Abstract
Immune thrombocytopenic purpura (ITP) is one of the complications of systemic lupus erythematosus (SLE). Although corticosteroids are usually selected for initial therapy, some patients are corticosteroid-resistant and, therefore, require other immunosuppressants or splenectomy. However, the best treatment approach in such patients remains unknown, and there is little evidence regarding which immunosuppressive agent can provide best results. We report the case of a patient with corticosteroid-resistant SLE-associated ITP (SLE-ITP) who was successfully treated with rituximab (RTX). RTX might be a therapeutic option for corticosteroid-resistant SLE-ITP.
Introduction
Immune thrombocytopenic purpura (ITP) is a thrombocytopenia caused by autoantibodies against platelet antigens and is classified into primary and secondary forms [Citation1]. Secondary ITP may occur with systemic lupus erythematosus (SLE), viral infections, including the human immunodeficiency and hepatitis C viruses, and chronic lymphocytic leukemia. Approximately 5%–20% of SLE patients develop ITP [Citation2,Citation3]. Usually, SLE-associated ITP (SLE-ITP) is treated with corticosteroids (CSs) and has a relatively good therapeutic response; however, some patients are CS-resistant [Citation4]. In such cases, intravenous immune globulin (IVIG) and splenectomy can be utilized, similar to that in primary ITP [Citation5], but this has not been well-studied in SLE-ITP. Recently, the efficacy of rituximab (RTX) for SLE-associated cytopenias, including ITP, has been reported [Citation6]. Here we report a case of CS-resistant SLE-ITP in a patient who was successfully treated with RTX. Hospital ethics committee approved these treatments. Written informed consent was obtained from the patient for the use of immunosuppressive agents and publication of this case report.
Case presentation
A 68-year-old Japanese woman presented with a 6-month history of anemia and 1-month history of fever, thrombocytopenia, and femoral lymph node swelling. She was hospitalized for further investigation and treatment. After hospitalization, bilateral knee and left shoulder pain with swelling occurred. Plain radiographs of the affected joints revealed no evidence of bone erosion. Laboratory findings are summarized in . Lymph node and bone marrow biopsies revealed no evidence of malignancy or hemophagocytosis. Whole leg ultrasonography and MRI of the head revealed no venous or arterial thrombotic events suspected antiphospholipid syndrome. Given the concomitant presence of synovitis, lymphopenia, thrombocytopenia, positive anti-nuclear antibody (ANA), hypocomplementemia, and a positive direct Coombs test, the patient was diagnosed with SLE according to the Systemic Lupus International Collaborating Clinics criteria. SLE disease activity index (SLEDAI) score was 7 points. The patient’s clinical course is summarized in . The patient’s thrombocytopenia worsened (platelet count, 6000/μL) on day 4 after admission. Therefore, induction therapy with oral prednisolone (PSL) 40 mg/day was initiated. However, this was ineffective because the platelet count remained low on day 8 (4000/μL). At this point, intravenous methylprednisolone (mPSL) pulse therapy (1 g daily for 3 days) was administered, and the thrombocytopenia improved by day 15 (platelet count, 12,500/μL). On day 21, the platelet count began to decrease again (8000/μL) and her thrombocytopenia became severe, thus requiring platelet transfusion. We considered her SLE-ITP to be resistant to CSs monotherapy and discussed adding other immunosuppressive agents as well as performing splenectomy. Splenectomy was ruled out, given the patient’s high perioperative infection and wound-healing risks secondary to high-dose CS therapy. Because she experienced general fatigue and polyarthritis associated SLE before treatment initiation, we added hydroxychloroquine (HCQ) on day 15; however, its effect on platelet count was limited. Serum immunological testing showed high positive ANA (1:2560) and high platelet-associated immunoglobulin G (PA-IgG) (708.3 ng/plt × 107). Therefore, we assumed that B-cell activation was pathophysiologically involved in our patient. Satisfactory RTX efficacy in primary ITP and SLE-ITP has been previously reported; therefore, we administered RTX (375 mg/m2 once weekly for 4 weeks) [Citation7]. The patient’s thrombocytopenia then improved rapidly and she achieved remission. Fever, lymph node swelling, and arthritis symptoms also improved. After remission, we gradually tapered the CS dose to betamethasone 2 mg daily on day 43, and the patient was discharged on day 50. The PA-IgG improved to 78.0 ng/plt × 107on day 47. At the time of discharge, her SLEDAI score was 2 points (hypocomplementemia only). One year after discharge, her CS dose was tapered to PSL 8 mg daily, and she was taking HCQ 200 mg daily. She has remained symptom-free without relapse of SLE-ITP and has normal PA-IgG (21.2 ng/plt × 107). RTX was not continued after hospital discharge because the SLE-ITP remained in remission.
Figure 1. Clinical course of the CS-resistant SLE-ITP patient. PSL: prednisolone; mPSL: methylprednisolone; HCQ: hydroxychloroquine; RTX: rituximab; SLEDAI: systemic lupus erythematosus disease activity index; C3: Complement 3; C4: Complement 4; PA-IgG: platelet-associated IgG.
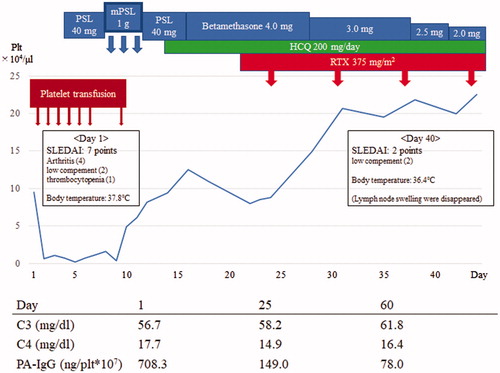
Table 1. Laboratory findings at admission.
Discussion
CSs are first-line therapy in SLE-ITP treatment; most patients respond relatively well and approximately 20% achieve remission after CS monotherapy [Citation4]. When SLE-ITP is resistant to CS monotherapy, splenectomy or IVIG are sometimes used, similar to primary ITP; however, this has not been well-studied and randomized controlled trials are lacking [Citation8]. In SLE-ITP patients with a high risk of hemorrhage, IVIG is a good therapeutic option because it results in a rapid increase in platelet count [Citation9]. However, its effectiveness may be temporary and induce difficulty of tapering CSs [Citation10]. In addition, IVIG is expensive and carries a small risk of anaphylactic and anaphylactoid reaction. On the other hand, splenectomy may induce a lupus flare or lupus nephritis, given that the spleen is responsible for clearance of circulating immune complexes [Citation11]. In addition, patients who undergo splenectomy are at risk for postsplenectomy infection. A previous study has reported a lifelong risk of severe and potentially fatal infection in patients with hereditary spherocytosis after splenectomy [Citation12]. This is a particular concern in SLE patients who may require long-term immunosuppressive therapy. Moreover, the splenectomy procedure itself may have a high risk in SLE patients receiving CSs because CSs increase the risk of perioperative infection and gastrointestinal hemorrhage and impair wound healing [Citation13–15].
HCQ has been reported to improve SLE-ITP, but it requires 8 months after introduction to exhibit its effects [Citation16]. Therefore, HCQ appears to be insufficient to treat acute thrombocytopenia in SLE but may be adequate for chronic thrombocytopenia.
Jovancevic et al. have reported that RTX is effective in treating SLE-ITP [Citation7]. RTX, a monoclonal antibody specific for human CD20, depletes B cells and is used as a therapeutic biologic agent in various autoimmune disorders. The role of B cells in inflammatory autoimmune diseases is immunoglobulin production to stimulate T cells and increase cytokine production. The clinical efficacy of RTX in inflammatory autoimmune disease is due to its reduction of memory B cells and subsequent reconstitution of the B-cell lineage [Citation17]. SLE is characterized by B-cell activation with production of pathogenic autoantibodies and deposition of immune complexes in various organs. SLE-ITP results from autoantibody-induced platelet destruction and impaired platelet production. Therefore, RTX is thought to be effective in both SLE-ITP and other SLE organ disorders. In the case presented here, laboratory testing suggested B-cell activation (elevated ANA and PA-IgG titers). Because RTX targets B cells, we hypothesized that it is effective in this patient. The RTX response time is approximately 20 days from initiation [Citation18]. Because of this relatively rapid response, RTX might be a good treatment option for patients with high hemorrhage risk due to active SLE-ITP. In addition, RTX might be able to maintain remission in refractory SLE-ITP without splenectomy; however, previous studies have reported that RTX discontinuation in maintenance phase could lead to be SLE-ITP flare [Citation19,Citation20]. In presented case, RTX administration for maintenance therapy did not perform, because platelet count did not decrease after 6 months from first RTX administration. HCQ might be useful for maintaining remission of SLE-ITP without RTX repeated administration.
The effectiveness of other immunosuppressive agents such as cyclophosphamide (CY), mycophenolate mofetil (MMF), or cyclosporine in SLE-ITP has been also reported [Citation20–22]. However, CY has considerable hematological toxicity and carries a risk of worsening thrombocytopenia. In addition, the level of evidence for using MMF and cyclosporine is low in refractory SLE-ITP, accounting only a small number of case series or single case reports.
Recently, thrombopoietin-receptor agonist agents also reported its effectiveness for steroid sparing therapy for SLE-ITP [Citation23]. Although, thrombopoietin-receptor agonist agents effects in acute phase of SLE-ITP are not established. Since, we did not use thrombopoietin-receptor agonist agents for this patients.
In conclusion, RTX might be a therapeutic option for refractory SLE-ITP patients who are resistant to CS therapy. Further studies are warranted to establish the safety and efficacy of RTX in SLE-ITP.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393.
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277.
- Ziakas PD, Poulou LS, Giannouli S, et al. Thrombocytopenia in lupus: baseline C3 as an independent risk factor for relapse. Ann Rheum Dis. 2007;66(1):130–131.
- Lurie DP, Kahaleh MB. Pulse corticosteroid therapy for refractory thrombocytopenia in systemic lupus erythematosus. J Rheumatol. 1982;9(2):311–314.
- Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–186.
- Serris A, Amoura Z, Canouï-Poitrine F, et al. Efficacy and safety of rituximab for systemic lupus erythematosus-associated immune cytopenias: a multicenter retrospective cohort study of 71 adults. Am J Hematol. 2018;93(3):424–429.
- Jovancevic B, Lindholm C, Pullerits R. Anti B-cell therapy against refractory thrombocytopenia in SLE and MCTD patients: long-term follow-up and review of the literature. Lupus. 2013;22(7):664–674.
- Alarcon-Segovia D. Splenectomy has a limited role in the management of lupus with. J Rheumatol. 2002;29(1):1–2.
- Maier WP, Gordon DS, Howard RF, et al. Intravenous immunoglobulin therapy in systemic lupus erythematosus-associated thrombocytopenia. Arthritis Rheum. 2010;33(8):1233–1239.
- Ter Borg EJ, Kallenberg CG. Treatment of severe thrombocytopenia in systemic lupus erythematosus with intravenous gammaglobulin. Ann Rheum Dis. 1992;51(10):1149–1151.
- Rivero SJ, Alger M, Alarcón-Segovia D. Splenectomy for hemocytopenia in systemic lupus erythematosus: a controlled appraisal. Arch Intern Med. 1979;139(7):773–776.
- Eber SW, Langendörfer CM, Ditzig M, et al. Frequency of very late fatal sepsis after splenectomy for hereditary spherocytosis: impact of insufficient antibody response to pneumococcal infection. Ann Hematol. 1999;78(11):524–528.
- Anstead GM. Steroids, retinoids, and wound healing. Adv Wound Care. 1998;11(6):277–285.
- Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954.
- Bollet AJ, Black R, Bunim JJ. Major undesirable side-effects resulting from prednisolone and prednisone. JAMA. 1955;158(6):459.
- Khellaf M, Chabrol A, Mahevas M, et al. Hydroxychloroquine is a good second-line treatment for adults with immune thrombocytopenia and positive antinuclear antibodies. Am J Hematol. 2014;89(2):194–198.
- Tanaka Y. Treatment of inflammatory immunologic disease 4. B cell targeting therapy using the anti-CD20 antibody rituximab in inflammatory autoimmune diseases. Intern Med. 2007;46(16):1313–1315.
- Brah S, Chiche L, Fanciullino R, et al. Efficacy of rituximab in immune thrombocytopenic purpura: a retrospective survey. Ann Hematol. 2012;91(2):279–285.
- Lindholm C, Börjesson-Asp K, Zendjanchi K, et al. Longterm clinical and immunological effects of anti-CD20 treatment in patients with refractory systemic lupus erythematosus. J Rheumatol. 2008;35(5):826–833.
- Boumpas DT, Barez S, Klippel JH, et al. Intermittent cyclophosphamide for the treatment of autoimmune thrombocytopenia in systemic lupus erythematosus. Ann Intern Med. 1990;112(9):674–677.
- Vasoo S, Thumboo J, Fong KY. Refractory immune thrombocytopenia in systemic lupus erythematosus: response to mycophenolate mofetil. Lupus. 2003;12(8):630–632.
- Quartuccio L, Sacco S, Franzolini N, et al. Efficacy of cyclosporin-A in the long-term management of thrombocytopenia associated with systemic lupus erythematosus. Lupus. 2006;15(2):76–79.
- Quartuccio L, Sacco S, Franzolini N, et al. Eltrombopag as steroid sparing therapy for immune thrombocytopenic purpura in systemic lupus erythematosus. Lupus. 2015;24:746–750.
