Abstract
Background and objectives: Access to safe and reliable contraception in the context of ARVs is essential. This study aimed to investigate the steady-state pharmacokinetics (PK) of ethinylestradiol/levonorgestrel (EE/LNG) 30/150 μg (Microgynon®) and atazanavir/cobicistat (ATV/COBI) 300/150 mg (Evotaz®), co-administered in HIV negative female volunteers, and assess its safety and tolerability.
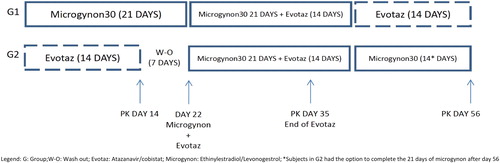
Methods: This phase 1, open label, 57-day, cross over, PK study randomized participants to one of two groups: (i) group 1 received EE/LNG alone on days 1–21, EE/LNG (21 days) + ATV/COBI (14 days) in the co-administration phase (days 22–42) and ATV/COBI alone on days 43–56; (ii) group 2 followed the same sequence but started with ATV/COBI and concluding with EE/LNG. Each group underwent intensive PK sampling on days 14, 35, and 56. EE/LNG and ATV/COBI concentrations were measured using validated LC-MS/MS methods.
Results: Of 14 healthy female volunteers screened, 11 attended baseline and six completed all PK phases (five withdrew secondary to side effects). Paired data were available for analysis in six subjects for EE/LNG and eight for ATV/COBI. Geometric mean ratios (GMR, with versus without ATV/COBI) and 90% confidence intervals (CI) for LNG Cmax, AUC0-24, C24 were 0.83 (0.68–1.02), 0.92 (0.71–1.18), 1.01 (0.73–1.38). GMR and 90% CI for EE Cmax, AUC0-24, C24 were 1.05 (0.92–1.19), 1.01 (0.83–1.22), 0.75 (0.60–0.93). No grade 3 or 4 adverse events or laboratory abnormalities were observed in the women who completed the study.
Conclusions: Our findings showed no significant changes in LNG concentrations and a 25% decrease in EE C24 when EE/LNG was co-administered with ATV/COBI.
Introduction
Women account for slightly more than half of the world’s 36.7 million people living with HIV/AIDS and the majority are of childbearing age.Citation1 Early and sustained HIV viral load suppression with antiretroviral (ARV) therapy now enables longer, healthier lives and improved fertility in women living with HIV (WLWH).Citation2–4 A significant number do, however, report requiring the flexibility to plan or prevent pregnancy,Citation3,Citation4 meaning that access to safe and reliable contraception in the context of ARVs is critical.Citation5,Citation6 The combined oral contraceptive pill (COCP) is a preferred method of contraception for many women worldwide.Citation7,Citation8 Nevertheless, WLWH have often been unable to use the COCP due to drug–drug interactions (DDIs) with some ARVs leading to altered drug levels and adverse events.Citation9 For instance, a reduction in the progestogen component of the COCP potentially runs the risk of contraceptive failure and unwanted pregnancyCitation9 whilst overexposure can lead to a number of side effects such as increased appetite, fluid retention, acne, and headaches.Citation10 Meanwhile, changes in oestrogen pharmacokinetics (PK) impact tolerability (e.g. breakthrough bleeding with underexposure, which can impact adherence) and toxicity (e.g. thrombotic events with increased concentrations).Citation11–13 There is now good evidence that an undetectable viral load equates to HIV being un-transmittableCitation14 and, hence, many HIV serodifferent couples choose to rely on hormonal contraception alone without condoms.Citation3,Citation15 It is therefore essential to define the pharmacokinetics and pharmacodynamics (PD) of hormonal contraception co-administered with individual ARVs in order to inform guidelines and ensure that the efficacy and safety of both are maintained.Citation11
Furthermore, exogenous oestrogens are also used by transgender women (TGW) as feminizing therapy.Citation16 TGW carry a disproportionate burden of HIV infection (estimated global prevalence 19.6% and incidence 3.4–7.8 per 100 person-years worldwide)Citation17,Citation18 and are less likely to engage into care or being compliant with ART.Citation19,Citation20 Qualitative studies have attributed this, in parts, to a limited understanding of the interactions between some ARVs and gender affirming hormones and to fears amongst TGW that ARVs will impede on hormonal efficacy, further underscoring the importance of having evidence-based data on DDIs.Citation16,Citation21 Ethinylestrodiol (EE), specifically, is not recommended for feminization therapy in guidelines;Citation22 however, it is the most studied oestrogen and the reference agent for drug interaction guidanceCitation16 and it is often used by TGW outside of healthcare settings for medical transition.Citation23
Despite very recently being removed from some major guidelines,Citation24–26 boosted atazanavir (ATV/b) remains the preferred second-line ARV agent in the World Health organization (WHO) guidelines and large numbers of patients, stable on treatment, continue therapy with ATV/b worldwide.Citation27 With its long-standing experience history, high genetic barrier and once daily dosing (OD), it remains an instrumental option in the management of HIV.Citation28 For over a decade, ATV was mainly co-administered with the only pharmacological booster available, ritonavir (RTV), a potent CYP3A4 inhibitor. Hormonal contraceptives are extensively metabolized by CYP enzymes (CYP3A and CYP2C9/19) and drug interactions between the COCP and ATV (with or without RTV) have been demonstrated.Citation29 The progestogens studied to date, norgestimate (NGM) and norethisterone (NET), are both increased in exposure with unboosted and with ritonavir-boosted ATV, through the inhibition of CYP3A4-mediated progestogen metabolism by both agents, thereby potentially leading to the side effects described above.Citation30,Citation31 Similarly ethinylestradiol concentrations also increase with unboosted ATV (48% rise in area under the curve, AUC) but, in contrast, decrease with ATV/RTV (16% decrease in EE maximum concentration (Cmax), 19% in AUC, and 37% in minimum concentration (Cmin)),Citation31 which is thought to result from RTV’s concomitant induction of CYP2C9 and of the glucuronidation responsible for EE clearance (via uridine-diphosphate glucuronosyltransferase 1A1–UGT1A1).Citation29 Therefore, according to guidelines and the atazanavir summary of product characteristics, if a combined oral contraceptive is administered with ATV/RTV, it must contain at least 35 μg of EE, strict compliance is necessary and a second method of contraception is recommended, considering the unknown effect of drug interactions.Citation31–33
Cobicistat (COBI), is the more recently approved alternative pharmacological booster to RTV. Its molecular properties offer the opportunity for co-formulation with PIs into fixed dose combinationsCitation34 and it is available co-formulated with ATV as Evotaz®.Citation35 It has no ARV activity and is a potent CYP3A4 inhibitor but, unlike ritonavir, it is neither a UGT1A1 nor a CYP2C9 inducerCitation34 and it may be better tolerated in some patients than RTV.Citation34 The product insert for Evotaz®Citation35 and the University of Liverpool’s HIV drug interactions websiteCitation36 state that no dosing recommendations can be made for COCP co-administration with ATV/COBI due to a lack of published data. It is currently suggested that additional or alternative (non-hormonal) forms of contraception should be considered.
ATV/COBI co-administration with the COCP has been investigated in an unpublished, phase I drug interaction study (Majeed et al, IWCPAT, Chicago 2017), which only assessed a single dose of drospirenone (DRSP)/EE 3 mg/20 μg administered prior to and at the end of a 14-day course of standard dose ATV/COBI in healthy volunteers (n = 14). The investigators found a 130% increase in DRSP AUC0-∞ and a 12% increase in Cmax. There was a nonclinically significant decrease in EE (22% reduction in AUC0-∞ and 18% in Cmax). The minimum concentration within the standard dosing interval of 24 h (C24) was not reported.Citation37 No COCP C24 or “steady state” (real life use) PK data are available; the latter are important since, progestogen serum levels are 2 to 3 fold higher in the steady state compared to a single administration, (after approximately 8–10 days of treatment) and EE steady state concentrations increase by a 30–40% rise in plasma level (5–6 days post initiation).Citation13,Citation38
EE/levonorgestrel (LNG) (Microgynon®) is the leading COCP prescribed in the UK.Citation39 The aim of this study was therefore to investigate the steady state PK of EE/LGN 30/150 μg and ATV/COBI 300/150 mg (Evotaz®) when co-administered in HIV negative female healthy volunteers and to assess the safety and tolerability of co-administration.
Participants and methods
Participants
Written informed consent was obtained from nonpregnant and nonlactating female healthy volunteers aged between 18 and 35 years with a body mass index (BMI) between 18 and 35 kg/m2. Participants were excluded if they had any significant acute or chronic medical illness; abnormal physical examination, ECG or clinical laboratory determinations; positive screens for HIV, hepatitis B or C; current or recent (within three months) gastrointestinal disease; clinically relevant alcohol or drug use that the investigator felt would adversely affect compliance with trial procedures; exposure to any investigational drug or placebo within three months of the first dose of the study drug; use of any other drugs, including over the counter medications and herbal preparations, within two weeks of the first dose of the study drug; and previous allergy to any of the constituents of the pharmaceuticals administered during the trial. Women of childbearing potential required a negative pregnancy test at screening and baseline.
Study design
This was an open-label, crossover, 57-day (excluding screening and follow-up) phase 1 PK trial carried out at the Clinical Trial Unit of the St. Stephen’s Centre, Chelsea and Westminster Hospital, London, UK.
At screening, clinical assessment and routine laboratory investigations were performed in all participants. The safety and tolerability of study medications were evaluated throughout the trial (on days 7, 28, 49, on PK days and at follow-up) using the National Institute of Allergy and Infectious Diseases (NIAID) Division of AIDS table for grading the severity of adult and pediatric adverse events (2004), that characterizes abnormal findings, vital signs, physical examinations, and clinical laboratory investigations.
The study design is illustrated in . After successful screening, volunteers were randomized to either (i) group 1, which received EE/LNG 30/150 μg alone on days 1–21, EE/LNG (21 days) + ATV/COBI 300/150 mg (14 days) in the co-administration period (days 22–42) and ATV/COBI alone on days 43–56 (14 days) or (ii) group 2, which followed the same structured sequence but started with ATV/COBI alone and finished with EE/LNG alone (14 or 21 days, patient choice). Each group underwent intensive PK sampling on study days 14, 35, and 56 to measure plasma concentrations of EE/LNG and/or ATV/COBI at 0 (predose), 2, 4, 8, 12, and 24 h postdose. On the PK days, study staff witnessed study medication intake with a standardized breakfast (626 kcal) and 240 mL of water.
Adherence
Adherence was assessed through direct questioning of dosing schedules, missed and late doses at each safety and PK visit. A pill count was also carried out at each PK visit.
Analytical and PK methods
Blood samples were collected into lithium heparin-containing blood tubes (12 mL) at each time-point, immediately inverted several times and then kept on ice or refrigerated until centrifugation. Within 30 min of blood collection, each blood sample was centrifuged for 10 min at 2000g at 4 °C. Plasma was then aliquoted equally into three 2 mL tubes (Sarstedt, Germany) and stored at –20 °C.
Samples were shipped on dry ice to the Liverpool Bioanalytical Facility for analysis. The laboratory is Good Clinical Laboratory Practice-accredited and participates in an external quality assurance scheme (KKGT, the Netherlands).Citation40,Citation41
Quantification of levonorgestrel, ethinylestradiol, atazanavir, and cobicistat
Concentrations of LNG, EE, ATV, and COBI in plasma were measured using validated high-pressure liquid chromatography–tandem mass spectrometry methods (LC-MS/MS).Citation42–44 The lower limits of quantification (LLQ) for the plasma analyses were 0.240 ng/mL for LNG, 5 pg/mL for EE, 10 ng/mL for ATV, and 5 ng/mL for COBI. For concentrations below the assay limit of quantification, a value of one-half of the quantification limit was used.
Accuracy (percentage bias) was between –0.42% and 1.5% (LNG), 0.61% and 3.28% (EE), 4.70% and 6.36% (ATV), and 6.45% and 8.07% (COBI) and precision was less than 3.1% (LNG), 8.0% (EE), 6.3% (ATV), and 8% (COBI).
Data analysis
The calculated PK parameters for plasma LNG, EE, ATV and COBI were plasma C24, Cmax and AUC from 0 to 24 h (AUC0–24). All PK parameters were calculated using actual blood sampling time and noncompartmental modeling techniques (WinNonlin Phoenix, version 6.1; Pharsight, Mountain View, CA). Descriptive statistics, including geometric mean (GM) and 95% confidence intervals (95% CI) were calculated for LNG, EE, ATV, and COBI plasma PK parameters. Each drug PK parameter during the co-administration period was compared to the unaccompanied drug PK parameter by calculating GM ratios (GMR) and 90% CI (co-administered/alone).
Inter-individual variability in drug PK parameters was expressed as a percentage coefficient of variation [CV, (standard deviation/mean)×100].
Statistical power
This is an exploratory study and, as such, no formal sample size calculation was performed. Sixteen participants completing the study were deemed appropriate to allow for relevant conclusions, and is standard for PK studies.
Ethics
The study protocol was approved by the Westminster Research Ethics Committee, London, United Kingdom, as well as by the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom. The study was conducted according to Good Clinical Practice and the Declaration of Helsinki (NCT02697851).
Results
Study population
Fourteen healthy female volunteers were screened, 13 were enrolled and 11 attended baseline (two withdrew prior to baseline for personal reasons). Nine subjects completed the intensive PK day 14 (five in group 1, four in group 2), eight completed day 35 (four in each group) and six completed day 56 (four in group 1, two in group 2). Overall, seven participants withdrew consent; five because of adverse events and two before starting the study medications. Of the six participants who completed all PK phases (up to day 56), the median (range) age and BMI were 31 (19–35) years, and 24 (19–29) kg/m2, respectively. Of the eleven participants who attended the baseline visit, four participants described themselves as Caucasian, six as black African, and one as Hispanic. Participant demographics, withdrawals and withdrawal reasons are summarized in .
Table 1 Participant demographics, withdrawals and withdrawal reasons
Pharmacokinetic results
Pharmacokinetic data for all four drugs in the two separate groups are detailed in . For each drug, only paired data from participants who completed the first period (either EE/LNG or ATV/COBI alone) and the co-administration period were used, so as to be able to assess and summarize intra-individual PK changes between the two periods. Groups 1 and 2 (G1 and G2) are grouped together in the results described below.
Table 2 Summary of pharmacokinetic data for all four drugs
Levonogestrel plasma pharmacokinetics
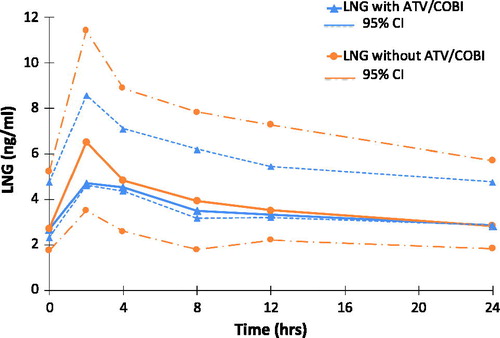
Six participants provided data for LNG intake with and without ATV/COBI (G1 n = 4, G2 n = 2). LNG GM plasma concentrations versus time are shown in . Geometric mean ratios (with ATV/COBI versus without) and 90% CI for LNG Cmax, AUC0-24, and C24 were 0.83 (0.68–1.02), 0.92 (0.71–1.18), and 1.01 (0.73–1.38) respectively.
Ethinylestradiol plasma pharmacokinetics
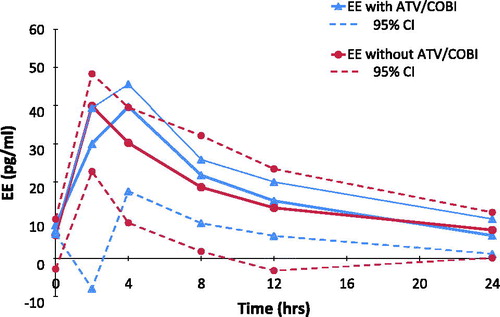
Six participants provided data for EE intake with and without ATV/COBI (G1 n = 4, G2 n = 2). GM plasma concentrations versus time curves are shown in . GMR (90% CI) for EE Cmax, AUC0-24, and C24 were 1.05 (0.92–1.19), 1.01 (0.88–1.22), and 0.75 (0.60–0.93) respectively.
Atazanavir plasma pharmacokinetics
Eight participants provided data for ATV intake with and without EE/LNG (G1 n = 4, G2 n = 4). GMR (90% CI) of ATV Cmax, AUC0-24, and C24 were 0.75 (0.60–0.95), 0.78 (0.64–0.96), and 0.89 (0.72–1.11) respectively.
Cobicistat plasma pharmacokinetics
Eight participants provided data for COBI intake with and without EE/LNG (G1 n = 4, G2 n = 4). GMR (90% CI) of COBI Cmax, AUC0-24, and C24 were 0.88 (0.8–0.97), 0.85 (0.77–0.95), and 0.89 (0.66–1.21) respectively.
summarizes our results together with currently reported findings in COCP interaction studies involving ATV/RTV and/or COBI.
Table 3 Summary of COCP drug interactions studies with atazanavir, ritonavir, and cobicistat
Safety and tolerability
Five participants withdrew consent from the study secondary to side effects; of those, data on the reason are available for three and are listed in . Full adherence was confirmed through regular direct questioning and pill counts in the women who completed the study and no grade 3 or 4 adverse events or laboratory abnormalities were observed in this group.
Discussion
To our knowledge, this is the first study to investigate the steady state PK of Microgynon® co-administered with ATV/COBI (Evotaz®).
The hormonal components of the COCP undergo extensive first-pass metabolism by phase I and II microsomal enzymes in the small intestinal mucosa and the liver before reaching the systemic circulation, meaning that they are highly susceptible to DDI.Citation45,Citation46
LNG is first hydroxylated in the liver, mainly by CYP3A4, and its metabolites are then excreted as glucuronide conjugates.Citation47 Our results showed only a small reduction in steady state LNG Cmax (17%) and no changes in C24 or AUC0-24 when it is administered with ATV/COBI (GMR range 0.92–1.01). Majeed et al. had also reported little interaction with progestogens, with only a 12% increase seen in DRSP Cmax when co-administered as a single dose with ATV/COBI. Both studies therefore suggest that ATV/COBI has a lesser impact on progestogen peak concentrations than ATV/RTV (co-administered with NGM or NET, GMR 1.33–1.68)Citation30,Citation31,Citation37 and when NGM is co-administered with COBI as part of Stribild® (tenofovir/emtricitabine/elvitegravir/COBI; GMR 2.08; Polina et al. 12th Clin Pharm of HIV Therapy Workshop 2011). Whilst the number of participants completing the relevant phases in our study (n = 6) was small and our data cannot be conclusive, a lack of clinically significant decrease in LNG minimum concentration and exposure is cautiously reassuring. This is because the progestogen-mediated suppression of the luteinizing hormone (LH) surge is one of the main contraceptive mechanisms of the COCPCitation48,Citation49 and LNG is the progestogen contained in the most commonly prescribed form of the emergency contraceptive pill.Citation47
Additionally, the lack of meaningful rise in progestogen C24 and AUC0-24 in our study differs to the increases seen with ATV/RTV co-administration (GMR 1.85 and 2.01 respectively) or with COBI co-administration when combined with DRV or EVG (GMR range 1.58–2.67). This finding is important since progestogen overexposure can lead to a number of significant side effects, as described above, including nausea, weight gain, and acne amongst others, that may impact tolerability and adherence.Citation10
Our study showed that EE C24 decreased by 25% with ATV/COBI compared to a 37% decrease reported with ATV/RTV. Furthermore, we found no clinically significant changes in Cmax or AUC0-24 (5% and 1% increases respectively), compared to the decreases seen with ATV/RTV (19% and 16% respectively).Citation31 This may be explained by the fact that, unlike RTV, COBI does not induce UGT1A1 or CYP2C9, both of which are involved in EE clearance.Citation34 As the EE component of the COCP is mainly responsible for endometrial stability and a significant reduction in C24 can lead to breakthrough bleeding potentially impacting adherence to contraception,Citation9 smaller decreases in PK parameters are likely to optimize tolerability and adherence. Our findings also compared well to EE co-administration with DRV/COBI in the Majeed et al. study, which lead to a 30% and a 14% decrease in EE AUC0-∞ and Cmax respectively, secondary to DRV-mediated induction of CYP2C9 and CYP2C19.Citation29,Citation37 In contrast, the induction of CYP2C9 and UGT through boosted EVG appears to overcome general COBI-medicated enzyme inhibition and leads to a decrease in EE PK parameters when it is co-administered with Stribild (GMR range 0.66–0.94; Polina et al. 12th Clin Pharm of HIV Therapy Workshop 2011).
As previously discussed, EE concentrations increase with unboosted ATV (AUC, Cmax, and Cmin 48%, 15%, and 91%, respectively), as do progestogen concentrations, through ATV mediated CYP3A4 and UGT1A1 inhibition. Yet, when co-administered with ATV/COBI in our study, EE AUC0-24, EE Cmax, LNG AUC0-24, and LNG C24 did not increase, whilst EE C24 and LNG Cmax even decreased. This is despite the lack of known COBI related enzyme or transporter induction (in vitro) to counteract ATV and COBI-mediated enzyme inhibition. This highlights that COBI’s in vivo metabolic effects may not yet be fully elucidated and warrant further investigation.
Of note, EE Cmax levels were consistently lower in our study (36–46 pg/mL regardless of the study phase), than in a majority of other EE PK studies (mean levels 80 pg/mL following a 30ug EE dose).Citation50 This could be explained by the sampling schedule used in this study – EE Tmax is usually reached at an average of 1.5 h; it is therefore possible that we missed the maximum concentration reached for some subjects by sampling 1- and 2-h postdose. Additionally, levels of EE have been shown to steadily increase within the first treatment cycle,Citation38 so concentrations measured at the end of the cycle (day 21) may be higher than mid-cycle (day 14), when our subjects underwent intensive PK sampling. It may also be higher again in subsequent cycles. This is however offset by our study design, which allowed for the intra-individual effect of co-administration to be measured. LNG PK parameters in our study were comparable to previous data.Citation10,Citation13
ATV and COBI concentrations were slightly reduced during the co-administration period (). For ATV, the changes in both groups combined were small and within the no-effect boundary (GMR Cmax was 0.79 and GMR C24 0.89). Interestingly, there was a significant decrease in ATV/COBI Cmax and AUC0-24 when combined with EE/LNG (GMR 0.64 and 0.68 respectively) in group 1 subjects only (i.e. those who received Microgynon30 alone for 21 days prior to combining with ATV/COBI) whereas no interaction was present with the group 2 subjects. Whilst this could be interpreted as being due to enzyme induction by EE, it is an observation and it is not possible to come to any conclusions based on the very small number of subjects in a subgroup (n = 4); C24 remained unchanged. Importantly, ATV concentrations in both groups remained reassuringly above the in vivo suggested minimum effective concentration for wild type HIV (MEC = 150 ng/mL).Citation28
The target recruitment was not reached and five/11 participants (45%) withdrew from the study secondary to side effects, three of which disclosed information on the reason (rash, deranged liver function tests, and gastrointestinal symptoms). This is a higher dropout rate than seen in Majeed et al. (22%), although participants in that study had only received one dose of COCP. Whilst high, it is in keeping with the combined published discontinuation rates secondary to any AE for boosted atazanavir (15%) and the COCP (29%).Citation49,Citation51 Both are drugs with relatively common side effects in the initial period and it is important that patients are counseled accordingly.
There are limitations to this study. The subjects were HIV negative healthy volunteers. As such, PK or pharmacodynamic comparisons with HIV positive women must be made cautiously and the practical implications of these PK observations are unknown. Clinical outcome data are required in large cohorts of HIV infected participants, and studies investigating pharmacodynamic endpoints (such as failure of viral suppression, HIV-related clinical disease progression, or unintended pregnancy) are needed in order to draw definite conclusions on how likely a contraceptive or an ARV is to fail in the context of co-prescription. It is also important to remember that efficacy rates of user-dependent contraception differ between perfect use (as seen in clinical studies) and real-life use.
The fact that our study involved healthy volunteers may also explain the low number of participants completing the study. In real-life settings, mild side effects associated with the initiation of COCP and/or of ARVs do not normally persist beyond three to six months, at which point an alternative would usually be offered. The option to persist and reassess was not available in the context of this study.
Nonetheless, our study provides the first steady state PK data on EE/LNG co-administered with ATV/COBI, demonstrating minimal changes in LNG concentrations and a smaller decrease in EE than seen with ATV/RTV. Whilst preliminary, these data are important in informing physicians, who need to discuss and choose safe and reliable contraception with their female patients living with HIV. Whether this minor difference between ATV/RTV and ATV/COBI will be clinically significant warrant further characterization in future studies.
Disclosure
ERE has received speaking and travel grants from Janssen, ViiV, Bristol-Myers Squibb, Merck Sharp & Dohme, and Gilead. EB, LE, and SDP have no conflicts of interest to declare. SK has received support from ViiV Healthcare, Merck, Janssen, Gilead and Bristol Myers Squibb for the HIV drug interactions website, and research grants from Merck, Janssen and ViiV Healthcare. GM has received speaker's and advisor's fees from Gilead Sciences, MSD, Janssen, BMS and has served as a member of the board of directors and on the scientific advisory board of Tobira Therapeutics. NN has received the following: Gilead Sciences (speaker fees, travel grants, conference registration, institutional research grant), BMS (speaker fees), Janssen (speaker and advisor fees, travel grants, conference registration), ViiV/GSK (speaker and advisor fees). MB had received travel and research grants from and has been advisor for Janssen, Roche, ViiV, Bristol-Myers Squibb, Merck Sharp & Dohme, Gilead, Mylan, Cipla, Teva.
Acknowledgments
The authors would like to thank the St Stephen’s Clinical Research team for their hard work and the volunteers who took part in the study. Part of this data was presented at the Conference on Retroviruses and Opportunistic Infections (CROI), 3 - 7 March 2018, Boston, MA, USA.
Additional information
Funding
Notes on contributors
Emilie R Elliot
Dr Emilie R Elliot is an HIV Physician at Chelsea and Westminster Hospital, London, UK with a special interest in ARV pharmacokinetics, pharmacodynamics and pharmacogenomics. She is currently finalising a PhD in ARV pharmacology at the Institute of Translational Medicine, University of Liverpool, UK.
Elisa Bisdomini
Elisa Bisdomini, RN. Previously Senior Clinical Trial Coordinator and currently Inflammatory Bowel Disease Clinical Nurse Specialist at Chelsea and Westminster Hospital. Currently pursuing MRes in Clinical Research at King's College London.
Sujan Dilly Penchala
Dr Sujan Dilly Penchala PhD is a postdoctoral research associate at University of Liverpool in Bioanalytical facility with extensive experience in the field of regulated quantitative bioanalysis of drugs and metabolites.
Saye Khoo
Prof Saye Khoo, FRCP is Professor in the Institute of Translational Medicine, University of Liverpool with an interest in Pharmacology of HIV treatment His research focuses on the pharmacology of HIV treatment failure and how therapy may be improved through individualised pharmacology.
Nneka Nwokolo
Dr Nneka Nwokolo, FRCP, is a Consultant Physician in HIV Medicine with an interest in the sexual and reproductive health of women.
Marta Boffito
Dr Marta Boffito, MD, PhD, FRCP is a Consultant Physician, Clinical Research Lead and HIV Service Director at Chelsea and Westminster Hospital and Reader at Imperial College, London, UK. Her clinical and research focus is on complex pharmacological issues in HIV and ARV pharmacology in older age HIV. She contributes to the educational, scientific, and guideline-formulation activities of national bodies including the British HIV Association and is involved in capacity building programmes for resource-limited settings (e.g. Uganda).
References
- UNAIDS. Data 2017. http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf.
- Haddad LB, Monsour M, Tepper NK, Whiteman MK, Kourtis AP, Jamieson DJ. Trends in contraceptive use according to HIV status among privately insured women in the United States. Am J Obstet Gynecol. 2017;217(6):676 e1–676.e11.
- Kaida A, Patterson S, Carter A, et al. Contraceptive choice and use of dual protection among women living with HIV in Canada: priorities for integrated care. Perspect Sex Reprod Health. 2017;49(4):223–236.
- Kennedy VL, Serghides L, Raboud JM, et al. The importance of motherhood in HIV-positive women of reproductive age in Ontario, Canada. AIDS Care. 2014;26(6):777–784.
- Nostlinger C, Desjardins F, Dec J, Platteau T, Hasker E, Eurosupport V. Child desire in women and men living with HIV attending HIV outpatient clinics: evidence from a European multicentre study. Eur J Contracept Reprod Health Care. 2013;18(4):251–263.
- Aizire J, Yende N, Nematadzira T, et al., eds. High frequency of unintended pregnancy and predictors of contraceptive choice among HIV-infected African women on life-long antiretroviral therapy (ART). The US-PEPFAR PROMOTE Cohort Study. Presented at: 22nd International AIDS Conference (AIDS 2018) abstract THAB0301; July 2018; Amsterdam.
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15–44: United States, 2011–2013. Natl Health Stat Report. No 86. Hyattsville, MD: National Center for Health Statistics.
- Statistics on sexual and reproductive health services; England 2015/16. Lifestyles Team, NHS Digital. Published 19 October 2016. Available at: https://files.digital.nhs.uk/publicationimport/pub21xxx/pub21969/srh-serv-eng-15-16-rep.pdf. Accessed 3rd July 2019.
- Thurman AR, Anderson S, Doncel GF. Effects of hormonal contraception on antiretroviral drug metabolism, pharmacokinetics and pharmacodynamics. Am J Reprod Immunol. 2014;71(6):523–530.
- McCann MF, Potter LS. Progestin-only oral contraception: a comprehensive review. Contraception. 1994;50(6 Suppl 1):S1–S195.
- Phillips S, Steyn P, Temmerman M. Contraceptive options for women living with HIV. Best Pract Res Clin Obstet Gynaecol. 2014;28(6):881–890.
- Pyra M, Heffron R, Mugo NR, et al. Effectiveness of hormonal contraception in HIV-infected women using antiretroviral therapy. AIDS. 2015;29(17):2353–2359.
- Microgynon 30ED. Summary of Product Characteristics. Bayer plc, 400 South Oak Way, Reading RG2 6AD. (Updated August 2017; Accessed August 2018). Available at: https://www.medicines.org.uk/emc/product/1131/smpc.
- Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–181.
- Patterson S, Carter A, Nicholson V, et al. Condomless sex among virally suppressed women with HIV with regular HIV-serodiscordant sexual partners in the era of treatment as prevention. J Acquir Immune Defic Syndr. 2017;76(4):372–381.
- Radix A, Sevelius J, Deutsch MB. Transgender women, hormonal therapy and HIV treatment: a comprehensive review of the literature and recommendations for best practices. J Int AIDS Soc. 2016;19(3 Suppl 2):20810.
- Baral SD, Poteat T, Stromdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):214–222.
- Kellogg TA, Clements-Nolle K, Dilley J, Katz MH, McFarland W. Incidence of human immunodeficiency virus among male-to-female transgendered persons in San Francisco. J Acquir Immune Defic Syndr. 2001;28(4):380–384.
- Baguso GN, Gay CL, Lee KA. Medication adherence among transgender women living with HIV. AIDS Care. 2016;28(8):976–981.
- Santos GM, Wilson EC, Rapues J, Macias O, Packer T, Raymond HF. HIV treatment cascade among transgender women in a San Francisco respondent driven sampling study. Sex Transm Infect. 2014;90(5):430–433.
- Sevelius JM, Patouhas E, Keatley JG, Johnson MO. Barriers and facilitators to engagement and retention in care among transgender women living with human immunodeficiency virus. Ann Behav Med. 2014;47(1):5–16.
- Pan American Health Organization [PAHO], J.S., Inc., World Professional Association for Transgender and e.a. Health, Blueprint for the Provision of Comprehensive Care for Trans Persons and Their Communities in the Caribbean and Other Anglophone Countries. Arlington, VA: John Snow Inc; 2014.
- Winter S, Doussantousse S. Transpeople, hormones, and health risks in Southeast Asia: a lao study. Int J Sex Health. 2009;21(1):35.
- Battegay M, Lundgren JD, Ryom L. European AIDS Clinical Society. Clinical management and treatment of HIV-infected patients in Europe. V 9.0. October 2017. Available from: http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html. Accessed 4 July 2018.
- U.S. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/0. Aids Info Updated May 2018. Accessed August 2018.
- Waters L. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015 (2016 interim update). http://www.bhiva.org/documents/Guidelines/Treatment/2016/treatment-guidelines-2016-interim-update.pdf. BHIVA. Accessed December 19, 2017.
- World Health Organisation: Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection recommendations for a public health approach. Second edition 2016. Available at: http://apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdf;jsessionid=06AB15554EB92AB1DD391D39FFBF642F?sequence=1. Accessed September 17, 2018.
- Elliot ER, Amara A, Pagani N, et al. Once-daily atazanavir/cobicistat and darunavir/cobicistat exposure over 72 h post-dose in plasma, urine and saliva: contribution to drug pharmacokinetic knowledge. J Antimicrob Chemother. 2017;72(7):2143.
- Tittle V, Bull L, Boffito M, Nwokolo N. Pharmacokinetic and pharmacodynamic drug interactions between antiretrovirals and oral contraceptives. Clin Pharmacokinet. 2015;54(1):23–34.
- Atrio J, Stanczyk FZ, Neely M, Cherala G, Kovacs A, Mishell DR Jr. Effect of protease inhibitors on steady-state pharmacokinetics of oral norethindrone contraception in HIV-infected women. J Acquir Immune Defic Syndr. 2014;65(1):72–77.
- Zhang J, Chung E, Yones C, et al. The effect of atazanavir/ritonavir on the pharmacokinetics of an oral contraceptive containing ethinyl estradiol and norgestimate in healthy women. Antivir Ther. 2011;16(2):157–164.
- Tackett D CM, Agarwala S, et al., eds. Atazanavir: a summary of two pharmacokinetic drug interaction studies in healthy subjects. Presented at: 10th Conference on Retrovirus and Opportunistic Infections; February 10–14, 2003; Boston.
- FSRH Clinical Effectiveness Unit. Hormonal contraception. Available from https://www.fsrh.org/standards-and-guidance/documents/combined-hormonal-contraception/. Update August 2012. Accessed August 2018)
- Marzolini C, Gibbons S, Khoo S, Back D. Cobicistat versus ritonavir boosting and differences in the drug-drug interaction profiles with co-medications. J Antimicrob Chemother. 2016;71(7):1755–1758.
- Evotaz: Atazanavir/cobicistat 300 mg/150 mg film-coated tablets summary of product characteristics. Bristol-Myers Squibb Pharmaceutical Limited, Uxbridge, UK. Availbale at: https://www.medicines.org.uk/emc/product/7006. Update February 2018. Accessed August 2018.
- University of Liverpool HIV Drug Interaction Checker. Available at: https://www.hiv-druginteractions.org Accessed May 11, 2019
- Majeed SR, West SK, Shuping J, et al., eds. Confirmation of the drug–drug interaction potential between cobicistat-boosted antiretroviral regimen. Paper presented at: IWCPAT; 2017; Chicago.
- Blode H, Wuttke W, Loock W, Roll G, Heithecker R. A 1-year pharmacokinetic investigation of a novel oral contraceptive containing drospirenone in healthy female volunteers. Eur J Contracept Reprod Health Care. 2000;5(4):256–264.
- Statista Report. Number of items of leading contraceptive pills prescribed in England in 2015. Available at: https://www.statista.com/statistics/573336/leading-contraceptive-pills-prescribed-in-england/. Accessed May 11, 2019.
- https://www.liverpool.ac.uk/translational-medicine/research/bioanalytical-facility/about/
- http://kkgt.nl/?page_id=248&lang=en.
- Cirrincione LR, Penchala SD, Scarsi KK, et al. Development, validation and utilization of a highly sensitive LC-MS/MS method for quantification of levonorgestrel released from a subdermal implant in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1084:106–112.
- Else L, Watson V, Tjia J, et al. Validation of a rapid and sensitive high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(19):1455–1465.
- Penchala SD, Fawcett S, Else L, et al. The development and application of a novel LC-MS/MS method for the measurement of Dolutegravir, Elvitegravir and Cobicistat in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1027:174–180.
- Watkins PB. Drug metabolism by cytochromes P450 in the liver and small bowel. Gastroenterol Clin North Am. 1992;21(3):511–526.
- Spatzenegger M, Jaeger W. Clinical importance of hepatic cytochrome P450 in drug metabolism. Drug Metab Rev. 1995;27(3):397–417.
- Levonelle 1500 microgram tablet Summary of Product Characteristics. Bayer plc, 400 South Oak Way, Reading RG2 6AD. Available at: https://www.medicines.org.uk/emc/product/133. Updated March 2018. Accessed August 2018.
- Rivera R, Yacobson I, Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol. 1999;181(5):1263–1269.
- Sondheimer SJ. Oral contraceptives: mechanism of action, dosing, safety, and efficacy. Cutis 2008;81(1 Suppl):19–22.
- Olsson B, Landgren BM. The effect of tolterodine on the pharmacokinetics and pharmacodynamics of a combination oral contraceptive containing ethinyl estradiol and levonorgestrel. Clin Ther. 2001;23(11):1876–1888.
- Teofilo E, Rocha-Pereira N, Kuhlmann B, et al. Long-term efficacy, tolerability, and renal safety of atazanavir/ritonavir-based antiretroviral therapy in a cohort of treatment-naive patients with HIV-1 infection: the REMAIN study. HIV Clin Trials. 2016;17(1):17–28.