Abstract
Sarcoidosis is a chronic inflammatory disease of unknown etiology that affects many systemic organs, including the eye. The eye is the second most frequently affected organ in patients with sarcoidosis after lung disease. Approximately 30–50% of patients with systemic sarcoidosis develop uveitis, which is a sight-threatening intraocular inflammatory disorder. Sarcoidosis is the leading cause of uveitis in Japan and is one of the major clinical entities in many countries. Therefore, uveitis in association with sarcoidosis (ocular sarcoidosis) is considered essential in clinical practice in ophthalmology. The current review focuses on distinguishing features of ocular sarcoidosis, diagnosis, management, and discussion of the etiology of ocular sarcoidosis.
1. Introduction
Sarcoidosis is a multi-system chronic inflammatory disorder of unknown etiology characterized by non-caseating granulomas in many systemic organs, such as the lung, skin, lymph nodes, heart, liver, and eye [Citation1]. Among the organs affected in sarcoidosis, the eye is the second most frequently affected organ after the lung [Citation2]. Because the eye is a very sensitive sensory organ, any symptoms of the eye, even if they are very small, lead patients to visit ophthalmologists soon after they occur. Thus, ophthalmologists will be the first gate where patients visit hospitals, and sarcoidosis is found by ophthalmic and systemic examinations thereafter. Thus, ophthalmologists play an important role in the clinical practice of sarcoidosis.
Approximately 30–50% of patients with sarcoidosis develop the intraocular inflammatory disease, that is, uveitis [Citation2–4]. Uveitis is an inflammatory condition in the eye that primarily affects uveal tissues, that is, the iris, ciliary body, and choroid, but also the entire intraocular tissue. In general, uveitis can be classified into four types according to the major site of inflammation: anterior uveitis affecting the iris and ciliary body, intermediate uveitis affecting the vitreous body and peripheral ocular fundus, posterior uveitis affecting the retina and choroid, and panuveitis affecting the entire intraocular tissues, as described by the Standardization of Uveitis Nomenclature working group [Citation5] (). Sarcoidosis can cause any type of uveitis; anterior uveitis, intermediate uveitis, posterior uveitis, or panuveitis. In addition, uveitis can be classified according to the etiology of the disease, that is, infectious uveitis (16% of whole uveitis), noninfectious uveitis (47%), and unclassified uveitis (37%) [Citation6]. Infectious uveitis includes intraocular inflammation caused by pathogenic infectious agents, including viruses, bacteria, fungi, and parasites. Noninfectious uveitis includes sarcoidosis, Vogt-Koyanagi-Harada disease, Behcet’s disease, sympathetic ophthalmia, inflammatory bowel disease-associated uveitis, juvenile idiopathic arthritis-associated uveitis, tubulointerstitial nephritis and uveitis syndrome associated uveitis, and HLA-B27 associated anterior uveitis. The precise pathogenic mechanisms of noninfectious uveitis are not fully understood, but immunological and autoimmune mechanisms are considered to play a significant role.
Figure 1. A schematic diagram of the eyeball. (Reproduced and translated from Jpn J Clin Med 2020;78:1303–1307 with permission.)
In Japan, sarcoidosis is the leading cause of uveitis, consisting of 10–13% of uveitis followed by Vogt-Koyanagi-Harada disease, herpetic anterior uveitis, and others [Citation6–8]. Similarly, sarcoidosis is one of the major clinical entities of uveitis in many countries worldwide [Citation9,Citation10]. Thus, sarcoidosis is considered important in the clinical practice of ophthalmology. Because uveitis is a sight-threatening disease, accurate diagnosis and initiation of appropriate treatment based on the diagnosis are essential for the best visual prognosis of patients with uveitis associated with sarcoidosis, that is, ocular sarcoidosis (OS). This review focuses on distinguishing the clinical features of OS, diagnosis, management, and some discussion on the etiology.
2. Distinguishing features of OS
OS is characterized by granulomatous uveitis and the formation of granulomas (or nodules) in various intraocular tissues. In this section, typical intraocular manifestations are described.
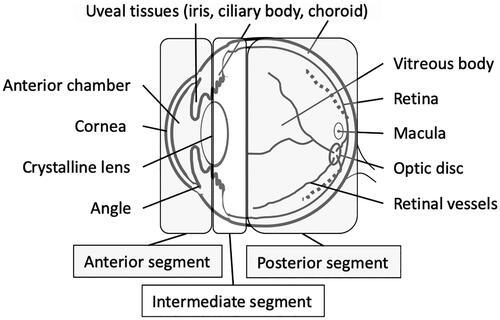
Previous studies compared various ocular manifestations between uveitis in biopsy-proven OS and control uveitis and analyzed the sensitivity and specificity of these intraocular manifestations for OS [Citation11]. Another international corroborative study also reported distinguishing features of OS [Citation12]. These include granulomatous anterior uveitis characterized by mutton-fat keratic precipitates (), nodules at the pupillary margin (Koeppe’s nodules) () or on the iris stroma (Busacca’s nodules) (), nodules at trabecular meshwork (TM) () or tent-shaped peripheral anterior synechia (); granulomatous intermediate uveitis characterized by ‘snowball vitreous opacities’ or ‘strings of pearls’ () or granulomatous posterior uveitis characterized by multiple small white active or atrophic chorioretinal nodular lesions (), nodular formation at the retinal veins but not arteries, that is, ‘segmental periphlebitis’ or ‘candle-wax drippings’ (), and optic disc granuloma and choroidal nodules.
Figure 2. Anterior segment findings of ocular sarcoidosis. (a) Mutton-fat keratic precipitates (arrows). (b) Koeppe nodules on the margin of the iris (arrows). (c) Busacca nodules on the stroma of the iris (arrows). (d) Trabecular meshwork nodule (arrows). (e) Tent-shaped peripheral anterior synechia of the iris (arrows).
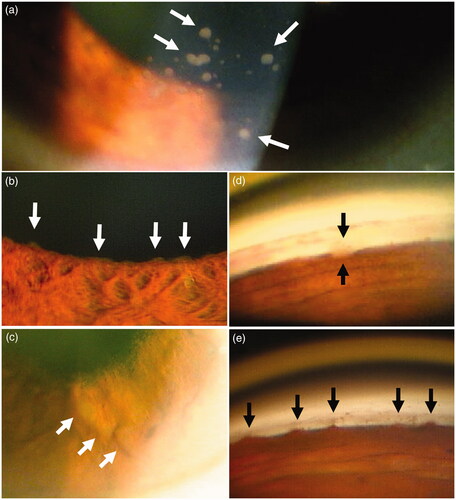
Figure 3. Posterior segment findings of ocular sarcoidosis. (a) String of pearl like vitreous opacity (arrows), and multiple chorioretinal lesions (arrowheads). (b) Fresh multiple chorioretinal lesions (arrows). (c) Nodular periphlebitis (arrows). (d) Multiple macroaneurysms surrounded by white hard exudates (arrows) and multiple chorioretinal lesions. (arrowheads).
These intraocular granulomatous findings can be seen in other uveitis entities, but the predictive values of such findings are higher in OS than in other types of granulomatous uveitis. Kawaguchi et al. reported that the sensitivity and specificity were 0.522 and 0.820 for mutton-fat KP/iris nodules, 0.806 and 0.838 for TM nodules/tent-shaped peripheral anterior synechia (PAS), 0.687 and 0.766 for snowball vitreous opacities, 0.448 and 0.883 for nodular periphlebitis, and 0.761 and 0.568 for multiple chorioretinal lesions [Citation11].
Other than granulomatous lesions, OS sometimes shows multiple macroaneurysms that may occur due to intraocular inflammation (). Bilaterality is also an important characteristic feature of OS [Citation1].
3. Diagnosis and etiological studies of OS using intraocular specimens
3.1. Etiological studies of systemic sarcoidosis
The ocular manifestations of OS are reported to be more common in Japanese patients compared with patients from other countries as shown by the high sensitivity of the ocular signs in Japanese patients [Citation12], and the genetic background of sarcoidosis patients also may differ by race and ethnicity. Although the exact disease mechanism of sarcoidosis is not known, the genetic factors of the patients that may affect the disease susceptibility, such as MHC class II [Citation13,Citation14], NOTCH4, which is involved in regulating the activity of T-cell immune responses have been reported in genome-wide association studies (GWAS) of African and European Americans [Citation13], whereas CCL24, also known as a C-C motif chemokine eotaxin-2, POR, which is cytochrome p450 oxidoreductase, and a cytokine receptor of interleukin-23, have been reported in GWAS of Japanese population [Citation14]. Meanwhile, it is believed that the allergic condition of the patients to certain pathogens plays an important role in granulomatous disease, including sarcoidosis. Various pathogens, such as mycobacteria [Citation15–17], mumps virus [Citation18], and human herpes viruses [Citation19] have been listed as candidates for sarcoidosis. While mycobacteria are regarded as an important candidate causative pathogen of sarcoidosis, especially in Western countries, Propionibacterium acnes (P. acnes), currently reclassified as Cutibacterium acnes, has been investigated as the causative agent of sarcoidosis in Japanese groups. P. acnes has been identified in the granulomas of sarcoidosis from various tissues, such as lung, lymph nodes, skin, conjunctiva, or intestine, by immunohistochemical methods [Citation20,Citation21], real-time PCR [Citation22], in situ hybridization [Citation23], or culture [Citation24].
3.2. Analysis of intraocular specimens
Some Japanese groups attempted to detect P. acnes. from the intraocular specimens. Yasuhara et al. analyzed the vitreous fluids from patients with sarcoidosis or control eyes and found DNA of Propionibacterium spp., but not mycobacterium was present in the vitreous from OS [Citation25]. Goto et al. performed the histological examination of the epiretinal membrane obtained during vitreous surgery and showed that four out of ten samples from OS showed epithelioid granuloma. Interestingly, granuloma in four samples and inflammatory cells in one sample that did not show granuloma showed positive immunohistochemistry using monoclonal antibodies against P. acnes [Citation26]. Furthermore, Nagata et al. used the same antibody to the granulomas detected in the biopsied retinas from ocular sarcoidosis [Citation27]. They found that 10 of 12 samples from ocular sarcoidosis showed positive staining against P. acnes within the granulomas but not in the control retinas obtained from other diseases. Therefore, it is possible that P. acnes may play a significant role in the pathogenesis of ocular sarcoidosis, but further investigation is necessary to reveal the detailed mechanisms of sarcoidosis.
4. Diagnosis of OS
The diagnosis of OS cannot be made based only on ocular findings. Histopathological proof of non-caseating granulomas in biopsy samples is the gold standard for diagnosis. However, a biopsy of these intraocular tissues, such as the iris, retina, or choroid, is rarely accepted by patients because of the possible risk of irreversible damage to these tissues, resulting in visual dysfunction. Therefore, the application of the results of systemic investigations is ideal. Previously, the basic diagnostic approaches were well described in the statements on sarcoidosis by the American Thoracic Society, the European Respiratory Society, and the World Association of Sarcoidosis and Other Granulomatous Disorders, [Citation28] but the detailed criteria for the clinical diagnosis of OS have not been documented. In Japan, guidelines for the diagnosis and management of sarcoidosis were established in 1976 by the Japan Society of Sarcoidosis and Other Granulomatous Disorders and revised several times to date [Citation11,Citation29–31]. The most current version of the Japanese guidelines states that OS is suspected when two of the six intraocular signs of granuloma are present (). For the clinical diagnosis of OS, two or more of the five systemic investigations should be positive (), and the clinical features of sarcoidosis must be present either in the lung or heart [Citation32].
Table 1. Ocular signs suggestive of sarcoidosis from guidelines by JSSOG [Citation32] (translated from Japanese).
Table 2. Systemic investigation characteristics of sarcoidosis by JSSOG [Citation32] (translated from Japanese).
To establish international criteria for the diagnosis of OS based on ocular findings and noninvasive systemic examinations, the 1st International Workshop on Ocular Sarcoidosis (IWOS) composed of ophthalmologists and pulmonologists from different countries was held in Tokyo in 2006. In the 1st IWOS, the international criteria for the diagnosis of OS (IWOS criteria) were proposed and were published in 2009 [Citation33]. Criteria were validated in some reports, [Citation12,Citation34,Citation35] and the criteria were revised in 2019 (revised IWOS criteria, ) [Citation36]. In the revised IWOS criteria, seven intraocular signs and eight systemic investigations, including bilateral hilar lymphadenopathy (BHL), negative tuberculin test or interferon-gamma releasing assay, elevated serum angiotensin-converting enzyme (ACE), elevated serum lysozyme, elevated CD4/CD8 ratio (>3.5) in bronchoalveolar lavage (BAL) fluid, abnormal accumulation of gallium-67 scintigraphy or 18 F-fluorodeoxyglucose PET imaging, lymphopenia, or parenchymal lung changes consistent with sarcoidosis, are listed (). By combining the intraocular signs and systemic investigations, three levels of diagnostic criteria were determined. Definite OS is a diagnosis supported by a biopsy with compatible uveitis. Presumed OS is a diagnosis that is not supported by biopsy, but BHL presents with two intraocular signs. Probable OS is a diagnosis that is not supported either by biopsy or BHL, but three intraocular signs and two systemic investigations are present.
Table 3. Revised IWOS Criteria for the Diagnosis of Ocular Sarcoidosis (2017) [Citation36].
We have validated the revised IWOS criteria in a single institute in Japan and found that the criteria showed high diagnostic values of 1.00 for sensitivity, 0.93 for specificity, 0.73 for positive predictive value, and 1.00 for negative predictive value [Citation37].
In both the revised IWOS criteria and Japanese guidelines, BHL is the most important extraocular sign for the clinical diagnosis of OS, and chest computed tomography (CT) is an important tool to detect BHL [Citation12,Citation38]. In addition to chest CT, most systemic examinations can be performed by ophthalmologists, except for bronchoalveolar lavage fluid examination. However, it is essential to consult other specialists, especially pulmonologists, to investigate the clinical signs and biopsy proof of sarcoidosis [Citation39] and to exclude other granulomatous disorders when OS is suspected.
5. Medical treatment of OS
The treatment of intraocular inflammation and its complications varies according to the site of the ocular disorder. The possible roots to deliver steroids to the ocular inflammatory site involve instillation, periocular injection, intraocular implantation, and systemic administration. Recently, recommendations for the management of OS were discussed for the first time in the 7th IWOS in 2019 and were published in 2020 [Citation40]. These recommendations describe the medical management of anterior, intermediate, and posterior uveitis in OS and the use of drugs including systemic steroids, immunosuppressive agents, and biologics. In this section, medical management of OS is highlighted based on recommendations for the management of OS by IWOS ().
Table 4. Recommendations for the management for ocular sarcoidosis by the International Workshop on Ocular Sarcoidosis (IWOS) [Citation40].
5.1. Medical treatment according to the anatomical site of uveitis
Anterior uveitis should be treated when new-onset mutton-fat KPs, Koeppe and Busacca iris nodules, new-onset posterior synechia, or TM nodules are found (). Prednisolone acetate 0.1% or similar drugs, such as betamethasone sodium phosphate 0.1% or dexamethasone sulfozenzoate 0.1%, are commonly used at least six times per day for moderate anterior uveitis and at least ten times per day for severe anterior uveitis. If these therapies are not sufficient, more frequent steroid eye drops, subconjunctival dexamethasone injection, periocular triamcinolone acetonide injection, and systemic corticosteroid are administered. Instillation of mydriatic drops is usually performed to avoid posterior synechia of the iris.
When active intermediate uveitis that may cause visual disorders, such as diffuse vitreous opacities, are present, periocular or intravitreal injections of triamcinolone acetonide, steroid implants (that is not available in Japan), or systemic corticosteroid therapy are administered, but if these therapies are insufficient, non-biologic immunosuppressive drugs are used.
For posterior uveitis, macular edema, optic disc granuloma, nodular periphlebitis, active chorioretinal lesions (), and choroidal lesions were the indicators for treatment. As the first-line therapy for such active posterior uveitis, systemic corticosteroids alone or along with non-biologic immunosuppressive drugs, periocular or intravitreal injection of triamcinolone injection, or steroid implants is recommended. If such therapies are insufficient, biological drugs are considered.
The recommended initial dose of systemic prednisolone is 0.5 − 1.0 mg/kg/day, up to 80 mg/day [Citation40], while the Japanese guideline recommends a maximum dose of 60 mg/day [Citation32]. The mean duration of the initial dose is 2–4 weeks and the mean duration of systemic steroid treatment is recommended to be 3–6 months.
5.2. Immunosuppressants and biologics
Although sarcoid lesions usually respond well to steroid therapy, it is reported that more than 70% of the disease, including uveitis, relapse when remission is induced by steroid therapy [Citation41]. If the disease is resistant to oral steroids or the inflammation recurs when oral steroids are tapered, steroid-sparing agents, including immunosuppressants or biologics, are considered as described above.
In Japan, cyclosporine and adalimumab are approved for refractory noninfectious intermediate uveitis, posterior uveitis, and panuveitis. Cyclosporine suppresses T-cell activation via inhibition of calcineurin, but the effectiveness of cyclosporine for OS has rarely been reported [Citation42]. In the recommendations by IWOS, the order of recommended steroid-sparing drugs is methotrexate, azathioprine, mycophenolate mofetil, and cyclosporine [Citation40]. Although methotrexate is an off-label use, it is widely used for ocular [Citation43,Citation44] as well as systemic sarcoidosis [Citation45], as a steroid-sparing agent in the world.
Adalimumab, a tumor necrosis factor (TNF) inhibitor, was approved for refractory noninfectious intermediate uveitis, posterior uveitis, or panuveitis several years ago; it is the only listed biologic in the recommendations by IWOS [Citation40]. The effectiveness of adalimumab for noninfectious uveitis as a steroid-sparing agent has been shown by the international prospective multicenter trials [Citation46–48], and OS was one of the major diseases in the study, although the number of patients was not large enough for subgroup analysis. The effectiveness, specifically not only for OS [Citation49,Citation50] but also for systemic refractory sarcoidosis [Citation51] has been shown in some reports, but the accumulation of more data is expected. Besides the effectiveness of TNF inhibitors for sarcoidosis, it is reported that TNF inhibitors could cause sarcoidosis or sarcoidosis-like granulomas during its treatment [Citation52]. Although it is rare, such paradoxical phenomenon by TNF inhibitors should also be noted when performing the anti-TNF treatment.
6. Complications and surgical interventions
OS may cause ocular complications that require surgical intervention. Before surgery, medical therapy should be administered to achieve complete remission of the disease. If prominent intraocular inflammation is still present or recurrence of intraocular inflammation is anticipated, but surgery is necessary, oral steroids or periocular injection of triamcinolone acetonide before and after surgery are encouraged.
Surgery for secondary cataract is often complicated by the presence of posterior synechia and fibrin membrane on the surface of the crystalline lens; however, surgery is well tolerated when intraocular inflammation is completely controlled [Citation53].
TM nodules or tent-shaped PAS may cause increased intraocular pressure (IOP), that is, secondary glaucoma, which may cause irreversible visual field defects and result in blindness. In addition to the IOP lowering medications, TM nodules require topical steroids to suppress inflammation and to reduce aqueous outflow resistance. Steroid-induced glaucoma also develops in patients treated with topical or systemic steroids. In the general population, 18–36% of patients treated with topical steroids show a moderate increase in IOP [Citation54]. If the IOP cannot be controlled and visual field defect progresses, surgical interventions, such as goniotomy, trabeculectomy, or glaucoma tube shunt surgery, is performed considering the mechanism of raised IOP [Citation42]. Steroid-induced glaucoma may be effectively treated by goniotomy, which reduces the aqueous outflow resistance in the trabecular meshwork [Citation54], but if the Schlemm canal is occluded by inflammatory cells [Citation55], trabeculectomy or tube shunt surgery may be necessary.
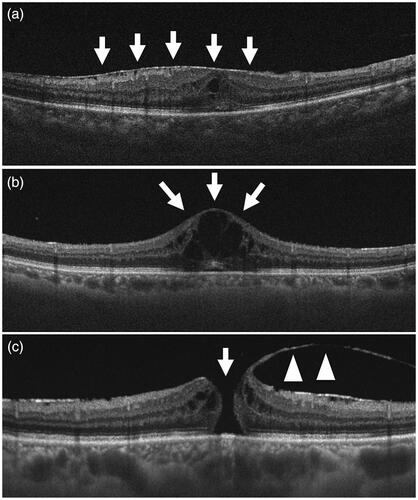
Chronic posterior uveitis may cause various complications in the posterior pole, such as the epiretinal membrane () [Citation56], cystoid macular edema (), or macular hole (), which may directly affect vision and require vitrectomy [Citation57,Citation58]. It has been reported that vitrectomy is also useful to ameliorate posterior intraocular inflammation, including cystoid macular edema [Citation59,Citation60] possibly by removing inflammatory mediators or epiretinal membrane.
Figure 4. Vertical section of the posterior fundus of ocular sarcoidosis by swept-source optical coherence tomography. (a) Epiretinal membrane (arrows) causing thickening of the central retina. (b) Cystoid macular edema (arrows) secondary to chronic retinal inflammation. (c) Macular hole (arrow) caused by the traction of the posterior hyaloid membrane (arrowheads).
Such surgical interventions, especially vitreous surgery, are an important opportunity to analyze intraocular specimens to diagnose and determine the etiology of OS. In addition to the exclusion of infectious uveitis [Citation61] or vitreoretinal lymphoma [Citation62] by cytology or polymerase chain reaction, it has been reported that analyzing T-cell population, that is, CD4/CD8 ratio, by flow cytometry has a high diagnostic value for OS [Citation63]. Analysis of causative agents of OS in the intraocular specimens may also be important as discussed in Section Diagnosis and etiological studies of OS using intraocular specimens.
7. Summary
The ocular manifestations, diagnosis, and management of ocular sarcoidosis were reviewed. It is important to understand the characteristic intraocular signs and look for them through comprehensive ocular examinations, including gonioscopy and fluorescein angiography, especially for uveitis with granulomatous signs. Suppose OS is suspected based on ocular findings. In that case, ophthalmologists should perform systemic investigations including lung chest X-ray or CT scan, serum tests for ACE, lysozyme, and soluble IL-2 receptor, if possible, and consult other specialists, especially pulmonologists. Although the opportunity is rare, intraocular samples collected during surgery should be intensively examined in terms of the presence of pathogens, including P. acnes and mycobacteria, to investigate the etiology of sarcoidosis.
Acknowledgements
The author thanks Professor Manabu Mochizuki, an emeritus professor at Tokyo Medical and Dental University, for critically reviewing this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ungprasert P, Tooley AA, Crowson CS, et al. Clinical characteristics of ocular sarcoidosis: a population-based study 1976-2013. Ocul Immunol Inflamm. 2019;27(3):389–395.
- Obenauf CD, Shaw HE, Sydnor CF, et al. Sarcoidosis and its ophthalmic manifestations. Am J Ophthalmol. 1978;86(5):648–655.
- Crick RP, Hoyle C, Smellie H. The eyes in sarcoidosis. Br J Ophthalmol. 1961;45(7):461–481.
- Jabs DA, Johns CJ. Ocular involvement in chronic sarcoidosis. Am J Ophthalmol. 1986;102(3):297–301.
- Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–516.
- Sonoda KH, Hasegawa E, Namba K, JOIS (Japanese Ocular Inflammation Society) Uveitis Survey Working Group, et al. Epidemiology of uveitis in Japan: a 2016 retrospective nationwide survey. Jpn J Ophthalmol. 2021;65(2):184–190.
- Goto H, Mochizuki M, Yamaki K, et al. Epidemiological survey of intraocular inflammation in Japan. Jpn J Ophthalmol. 2007;51(1):41–44.
- Ohguro N, Sonoda KH, Takeuchi M, et al. The 2009 prospective multi-center epidemiologic survey of uveitis in Japan. Jpn J Ophthalmol. 2012;56(5):432–435.
- Bajwa A, Osmanzada D, Osmanzada S, et al. Epidemiology of uveitis in the mid-Atlantic United States. Clin Ophthalmol. 2015;9:889–901.
- Tran VT, Auer C, Guex-Crosier Y, et al. Epidemiology of uveitis in Switzerland. Ocul Immunol Inflamm. 1994;2(3):169–176.
- Kawaguchi T, Hanada A, Horie S, et al. Evaluation of characteristic ocular signs and systemic investigations in ocular sarcoidosis patients. Jpn J Ophthalmol. 2007;51(2):121–126.
- Acharya NR, Browne EN, Rao N, International Ocular Sarcoidosis Working Group, et al. Distinguishing features of ocular sarcoidosis in an international cohort of uveitis patients. Ophthalmology. 2018;125(1):119–126.
- Adrianto I, Lin CP, Hale JJ, et al. Genome-wide association study of African and European Americans implicates multiple shared and ethnic specific loci in sarcoidosis susceptibility. PLoS One. 2012;7(8):e43907:
- Meguro A, Ishihara M, Petrek M, et al. Genetic control of CCL24, POR, and IL23R contributes to the pathogenesis of sarcoidosis. Commun Biol. 2020;3(1):465.
- Fang C, Huang H, Xu Z. Immunological evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. PLoS One. 2016;11(8):e0154716.
- Lee H, Eom M, Kim SH, et al. Identification of Mycobacterium tuberculosis and non-tuberculous mycobacteria from cutaneous sarcoidosis lesions by reverse blot hybridization assay. J Dermatol. 2019;46(10):917–921.
- Li QH, Zhang Y, Zhao MM, et al. Simultaneous amplification and testing method for Mycobacterium tuberculosis rRNA to differentiate sputum-negative tuberculosis from sarcoidosis. Am J Physiol Lung Cell Mol Physiol. 2019;316(3):L519–l524.
- Uzun L, Savranlar A, Altin R, et al. Mumps virus: a trigger for sarcoidosis? Sarcoidosis Vasc Diffuse Lung Dis. 2004;21(3):237.
- Nikoskelainen J, Hannuksela M, Palva T. Antibodies to Epstein-Barr virus and some other herpesviruses in patients with sarcoidosis, pulmonary tuberculosis and erythema nodosum. Scand J Infect Dis. 1974;6(3):209–216.
- Eishi Y. Etiologic link between sarcoidosis and Propionibacterium acnes. Respir Investig. 2013;51(2):56–68.
- Negi M, Takemura T, Guzman J, et al. Localization of propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod Pathol. 2012;25(9):1284–1297.
- Ishige I, Usui Y, Takemura T, et al. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet. 1999;354(9173):120–123.
- Yamada T, Eishi Y, Ikeda S, et al. In situ localization of Propionibacterium acnes DNA in lymph nodes from sarcoidosis patients by signal amplification with catalysed reporter deposition. J Pathol. 2002;198(4):541–547.
- Ishige I, Eishi Y, Takemura T, et al. Propionibacterium acnes is the most common bacterium commensal in peripheral lung tissue and mediastinal lymph nodes from subjects without sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22(1):33–42.
- Yasuhara T, Tada R, Nakano Y, et al. The presence of Propionibacterium spp. in the vitreous fluid of uveitis patients with sarcoidosis. Acta Ophthalmol Scand. 2005;83(3):364–369.
- Goto H, Usui Y, Umazume A, et al. Propionibacterium acnes as a possible pathogen of granuloma in patients with ocular sarcoidosis. Br J Ophthalmol. 2017;101(11):1510–1513.
- Nagata K, Eishi Y, Uchida K, et al. Immunohistochemical Detection of Propionibacterium acnes in the Retinal Granulomas in Patients with Ocular Sarcoidosis. Sci Rep. 2017;7(1):15226:
- Statement on sarcoidosisJoint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755.
- Asukata Y, Ishihara M, Hasumi Y, et al. Guidelines for the diagnosis of ocular sarcoidosis. Ocul Immunol Inflamm. 2008;16(3):77–81.
- Shijubo N, Yamaguchi T. Diagnosis criteria and classification of disease severity for sarcoidosis in Japan. JJSOGD. 2015;35(1):3–8.
- Yamamoto M. The concept, definition and diagnostic criteria of sarcoidosis (in Japanese, with English abstract. Nihon Rinsho. 1994;52(6):1426–1432. ).
- Consensus statements for the management of sarcoidosis 2020. by Japan Society of Sarcoidosis and other Granulomatous Disorders (in Japanese). https://wwwjssogcom/wp/wp-content/themes/jssog/images/system/guidance/2-4-2pdf.
- Herbort CP, Rao NA, Mochizuki M. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS). Ocul Immunol Inflamm. 2009;17:160–169.
- Takase H, Shimizu K, Yamada Y, et al. Validation of international criteria for the diagnosis of ocular sarcoidosis proposed by the first international workshop on ocular sarcoidosis. Jpn J Ophthalmol. 2010;54(6):529–536.
- Jones NP. Liver function testing is not useful in the diagnosis of sarcoidosis in patients presenting with uveitis. Ocul Immunol Inflamm. 2017;25(3):333–337.
- Mochizuki M, Smith JR, Takase H, International Workshop on Ocular Sarcoidosis Study Group, et al. Revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. Br J Ophthalmol. 2019;103(10):1418–1422.
- Handa-Miyauchi M, Takase H, Tanaka M, et al. A Validation Study of the Revised Diagnostic Criteria from the International Workshop on Ocular Sarcoidosis at a Single Institute in Japan. Ocul Immunol Inflamm. 2020. DOI:https://doi.org/10.1080/09273948.2020.1758159.
- Babu K, Shukla SB, Philips M. High resolution chest computerized tomography in the diagnosis of ocular sarcoidosis in a high TB endemic population. Ocul Immunol Inflamm. 2017;25(2):253–258.
- Ohara K, Okubo A, Kamata K, et al. Transbronchial lung biopsy in the diagnosis of suspected ocular sarcoidosis. Arch Ophthalmol. 1993;111(5):642–644.
- Takase H, Acharya NR, Babu K, et al. Recommendations for the management of ocular sarcoidosis from the International Workshop on Ocular Sarcoidosis. Br J Ophthalmol. 2020. DOI:https://doi.org/10.1136/bjophthalmol-2020-317354.
- Reich JM. Corticosteroid therapy and relapse in sarcoidosis. Chest. 1998;113(2):559–561.
- Palestine AG, Nussenblatt RB, Chan CC. Treatment of intraocular complications of sarcoidosis. Ann N Y Acad Sci. 1986;465:564–574.
- Mayer GN, Longo M, Gomes BB, et al. Low dose corticosteroid in association with methotrexate for therapy of ocular sarcoidosis: report of a case. Int J Retina Vitreous. 2015;1:7.
- Baughman RP, Lower EE, Kaufman AH. Ocular sarcoidosis. Semin Respir Crit Care Med. 2010;31(4):452–462.
- Cremers JP, Drent M, Bast A, et al. Multinational evidence-based World Association of Sarcoidosis and Other Granulomatous Disorders recommendations for the use of methotrexate in sarcoidosis: integrating systematic literature research and expert opinion of sarcoidologists worldwide. Curr Opin Pulm Med. 2013;19(5):545–561.
- Jaffe GJ, Dick AD, Brezin AP, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375(10):932–943.
- Nguyen QD, Merrill PT, Jaffe GJ, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet. 2016;388(10050):1183–1192.
- Suhler EB, Adan A, Brezin AP, et al. Safety and efficacy of adalimumab in patients with noninfectious uveitis in an ongoing open-label study: VISUAL III. Ophthalmology. 2018;125(7):1075–1087.
- Riancho-Zarrabeitia L, Calvo-Rio V, Blanco R, et al. Anti-TNF-α therapy in refractory uveitis associated with sarcoidosis: Multicenter study of 17 patients. Semin Arthritis Rheum. 2015;45(3):361–368.
- Erckens RJ, Mostard RL, Wijnen PA, et al. Adalimumab successful in sarcoidosis patients with refractory chronic non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2012;250(5):713–720.
- Drent M, Cremers JP, Jansen TL, et al. Practical eminence and experience-based recommendations for use of TNF-alpha inhibitors in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(2):91–107.
- Massara A, Cavazzini L, La Corte R, et al. Sarcoidosis appearing during anti-tumor necrosis factor alpha therapy: a new "class effect" paradoxical phenomenon. Two case reports and literature review. Semin Arthritis Rheum. 2010;39(4):313–319.
- Akova YA, Foster CS. Cataract surgery in patients with sarcoidosis-associated uveitis. Ophthalmology. 1994;101(3):473–479.
- Razeghinejad MR, Katz LJ. Steroid-induced iatrogenic glaucoma. Ophthalmic Res. 2012;47(2):66–80.
- Hamanaka T, Takei A, Takemura T, et al. Pathological study of cases with secondary open-angle glaucoma due to sarcoidosis. Am J Ophthalmol. 2002;134(1):17–26.
- Maitra P, Kumar DA, Agarwal A. Epiretinal membrane profile on spectral domain optical coherence tomography in patients with uveitis. Indian J Ophthalmol. 2019;67(3):376–381.
- Takai N, Kobayashi T, Kida T, et al. Clinical features of Japanese patients with ocular inflammation and their surgical procedures over the course of 20 years. Clin Ophthalmol. 2020;14:2799–2806.
- Kiryu J, Kita M, Tanabe T, et al. Pars plana vitrectomy for epiretinal membrane associated with sarcoidosis. Jpn J Ophthalmol. 2003;47(5):479–483.
- Kiryu J, Kita M, Tanabe T, et al. Pars plana vitrectomy for cystoid macular edema secondary to sarcoid uveitis. Ophthalmology. 2001;108(6):1140–1144.
- Takayama K, Tanaka A, Shibata M, et al. Evaluation of microincision vitrectomy surgery using wide-viewing system for complications with ocular sarcoidosis. Medicine (Baltimore). 2015;94(7):e559:
- Mochizuki M, Sugita S, Kamoi K, et al. A new era of uveitis: impact of polymerase chain reaction in intraocular inflammatory diseases. Jpn J Ophthalmol. 2017;61(1):1–20.
- Sugita S, Takase H, Sugamoto Y, et al. Diagnosis of intraocular lymphoma by polymerase chain reaction analysis and cytokine profiling of the vitreous fluid. Jpn J Ophthalmol. 2009;53(3):209–214.
- Maruyama K, Inaba T, Tamada T, et al. Vitreous lavage fluid and bronchoalveolar lavage fluid have equal diagnostic value in sarcoidosis. Medicine (Baltimore). 2016;95(49):e5531:
