Abstract
Systemic sclerosis (SSc) is a complex disease which consists of autoimmunity, fibrosis and vasculopathy. Most organ involvement in SSc patients is related to progressive fibrosis. Once fibrosis progresses, it becomes impossible to maintain a normal structure histologically. Therefore, treatment in cases with advanced fibrosis is quite difficult. On the other hand, the role of vasculopathy is clear in the pathogenesis of SSc. Lethal organ disorders of SSc, such as pulmonary arterial hypertension and scleroderma renal crisis, include severe signs of advanced vasculopathy. Nailfold videocapillaroscopy (NVC) is a safe and crucial method for evaluating microvasculopathy. The morphological changes and their progressions can be detected and scored by NVC. Indeed, the microvascular damage and dysfunction represent early markers of systemic sclerosis. Systemic sclerosis has clinical heterogeneity and the use of NVC has been validated to help with early diagnosis and even treatment. Finally, NVC may be useful in evaluating the pathogenesis of systemic sclerosis and its progression in connection with other early biomarkers and functional tools.
1. Introduction
In the treatment of rheumatoid arthritis (RA), the rise of molecular targeted therapy, including biologics, has dramatically improved the prognosis [Citation1]. In contrast, there is very little evidence in the treatment for skin sclerosis, which is the main symptom of systemic sclerosis (SSc). Therefore, SSc is one of the most refractory of autoimmune diseases. This is seen in the cause of death in each disease. For example, the main causes of death in patients with RA are infectious diseases and malignancy, which are the same as important causes of death in the general population. However, more than half of the patients diagnosed with SSc die as a direct consequence of it [Citation2]. In terms of the pathology, RA is composed of bone metabolic abnormality and accompanying autoimmunity and inflammation. Meanwhile, SSc is a complex disease in which autoimmunity, fibrosis and vasculopathy lead to a complex pattern of organ-based complications with high mortality and morbidity [Citation3]. This complicated pathology has made the development of treatment difficult.
It has been pointed out that composite tools are needed to accurately assess autoimmunity, fibrosis and vasculopathy [Citation4]. However, it is difficult to evaluate the disease activity in such a complex disease, and there are no validated methods accepted globally. The establishment of a disease activity score is ongoing [Citation5].
Furthermore, regarding vasculopathy among the three main pathologies of SSc, the evaluation of microvascular damage using nailfold videocapillaroscopy (NVC) has been regarded as important [Citation6]. In 2013, a collaboration of the American College of Rheumatology and the European League Against Rheumatism (ACR/EULAR) proposed a new set of criteria for systemic sclerosis for the first time in 30 years [Citation7,Citation8]. Further items are given a weighted score, and a score of more than 9 is diagnostic of systemic sclerosis. These classification criteria encompass a broader spectrum of systemic sclerosis, including patients whose disease is in the early stages, as well as those in the later stages, and have excellent sensitivity and specificity. Of note, nailfold capillary abnormalities were one of the new items in these criteria. With this scoring system, it has become possible to diagnose systemic sclerosis before skin thickening of the fingers is detected.
In this article, we make a commentary on the method and significance of NVC in SSc.
2. Pathogenesis of systemic sclerosis
As described above, SSc consists of autoimmunity, fibrosis and vasculopathy. The presence of autoantibodies, which suggests the involvement of autoimmunity, is one of the findings in systemic sclerosis. In fact, antinuclear antibodies are detected in the serum of most SSc patients, although the mechanism by which antinuclear antibodies are produced has not been elucidated. SSc-related autoantibodies are commonly used and have been well investigated as prognostic factors in patients with SSc [Citation9]. Since clinical application of rituximab, which is the B cell depletion therapy, has certain effects [Citation10–12], it has been suggested that autoimmunity contributes to organ involvement. Thus, autoimmunity could be considered as the treatment target.
Most organ involvement in SSc patients is related to fibrosis. Typical complications of SSc, such as skin sclerosis and interstitial lung disease, are based on the final fibrosis. As antifibrotic treatment, pirfenidone and nintedanib (both are approved for idiopathic pulmonary fibrosis) are expected to be applied as drugs for SSc, however, it is challenging to target fibrosis. Once fibrosis progresses, it becomes impossible to maintain a normal structure histologically. Therefore, the treatment in cases with progressive fibrosis is difficult.
Vasculopathy is the last factor. For instance, the prevalence of Raynaud’s phenomenon is over 90% in patients with SSc. Moreover, lethal organ disorders of SSc, such as pulmonary arterial hypertension and scleroderma renal crisis, have characteristics of vasculopathy. Furthermore, vasculopathy not only causes such organ involvement but also tissue hypoxia associated with ischemia. It contributes to promote dysfunction and fibrosis in each organ. Therefore, improvement of vasculopathy in SSc patients is one of the key treatment targets. NVC is established as a validated method to evaluate microvascular damage and is considered to be indispensable in the diagnosis and treatment of SSc [Citation13].
3. Evaluating microvascular damage by NVC
NVC represents the best and safest method to detect and analyze morphological microvascular abnormalities. Nailfold is the skin that overlaps the edge of a fingernail. The dermis is thin at this site anatomically, and the capillary can be seen directly. Capillary abnormalities of the nailfold are often seen in patients with SSc. Since dermatoscopy (30 magnifications) suffices for distinguishing between normal and abnormal nailfold capillaries, dermatoscopy has been used to detect the nailfold capillaries in Japan. Although dermatoscopy is convenient to carry out, inexpensive and simple, it is not suitable for detailed analysis of the capillary. On the other hand, NVC can allow analyzing of the shape of a capillary in detail at a high magnification of 200 or more and is superior to dermatoscopy in that it can accurately measure the length, thickness and twist, although training for evaluation is required. From this point of view, clinical research by NVC has been recognized as useful for understanding the pathogenesis. It is desirable that physicians caring for SSc patients use videocapillaroscopy to take an accurate measurement of the fineness and the number of capillaries.
Practically, NVC is performed using optical videocapillaroscopy equipped with a 200 magnification probe and connected to image analysis software such as Videocap (DS Medica, Italy), after the immersion oil is applied to the nailfold. In order to prevent vasospasm, the patient should be inside a room with a temperature of 20–22 degrees for a minimum of 15 minutes before the NVC is examined. Nailfolds of the second, third, fourth and fifth fingers are examined.
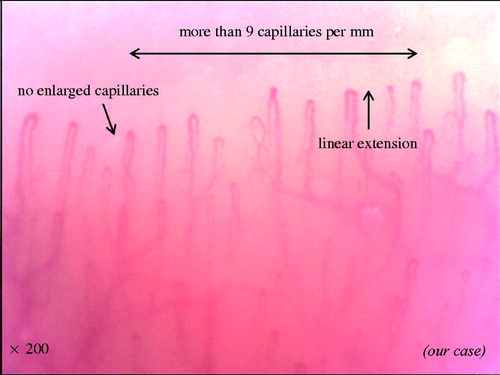
This way is a safe and non-invasive manner to capture the nailfold capillaries. In a healthy subject or in primary Raynaud’s phenomenon, there are ‘lots of thin and linear capillaries’ (). This describes the normal morphology of a capillary. First, the normal vessel’s diameter is less than 20 μm. The diameter could exceed 20 μm in some capillaries in healthy individuals. However, the detection of even a single loop with a homogeneous increase in diameter to more than 50 µm should be considered a potential marker of microangiopathy. Second, capillaries form a straight line toward the distal. Finally, the number of capillaries is more than nine within 1 mm of the row of the nailfold bed. In addition, abnormalities of these findings (the fineness, direction and the number of capillaries) are seen in patients with SSc. These sequential capillaroscopic changes, which are the so-called NVC scleroderma spectrum abnormality, are typical of microvascular involvement in SSc.
4. Diagnosis of systemic sclerosis by NVC
The significance of NVC findings in the diagnosis of SSc has been known for a long time [Citation14]. In 2013, a collaboration of the American College of Rheumatology and the European League Against Rheumatism (ACR/EULAR) proposed a new set of criteria for systemic sclerosis for the first time in 30 years [Citation7,Citation8]. One the most significant changes of these criteria was that nailfold capillary abnormalities were listed as one of the new items. The new classification criteria for systemic sclerosis encompass a broader spectrum of systemic sclerosis and focus on early diagnosis [Citation7,Citation8]. Sensitivity and specificity were 91% and 92% for the new classification criteria and 75% and 72% for the 1980 ACR classification criteria. These criteria include ‘abnormal nailfold capillaries’. The definition of ‘abnormal nailfold capillaries’ is enlarged capillaries and/or capillary loss with or without pericapillary hemorrhages at the nailfold. These findings will be described in detail later.
5. Scoring of NVC scleroderma spectrum abnormalities by NVC
In addition to the usefulness in diagnosis, pattern classification of nailfold capillary changes in SSc has a strong correlation with organ involvement [Citation15]. One problem is that the way to evaluate the NVC scleroderma spectrum abnormalities has not been integrated yet. Standardization regarding the findings of NVC has been proposed by the EULAR study group on microcirculation in Rheumatic diseases [Citation16,Citation17]. In general, qualitative interpretation of capillaroscopy can be applied (see scleroderma patterns by M Cutolo et al. below) and quantitative interpretation is also being applied. In this way standardly number of capillaries (density), measurement of apical diameter (dimension), presence or absence of hemorrhages and number of abnormal shapes is being evaluated. This standard approach is applicable to all rheumatic diseases [Citation18].
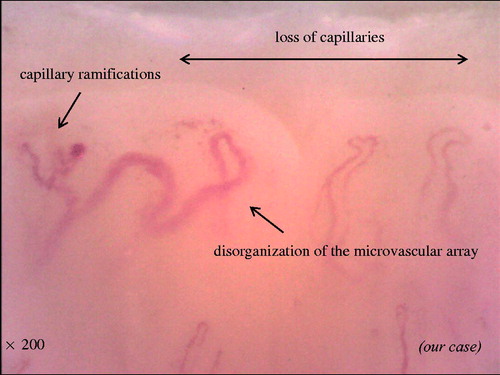
Previous literature had described in SSc the following six capillaroscopic parameters [Citation19]: (a) irregularly enlarged capillary (): an increase in capillary diameter (homogeneous or irregular) > 20μm; (b) giant capillary (): homogeneously enlarged loops with a diameter >50μm; (c) micro hemorrhage (): dark mass due to hemosiderin deposit; (d) loss of capillaries (): reduction of the capillary number below normal range (the normal range was adopted from the literature: average of nine capillaries per linear millimeter, counted at distal row of the nailfold); (e) disorganization of the microvascular array (): irregular capillary distribution and orientation, together with shape heterogeneity of the loops and (f) capillary abnormal shapes, i.e., ramifications (): branching, bushy or coiled capillaries, often originating from a single normal sized capillary. These parameters, excepting irregularly enlarged capillaries, characterize overt systemic sclerosis.
Figure 2. Representative photograph of irregularly enlarged capillary, giant capillary, and microhemorrhage.
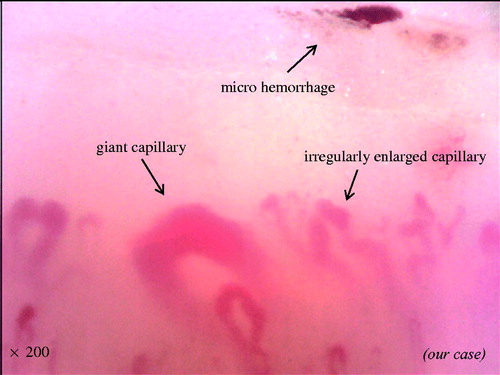
Figure 3. Representative photograph of disorganization of loss of capillaries, microvascular array, and capillary ramifications.
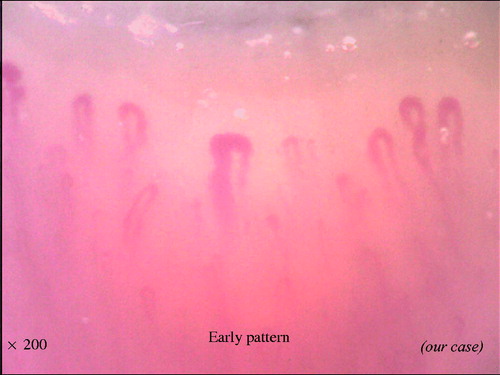
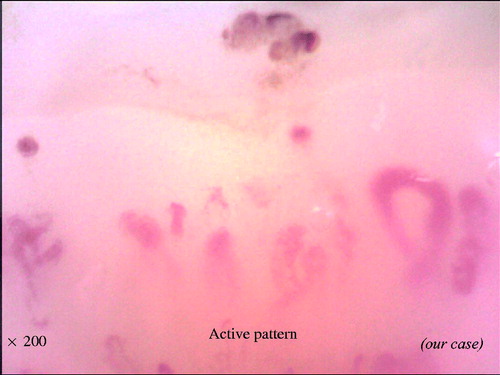
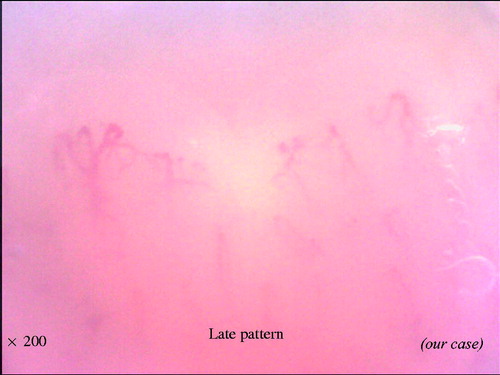
With the combination of these findings, the NVC can be categorized into three different qualitative patterns: ‘early’, ‘active’ and ‘late’, which are clearly distinct from the ‘normal’ pattern (). The early pattern is characterized by few giant capillaries, few capillary microhemorrhages and no evident loss of capillaries (). The active pattern comprises frequent giant capillaries, frequent capillary microhemorrhages and moderate loss of capillaries (). The late pattern is characterized by irregular enlargement of capillaries, almost absent giant capillaries and microhemorrhages, severe loss of capillaries with extensive avascular areas, ramified capillaries and intense disorganization of the normal capillary array (). Natural progression of microvascular damage starts from the early pattern and proceeds to the late pattern [Citation20]. These concepts are similar to the Steinbrocker radiographic stage score in RA (). The presence of anti-Scl70 antibodies seems to be related to the earlier expression of the active and late patterns. On the other hand, the anti-centromere antibody seems to be related to the delayed expression of the late NVC pattern [Citation21].
Figure 7. Comparison between NVC scleroderma spectrum abnormality and Steinbrocker radiographic stage score.
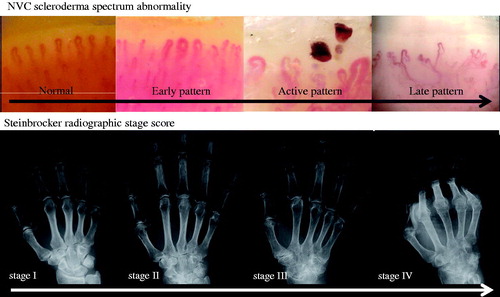
Table 1. Definition of each pattern of NVC scleroderma spectrum abnormalities.
6. Correlation between patterns of NVC scleroderma spectrum abnormalities and organ involvement
It is well known that the patterns of NVC scleroderma spectrum abnormalities are closely correlated to organ involvement in patients with SSc [Citation22]. Interstitial lung disease and pulmonary artery hypertension [Citation23] and skin ulcers [Citation24] are common in cases with NVC scleroderma spectrum abnormalities. In particular, the loss of capillaries with extensive avascular areas is an important finding, and there are many reports that a late NVC pattern suggests the presence of organ involvement. Among them, the largest retrospective multicenter (110 facilities) collaborative study by EULAR scleroderma trials and research (EUSTAR) database showed a correlation between organ involvement and vasculopathy [Citation25]. In this study, the researchers investigated the correlation between the pattern of NVC scleroderma spectrum abnormalities and clinical findings. In 494 early pattern cases, 778 active pattern cases and 598 late pattern cases, complications of organ involvement, such as skin ulcer, pulmonary artery hypertension, interstitial lung disease and joint contractures increased with the progression of NVC scleroderma spectrum abnormalities. This can be thought of as indirect evidence that organ involvement of SSc occurs, based on vasculopathy.
The prognostic value of NVC scleroderma spectrum abnormalities in predicting SSc disease progression has been also reported. For example, the strength of the association between NVC scleroderma spectrum abnormalities and future digital ulcer has been indicated [Citation26]. It has been reported that NVC abnormalities predict skin ulcers [Citation27]. However, most of the other studies contain retrospective bias that cannot be ignored, and it is difficult to say whether NVC abnormality can predict future organ involvement. One prospective study has indicated the possibility of prognostic prediction by NVC findings [Citation28], and prospective trials in a multicenter collaboration are desired in the future.
7. Conclusion
Here, we described the practical evaluation methods of microvascular damage and the significance of NVC scleroderma spectrum abnormality in patients with SSc. For the development of disease-specific treatment, it is necessary to overcome the heterogeneity of SSc patients. Multilateral, comprehensive and accurate evaluation, including vasculopathy, autoimmunity and fibrosis, can lead to elucidate the pathogenesis of SSc.
Disclosure statement
S. Kubo has received speaking fees from Bristol-Myers, Pfizer, Takeda, and Eli Lilly. V. Smith has received research grants from Actelion Pharmaceuticals, Boehringer Ingelheim, Bayer, and Hoffmann-La Roche. M. Cutolo has received research grants from Actelion Pharmaceuticals, Bristol-Myers, Celgene Corporation, and Boehringer Ingelheim. Y. Tanaka, has received speaking fees and/or honoraria from Daiichi-Sankyo, Astellas, Eli Lilly, Chugai, Sanofi, Abbvie, YL Biologics, Bristol-Myers, Glaxo-Smithkline, UCB, Mitsubishi-Tanabe, Novartis, Eisai, Takeda, Janssen, Asahi-kasei and has received research grants from Mitsubishi-Tanabe, Bristol-Myers, Eisai, Chugai, Takeda, Abbvie, Astellas, Daiichi-Sankyo, Ono, MSD, Taisho-Toyama.
Additional information
Funding
References
- Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75:3–15.
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–1815.
- Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–1699.
- Denton CP, Ong VH. Targeted therapies for systemic sclerosis. Nat Rev Rheumatol. 2013;9:451–464.
- Valentini G, Iudici M, Walker UA, et al. The European scleroderma trials and research group (EUSTAR) task force for the development of revised activity criteria for systemic sclerosis: derivation and validation of a preliminarily revised EUSTAR activity index. Ann Rheum Dis. 2017;76:270–276.
- Burmester GR, Bijlsma JWJ, Cutolo M, et al. Managing rheumatic and musculoskeletal diseases – past, present and future. Nat Rev Rheumatol. 2017;13:443–448.
- van den Hoogen F, Khanna D, Fransen J, et al. Classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–2747.
- van den Hoogen F, Khanna D, Fransen J, et al. Classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72:1747–1755.
- Kuwana M, Kaburaki J, Okano Y, et al. Clinical and prognostic associations based on serum antinuclear antibodies in Japanese patients with systemic sclerosis. Arthritis Rheum. 1994;37:75–83.
- Jordan S, Distler JH, Maurer B, et al. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis. 2015;74:1188–1194.
- Smith V, Van Praet JT, Vandooren B, et al. Rituximab in diffuse cutaneous systemic sclerosis: an open-label clinical and histopathological study. Ann Rheum Dis. 2010;69:193–197.
- Smith V, Piette Y, van Praet JT, et al. Two-year results of an open pilot study of a 2-treatment course with rituximab in patients with early systemic sclerosis with diffuse skin involvement. J Rheumatol. 2013;40:52–57.
- Cutolo M, Sulli A, Smith V. Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat Rev Rheumatol. 2010;6:578–587.
- Maricq HR, LeRoy EC, D'Angelo WA, et al. Diagnostic potential of in vivo capillary microscopy in scleroderma and related disorders. Arthritis Rheum. 1980;23:183–189.
- Cutolo M, Smith V. State of the art on nailfold capillaroscopy: a reliable diagnostic tool and putative biomarker in rheumatology?. Rheumatology (Oxford). 2013;52:1933–1940.
- Cutolo M, Melsens K, Herrick AL, et al. Reliability of simple capillaroscopic definitions in describing capillary morphology in rheumatic diseases. Rheumatology (Oxford). 2018;157:757–759.
- Smith V, Beeckman S, Herrick AL, et al. An EULAR study group pilot study on reliability of simple capillaroscopic definitions to describe capillary morphology in rheumatic diseases. Rheumatology (Oxford). 2016;55:883–890.
- Cutolo M, Melsens K, Wijnant S, et al. Nailfold capillaroscopy in systemic lupus erythematosus: a systematic review and critical appraisal. Autoimmun Rev. 2018;17:344–352.
- Sulli A, Secchi ME, Pizzorni C, et al. Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Ann Rheum Dis. 2008;67:885–887.
- Sulli A, Pizzorni C, Smith V, et al. Timing of transition between capillaroscopic patterns in systemic sclerosis. Arthritis Rheum. 2012;64:821–825.
- Cutolo M, Pizzorni C, Tuccio M, et al. Nailfold videocapillaroscopic patterns and serum autoantibodies in systemic sclerosis. Rheumatology (Oxford). 2004;43:719–726.
- Smith V, Decuman S, Sulli A, et al. Do worsening scleroderma capillaroscopic patterns predict future severe organ involvement? A pilot study. Ann Rheum Dis. 2012;71:1636–1639.
- Markusse IM, Meijs J, de Boer B, et al. Predicting cardiopulmonary involvement in patients with systemic sclerosis: complementary value of nailfold videocapillaroscopy patterns and disease-specific autoantibodies. Rheumatology (Oxford). 2017;156:1081–1088.
- Ghizzoni C, Sebastiani M, Manfredi A, et al. Prevalence and evolution of scleroderma pattern at nailfold videocapillaroscopy in systemic sclerosis patients: clinical and prognostic implications. Microvasc Res. 2015;99:92–95.
- Ingegnoli F, Ardoino I, Boracchi P, et al. Nailfold capillaroscopy in systemic sclerosis: data from the EULAR scleroderma trials and research (EUSTAR) database. Microvasc Res. 2013;89:122–128.
- Smith V, De Keyser F, Pizzorni C, et al. Nailfold capillaroscopy for day-to-day clinical use: construction of a simple scoring modality as a clinical prognostic index for digital trophic lesions. Ann Rheum Dis. 2011;70:180–183.
- Cutolo M, Herrick AL, Distler O, et al. Nailfold videocapillaroscopic features and other clinical risk factors for digital ulcers in systemic sclerosis: a multicenter, prospective cohort study. Arthritis Rheumatol. 2016;68:2527–2539.
- Avouac J, Lepri G, Smith V, et al. Sequential nailfold videocapillaroscopy examinations have responsiveness to detect organ progression in systemic sclerosis. Semin Arthritis Rheum. 2017;47:86–94.