Abstract
Background: Chronic HIV is associated with increased inflammation and tissue fibrosis despite suppressive antiretroviral therapy (ART). Monocytes and macrophages have been implicated in the pathogenesis of fibrosis, facilitated by chemokine receptor interactions.
Methods: We assessed systemic fibrotic biomarkers (transforming growth factor beta-1 [TGF-β1], thrombospondin-1 [TSP-1], C-terminal pro-peptide of collagen type I [CICP], and IL-11) in banked plasma from a previously published 24-week open-label trial of cenicriviroc (CVC), a dual CCR2/CCR5 antagonist, among persons living with HIV (PLWH) on stable ART with undetectable plasma HIV RNA (<50 copies/mL). Fibrotic markers were assessed by ELISA and Luminex. Untreated HIV-seronegative individuals (n = 6) of similar age and demographics served as a comparator group.
Results: Median age of PLWH was 55 years. At baseline, PLWH had higher median TGF-β1 (2.11 vs 1.62 ng/mL, p = 0.01), TSP-1 (236.74 vs 83.29 ng/mL, p < 0.0001), and CICP (200.46 vs 111.28 ng/mL, p = 0.01), but lower IL-11 (36.00 vs 53.74 pg/mL, p = 0.01) compared to HIV-uninfected individuals. Over 24 weeks, median TGF-β1 (-0.74 ng/mL, p = 0.006), TSP-1 (-52.12 ng/mL, p < 0.0001), and CICP (-28.12 ng/mL, p < 0.0001) decreased and IL-11 (28.98 pg/mL, p < 0.0001) increased in PLWH. At week 24, TGF-β1, CICP, and IL-11 were similar between the two groups (p > 0.05), while TSP-1 remained elevated in PLWH (p = 0.009) compared to controls.
Conclusions: PLWH had higher levels of the plasma fibrotic markers TGF-β1, TSP-1, and CICP. After 24 weeks of CVC, fibrotic markers generally returned to levels comparable to HIV-uninfected controls. Dual CCR2 and CCR5 blockade may ameliorate the detrimental fibrotic events that persist in treated HIV.
Background
Chronic human immunodeficiency virus (HIV) infection is associated with increased risk of fibrosis of various tissues including lung, kidney, liver, and lymph node.Citation1–4 The link between inflammation and fibrosis has been well reviewed in the general population.Citation5 Tissue recruitment of monocytes, a hallmark of inflammation, is mediated through the interaction of chemokines and their receptors. C-C chemokine receptor type 2 (CCR2) and type 5 (CCR5) are two receptors important for monocyte recruitment and migration,Citation6,Citation7 making them potential therapeutic targets for intervention in disease states associated with inflammation and fibrosis.
Maraviroc, the first CCR5 antagonist approved for the treatment of HIV-1, was shown to reduce migratory tendencies of monocytes in persons living with HIV (PLWH).Citation8 Furthermore, treatment with maraviroc has been reported to decrease matrix metalloproteinases, which degrade extracellular matrix components.Citation9 Cenicriviroc, a dual CCR2 and CCR5 antagonist, was shown by our group to reduce in vitro monocyte trans-endothelial migration.Citation10 We have also reported in a small clinical trial among PLWH on antiretroviral therapy (ART) that CVC decreased monocyte activation and inflammatory tendencies as assessed by plasma sCD163, sCD14, and neopterin levels.Citation11 The observed inflammatory reduction was associated with improvement in neurocognitive function widely believed to be partially mediated by monocyte-induced inflammation. CVC has been reported to have beneficial effects on non-alcoholic steatohepatitis (NASH) and liver fibrosis in association with marked reduction in circulating biomarkers of systemic inflammation including high‐sensitivity C‐reactive protein (hs‐CRP), interleukin (IL)‐6, fibrinogen, IL‐1ß, and the monocyte activation marker sCD14.Citation12,Citation13 Recently it has been reported that 48 weeks of CVC treatment resulted in a reduction in liver fibrosis in PLWH, as measured by the Enhanced Liver Fibrosis (ELF) index.Citation14 A phase 2 b trial showed comparable efficacy between CVC and efavirenz as an HIV antiretroviral drug.Citation15
Transforming growth factor beta (TGF-β) is a member of a superfamily of related growth factors consisting of three different isoforms, TGF-β1, TGF-β2, and TGF-β3.Citation16 Nearly all cells produce TGF-β and have receptors for it.Citation17 Several studies have implicated the over-production of this anti-inflammatory cytokine to have a direct impact on HIV-disease progression during chronic HIV.Citation18 The role of TGF-β1 in fibrogenesis has been well described.Citation16 TGF-β1 is a critical factor in fibrotic diseases resulting in the deposition of collagen and fibronectin through the activation of the Smad pathway and myofibroblast activationCitation16 and remains elevated in both treated and ART-naïve PLWH.Citation19 The majority of TGF-β1 exists in a latent form and requires activation before resulting in biological function. Current assays measure total TGF-β1, which can be confounded through the inclusion of the latent form.Citation20 Thrombospondin-1 (TSP-1), a secreted extracellular matrix protein and perhaps the best studied activator of latent TGF-β1, has been suggested as a more accurate measure of biologically active TGF-β1.Citation21 Type I collagen is the most abundant type of collagen found in connective and bone tissues, the synthesis of which produces C-terminal pro-peptide of collagen type I (CICP),Citation22 a potential soluble marker for lymphoid fibrosis in HIV.Citation23 RNAseq analysis showed primary cardiac fibroblasts exposed to TGF-β1 drastically upregulated IL-11 transcription.Citation24 Furthermore, this response, and the resulting collagen deposition, was inhibited with the use of IL-11 blocking monoclonal antibodies.
We hypothesized that the known anti-fibrotic effects of CVC may be reflected in circulating plasma fibrotic biomarkers. Here we report the changes of plasma fibrotic biomarkers (TGF-β1, TSP-1, and CICP and IL-11), utilizing banked plasma from our previously published clinical trial of ART-suppressed HIV infected adults receiving 24 weeks of CVC.Citation11 We also report the relationship between these fibrotic biomarkers and previously assessed plasma markers of monocyte activation including levels of monocyte CCR2 and CCR5 expression.Citation11
Methods
Study design
This study accessed biological specimens and clinical data from a completed clinical trial of CVC thathas been previously reported.Citation11 Briefly, this was a single-arm, open-label, 24-week trial of CVC in chronically infected PLWH (n = 17), ages 18-70 years, on stable ART for at least 1 year with plasma HIV RNA < 50 copies/mL. Details on the study design, including inclusion and exclusion criteria and the primary study results have previously been reported.Citation11 We excluded individuals who had ongoing need for medications with known CVC interactions, had hepatitis B or C or evidence of Child-Pugh class B or C hepatic impairment, creatinine clearance ≤ 30 mL/min, evidence of infection, clinically significant cardiovascular or cerebrovascular disease or uncontrolled chronic illnesses or cancer.
The CVC dosage used for each participant varied by their ART regimen and other drugs affecting the CYP450 system (either 50, 200, or 400 mg QD) adjusted in accordance with the manufacturer’s recommendations. Blood specimens were drawn at baseline and at weeks 4, 8, 12, and 24. The informed consent document included permission to utilize residual banked blood for other research questions related to HIV pathogenesis. Monocyte and T cell subsets, as well as cytokine receptor expression, were available for analysis at entry and week 24 after CVC as previously reported.Citation11
A convenience sample of HIV uninfected individuals (n = 6) broadly matched for age and gender were separately consented and evaluated for plasma fibrotic biomarkers and used as controls.
Quantification of soluble biomarkers
Plasma fibrotic biomarkers (TGF-β1, CICP, TSP-1 and IL-11) were assessed at entry for both PLWH and HIV-seronegative groups. In addition, fibrotic markers were assessed at week 12 and week 24 in PLWH. CICP and IL-11 were quantified by single-analyte ELISA (Quidel Corporation, San Diego, CA, and R&D Systems, Minneapolis, MN, respectively). TSP-1 and TGF-β1 were measured by Luminex (EMD Millipore, Burlington, MA).
Statistical analyses
Variables were summarized using descriptive statistics. Medians and interquartile ranges were used for continuous measures, whereas frequencies and proportions were used for categorical measures. Wilcoxon matched pair signed-rank test was used to assess changes from entry to week 24, Mann-Whitney U test was used to evaluate differences between HIV-infected and uninfected samples, and Spearman correlation coefficient was used to assess the pairwise relationship between numerical variables. All statistical analyses were performed using SPSS software version 25 (IBM, Armonk, NY). A two-sided P-value < 0.05 was regarded as statistically significant for all tests. P-values provided were adjusted by the Benjamini-Hochberg procedure.
Results
Study participant baseline clinical and demographic characteristics
Banked plasma for quantification of soluble fibrotic biomarkers were available for entry, week 12, and week 24 timepoints from all 17 participants who enrolled and completed the study (). Participants were generally older (median = 55 years) males with a long history (median = 21 years) of HIV infection. A convenience sample of healthy HIV seronegative consisting of 6 individuals did not differ by age or gender as compared to the CVC study group (p = 0.90 and ∼1.000, respectively). As previously published, CVC was well tolerated.Citation11
Table 1 Characteristics of people living with HIV at entry (n = 17)
Baseline assessment of fibrotic markers and their association with other immunologic parameters
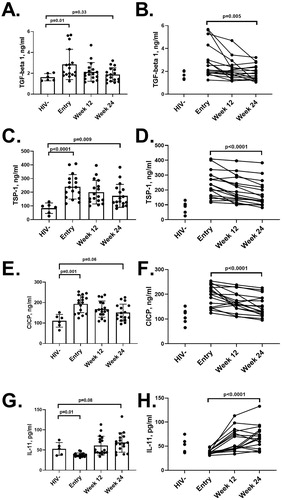
Median baseline TGF-β1 (2.11 vs 1.62 ng/mL, p = 0.01, ), TSP-1 (236.74 vs 83.29 ng/mL, p < 0.0001, ), and CICP (200.46 vs 111.28 ng/mL, p = 0.001, ) levels were elevated and IL-11 (36.00 vs 53.74 pg/mL, p = 0.01, ) levels were reduced in PLWH compared to healthy individuals. When stratified by non-nucleoside reverse transcriptase inhibitor-, protease inhibitor-, or integrase strand transfer inhibitor –containing ART regimens (yes vs no) or by the use of tenofovir (yes or no), no drug-specific differences were observed with levels of fibrotic biomarkers at base, week 12, or week 24. Furthermore, no associations were seen between fibrotic biomarkers and baseline CD4:CD8 T cell ratio or with nadir CD4.
Figure 1 Plasma soluble biomarkers. Plasma soluble biomarkers were measured at baseline, week 12, and week 24 in people living with HIV and compared with seronegative individuals. Biomarker levels are depicted by visit and individually for TGF-β1 (Fig. A and B, respectively), TSP-1 (C and D), CICP (E and F), and IL-11 (G and H). P-values presented were obtained by Mann-Whitney U test (comparing HIV + and HIV- in the left side graphs A, C, E and G) and Wilcoxon signed rank test (comparing entry to week 24 within the HIV + cohort in the right side graphs B, D, F, and H), with adjustments by the Benjamini-Hochberg procedure. Error bars indicate mean +/- standard deviation
Within the CVC cohort, baseline TSP-1 levels were negatively associated with IL-11 (rho=-0.58, p = 0.02) and trended towards significance with CICP (rho = 0.46, p = 0.06). No associations were seen between TGF-β1 and the other fibrotic biomarkers or between CICP and Il-11.
Positive correlations were seen between TGF-β1 and the markers of monocyte activation neopterin (rho = 0.66, p = 0.003) and sCD163 (rho = 0.49, p = 0.05), but not sCD14 (rho = 0.18, p = 0.50). There were no other associations found between fibrotic and monocyte activation biomarkers at baseline.
CICP levels were negatively associated with CCR5+ CD8+ T cells (rho = –0.58, p = 0.05) and showed a borderline positive association with frequencies of CCR2+ non-classical (CD14low/+CD16++) monocytes (rho = 0.55, p = 0.097). IL-11 correlated positively with frequencies of CCR2+ CD4+ T cells (rho = 0.68, p = 0.01) and CCR5+ CD4+ T cells (rho = 0.62, p = 0.04). No associations were observed between TGF-β1 or TSP-1 and expression of CCR2 or CCR5 on subsets of monocytes or T cells.
Changes in fibrotic biomarkers after 24 weeks in HIV infected individuals on ART
Over 24 weeks, decreases (Week 24 - Entry) in median TGF-β1 (-0.74 ng/mL, p = 0.006), TSP-1 (-52.12 ng/mL, p < 0.0001), and CICP (-28.12 ng/mL, p < 0.0001) were seen, as well as an increase in median IL-11 concentration (28.98 pg/mL, p < 0.0001). Some differences in response was seen for TGF-β1 responses. Thirteen individuals had decreased TGF-β1 values from entry to week 24 with a median absolute decrease of 1.00 (0.40, 2.44) ng/mL, representing a median percent decrease of 42.6% (22.8, 51.8) from baseline [(Week 24 – Entry)/Entry]. Four individuals had increased TGF-β1 after 24 weeks of CVC treatment, with a median increase of 0.20 (0.04, 0.55) ng/mL, representing an 11.0% (2.9, 30.1) increase from baseline measurements. Longitudinal changes of fibrotic biomarkers as a group and of individual participants are shown in .
Week 24 assessment of fibrotic biomarkers and their association with cellular and soluble immunologic parameters
At week 24, TGF-β1, CICP, and IL-11 in PLWH showed no significant differences compared to healthy individuals (p > 0.05), while TSP-1 remained elevated (p = 0.01). TSP-1 was associated with CICP (rho = 0.57, p = 0.02), however we could not observe the significant association seen at baseline with IL-11 (p = 0.14). No other associations between fibrotic biomarkers were observed at 24 weeks with CVC treatment.
TGF-β1 maintained its association with sCD163 (rho = 0.52, p = 0.03) at 24 weeks, however we did not observe the significant association observed at baseline with neopterin (p = 0.26). Similar to baseline, no other associations were observed between fibrotic and inflammatory biomarkers.
As previously reported, an increase over 24 weeks of CVC in CCR2 density was observed on classical (CD14++CD16-) monocytes and CD4+ T cells, as well as an increased density of CCR5 on CD4+ and CD8+ T cells.Citation11 At week 24 of CVC treatment, TGF-β1 correlated with CCR5 expression on non-classical monocytes (rho = –0.58, p = 0.05) and IL-11 showed an association with CCR2+ classical monocytes (rho = 0.59, p = 0.04). There were no other associations between fibrotic biomarkers and CCR2 or CCR5 density on monocyte subsets or T cells.
Discussion
The persistent immune activation and heightened inflammatory state characterized by chronic HIV-infection is believed to lead to tissue fibrosis, such as in the liver,Citation25 heart,Citation3 lungs,Citation26 and lymphoid tissues.Citation23 In this 24-week, single-arm study of CVC in an aviremic, chronically HIV-infected population on ART, we demonstrate a decrease in plasma fibrotic biomarkers TGF-β1, TSP-1, and CICP, as well as an increase in IL-11.
Our group previously reported that in vitro CVC treatment resulted in reduced trans-endothelial monocyte migration.Citation10 When monocytes and endothelial cells were treated with a single antagonist, maraviroc (CCR5) or BMS-22 (CCR2), a more modest inhibitory effect was observed. We have also reported that antagonism of CCR2 and CCR5 impacts monocyte activation.Citation11 Human hepatic stellate cells treated with maraviroc to inhibit CCR5 has been shown to reduce the production of fibrotic collagens and extracellular matrix proteins.Citation27 Additionally, the accumulation of CCR2+ monocytes within renal tissue of mice resulted in tissue injury and fibrotic remodeling, the effect of which was significantly reduced in CCR2-deficient mice.Citation28 These results suggest the dual inhibitory effect of CVC may have contributed to the significant decrease in systemic fibrotic biomarkers observed in our study.
We found a global decrease in the plasma fibrotic markers TGF-β1, TSP-1, and CICP after 24 weeks of CVC treatment. Interestingly, a previous study by Sherman et al among ART-naïve PLWH treated with 48 weeks of CVC intensification showed an increase in serum TGF-β1.Citation14 The discrepancy in the results between the two studies is perplexing. We hypothesize that the opposing results may be attributed to the difference in patient characteristics such as ART-naïve versus long-term virologic suppression. In our study, we note that the decrease in TGF-β1 was not uniform in that 4 of 17 participants had a small increase rather than decrease in their levels over 24 weeks.
Contrary to our expectations, IL-11 increased after 24 weeks of CVC treatment. Initially, we expected IL-11 to exacerbate fibrosis through IL-11-dependent TGF-β1 activation.Citation24 Increases in IL-11 has been associated with severity of disease state in individuals with idiopathic pulmonary fibrosis and differentiation of lung fibroblasts into collagen-secreting myofibroblasts.Citation29 IL-11 blockade reduced fibrosis and inflammation in mouse NASH models.Citation30 Conversely, IL-11 administration in culture, reduced mouse CD4+ T cell effector cytokine production hinting at an anti-inflammatory function of IL-11.Citation31 In the context of HIV, IL-11 has been reported to restore natural killer cell function which has been associated with delayed HIV-mediated immunodeficiency.Citation32 Further research is needed to elucidate CVC’s effect on IL-11 production, fibrosis, and inflammation. Interestingly, TGF-β1, CICP and IL-11 returned to levels observed in the comparative group while TSP-1 did not.
The results of this study are intriguing given the increased risk in PLWH of fibrosis-related diseases. Combined with our previous reports that CVC treatment reduces monocyte trafficking and inflammation, these decreases in plasma fibrotic biomarkers may indicate that CVC may have the ability to reduce tissue fibrosis. It should be noted that CVC’s mechanism of action includes HIV entry inhibition through CCR5 blockade. It is possible that better control of ongoing viral replication despite ART may account for the decreases in fibrotic markers while on CVC. This may be a less likely explanation than reduced monocyte migration given that all participants at entry had undetectable HIV RNA <50 copies/mL. However, an effect on residual low-grade viral replication cannot be excluded.
This study is limited by its small sample size. Nonetheless, our findings suggest that CVC may play a role in mitigating HIV-associated fibrosis. The inclusion of CVC as an integral part of a combination ART regimen could be re-visited as the additional anti-fibrotic effects of CVC may be of considerable benefit in this HIV population with increased risk of liver, lung, cardiac, and lymphoid fibrosis. Future tissue-based trials of CVC are needed to further elucidate the relationship between tissue fibrosis and these plasma biomarkers.
Acknowledgement
The authors would like to thank Tobira Therapeutics (currently Allergan) for providing cenicriviroc used in this study, as well as the Edwin C. Cadman endowment for providing financial support.
Additional information
Notes on contributors
S. Bowler
S. Bowler is a graduate student in the Department of Tropical Medicine, Medical Microbiology & Pharmacology at the John A. Burns School of Medicine (JABSOM), University of Hawaii - Manoa (UHM). He was primarily responsible for the analyses of the fibrotic markers in the context of our cenicriviroc clinical trial and for manuscript preparation.
C. Siriwardhana
C. Siriwardhana is Assistant Professor and Biostatistician in the Department of Quantitative Health Sciences at JABSOM, UHM. He supervised the biostatistical analyses of the study results.
B. L. Mitchell
B. I. Mitchell is a post-doctoral researcher with the Hawaii Center for AIDS (HICFA), JABSOM, UHM. He was responsible for planning and conducting the fibrotic assays and contributed intellectually to the study.
M. L. D’Antoni
M. L. D'Antoni was previously a post-doctoral researcher at JABSOM, UHM. She is currently employed as a Research Scientist in Clinical Virology at Gilead Sciences. She assisted with the planning of the study.
D. Ogata-Arakaki
D. Ogata-Arakaki is Nurse Unit Coordinator for HICFA, JABSOM, UHM. She assisted with the development of the cenicriviroc clinical trial protocol and was the clinical trial nurse responsible for the implementation of the trial.
S. Souza
S. Souza is Research Pharmacist, HICFA, JABSOM, UHM who was responsible for the pharmacy aspects of the cenicriviroc clinical trial and assisted intellectually in the drafting of the manuscript.
R. Yee
R. Yee is a medical student, JABSOM, UHM who was responsible for the IL-11 assay and assisted with manuscript preparation.
L. M. A. Gangcuangco
L. M. A. Gangcuangco is a graduate student in the Department of Tropical Medicine, Medical Microbiology & Pharmacology, JABSOM, UHM. He assisted intellectually in the drafting of the manuscript.
D. C. Chow
D. C. Chow is Professor of Medicine, JABSOM, UHM. He assisted in supervising the cenicriviroc clinical trial and intellectually in the drafting of the manuscript.
L. C. Ndhlovu
L. C. Ndhlovu at the time of the study was Professor in the Department of Tropical Medicine, Medical Microbiology & Pharmacology, JABSOM, UHM. He is currently Professor, Medicine at Weill Cornell Medicine. He was responsible overall for ensuring that the assays were conducted properly and contributed intellectually to the drafting of the manuscript.
C. Shikuma
C. Shikuma is Professor of Medicine and Director of HICFA, JABSOM, UHM and Principal Investigator of the clinical cenicriviroc study. She was overall responsible for the integrity of the study results and its reporting in the manuscript.
References
- Zeng M, Smith AJ, Wietgrefe SW, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121(3):998–1008.
- Deeks SG. HIV infection, lymphoid fibrosis, and disease. Blood. 2012;120(9):1753–1754.
- Hsue PY, Tawakol A. Inflammation and Fibrosis in HIV: Getting to the Heart of the Matter. Circ Cardiovasc Imaging. 2016;9(3):e004427.
- Papagianni M, Tziomalos K. Non-Alcoholic Fatty Liver Disease in Patients with HIV Infection. AIDS Rev. 2018;20(3):171–173.
- Mack M. Inflammation and fibrosis. Matrix Biol. 2018;68–69:106–121.
- Charo IF, Peters W. Chemokine receptor 2 (CCR2) in atherosclerosis, infectious diseases, and regulation of T-cell polarization. Microcirculation. 2003;10(3–4):259–264.
- Ness TL, Kunkel SL, Hogaboam CM. CCR5 antagonists: the answer to inflammatory disease?. Expert Opin Ther Pat. 2006;16(8):1051–1065.
- Rossi R, Lichtner M, Sauzullo I, et al. Downregulation of Leukocyte Migration After Treatment With CCR5 Antagonist Maraviroc. J Acquired Immune Deficiency Syndromes. 2010;54(5):e13–e14.
- Gramegna P, Latronico T, Brana MT, et al. In vitro downregulation of matrix metalloproteinase-9 in rat glial cells by CCR5 antagonist maraviroc: therapeutic implication for HIV brain infection. PloS One.2011;6(12):e28499.
- D’Antoni ML, Mitchell BI, McCurdy S, et al. Cenicriviroc inhibits trans-endothelial passage of monocytes and is associated with impaired E-selectin expression. J Leukoc Biol. 2018;104(6):1241–1252.
- D’Antoni ML, Paul RH, Mitchell BI, et al. Improved Cognitive Performance and Reduced Monocyte Activation in Virally Suppressed Chronic HIV After Dual CCR2 and CCR5 Antagonism. J Acquir Immune Defic Syndr.2018;79(1):108–116.
- Friedman SL, Ratziu V, Harrison SA, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67(5):1754–1767.
- Lefebvre E, Moyle G, Reshef R, et al. Antifibrotic Effects of the Dual CCR2/CCR5 Antagonist Cenicriviroc in Animal Models of Liver and Kidney Fibrosis. PloS One. 2016;11(6):e0158156.
- Sherman KE, Abdel-Hameed E, Rouster SD, et al. Improvement in hepatic fibrosis biomarkers associated with chemokine receptor inactivation through mutation or therapeutic blockade. Clin Infectious Dis. 2019;68(11):1911–1918.
- Thompson M, Saag M, DeJesus E, et al. A 48-week randomized phase 2b study evaluating cenicriviroc versus efavirenz in treatment-naive HIV-infected adults with C-C chemokine receptor type 5-tropic virus. Aids. 2016;30(6):869–878.
- Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325–338.
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–1358.
- Maina EK, Abana CZ, Bukusi EA, Sedegah M, Lartey M, Ampofo WK. Plasma concentrations of transforming growth factor beta 1 in non-progressive HIV-1 infection correlates with markers of disease progression. Cytokine. 2016;81:109–116.
- Theron AJ, Anderson R, Rossouw TM, Steel HC. The role of transforming growth factor beta-1 in the progression of HIV/AIDS and development of non-AIDS-defining fibrotic disorders. Front Immunol. 2017;8:1461.
- Mancini D, Monteagudo J, Suarez-Farinas M, et al. New methodologies to accurately assess circulating active transforming growth factor-beta1 levels: implications for evaluating heart failure and the impact of left ventricular assist devices. Transl Res. 2018;192:15–29.
- Murphy-Ullrich JE, Suto MJ. Thrombospondin-1 regulation of latent TGF-beta activation: A therapeutic target for fibrotic disease. Matrix Biol. 2018;68–69:28–43.
- Kallergis EM, Manios EG, Kanoupakis EM, et al. Extracellular matrix alterations in patients with paroxysmal and persistent atrial fibrillation: biochemical assessment of collagen type-I turnover. J Am Coll Cardiol. 2008;52(3):211–215.
- Estes JD. Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol Rev. 2013;254(1):65–77.
- Schafer S, Viswanathan S, Widjaja AA, et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature 2017;552(7683):110–115.
- Stabinski L, Reynolds SJ, Ocama P, et al. High prevalence of liver fibrosis associated with HIV infection: a study in rural Rakai, Uganda. Antivir Ther. 2011;16(3):405–411.
- Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183(3):388–395.
- Coppola N, Perna A, Lucariello A, et al. Effects of treatment with Maraviroc a CCR5 inhibitor on a human hepatic stellate cell line. J Cell Physiol. 2018;233(8):6224–6231.
- Wilkening A, Krappe J, Muhe AM, et al. C-C Chemokine Receptor Type 2 Mediates Glomerular Injury and Interstitial Fibrosis in Focal Segmental Glomerulosclerosis. Nephrol Dialysis Transplant. Official Publication of the European Dialysis and Transplant Association - European Renal Association 2018.
- Ng B, Dong J, D’Agostino G, et al. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci Transl Med 2019;11(511): eaaw1237.
- Widjaja AA, Singh BK, Adami E, et al. Inhibiting interleukin 11 signaling reduces hepatocyte death and liver fibrosis, inflammation, and steatosis in mouse models of nonalcoholic steatohepatitis. Gastroenterology. 2019;157(3):777–792 e14.
- Gurfein BT, Zhang Y, Lopez CB, et al. IL-11 regulates autoimmune demyelination. J Immunol. 2009;183(7):4229–4240.
- Favors SE, Curd LM, Gregg RK. Use of the anti-inflammatory cytokine interleukin-11 to reverse HIV-1gp120 repression of a natural killer cell line. Cell Immunol. 2012;276(1–2):1–5.
