobacterial performance is mostly evaluated in terms of productivity, whereas issues such as contamination, material degradation, and the sustainability and the reliability of the production process, which can determine the success or failure of the large-scale activity more than the yields, are slighted [Citation11]. Also, when specific rates or the yields are compared, the expectations are based on the promising results obtained in the laboratory under sterile conditions which are never attained in large-scale.
Even though the importance of full optimization study in lab-scale before considering the scale-up has been emphasized [Citation22] it seems crucial to reevaluate the optimized parameters during the pilot-scale studies. Provided that the design or shape of the pilot reactor varies from the lab-scale reactor or the water quality, the temperature or pH control differ, the assessment of the parameters and the determination of productivities need to be redone.
More importantly, the research needs to focus in the future to advance the understanding and link between glycogen and PHB synthesis and the carbon partitioning and the switch between glycogen and PHB metabolism. The control of glycogen production and the shift toward PHB synthesis, which finally leads to high titres of the polymer are certainly required. The mutant strain MT_a24 accumulates glycogen of more than 50 % (DCW) [Citation7] generally from the onset of the nitrogen limitation. PHB is considered as a secondary energy and electron sink [Citation23] which accumulates mainly at much lower contents. Blocking the glycogen synthetic pathways might not be a practical option as it has already been reported that the glycogen deficient cyanobacteria are impaired with growth and survival [Citation24]. In order to achieve high PHB titres during large-scale cyanobacterial cultivations, the pathway needs to be functionally bridged leading to glycogen conversion to produce higher PHB contents. This might be achievable using genetic engineering tools or the modification of process parameters.
The current study tries to fill the gap between industry and research, the main issues regarding tech-transfer for the scale-up scenario of the cyanobacterial PHB production process are discussed here. The possible routes to overcome the bottlenecks of scale-up operation are proposed which may result in sensible preparation for a viable process. The strategies listed are: i) Cyanobacterial cultures need to be adapted to outdoor cultivation conditions (light, temperature, and pH changes) before the start of scale-up process ii) The expected outdoor environment need to be evaluated in advance iii) Experiments are required in pilot-scale to mimic the physiological response of the cells to the final large-scale outdoor process iv) The experiments need to be done and productivities have to be determined using the real media and water (waste or recycled water) used for the actual process. Like algae-based industries, PHB production from cyanobacteria requires the optimum combination of technical innovations in systems and processes, coupled with economic feasibility in the practical application and integrated scale-up for industrial production and marketing [Citation13].
Conclusions
The cyanobacterial derived bioplastics can be economically feasible and competitive in the market with other bioplastics only with a long term considerable research and development work with the starting point being the pilot-scale production. The optimized strains, synthetic media and cultivation parameters used under sterile conditions of the lab may not be applicable for large-scale outdoor production systems. The research should focus on the development of more versatile and industrially suitable cyanobacterial production systems considering the challenges of outdoor processes. Further, to obtain the productivity values from the lab in large-scale the re-optimization of media and process seem obligatory.
Highlights
Pilot-scale trials aid in the selection of the significant criteria for scale-up
Mutant MT_a24 has a potential for PHB production under unsterile outdoor conditions
Optimization studies performed in lab-scale may not apply to the pilot production
Cyanobacteria need to be adapted to the outdoor conditions prior to scale-up
During scale-up media plays an important role in obtaining high productivities
Authors’ contributions
DKA and CH have planned this study. DKA has carried out the experiments in the lab-scale and DKI performed the experiments in pilot trials with the supervision of SF. DKA has performed the analytics and written this manuscript. The study has been initiated by ML and CH and CH has supervised this project.
Availability of data and materials
The data sets supporting the conclusions of this article are included in the main article. The raw data would remain confidential and will not be shared due to a filed patent application (No. A68/2018).
Statement of informed consent, human/animal rights
No conflicts, informed consent, human or animal rights applicable
Author agreement to authorship
All authors have approved the submission of this work and warrant that it is original work not under consideration by any other journal nor has it previously been published.
Acknowledgments
The authors would like to thank their institutions for funding this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- de Jaeger L. Strain improvement of oleaginous microalgae. Vol. PhD. Netherlands: Wageningen University; 2015. p. 200.
- Melnicki MR, Eroglu E, Melis A. Changes in hydrogen production and polymer accumulation upon sulfur-deprivation in purple photosynthetic bacteria. Int J Hydrogen Energy. 2009;34(15):6157–6170.
- Knoot CJ, Ungerer JL, Wangikar PP, et al. Cyanobacteria: promising biocatalysts for sustainable chemical production. J Biol Chem. 2017. DOI:10.1074/jbc.R117.815886
- Wang B, Pugh S, Nielsen DR, et al. Engineering cyanobacteria for photosynthetic production of 3-hydroxybutyrate directly from CO2. Metab Eng. 2013;16:68–77.
- Wang B, Xiong W, Yu J, et al. Unlocking the photobiological conversion of CO2 to (R)-3-hydroxybutyrate in cyanobacteria. Green Chem. 2018;20(16):3772–3782.
- Kamravamanesh D, Kovacs T, Pflügl S, et al. Increased poly-β-hydroxybutyrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: mutant generation and characterization. Bioresour Technol. 2018;266:34–44.
- Kamravamanesh D, Slouka C, Limbeck A, et al. Increased carbohydrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: bioprocess understanding and evaluation of productivities. Bioresour Technol. 2019;273:277–287.
- Troschl C, Meixner K, Fritz I, et al. Pilot-scale production of poly-β-hydroxybutyrate with the cyanobacterium Synechocytis sp. CCALA192 in a non-sterile tubular photobioreactor. Algal Res. 2018;34:116–125.
- Millili GP 2011. Scale-up & technology transfer as a part of pharmaceutical quality systems. Pharmaceutical Quality System (ICH Q10) Conference, Arlington, Virginia. fda.gov. pp. 14–16.
- Borowitzka MA, Avigad V. Scaling up microalgae cultures to commercial scale. Eur J Phycol. 2017;52(4):407–418.
- da Silva TL, Reis A. Scale-up problems for the large scale production of algae. In: D. Das (Ed.). Algal biorefinery: an integrated approach. New Delhi (India): Capital Publishing Company; 2015, p. 125–149.
- Kamravamanesh D, Pflügl S, Nischkauer W, et al. Photosynthetic poly-β-hydroxybutyrate accumulation in unicellular cyanobacterium Synechocystis sp. PCC 6714. AMB Express. 2017;7(1):143.
- Gendy TS, El-Temtamy SA. Commercialization potential aspects of microalgae for biofuel production: an overview. Egypt J Petrol. 2013;22(1):43–51.
- Zittelli GC, Biondi N, Rodolfi L, et al. Photobioreactors for mass production of microalgae. In: A.R.a.Q. Hu (Ed.). Handbook of microalgal culture. Hoboken (NJ): John Wiley & Sons, Ltd; 2013.
- Tredici MR. Mass production of microalgae: photobioreactors. In: Richmond A, editor. Handbook of microalgal culture. Hoboken (NJ): Wiley-Blackwell; 2007, p. 178–214.
- Krzemińska I, Pawlik-Skowrońska B, Trzcińska M, et al. Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess Biosys Eng. 2014;37(4):735–741.
- Richmond A. Handbook of microalgal culture: biotechnology and applied phycology. Hoboken (NJ): Wiley Online Library; 2004.
- Nedbal L, Tichý V, Xiong F, et al. Microscopic green algae and cyanobacteria in high-frequency intermittent light. J Appl Phycol. 1996;8(4–5):325–333.
- Vonshak A, Torzillo G, Masojidek J, et al. Sub‐optimal morning temperature induces photoinhibition in dense outdoor cultures of the alga Monodus subterraneus (Eustigmatophyta). Plant Cell Environ. 2001;24(10):1113–1118.
- Qiang H. Environmental effects on cell composition. Oxford, UK: Blackwell Science Ltd.; 2004.
- Goldman JC, Riley CB, Dennett MR. The effect of pH in intensive microalgal cultures. II. Species competition. J Exp Mar Biol Ecol. 1982;57(1):15–24.
- Rawat I, Ranjith Kumar R, Mutanda T, et al. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl Energy. 2013;103:444–467.
- Stal JL. Poly (hydroxyalkanoate) in cyanobacteria: an overview. FEMS Microbiol Lett. 1992;103:169–180.
- Gründel M, Scheunemann R, Lockau W, et al. Impaired glycogen synthesis causes metabolic overflow reactions and affects stress responses in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology. 2012;158(12):3032–3043.
