Abstract
Natural killer (NK) cells express various Toll-like receptors (TLRs). Little is known about the role of TLRs in direct NK cell activation. To clarify possible synergistic roles of cytokine and TLR signaling, human NK cell line KHYG-1 was stimulated with agonists for TLR1-9. IFN-γ production was not significantly induced following stimulation with single TLR agonists. Of the nine TLR agonists tested, only poly(I:C) strongly upregulated IFN-γ production by synergistic interleukin-2 (IL-2) and IL-12 stimulation. The role of TLR3 signaling was also examined. An inhibitor of Toll-IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) blocked this synergistic action. However, TLR3 expression was unchanged in the presence of IL-2 and IL-12. Our findings suggest a possible role for type 1 cytokines in NK cell IFN-γ production against viral infections.
1. Introduction
Natural killer (NK) cells are primitive immune cells, which trigger first-line defense against viral infection and neoplasms. In mucosal tissues, such as the intestinal and genital surfaces, localized NK cells often encounter pathogenic microorganisms including viruses, bacteriae and fungi. Generally accepted idea is these pathogens activate antigen-presenting cells (APCs) during innate immune responses. Following pathogen exposure, APCs recognize pathogen-associated molecular patterns (PAMPs) through Toll-like receptors (TLRs), enabling them to secrete cytokines, such as Interleukin (IL)-12 [Citation1].
Recently, NK cells have been demonstrated to express TLRs and to directly recognize PAMPs [Citation2–5]. Although both human and murine NK cells have been reported to express the majority of TLRs, whether direct, ligand-mediated TLR activation occurs in NK cells remains controversial [Citation6–8]. Of interest, the requirement of IL-12 for IFN-γ production by TLR stimulation has been reported [Citation5,Citation9]. We and others have reported indispensable roles for IL-2 and IL-12 in the stimulation of lymphokine-activated killer (LAK) cells against choriocarcinoma and autologous tumor cells [Citation10–12]. Therefore, we asked whether the presence of IL-2 and IL-12 would affect TLR3 expression and subsequent TLR3-mediated NK cell activation. In addition, the intracellular signaling of direct NK cell activation via TLR3 has not been revealed yet. Toll-IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) is an adaptor mediating TLR3 signaling in innate immune cells. Thus, in order to investigate the involvement of TRIF, we used a TRIF inhibitor to block the TRIF pathway.
In the present study, we demonstrated that IL-2 and IL-12 enhanced poly(I:C)-induced IFN-γ production in the human mucosal NK cell line KHYG-1 without enhanced TLR3 expression.
2. Materials and methods
2.1. Natural killer cell line
The NK cell line KHYG-1 [Citation13] was purchased from The Human Science Research Resources Bank (JCRB0156; Tokyo, Japan) and cultured in RPMI 1640 medium (Gibco, Carlsbad, CA) supplemented with 10% (v/v) FBS, 100 U/mL penicillin, 100 U/mL streptomycin, and maintenance dose (50 U/mL) of recombinant human IL-2 (BD Biosciences, Franklin Lakes, NJ). The cells were cultured at 37 °C and 5% CO2.
2.2. Flow cytometry
Fluorochrome-conjugated mouse monoclonal antibody (mAbs) against human CD69 was purchased from BD Biosciences (Franklin Lakes, NJ). The mouse mAb against human TLR3 was purchased from IMGENEX (San Diego, CA). Direct staining was performed by adding 20 µL of the mAbs to each sample and incubating the cells at 4 °C for 30 min. KHYG-1 cells were fixed and permeabilized using BD Cytofix/Cytoperm Kit (BD Biosciences) according to the manufacturer’s instructions. Expression of TLR3 in KHHG-1 was determined by adding 5 µL of the anti-TLR3 antibody conjugated with PE at 4 °C for 30 min. Flow cytometry was performed using CellQuest software on a BD FACSCalibur (BD Biosciences) instrument.
2.3. TLR agonists treatment
The KHYG-1 cells were incubated with or without TLR1-9 agonists (Human TLR1-9 agonist Kit, InvivoGen, San Diego, CA) in the presence or absence of IL-2 (105 U/mL) and IL-12 (Recombinant Human IL-12 (p70), BD Biosciences) (104 U/mL) for 24 h. The optimal concentrations suggested by the manufacturer were utilized throughout the study. Before these experiments, we examined the baseline mRNA expression of TLR1-9 in unstimulated KHYG-1 cells by RT-PCR as described previously (data not shown).
2.4. ELISPOT IFN-γ detection
The number of IFN-γ producing cells was examined using an ELISpot for Human Interferon-γ Kit (MABTECH AB, Nacka Strand, Sweden); all protocols were performed according to the manufacturer’s instructions. Briefly, the KHYG-1 cells that had been stimulated with the TLR1-9 agonists for 24 h with or without IL-2 and IL-12 were seeded at 1 × 105 cells/well and incubated for 5 h on membranes coated with mouse anti-human IFN-γ capture mAb. After washing, the plates were incubated with biotinylated anti-human IFN-γ detection mAb and treated with streptavidin-ALP and BCIP/NBT substrate (Bio-Rad, Richmond, CA). The plates were then washed to stop the color development. The colored spots were counted using the ImmunoScan system (Cellular Technology Ltd., Shaker Heights, OH).
2.5. TRIF inhibitor administration
To examine the indispensable role of TLR3 on IL-2 + IL-12-induced IFN-γ production, KHYG-1 cells were treated with a TRIF inhibitor. KHYG-1 cells were seeded into 24-well plates (5 × 105 cells/well) and incubated with 12.5 µg/mL poly(I:C) and 50 µM of the TRIF inhibitor peptide (Pepinh-TRIF, InvivoGen, San Diego, CA) for 24 h under presence or absence of IL-2 and IL-12. The optimal concentration of Pepinh-TRIF was suggested by the manufacturer and we confirmed Pepinh-TRIF had no cytotoxic effect on KHYG-1 cells by preliminary experiment (data not shown). The culture supernatants were collected and centrifuged at 3000g at 4 °C for 5 min to remove cellular debris; samples were stored at −80 °C until use.
2.6. IFN-γ ELISA
The IFN-γ levels in the culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA) using the Human IFN-γ ELISA Kit (Thermo, Rockford, IL), which was utilized according to the manufacturer’s instructions. Briefly, 50 µL of the biotinylated antibody reagent and 50 µL of the IFN-γ standards or culture supernatant samples were added to 96-well plates coated with the anti-IFN-γ antibody and incubated for 2 h at room temperature. After the incubation, the plates were washed and incubated with 100 µL of streptavidin-HRP for 30 min at room temperature. After washing, the plates were incubated with the TMB substrate for 30 min. Reactions were stopped through the addition of 100 µL of stop solution. Sample absorbance was read at 450 nm using a microplate reader.
2.7. Statistical analysis
The data were analyzed using the Tukey-Kramer test and Welch’s t-test using Statcel 2 software (OMS Publishing Inc, Tokorozawa, Saitama, Japan). A probability level of 5% (p < .05) was considered to be statistically significant.
3. Results
3.1. TLR agonist stimulation is insufficient to induce KHYG-1 cell IFN-γ production
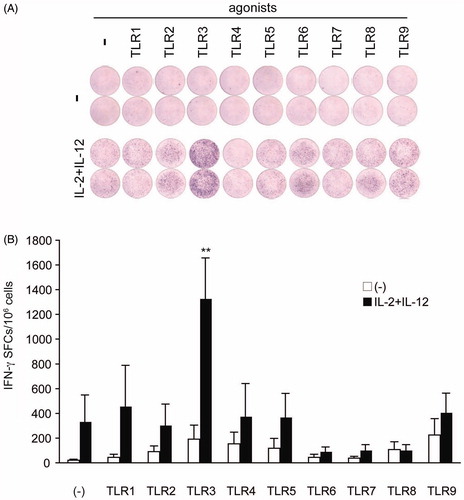
We treated KHYG-1 cells with TLR1-9 agonists for 24 h under presence or absence of IL-2 and IL-12 and examined IFN-γ production using a highly sensitive ELISPOT assay. IFN-γ production was not significantly induced following TLR agonist stimulation without IL-2 or IL-12 for 24 h (). Under the combination of IL-2 and IL-12, of the nine TLR agonists, only poly(I:C) increased IFN-γ production (). This finding suggests that IL-2 and IL-12 are essential for TLR3-mediated IFN-γ production in KHYG-1 cells.
Figure 1. IFN-γ-producing KHYG is increased by poly (I:C) stimulation with IL-2 and IL-12. The KHYG-1 cells were stimulated with the TLR1-9 agonists for 24 h with or without IL-2 (105 U/mL) and IL-12 (104 U/mL). (A) The purple dots in each well indicate the IFN-γ-producing cells. Although almost no response was observed to the TLR agonists alone, a strong induction was observed in the presence of IL-2 and IL-12. Representative ELISPOT patterns are shown. (B) The number of IFN-γ-producing cells out of 1 × 106 cells are plotted as open (TLR agonist alone) or closed (in the presence of IL-2 and IL-12) bars. The mean levels and standard deviations obtained from experiments performed in triplicate are shown. There were less than 200 IFN-γ-producing cells in the presence of the TLR ligands alone. A significant increase (greater than six-fold) was observed in response to poly(I:C) stimulation (p < .01). Data represent three independent experiments. Error bars represent SEM.
3.2. IL-2 and IL-12 do not affect KHYG-1 cell TLR3 expression
Recently, cytokine-induced upregulation of TLRs has been reported in various inflammatory cell types, including human neutrophils [Citation14] and monocytes [Citation15], murine monocytes [Citation16] and mast cells [Citation17]. However, whether cytokine-mediated induction of TLRs occurs in human mucosal NK cells remains unknown.
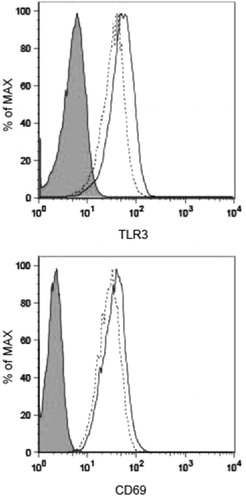
Thus, TLR3 expression levels in KHYG-1 were examined by flow cytometry. The TLR3-positive cell population did not change after 24 h of culture in the presence of IL-2 and IL-12 (MFI: 48.6 ± 14.8 vs. 40.9 ± 7.4, p = .65) (). While expression of the NK cell activation marker CD69 was slightly increased after 24 h in the presence of IL-2 and IL-12 (MFI: 22.2 ± 1.5 vs. 31.1 ± 1.5, p = .00114) ().
Figure 2. Flow cytometric analysis of KHYG-1 cells treated with IL-2 and IL-12. TLR3 and CD69 expression levels in KHYG-1 were examined by flow cytometry. The frequency and intensity of positive staining for TLR3 and the activation marker CD69 were unchanged after 24 h of culture in the presence (solid line) or absence (dashed line) of IL-2 and IL-12. Filled histogram: isotype control mAb. Results are representative of six independent experiments.
3.3. Synergistic upregulation of IFN-γ production by IL-2/IL-12 and poly(I:C) is mediated through the TLR3/TRIF pathway
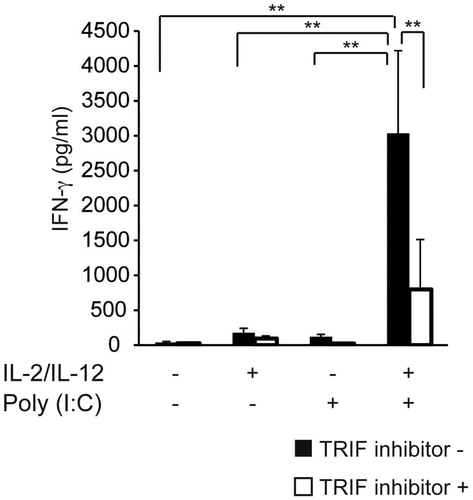
On the basis of the above results, we hypothesized that the synergistic upregulation of IFN-γ by IL-2/IL-12 and poly(I:C) in KHYG-1 cells is mediated by enhanced signaling through the TLR3/TRIF cascade. To test this hypothesis, we treated KHYG-1 cells with TRIF inhibitory peptides. As shown in , the TRIF inhibitory peptide Pepinh-TRIF significantly suppressed the effects of IL-2 and IL-12 on poly(I:C)-induced IFN-γ production.
Figure 3. Synergistic upregulation of IFN-γ production by IL-2/IL-12 and poly(I:C) is mediated through the TLR3/TRIF pathway. KHYG-1 cells were seeded into 24-well plates (5 × 105 cells/well) and incubated with 12.5 μg/mL poly(I:C) and 50 μM of the TRIF inhibitor peptide for 24 h under presence or absence of IL-2 and IL-12. The IFN-γ levels in the culture supernatants were measured by ELISA. Treatment with IL-2 and IL-12 sensitizes KHYG-1 cells to poly(I:C) stimulation, resulting in TLR3-mediated IFN-γ production. This effect was completely reversed by the inclusion of TRIF inhibitor peptides. Data represent three independent experiments. Error bars represent SEM.
4. Discussion
Natural killer cells are an important part of the innate immune system to kill tumors and virally infected cells without MHC restriction or previous antigen exposure [Citation18]. NK cells also produce cytokines that contribute to early host defense responses against pathogens [Citation19]. Mucosal NK cells, including uterine NK (uNK) cells, frequently contact pathogens and virus-infected cells. We have recently reported that IL-2 is important for lipopolysaccharide-induced IFN-γ production by human decidual mononuclear cells [Citation20]. However, because of the difficulty of maintaining freshly prepared decidual NK cells, a detailed analysis has not been possible. Thus, we employed the large granular human NK cell line KHYG-1 in this study as a model for mucosal NK cells. The KHYG-1 cell line was established from a patient with NK cell leukemia [Citation13], and it has been employed as a human NK cell model in multiple studies [Citation21–25].
Toll-like receptors are an evolutionarily conserved family of transmembrane proteins that play a primary role in innate immunity. In mammals, TLRs are expressed on mucosal innate immune cells that recognize PAMPs. TLR binding of PAMPs triggers the innate immune response through nuclear factor-κB (NF-κB)-dependent and interferon regulatory factor (IRF)-dependent signaling pathways [Citation1]. TLRs play physiological roles in dermal and mucosal immune cells, which cope with multiple types of pathogens, including viruses.
In the present study, we asked whether NK cells use TLR3 to recognize PAMPs and to regulate cell function. We confirmed TLR3 expression in the NK cell line KHYG-1, implying that they are poised to rapid response to local viral exposure.
Of interest, though the TLR3 agonist poly(I:C) alone resulted in weak IFN-γ production, simultaneous stimulation with IL-2 and IL-12 strongly induced IFN-γ production. Importantly IL-2 and IL-12 treatment induced slightly upregulation of CD69 expression, TLR3 expression was unchanged. These findings indicate enhanced IFN-γ production does not simply reflect NK cell activation. Our results suggest that the cytokine signals IL-2 and IL-12 may be essential for the regulation of direct activation of NK cells by TLR3, which increases the initial potency of these cells at the site of infection and promotes the rapid development of subsequent adaptive immune responses. Our hypothesis was confirmed through experiments with TRIF inhibitory peptides. These results are partially consistent with our previous study employing primary human decidual mononuclear cell cultures that failed to detect IL-12-induced TLR4 upregulation [Citation20]. However, decidual mononuclear cells contain various cell types including dendritic cells, macrophages and neutrophils. This fact possibly explains the discrepancy between the results obtained in this study and our previous work.
In conclusion, our results demonstrate that a NK cell line KHYG-1 constitutively express intracellular TLR3. IL-2 and IL-12 enhance TLR3 signaling in KHYG-1 cells to produce IFN-γ. These findings suggest possible roles of type 1 cytokines for NK cells during mucosal inflammatory response against viral infections.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Akira S, Uematsu S, Takeuchi O, et al. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
- Lauzon NM, Mian F, MacKenzie R, et al. The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cell Immunol. 2006;241:102–112.
- Schmidt KN, Leung B, Kwong M, et al. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172:138–143.
- Chalifour A, Jeannin P, Gauchat J-F, et al. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104:1778–1783.
- Duluc D, Tan F, Scotet M, et al. PolyI:C plus IL-2 or IL-12 induce IFN-gamma production by human NK cells via autocrine IFN-beta. Eur J Immunol. 2009;39:2877–2884.
- Hart OM, Athie-Morales V, O'Connor GM, et al. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–1642.
- Motegi A, Kinoshita M, Sato K, et al. An in vitro Shwartzman reaction-like response is augmented age-dependently in human peripheral blood mononuclear cells. J Leukoc Biol. 2006;79:463–472.
- Sivori S, Falco M, Della Chiesa M, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004;101:10116–10121.
- Girart MV, Fuertes MB, Domaica CI, et al. Engagement of TLR3, TLR7, and NKG2D regulate IFN-gamma secretion but not NKG2D-mediated cytotoxicity by human NK cells stimulated with suboptimal doses of IL-12. J Immunol. 2007;179:3472–3479.
- DeBlaker-Hohe DF, Yamauchi A, Yu CR, et al. IL-12 synergizes with IL-2 to induce lymphokine-activated cytotoxicity and perforin and granzyme gene expression in fresh human NK cells. Cell Immunol. 1995;165:33–43.
- Ohta Y, Hayakawa S, Karasaki-Suzuki M, et al. Granulocyte colony-stimulating factor suppresses autologous tumor killing activity of the peripheral blood lymphocytes in the patients with ovarian carcinoma. Am J Reprod Immunol. 2004;52:81–87.
- Sugita K, Hayakawa S, Karasaki-Suzuki M, et al. Granulocyte colony stimulation factor (G-CSF) suppresses interleukin (IL)-12 and/or IL-2 induced interferon (IFN)-gamma production and cytotoxicity of decidual mononuclear cells. Am J Reprod Immunol. 2003;50:83–89.
- Yagita M, Huang CL, Umehara H, et al. A novel natural killer cell line (KHYG-1) from a patient with aggressive natural killer cell leukemia carrying a p53 point mutation. Leukemia. 2000;14:922–930.
- Kurt-Jones EA, Mandell L, Whitney C, et al. Role of toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood. 2002;100:1860–1868.
- O'Mahony DS, Pham U, Iyer R, et al. Differential constitutive and cytokine-modulated expression of human Toll-like receptors in primary neutrophils, monocytes, and macrophages. Int J Med Sci. 2008;5:1–8.
- An H, Xu H, Yu Y, et al. Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activation of NF-kappaB, ERK and p38 MAPK signal pathways. Immunol Lett. 2002;81:165–169.
- Yang H, Wei J, Zhang H, et al. Upregulation of Toll-like receptor (TLR) expression and release of cytokines from P815 mast cells by GM-CSF. BMC Cell Biol. 2009;10:37.
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376.
- Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–2438.
- Negishi M, Izumi Y, Aleemuzzaman S, et al. Lipopolysaccharide (LPS)-induced Interferon (IFN)-gamma production by decidual mononuclear cells (DMNC) is interleukin (IL)-2 and IL-12 dependent. Am J Reprod Immunol. 2011;65:20–27.
- Taguchi Y, Kondo T, Watanabe M, et al. Interleukin-2-induced survival of natural killer (NK) cells involving phosphatidylinositol-3 kinase-dependent reduction of ceramide through acid sphingomyelinase, sphingomyelin synthase, and glucosylceramide synthase. Blood. 2004;104:3285–3293.
- Yamasaki S, Maeda M, Ohshima K, et al. Growth and apoptosis of human natural killer cell neoplasms: role of interleukin-2/15 signaling. Leuk Res. 2004;28:1023–1031.
- Kishimoto S, Muramatsu M, Gokoh M, et al. Endogenous cannabinoid receptor ligand induces the migration of human natural killer cells. J Biochem. 2005;137:217–223.
- Choi YL, Moriuchi R, Osawa M, et al. Retroviral expression screening of oncogenes in natural killer cell leukemia. Leuk Res. 2005;29:943–949.
- Suck G, Branch DR, Aravena P, et al. Constitutively polarized granules prime KHYG-1 NK cells. Int Immunol. 2006;18:1347–1354.
