Abstract
Rheumatoid arthritis (RA) is an autoimmune disease characterized by tumor-like hyperplasia and inflammation of the synovium, which causes synovial cell invasion into the bone and cartilage. In RA pathogenesis, various molecules in effector cells (i.e., immune cells and mesenchymal cells) are dysregulated by genetic and environmental factors. Consistent with the early stages of RA, these pathogenic cells cooperate and activate each other directly by cell-to-cell contact or indirectly via humoral factors. Recently, growing evidence has revealed essential role of adipokines, which are multifunctional signal transduction molecules, in the immune system. In this review, we summarize the current understanding of the cross-talk between leptin, one of the most well-known and best-characterized adipokines, and osteoimmunology. Furthermore, we discuss the contribution of leptin to the pathogenesis of RA and its potential mechanisms.
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that can impair physical function by causing persistent synovial inflammation leading to joint destruction. In RA pathogenesis, various molecules in immune cells (e.g., T cells, B cells, and monocytes) and mesenchymal cells are dysregulated through the influence of genetic predisposition and environmental factors. From the onset of early RA to the progression of destructive synovitis, these pathogenic cells cooperate and activate each other directly via cell-to-cell contact or indirectly via humoral factors (e.g., cytokines and chemokines). For instance, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β play major roles in joint inflammation, and biological agents against these molecules have been developed in the field of RA therapy [Citation1]. Adipokines are multifunctional signal transduction molecules, and their relationship with osteoimmunology has attracted attention in recent years [Citation2–11]. In this review, we discuss the role and contribution of leptin, one of the best-characterized adipokines, in the pathogenesis of RA.
2. Source and signal transduction of leptin
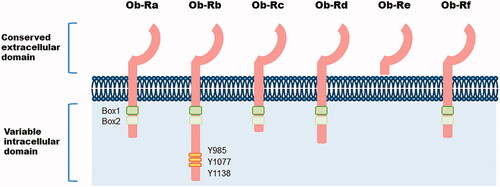
Leptin is a 16-kDa non-glycosylated cytokine-like hormone encoded by LEP and is mainly secreted by adipocytes [Citation12]; thus, its concentration often correlates with the white adipose tissue volume. However, leptin is produced not only in adipocytes, but also in other cell types (e.g., skeletal muscle cells, epithelial cells, macrophages, basophils, mast cells, and T cells) [Citation13–18] and is modulated by factors affecting energy metabolism, including pro-inflammatory cytokines (e.g., TNF-α, IL-6, and IL-1β) [Citation19]. For instance, human Foxp3+CD4+CD25+ T regulatory (Treg) cells and CD4+ effector T cells have been observed to produce leptin even in the steady-state and are enhanced under CD3/CD28 stimulation [Citation20]. Furthermore, human lung tissue taken after infectious pneumonia has shown significantly greater leptin staining in epithelial cells compared with uninjured lungs [Citation21]. Its primary function is to regulate food intake and energy consumption by inducing anorexigenic factors and suppressing orexigenic neuropeptides in the hypothalamus [Citation22]. Moreover, leptin can function in the periphery by entering the bloodstream and modifying various biological activities such as basal metabolism, insulin production, atherosclerosis, bone and cartilage metabolism, immune responses, inflammation, and reproduction [Citation23–29]. Synthesized leptin binds to the leptin receptor (Ob-R), a member of the class I cytokine receptor superfamily [Citation30]. There are at least six receptor isoforms with common extracellular domains but have cytoplasmic domains of different lengths: a soluble isoform (Ob-Re), four short isoforms (Ob-Ra, Ob-Rc, Ob-Rd, and Ob-Rf), and a long isoform (Ob-Rb) (). Ob-Rb is the only receptor capable of transmitting canonical Janus kinase (JAK) and signal transducer and activator of transcription (STAT) signaling [Citation31], while the short and soluble receptors are thought to be pivotal for leptin transport and degradation [Citation32]. Additionally, various alternative pathways are known to be involved in leptin signaling, such as the mitogen-activated protein kinase (MAPK), protein kinase C, Src homology region 2-containing protein tyrosine phosphatase 2/growth factor receptor-bound protein 2, and phosphoinositide 3-kinases (PI3K)/Akt pathways [Citation22]. These receptors are expressed in a wide range of cell types, such as immune cells, chondrocytes, synovial fibroblasts, and osteoblasts, suggesting that leptin is an important factor linking inflammation and joint destruction [Citation33,Citation34].
Figure 1. Structure of leptin receptor isoforms. Six different spliced isoforms of the leptin receptor Ob-R (a-f). Ob-R share extracellular ligand-bind domain. The intracellular domain of Ob-R varies between isoforms. Ob-Rb, the longest isoform, includes two box domains (box1 and box2) and some tyrosine residues crucial for downstream signalling.
3. Leptin in immune systems
Leptin has been shown to regulate immune responses and inflammation in various manners [Citation3,Citation5,Citation6]. Leptin has a positive effect on cell proliferation and survival, pro-inflammatory mediator secretion, and migration/chemotaxis of natural killer (NK) cells, monocytes/macrophages, dendritic cells, neutrophils, eosinophils, and basophils. Therefore, leptin appears to induce and amplify inflammation by modifying the innate immune system.
3.1. Innate immunity
3.1.1. Natural killer (NK) cells
Leptin has also been reported to affect NK cell proliferation and cytotoxicity. Short-term leptin stimulation augmented the proliferation of human NK cells in an in vitro study [Citation35], and in response to leptin administration, the number of NK cells in the peripheral circulation was markedly increased in both lean and obese rats [Citation36]. In the mouse bone marrow, leptin signaling regulates NK cell development by enhancing immature NK cell survival via downregulating Bcl-2 expression and upregulating Bax transcripts [Citation37]. Moreover, leptin has been reported to decrease IL-4 and IL-10 expression [Citation38] and increase IL-2 and IFN-γ production in human NK cells [Citation39,Citation40]. In addition, leptin increased the cytotoxic activity of human NK cells via STAT3 phosphorylation and expression of IL-2 and perforin [Citation39], promoted actin rearrangement, which is essential for cell migration [Citation41], and enhanced the expression of adhesion molecules represented by L-selectin [Citation38].
3.1.2. Monocytes/macrophages
Leptin has been reported to enhance the inflammatory phenotype of monocytes/macrophages [Citation42,Citation43]. For instance, leptin promotes the phagocytic activity of macrophages by modifying the expression levels of cAMP [Citation44] and reactive oxygen species [Citation45]. In addition, chemotaxis of macrophages was enhanced by leptin via signal transduction pathways including JAK/STAT, MAPK, and PI3K/Akt signaling [Citation46]).
3.1.3. Dendritic cells (DCs)
Leptin has been reported to promote the survival of DCs by inhibiting apoptosis [Citation47,Citation48]. In addition, leptin induces the expression of some pro-inflammatory molecules such as IL-1β, IL-6, IL-12, TNF-α, and MIP-1α in human DCs as well as stimulates the migration and chemotaxis of immature DCs [Citation47,Citation49–51].
3.1.4. Neutrophils
Leptin induces the survival of neutrophils by delaying the cleavage of pro-apoptotic factors (i.e., Bid and Bax), mitochondrial release of cytochrome C, and activation of caspases through PI3K, NF-κB, and MAPK signaling [Citation52]. Moreover, leptin mediates the migration and chemotaxis of neutrophils via p38 MAPK and Src kinases [Citation53]. Accordingly, in individuals who are obese, neutrophils show enhanced release of superoxide and chemotactic activity [Citation54].
3.1.5. Eosinophils
In eosinophils, leptin has been reported to act as a survival factor by delaying apoptosis [Citation55]. Leptin also increased the secretion of the pro-inflammatory factors IL-1β, IL-6, IL-8, and MCP-1 and modulated the expression of adhesion molecules; that is, it upregulated ICAM-1 and CD18 and downregulated ICAM-3 and L-selectin [Citation55]. In individuals who are obese, eosinophils demonstrated increased adhesive and migratory capacities towards eotaxin and CCL5 [Citation56].
3.1.6. Basophils
In basophils, leptin promotes migratory activity, induces cytokine secretion (i.e., IL-4 and IL-13), increases the expression of CD63, one of the activation markers, and augments degranulation in response to aggregation of the high-affinity receptor for IgE [Citation16].
3.2. Adaptive immunity
Studies have also focused on the effects of leptin on the adaptive immune system. Interestingly, mice deficient in leptin (ob/ob) and leptin receptors (db/db) demonstrated thymus atrophy and T cell lymphopenia, which were rescued by leptin treatment in ob/ob mice [Citation57,Citation58].
3.2.1. T cells
Leptin promotes the differentiation of double-positive CD4+CD8+ thymocytes into single-positive CD4+ T cells [Citation59] and stimulates the production of IL-2, IFN-γ, and granulocyte-macrophage colony-stimulating factor from thymocytes [Citation60]. In addition, leptin enhances the proliferation of human naïve CD4+ T cells [Citation57], while inhibiting that of Treg cells [Citation14,Citation61–63] and memory T cells [Citation64]. Interestingly, in co-culture of CD8+ T cells with leptin-treated Treg cells, leptin impaired Treg cells activity and restored CD8+ T cell proliferation [Citation65]. Moreover, leptin decreased the secretion of transforming growth factor-β and IL-10 from Treg cells. Accordingly, congenitally leptin-deficient children showed decreased numbers of circulating CD4+ T cells and impaired T cell proliferation and cytokine expression, which were rescued by administration of recombinant human leptin [Citation66]. In contrast, subjects who were obese demonstrated a reduced number of Treg cells [Citation67].
Leptin can also polarize CD4+ T cells into the Th1 phenotype through the synthesis of Th1-cytokines (i.e., IL-2 and IFN-γ) [Citation68–71]. In fact, CD4+ T cells derived from ob/ob mice showed significantly decreased expression of Th1 cell-specific T-box transcription factor [Citation72]. The expression of retinoic acid-related orphan receptor γ t, a transcription factor crucial for the differentiation of Th17 cells, was enhanced by leptin in CD4+ T cells [Citation73]. In contrast, neutralizing the leptin antibody reduced the number of Th17 cells and expression of retinoic acid-related orphan receptor γ t [Citation74]. Another study demonstrated that CD4+ T cells derived from ob/ob mice show diminished polarization of these cells into Th17 cells as a consequence of STAT3 signaling inhibition [Citation75]. Similarly, Th17 cells from fasted mice showed reduced proliferative activity that was rescued by leptin administration [Citation76].
3.2.2. B cells
In B cells, leptin suppresses apoptotic activity and induces cell proliferation by activating Bcl-2 and cyclin D [Citation77]. In addition, leptin has been shown to augment the expression of pro-inflammatory molecules (e.g., TNF-α, IL-4, IL-6, and CXCL8) and anti-inflammatory cytokines (e.g., IL-10) through the JAK/STAT, p38 MAPK, and ERK1/2 pathways [Citation78–81]. Accordingly, db/db and ob/ob mice showed reduced numbers of B cells in the circulation and bone marrow. Moreover, in the bone marrow of fasted mice, the number of pro-B, pre-B, and immature B cells decreased while that of mature B cells increased, which were all modified by leptin treatment [Citation82–84].
4. Leptin as a potential regulator of RA pathophysiology
Numerous studies have shown that the concentrations of leptin are elevated in blood samples from patients with RA compared to in those from control subjects [Citation85–93], independent of BMI [Citation90]. Furthermore, several studies reported a correlation between leptin levels and disease activity measures (i.e., DAS28 and CRP), whereas other did not show similar results [Citation94–97]. In other studies, leptin was reported to be expressed in the synovium [Citation98], and its concentration in the synovial fluid was higher in patients with RA than in patients with osteoarthritis (OA) [Citation86,Citation99]. The role of leptin in joint destruction in RA has also been examined. One study reported that patients with RA showing advanced erosion had higher serum leptin concentrations [Citation100], whereas other studies showed inconsistent results [Citation101–105]. The concentrations of leptin were lower in the synovial fluid than in paired serum from patients with non-erosive RA, but not in patients with advanced joint destruction [Citation86]. This result proposed that local consumption of leptin in the joint could protect against erosive progression.
In addition to its effects on the immune system, leptin can modulate metabolism in the bone, cartilage, and synovium [Citation106,Citation107]. Leptin may promote the influx of immune cells into the synovium by regulating the expression of adhesion molecules on endothelial cells as well as on immune cells [Citation108,Citation109]. In addition, leptin stimulates chondrocytes to produce proteases such as matrix metalloproteinases, pro-inflammatory mediators including IL-1β, TNF-α, IL-6, IL-8, and MCP-1, and adhesion molecules such as VCAM-1 [Citation110,Citation111]. Chondrocytes of OA, a representative degenerative disease that is also known to be a common comorbidity of RA, produce higher levels of leptin compared to healthy donors [Citation112] and are related to the severity of cartilage destruction [Citation113,Citation114]. Leptin also modulates osteoblast function and bone metabolism to lead to joint destruction in OA patients [Citation107]. Moreover, leptin induces the expression of some cytokines (e.g., IL-6) and chemokines (e.g., IL-8) via JAK2/STAT3, NF-κB, and AP-1 signaling in synovial fibroblasts [Citation115,Citation116].
The relationship between leptin and RA pathophysiology has been demonstrated in animal models. ob/ob mice showed less severe arthritis accompanied by reduced IL-1β and TNF-α levels [Citation117]. In addition, articular injection of leptin enhanced Th17 cells in the joints of a collagen-induced arthritis mouse model, which was associated with the severity of inflammation and development of arthritis [Citation118]. A positive correlation between leptin and IL-17 in the plasma was reported in patients with RA [Citation119].
Overall, these data indicate that leptin is a crucial modulator of joint inflammation and bone and cartilage remodeling associated with the RA pathophysiology. If the disease activity of RA remaining under the use of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) or biologic DMARDs is associated with leptin signaling, at least part of the clinical effect of JAK inhibitors on multidrug-resistant cases could be mediated by blocking leptin signaling. Furthermore, differences in JAK selectivity have also been reported among JAK inhibitors. As described above, among the JAK/STAT pathways downstream of leptin, JAK2/STAT3 is particularly important, and the difference in immunological properties between drugs with high JAK1 selectivity (e.g., upadacitinib, filgotinib) and JAK inhibitors including JAK2 (e.g., tofacitinib、baricitinib、peficitinib) is interesting. The blocking efficiency of leptin signaling by each JAK inhibitor and the resulting difference in clinical outcomes are for further study.
5. Conclusions
The risk of developing moderate-to-severe RA is reportedly higher in patients with obesity than in non-obese subjects [Citation120–122]. However, the amount of subcutaneous and visceral fat does not necessarily correlate with the amount of intra-articular fat. Further research is required to clarify the effects of systemic hyperleptinemia on the immune system, as well as provide mechanistic insights into leptin-induced inflammation and local destruction of RA joints.
Author contributions
H.T. wrote the manuscript. K.F. supervised and revised the manuscript. All authors read and approved the final manuscript.
Disclosure statement
The authors declare that they have no competing interests.
References
- Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001.
- Neumann E, Junker S, Schett G, et al. Adipokines in bone disease. Nat Rev Rheumatol. 2016;12(5):296–302.
- Abella V, Scotece M, Conde J, et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 2017;13(2):100–109.
- Procaccini C, La Rocca C, Carbone F, et al. Leptin as immune mediator: interaction between neuroendocrine and immune system. Dev Comp Immunol. 2017;66:120–129.
- Francisco V, Pino J, Campos-Cabaleiro V, et al. Obesity, fat mass and immune system: role for leptin. Front Physiol. 2018;9:640.
- Francisco V, Pino J, Gonzalez-Gay MA, et al. Adipokines and inflammation: is it a question of weight? Br J Pharmacol. 2018;175(10):1569–1579.
- MacDonald IJ, Liu SC, Huang CC, Kuo SJ, et al. Associations between adipokines in arthritic disease and implications for obesity. IJMS. 2019;20(6):1505.
- Francisco V, Ruiz-Fernández C, Pino J, et al. Adipokines: linking metabolic syndrome, the immune system, and arthritic diseases. Biochem Pharmacol. 2019;165:196–206.
- Carrión M, Frommer KW, Pérez-García S, et al. The adipokine network in rheumatic joint diseases. IJMS. 2019;20(17):4091.
- Żelechowska P, Brzezińska-Błaszczyk E, Kusowska A, et al. The role of adipokines in the modulation of lymphoid lineage cell development and activity: an overview. Obes Rev. 2020;21(10):e13055.
- Toussirot E. Mini-review: the contribution of adipokines to joint inflammation in inflammatory rheumatic diseases. Front Endocrinol (Lausanne). 2020;11:606560.
- Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432.
- Smith-Kirwin SM, O'Connor DM, De Johnston J, et al. Leptin expression in human mammary epithelial cells and breast milk. J Clin Endocrinol Metab. 1998;83(5):1810–1813.
- De Rosa V, Procaccini C, Calì G, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26(2):241–255.
- Taildeman J, Pérez-Novo CA, Rottiers I, et al. Human mast cells express leptin and leptin receptors. Histochem Cell Biol. 2009;131(6):703–711.
- Suzukawa M, Nagase H, Ogahara I, et al. Leptin enhances survival and induces migration, degranulation, and cytokine synthesis of human basophils. J Immunol. 2011;186(9):5254–5260.
- Wolsk E, Mygind H, Grøndahl TS, et al. Human skeletal muscle releases leptin in vivo. Cytokine. 2012;60(3):667–673.
- Zhou Y, Yu X, Chen H, et al. Leptin deficiency shifts mast cells toward anti-inflammatory actions and protects mice from obesity and diabetes by polarizing M2 macrophages. Cell Metab. 2015;22(6):1045–1058.
- Behnes M, Brueckmann M, Lang S, et al. Alterations of leptin in the course of inflammation and severe sepsis. BMC Infect Dis. 2012;12(1):217.
- Procaccini C, De Rosa V, Galgani M, et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33(6):929–941.
- Ubags ND, Vernooy JH, Burg E, et al. The role of leptin in the development of pulmonary neutrophilia in infection and acute lung injury. Crit Care Med. 2014;42(2):e143–e151.
- Zhou Y, Rui L. Leptin signaling and leptin resistance. Front Med. 2013;7(2):207–222.
- Tang CH, Lu DY, Yang RS, et al. Leptin-induced IL-6 production is mediated by leptin receptor, insulin receptor substrate-1, phosphatidylinositol 3-kinase, Akt, NF-kappaB, and p300 pathway in microglia. J Immunol. 2007;179(2):1292–1302.
- Gómez R, Conde J, Scotece M, et al. What's new in our understanding of the role of adipokines in rheumatic diseases? Nat Rev Rheumatol. 2011;7(9):528–536.
- Scotece M, Conde J, Gómez R, et al. Role of adipokines in atherosclerosis: interferences with cardiovascular complications in rheumatic diseases. Mediators Inflamm. 2012;2012:125458.
- Conde J, Scotece M, Abella V, et al. An update on leptin as immunomodulator. Expert Rev Clin Immunol. 2014;10(9):1165–1170.
- Scotece M, Conde J, Vuolteenaho K, et al. Adipokines as drug targets in joint and bone disease. Drug Discovery Today. 2014;19(3):241–258.
- Feijóo-Bandín S, Portolés M, Roselló-Lletí E, et al. 20 years of leptin: role of leptin in cardiomyocyte physiology and physiopathology. Life Sci. 2015;140:10–18.
- Ramos-Lobo AM, Donato J. Jr. The role of leptin in health and disease. Temperature (Austin). 2017;4(3):258–291.
- Münzberg H, Morrison CD. Structure, production and signaling of leptin. Metabolism. 2015;64(1):13–23.
- Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393(Pt 1):7–20.
- Wada N, Hirako S, Takenoya F, et al. Leptin and its receptors. J Chem Neuroanat. 2014;61–62:191–199.
- Conde J, Scotece M, López V, et al. Adiponectin and leptin induce VCAM-1 expression in human and murine chondrocytes. PloS One. 2012;7(12):e52533.
- Procaccini C, Jirillo E, Matarese G. Leptin as an immunomodulator. Mol Aspects Med. 2012;33(1):35–45.
- Wrann CD, Laue T, Hübner L, et al. Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. Am J Physiol Endocrinol Metab. 2012;302(1):E108–16.
- Nave H, Mueller G, Siegmund B, et al. Resistance of Janus kinase-2 dependent leptin signaling in natural killer (NK) cells: a novel mechanism of NK cell dysfunction in diet-induced obesity. Endocrinology. 2008;149(7):3370–3378.
- Lo CK, Lam QL, Yang M, et al. Leptin signaling protects NK cells from apoptosis during development in mouse bone marrow. Cell Mol Immunol. 2009;6(5):353–360.
- Shirshev SV, Nekrasova IV, Orlova EG, et al. Roles of leptin and ghrelin in the regulation of the phenotype and cytokine production by NK cells from peripheral blood. Dokl Biol Sci. 2016;470(1):249–252.
- Zhao Y, Sun R, You L, et al. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun. 2003;300(2):247–252.
- Lamas B, Goncalves-Mendes N, Nachat-Kappes R, et al. Leptin modulates dose-dependently the metabolic and cytolytic activities of NK-92 cells. J Cell Physiol. 2013;228(6):1202–1209.
- Oswald J, Büttner M, Jasinski-Bergner S, et al. Leptin affects filopodia and cofilin in NK-92 cells in a dose- and time-dependent manner. Eur J Histochem. 2018;62(1):2848.
- Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194(1):6–11.
- Iikuni N, Lam QL, Lu L, et al. Leptin and inflammation. Curr Immunol Rev. 2008;4(2):70–79.
- Amarilyo G, Iikuni N, Liu A, et al. Leptin enhances availability of apoptotic cell-derived self-antigen in systemic lupus erythematosus. PloS One. 2014;9(11):e112826.
- Dayakar A, Chandrasekaran S, Veronica J, et al. Leptin induces the phagocytosis and protective immune response in Leishmania donovani infected THP-1 cell line and human PBMCs. Exp Parasitol. 2016;160:54–59.
- Gruen ML, Hao M, Piston DW, et al. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am J Physiol Cell Physiol. 2007;293(5):C1481–C1488.
- Lam QL, Liu S, Cao X, et al. Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. Eur J Immunol. 2006;36(12):3118–3130.
- Mattioli B, Giordani L, Quaranta MG, et al. Leptin exerts an anti-apoptotic effect on human dendritic cells via the PI3K-Akt signaling pathway. FEBS Lett. 2009;583(7):1102–1106.
- Mattioli B, Straface E, Quaranta MG, et al. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174(11):6820–6828.
- Mattioli B, Straface E, Matarrese P, et al. Leptin as an immunological adjuvant: enhanced migratory and CD8+ T cell stimulatory capacity of human dendritic cells exposed to leptin. Faseb J. 2008;22(6):2012–2022.
- Moraes-Vieira PM, Larocca RA, Bassi EJ, Peron JP, et al. Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells. Eur J Immunol. 2014;44(3):794–806.
- Bruno A, Conus S, Schmid I, et al. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol. 2005;174(12):8090–8096.
- Montecucco F, Bianchi G, Gnerre P, et al. Induction of neutrophil chemotaxis by leptin: crucial role for p38 and Src kinases. Ann N Y Acad Sci. 2006;1069:463–471.
- Brotfain E, Hadad N, Shapira Y, et al. Neutrophil functions in morbidly obese subjects. Clin Exp Immunol. 2015;181(1):156–163.
- Wong CK, Cheung PF, Lam CW. Leptin-mediated cytokine release and migration of eosinophils: implications for immunopathophysiology of allergic inflammation. Eur J Immunol. 2007;37(8):2337–2348.
- Grotta MB, Squebola-Cola DM, Toro AA, et al. Obesity increases eosinophil activity in asthmatic children and adolescents. BMC Pulm Med. 2013;13:39.
- Lord GM, Matarese G, Howard JK, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394(6696):897–901.
- Howard JK, Lord GM, Matarese G, et al. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104(8):1051–1059.
- Kim SY, Lim JH, Choi SW, et al. Preferential effects of leptin on CD4 T cells in central and peripheral immune system are critically linked to the expression of leptin receptor. Biochem Biophys Res Commun. 2010;394(3):562–568.
- Lamas A, Lopez E, Carrio R, et al. Adipocyte and leptin accumulation in tumor-induced thymic involution. Int J Mol Med. 2016;37(1):133–138.
- Zeng H, Chi H. The interplay between regulatory T cells and metabolism in immune regulation. Oncoimmunology. 2013;2(11):e26586.
- Lourenço EV, Liu A, Matarese G, et al. Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc Natl Acad Sci U S A. 2016;113(38):10637–10642.
- Orlova EG, Shirshev SV. Role of PKA and PI3K in leptin and ghrelin regulation of adaptive subpopulations of regulatory CD4+ T-lymphocyte formation. Biochemistry (Mosc). 2017;82(9):1061–1072.
- Lord GM, Matarese G, Howard JK, et al. Leptin inhibits the anti-CD3-driven proliferation of peripheral blood T cells but enhances the production of proinflammatory cytokines. J Leukocyte Biol. 2002;72(2):330–338.
- Wei R, Hu Y, Dong F, et al. Hepatoma cell-derived leptin downregulates the immunosuppressive function of regulatory T-cells to enhance the anti-tumor activity of CD8+ T-cells. Immunol Cell Biol. 2016;94(4):388–399.
- Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110(8):1093–1103.
- Wagner NM, Brandhorst G, Czepluch F, et al. Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity (Silver Spring, MD). 2013;21(3):461–468.
- Martín-Romero C, Santos-Alvarez J, Goberna R, et al. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199(1):15–24.
- Oral EA, Javor ED, Ding L, et al. Leptin replacement therapy modulates circulating lymphocyte subsets and cytokine responsiveness in severe lipodystrophy. J Clin Endocrinol Metab. 2006;91(2):621–628.
- Saucillo DC, Gerriets VA, Sheng J, et al. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol. 2014;192(1):136–144.
- Scotece M, Pérez T, Conde J, et al. Adipokines induce pro-inflammatory factors in activated Cd4+ T cells from osteoarthritis patient. J Orthop Res. 2017;35(6):1299–1303.
- Batra A, Okur B, Glauben R, et al. Leptin: a critical regulator of CD4+ T-cell polarization in vitro and in vivo. Endocrinology. 2010;151(1):56–62.
- Yu Y, Liu Y, Shi FD, et al. Cutting edge: leptin-induced RORγt expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus. J Immunol. 2013;190(7):3054–3058.
- Wang S, Baidoo SE, Liu Y, et al. T cell-derived leptin contributes to increased frequency of T helper type 17 cells in female patients with Hashimoto's thyroiditis. Clin Exp Immunol. 2013;171(1):63–68.
- Reis BS, Lee K, Fanok MH, et al. Leptin receptor signaling in T cells is required for Th17 differentiation. J Immunol. 2015;194(11):5253–5260.
- Gerriets VA, Danzaki K, Kishton RJ, et al. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur J Immunol. 2016;46(8):1970–1983.
- Lam QL, Wang S, Ko OK, et al. Leptin signaling maintains B-cell homeostasis via induction of Bcl-2 and Cyclin D1. Proc Natl Acad Sci USA. 2010;107(31):13812–13817.
- Agrawal S, Gollapudi S, Su H, et al. Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J Clin Immunol. 2011;31(3):472–478.
- Gupta S, Agrawal S, Gollapudi S. Increased activation and cytokine secretion in B cells stimulated with leptin in aged humans. Immun Ageing. 2013;10(1):3.
- Xu T, Xie W, Ma Y, et al. Leptin/OB-R pathway promotes IL-4 secretion from B lymphocytes and induces salivary gland epithelial cell apoptosis in Sjögren's syndrome. Oncotarget. 2017;8(38):63417–63429.
- Frasca D, Diaz A, Romero M, et al. Leptin induces immunosenescence in human B cells. Cell Immunol. 2020;348:103994.
- Bennett BD, Solar GP, Yuan JQ, et al. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol. 1996;6(9):1170–1180.
- Claycombe K, King LE, Fraker PJ. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci USA. 2008;105(6):2017–2021.
- Tanaka M, Suganami T, Kim-Saijo M, et al. Role of central leptin signaling in the starvation-induced alteration of B-cell development. J Neurosci. 2011;31(23):8373–8380.
- Anders HJ, Rihl M, Heufelder A, et al. Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metabolism. 1999;48(6):745–748.
- Bokarewa M, Bokarew D, Hultgren O, et al. Leptin consumption in the inflamed joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62(10):952–956.
- Härle P, Pongratz G, Weidler C, et al. Possible role of leptin in hypoandrogenicity in patients with systemic lupus erythematosus and rheumatoid arthritis. Ann Rheum Dis. 2004;63(7):809–816.
- Toussirot E, Nguyen NU, Dumoulin G, et al. Relationship between growth hormone-IGF-I-IGFBP-3 axis and serum leptin levels with bone mass and body composition in patients with rheumatoid arthritis. Rheumatology (Oxford). 2005;44(1):120–125.
- Popa C, Netea MG, Radstake TR, et al. Markers of inflammation are negatively correlated with serum leptin in rheumatoid arthritis. Ann Rheum Dis. 2005;64(8):1195–1198.
- Otero M, Lago R, Gomez R, et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65(9):1198–1201.
- Olama SM, Senna MK, Elarman M. Synovial/serum leptin ratio in rheumatoid arthritis: the association with activity and erosion. Rheumatol Int. 2012;32(3):683–690.
- Toussirot E, Grandclément E, Gaugler B, et al. Serum adipokines and adipose tissue distribution in rheumatoid arthritis and ankylosing spondylitis. A comparative study. Front Immunol. 2013;4:453.
- Tian G, Liang JN, Pan HF, et al. Increased leptin levels in patients with rheumatoid arthritis: a meta-analysis. Ir J Med Sci. 2014;183(4):659–666.
- Lee SW, Park MC, Park YB, et al. Measurement of the serum leptin level could assist disease activity monitoring in rheumatoid arthritis. Rheumatol Int. 2007;27(6):537–540.
- Yoshino T, Kusunoki N, Tanaka N, et al. Elevated serum levels of resistin, leptin, and adiponectin are associated with C-reactive protein and also other clinical conditions in rheumatoid arthritis. Intern Med. 2011;50(4):269–275.
- Lee YH, Bae SC. Circulating leptin level in rheumatoid arthritis and its correlation with disease activity: a meta-analysis. Z Rheumatol. 2016;75(10):1021–1027.
- Cao H, Lin J, Chen W, et al. Baseline adiponectin and leptin levels in predicting an increased risk of disease activity in rheumatoid arthritis: a meta-analysis and systematic review. Autoimmunity. 2016;49(8):547–553.
- Toussirot E, Streit G, Wendling D. The contribution of adipose tissue and adipokines to inflammation in joint diseases. Curr Med Chem. 2007;14(10):1095–1100.
- Schäffler A, Ehling A, Neumann E, et al. Adipocytokines in synovial fluid. JAMA. 2003;290(13):1709–1710.
- Targońska-Stepniak B, Dryglewska M, Majdan M. Adiponectin and leptin serum concentrations in patients with rheumatoid arthritis. Rheumatol Int. 2010;30(6):731–737.
- Rho YH, Solus J, Sokka T, et al. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum. 2009;60(7):1906–1914.
- Giles JT, Allison M, Bingham CO, 3rd, et al. Adiponectin is a mediator of the inverse association of adiposity with radiographic damage in rheumatoid arthritis. Arthritis Rheum. 2009;61(9):1248–1256.
- Giles JT, van der Heijde DM, Bathon JM. Association of circulating adiponectin levels with progression of radiographic joint destruction in rheumatoid arthritis. Ann Rheum Dis. 2011;70(9):1562–1568.
- Klein-Wieringa IR, van der Linden MP, Knevel R, et al. Baseline serum adipokine levels predict radiographic progression in early rheumatoid arthritis. Arthritis Rheum. 2011;63(9):2567–2574.
- Meyer M, Sellam J, Fellahi S, et al. Serum level of adiponectin is a surrogate independent biomarker of radiographic disease progression in early rheumatoid arthritis: results from the ESPOIR cohort. Arthritis Res Ther. 2013;15(6):R210.
- Neumann E, Frommer KW, Vasile M, et al. Adipocytokines as driving forces in rheumatoid arthritis and related inflammatory diseases? Arthritis Rheum. 2011;63(5):1159–1169.
- Francisco V, Pérez T, Pino J, et al. Biomechanics, obesity, and osteoarthritis. the role of adipokines: when the levee breaks. J Orthop Res. 2018;36(2):594–604.
- Schroeter MR, Leifheit M, Sudholt P, et al. Leptin enhances the recruitment of endothelial progenitor cells into neointimal lesions after vascular injury by promoting integrin-mediated adhesion. Circ Res. 2008;103(5):536–544.
- Manuel-Apolinar L, López-Romero R, Zarate A, et al. Leptin mediated ObRb receptor increases expression of adhesion intercellular molecules and cyclooxygenase 2 on murine aorta tissue inducing endothelial dysfunction. Int J Clin Exp Med. 2013;6(3):192–196.
- Gómez R, Scotece M, Conde J, et al. Adiponectin and leptin increase IL-8 production in human chondrocytes. Ann Rheum Dis. 2011;70(11):2052–2054.
- Conde J, Gomez R, Bianco G, et al. Expanding the adipokine network in cartilage: identification and regulation of novel factors in human and murine chondrocytes. Ann Rheum Dis. 2011;70(3):551–559.
- Liu B, Gao YH, Dong N, et al. Differential expression of adipokines in the synovium and infrapatellar fat pad of osteoarthritis patients with and without metabolic syndrome. Connect Tissue Res. 2019;60(6):611–618.
- Simopoulou T, Malizos KN, Iliopoulos D, et al. Differential expression of leptin and leptin's receptor isoform (Ob-Rb) mRNA between advanced and minimally affected osteoarthritic cartilage; effect on cartilage metabolism. Osteoarthr Cartil. 2007;15(8):872–883.
- Ku JH, Lee CK, Joo BS, et al. Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clin Rheumatol. 2009;28(12):1431–1435.
- Yang WH, Liu SC, Tsai CH, et al. Leptin induces IL-6 expression through OBRl receptor signaling pathway in human synovial fibroblasts. PloS One. 2013;8(9):e75551.
- Muraoka S, Kusunoki N, Takahashi H, et al. Leptin stimulates interleukin-6 production via janus kinase 2/signal transducer and activator of transcription 3 in rheumatoid synovial fibroblasts. Clin Exp Rheumatol. 2013;31(4):589–595.
- Busso N, So A, Chobaz-Péclat V, et al. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol. 2002;168(2):875–882.
- Deng J, Liu Y, Yang M, et al. Leptin exacerbates collagen-induced arthritis via enhancement of Th17 cell response. Arthritis Rheum. 2012;64(11):3564–3573.
- Xibillé-Friedmann D, Bustos-Bahena C, Hernández-Góngora S, et al. Two-year follow-up of plasma leptin and other cytokines in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69(5):930–931.
- Karvounaris SA, Sidiropoulos PI, Papadakis JA, et al. Metabolic syndrome is common among middle-to-older aged Mediterranean patients with rheumatoid arthritis and correlates with disease activity: a retrospective, cross-sectional, controlled, study. Ann Rheum Dis. 2007;66(1):28–33.
- Crowson CS, Myasoedova E, Davis JM, 3rd, et al. Increased prevalence of metabolic syndrome associated with rheumatoid arthritis in patients without clinical cardiovascular disease. J Rheumatol. 2011;38(1):29–35.
- Ferraz-Amaro I, González-Juanatey C, López-Mejias R, et al. Metabolic syndrome in rheumatoid arthritis. Mediators Inflamm. 2013;2013:710928.
