Abstract
We examined whether serum B cell activating factor (BAFF) is useful for predicting the remission of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) following rituximab treatment. We used the Birmingham Vasculitis Activity Score (BVAS) 2008 version 3 for the evaluation of 27 patients with AAV 6 months after rituximab treatment. Those with BVAS = 0 achieved remission, whereas those with BVAS score > 0 did not achieve remission. We considered changes in serum BAFF before rituximab treatment, 1 month after treatment, and 6 months after treatment. In the remission group, the serum BAFF increased consistently. In the non-achieved group, serum BAFF was within the normal range. In addition, there was no statistically significant difference between the two groups in terms of serum BAFF before and 1 month after rituximab treatment. However, the serum BAFF level at 6 months after rituximab treatment was significantly higher in the remission group than in the non-achieved group. If serum BAFF does not increase after 6 months of rituximab in AAV, it may be assumed that there are residual B cells and plasma cells in the tissues. Enhanced treatment targeting B cells, including re-administration of rituximab or the addition of other immunosuppressive drugs, should be considered.
Introduction
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is characterized by necrotizing vasculitis of small vessels [Citation1]. Microscopic polyangiitis (MPA) is a member of the AAV family. The characteristic histology of MPA shows necrotizing vasculitis without granulomatosis; the major organs involved are the kidneys and lungs [Citation1]. Myeloperoxidase (MPO)-ANCA is observed in almost 80% of patients with MPA [Citation2]. Granulomatosis with polyangiitis (GPA) is another member of the AAV family. The characteristic histology of GPA shows necrotizing vasculitis with granulomatosis. The paranasal sinuses, kidneys, and lungs are the major organs involved in GPA [Citation1]. In Japan, MPO-ANCA and proteinase 3 (PR3)-ANCA are observed in approximately the same number of patients with GPA [Citation3]. High-dose corticosteroids and rituximab (RTX) or cyclophosphamide are used as remission-induction therapies for MPA and GPA [Citation4].
RTX, a B-cell-depleting monoclonal antibody, has high remission-induction sustained-maintenance rates for patients with MPA and GPA. Administration of four weekly doses of 375 mg/m2 RTX is commonly used as a remission-induction therapy for AAV. A recent study that compared this dose with two once-weekly doses of 375 mg/m2 showed no differences in 1-year cumulative complete remission rates, peripheral B cell counts, and the incidence of serious adverse events [Citation5]. The Birmingham Vasculitis Activity Score (BVAS) is an indicator of AAV activity and a predictor of treatment response to RTX. There have been various references to markers for the achievement of RTX-induced remission, but no consensus has been reached. Objective indicators, especially those using serum markers, are required. The CD19-positive B lymphocyte count has been used as a standard indicator of RTX remission; however, it can only assess B cell depletion at the time of measurement, making it difficult to evaluate the effect of RTX over time.
B cell activating factor (BAFF), which belongs to the tumor necrosis factor (TNF) family, is a member of the tumor necrosis factor superfamily and is expressed on monocytes, macrophages, dendritic cells, neutrophils, and activated T cells. A proliferation-inducing ligand (APRIL) is also expressed on monocytes, macrophages, dendritic cells, neutrophils, and activated T cells, and is highly homologous to BAFF. BAFF and APRIL have two forms: a membrane-bound form and a soluble form. Soluble BAFF and APRIL are produced by the cleavage of membrane-bound forms with furin convertase. BAFF and APRIL are ligands for three TNF receptor superfamily members (B-cell maturation antigen [BCMA], transmembrane activator and calcium-modulator and cyclophilin ligand interactor [TACI], and BAFF receptor [BAFF-R]) and play a role in the survival and maturation of B cells [Citation6]. These receptors also have a soluble form. Soluble BAFF-R and TACI are produced by ADAM10 and ADAM17; soluble BCMA is produced by γ-secretase [Citation7]. Furthermore, excessive BAFF rescues self-reactive B cells from energy, which may play a crucial role in the induction and development of autoimmunity. Excessive BAFF has also been reported to be associated with AAV pathology [Citation8]. We investigated the usefulness of serum BAFF as a biomarker for RTX remission.
Materials and methods
Patient details and serum collection
This study was conducted in accordance with the ethical guidelines for medical research involving human subjects and was approved by the Ethical Review Committee of the Hyogo Medical University, (Ethical Review No. 1647). Written consent was obtained from each subject at the time of study participation. For this retrospective study, we included 9 and 18 patients with MPA and GPA, respectively, who had been treated with RTX at the Hyogo College of Medicine between 2012 and 2019 and were followed up for at least 6 months. Patients were treated with RTX for remission-induction due to refractoriness to multidrug therapy or to hasten prednisolone tapering due to advanced age. BVAS 2008 version 3 was used to classify the patients at 6 months after RTX treatment; BVAS = 0 and > 0 defined the remission-achieved and non-achieved groups, respectively.
Age, sex, disease phenotype, disease duration, relapse time, number of RTX induction treatments, previous treatment, prednisolone dose, serum C-reactive protein (CRP) level, serum ANCA level, blood CD19 positive B cell count before and 6 months after RTX induction, and serum BAFF and serum APRIL before and 1 and 6 months after RTX induction were retrospectively examined for remission- achieved and unachieved groups. Serum BAFF and serum APRIL levels were measured in serum stocks.
Enzyme-linked immunosorbent assay (ELISA)
We used the human BAFF/BLyS Quantikine® ELISA immunoassay (R&D Systems, Minneapolis, MN, USA) and the human APRIL/TNFSF13 duo set ELISA (R&D Systems, Minneapolis, MN, USA). The detection sensitivities were 6.44 pg/mL and 31.25 pg/mL, respectively, and the reference values in serum were 584–1186 pg/mL (according to the reagent package) and 2.00 ± 1.74 ng/mL (according to Phatak et al. [Citation9]), respectively.
Statistics
Statistical analysis was performed using JMP Pro 13.1.0 (SAS Institute Inc., Cary, NC, USA) software. Data are presented as the mean ± standard deviation. Differences between the two groups were analyzed using Mann-Whitney's U test, and changes between the two groups were analyzed using Welch's t-test. Statistical significance was set at p < .05. Correlations between serum CRP, serum ANCA, blood CD19 positive B cell count, BVAS, and serum BAFF were evaluated using Pearson's correlation coefficient.
Results
Background factors
There were no statistically significant differences between the remission and non-remission groups in terms of current age, sex, disease phenotypes, disease duration, relapse time, number of RTX induction treatments, prednisolone dose, serum CRP level, serum ANCA level, and blood CD19-positive B cell count before and 6 months after RTX induction; correlation with CD19-positive B cell counts became negative in both groups at 6 months after RTX induction (). There was no statistically significant difference between the two groups in terms of the affected organs (). Furthermore, there was no significant difference between the two groups in terms of the use of immunosuppressive drugs and biological therapies ().
Table 1. Baseline characteristics.
Table 2. Frequency of affected organs in each group.
Table 3. Frequency of previous treatments in each group.
Serum BAFF
As shown in and , the mean values of BAFF in the remission-achieved and non-achieved groups before RTX induction were 817.6 ± 259.6 pg/mL (n = 13) and 652.8 ± 240.8 pg/mL (n = 5), respectively. One month after RTX induction, the mean values of BAFF in the remission-achieved and non-achieved groups were 1670.6 ± 623.2 pg/mL (n = 5) and 722.0 ± 613.8 pg/mL (n = 2), respectively. There was no statistically significant difference between the two groups in terms of serum BAFF before and 1 month after RTX induction (p = .240 and .234, respectively). Six months after RTX induction, the mean values of BAFF in the remission-achieved and non-achieved groups were 2642.0 ± 971.6 pg/mL (n = 13) and 948.8 ± 191.2 pg/mL (n = 5), respectively. Serum BAFF at 6 months after RTX induction was significantly lower in the non-achieved group (p < .0001), and the mean values were within the normal range. On observing BAFF values before, 1 month, and 6 months after RTX induction, the mean values increased uniformly in the remission-achieved group, whereas they were all within the normal range in the non-achieved group.
Figure 1. Change in BAFF levels over the 6-month study period. Remission-achieved group (●), non-achieved group (■). The mean values of BAFF in the remission-achieved group increased uniformly over the period before, 1 month after, and 6 months after RTX induction; however, the mean values of BAFF in the non-achieved group were all within the normal range. BAFF, B cell activating factor; RTX, rituximab.
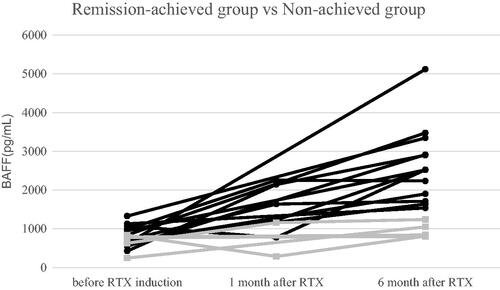
There was no correlation between BAFF and CRP levels, ANCA levels, or CD19-positive B-cell counts. In the remission-achieved group, there was a significant negative correlation between BVAS and BAFF (r = − 0.61, p < .05).
Serum APRIL
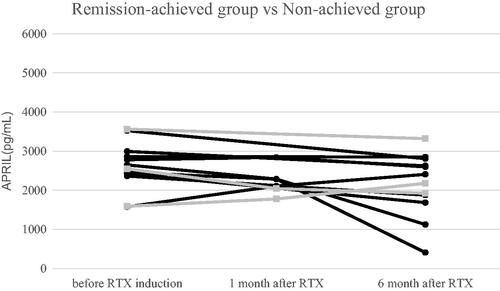
As shown in , the mean values of APRIL in the remission-achieved and non-achieved groups before RTX induction were 2624.7 ± 530.9 pg/mL (n = 9) and 2567.7 ± 986.1 pg/mL (n = 3), respectively. One month after RTX induction, the mean values of APRIL in the remission-achieved and non-achieved groups were 2380.5 ± 318.1 pg/mL (n = 4) and 1911.5 ± 187.4 pg/mL (n = 2), respectively. Six months after RTX induction, the mean values of APRIL in the remission-achieved and non-achieved groups were 2041.9 ± 842.9 pg/mL (n = 9) and 2474.7 ± 742.8 pg/mL (n = 3), respectively. There were no statistically significant between-group differences in serum APRIL levels before and at 1 and 6 months after RTX induction (p = .931, .095, and .447, respectively).
Figure 2. Change in APRIL levels over the 6-month study period. Remission-achieved group (●), non-achieved group (■). There was no statistically significant difference in serum APRIL levels between the two groups before, 1 month after, or 6 months after RTX induction. APRIL, A proliferation-inducing ligand; RTX, rituximab.
Discussion
It has been reported that serum B lymphocyte stimulator (BLyS) levels increase in patients with AAV [Citation10]. We measured serum BAFF levels in patients with untreated GPA and MPA. Serum BAFF levels increased in these patients compared to the reference values.
Regarding the change in serum BAFF after treatment for AAV, it has been reported that serum BAFF in patients with GPA who are treated with glucocorticoids decreases to almost the same level as that in healthy subjects [Citation11]. In our cases, serum BAFF levels may have been within the normal range before RTX administration because of previous treatment, including prednisolone (PSL).
However, it has been reported that serum BAFF levels increase in patients with AAV after 1–3 months of treatment with RTX compared to pre-treatment levels [Citation10]. Anti-myelin-associated glycoprotein (MAG) polyneuropathy is a slowly progressive distal form of mixed motor sensory polyneuropathy, and RTX has shown promise as an effective treatment. Regarding the difference in serum BAFF levels between responders and non-responders to RTX, it has been reported that serum BAFF levels in anti-MAG polyneuropathy are significantly higher in responders than in non-responders after 1 month of treatment with RTX [Citation12].
Our results showed that serum BAFF after 6 months of treatment with RTX was significantly higher in the remission-achieved group than in the non-achieved group. In the aforementioned study on anti-MAG polyneuropathy, the mechanisms underlying serum BAFF increase after B cell depletion may have relied on two phenomena: the unavailability of BAFF receptors on B cells, with subsequent increases in circulating unbound BAFF, and the lack of negative feedback on the BAFF-producing cells owing to B cell depletion [Citation12]. We speculate that serum BAFF levels in the remission-achieved group increased with similar mechanisms. In contrast, in the non-achieved group, serum BAFF levels after 6 months of treatment with RTX were significantly lower; the mean value was within the normal range.
First, we speculated that B cells did not deplete completely in the non-achieved group after 6 months of treatment with RTX. In a previous study, the presence or absence of B cells was compared with BLyS levels before and after RTX administration in patients with active AAV who received two cycles of RTX. Patients who had detectable peripheral B cells prior to receiving RTX on the first occasion had significantly lower serum concentrations of BLyS than those with no detectable B cells [Citation10]. In a previous study comparing lymph node tissues before and after RTX administration in rheumatoid arthritis, CD27+ IgD-switched memory B cells remained after RTX administration [Citation13]. In a previous study, memory B cells remained after RTX in patients with AAV who were tested using EuroFlow-based highly sensitive flow cytometry (HSFC) [Citation14]. In our study, it is possible that the feedback mechanism was not affected by BAFF-R in memory B cells, and serum BAFF did not increase.
Second, we considered that plasma cells were activated in the non-achieved group after 6 months of treatment with RTX. In general, plasma cells do not have CD20, the target of RTX, and remain after RTX introduction. In a previous study, plasma cells remained after RTX in patients with AAV who were tested using HSFC [Citation14]. In this context, BAFF-R is not expressed in plasma cells, but TACI and BCMA are expressed and are involved in the survival of plasma cells [Citation15]. In another study, soluble TACI and soluble BCMA were secreted when plasma cells were activated; soluble TACI bound to BAFF and acted as a decoy [Citation7,Citation16]. In our case, it is possible that soluble TACI acted as a decoy, and the feedback mechanism did not function. Furthermore, a previous study demonstrated that while BCMA displays specificity for B cells, BAFF-R and TACI can also be expressed by T cells. Therefore, we speculate that T cells expressing BAFF-R or TACI may have been activated in the non-achieved group after 6 months of treatment with RTX [Citation17].
Conversely, soluble TACI has been reported to act as a decoy for APRIL [Citation7]. We also compared the serum APRIL levels in the remission and non-remission groups, but found no difference; this was unlike the case of serum BAFF. We estimate that soluble BCMA also acts as a decoy for APRIL and that feedback to APRIL will not work [Citation18]. In a previous study, APRIL levels were not significantly different in patients with active vasculitis and inactive MPO-ANCA-associated renal vasculitis [Citation19]. In another study, APRIL genes in patients with AAV had significantly higher expression in those who achieved remission, compared to those with active vasculitis [Citation20].
There are a few limitations to this study. The sample size was small. We, therefore, believe that it is essential that serum APRIL levels are compared between remission and non-remission groups in future studies with larger sample sizes, and that the presence of memory B cells and soluble TACI are evaluated in the non-achieved group.
In summary, we showed the feasibility of using serum BAFF as a biomarker for induction of remission with RTX in AAV. If serum BAFF does not increase after 6 months of RTX in AAV, it may be assumed that there are residual B cells and plasma cells in the tissues; enhanced treatment targeting B cells, including re-administration of RTX or the addition of other immunosuppressive drugs, should be considered. Conversely, if serum BAFF increases consistently after treatment with RTX, maintenance with RTX may be considered when ANCA-specific memory B-cells persist on HSFC.
Disclosure statement
Kiyoshi Matsui has received research grants from Chugai and Asahi Kasei.
References
- Nakazawa D, Masuda S, Tomaru U, et al. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat Rev Rheumatol. 2019;15(2):91–101.
- Sada KE, Yamamura M, Harigai M, et al. Classification and characteristics of Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis in a nationwide, prospective, inception cohort study. Arthritis Res Ther. 2014;16(2):R101.
- Furuta S, Chaudhry AN, Arimura Y, et al. Comparison of the phenotype and outcome of granulomatosis with polyangiitis between UK and Japanese cohorts. J Rheumatol. 2017;44(2):216–222.
- Nagasaka K, Harigai M, Hagino N, et al. Systematic review and meta-analysis for 2017 clinical practice guidelines of the Japan research committee of the ministry of health, labour, and welfare for intractable vasculitis for the management of ANCA-associated vasculitis. Mod Rheumatol. 2019;29(1):119–129.
- Takakuwa Y, Hanaoka H, Kiyokawa T, et al. Low-dose rituximab as induction therapy for ANCA-associated vasculitis. Clin Rheumatol. 2019;38(4):1217–1223.
- Tsukamoto H. BAFF and APRIL in systemic lupus erythematosus. Clin Rheumatol. 2015;27:72–75.
- Sakai J, Akkoyunlu M. The role of BAFF system molecules in host response to pathogens. Clin Microbiol Rev. 2017;30(4):991–1014.
- Matsushita T, Sato S. The role of BAFF in autoimmune diseases. Nihon Rinsho Meneki Gakkai Kaishi. 2005;28(5):333–342.
- Phatak S, Chaurasia S, Mishra SK, et al. Urinary B cell activating factor (BAFF) and a proliferation‐inducing ligand (APRIL): potential biomarkers of active lupus nephritis. Clin Exp Immunol. 2017;187(3):376–382.
- Holden NJ, Williams JM, Morgan MD, et al. ANCA-stimulated neutrophils release BLyS and promote B cell survival: a clinically relevant cellular process. Ann Rheum Dis. 2011;70(12):2229–2233.
- Krumbholz M, Specks U, Wick M, et al. BAFF is elevated in serum of patients with Wegener's granulomatosis. J Autoimmun. 2005;25(4):298–302.
- Benedetti L, Zardini E, Briani C, et al. B-cell-activating factor in rituximab-treated patients with anti-MAG polyneuropathy. J Neurol Neurosurg Psychiatry. 2011;82(11):1291–1294.
- Ramwadhdoebe TH, van Baarsen LGM, Boumans MJH, et al. Effect of rituximab treatment on T and B cell subsets in lymph node biopsies of patients with rheumatoid arthritis. Rheumatology. 2019;58(6):1075–1085.
- van Dam LS, Oskam JM, Kamerling SWA, et al. Highly sensitive flow cytometric detection of residual B-cells after rituximab in anti-neutrophil cytoplasmic antibodies-associated vasculitis patients. Front Immunol. 2020;11(566732):566732.
- Kitagori K, Kawabata D. B cell abnormality in systemic lupus erythematosus. Jpn J Clin Immunol. 2012;35(6):495–502.
- Zhang Y, Li J, Zhang YM, et al. Effect of TACI signaling on humoral immunity and autoimmune diseases. J Immunol Res. 2015;2015:247426.
- Seyler TM, Park YW, Takemura S, et al. BLyS and APRIL in rheumatoid arthritis. J Clin Invest. 2005;115(11):3083–3092.
- Laurent SA, Hoffmann FS, Kuhn PH, et al. γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun. 2015;6(1):7333.
- Nagai M, Hirayama K, Ebihara I, et al. Serum levels of BAFF and APRIL in myeloperoxidase anti-neutrophil cytoplasmic autoantibody-associated renal vasculitis: association with disease activity. Nephron Clin Pract. 2011;118(4):c339–45.
- Jha S, Singh J, Minz RW, et al. Increased gene expression of B cell-activating factor of tumor necrosis factor family, in remitting antineutrophil cytoplasmic antibody-associated vasculitis patients. Int J of Rheum Dis. 2022;25(2):218–227.
