Abstract
Introduction
Recent results from Phase 3 clinical trials with cabotegravir (CAB) and rilpivirine (RPV) long-acting (LA) have shown that a monthly regimen is non-inferior to daily oral antiretroviral therapy (ART). Additional insights are necessary to prepare for LA ART roll-out, including identifying the appropriate patients.
Methods
Within the ATLAS-2M trial, an online survey was administered to 329 health care providers (HCPs) in 13 countries. Multivariate logistic regression was conducted to identify factors associated with providers considering a greater proportion of patients as appropriate LA ART candidates.
Results
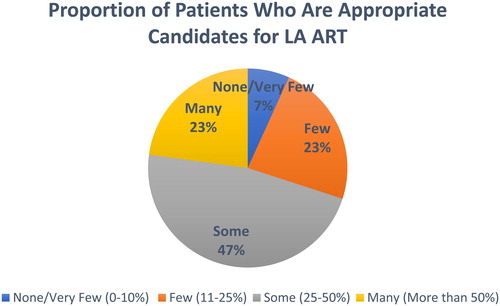
Forty-seven percent of HCPs believed that “some” patients (25–50%) would be appropriate while nearly one-quarter of HCPs (23%) felt that “many” patients (more than 50%) would be appropriate candidates for LA ART. Providers in the African region had a greater odds of identifying a greater proportion of their patients as appropriate candidates (AOR 8.97; p < 0.001) vs. other regions. Nurses/physician assistants and research staff/pharmacists had a higher odds of perceiving a greater proportion of their patients as appropriate candidates vs. physicians, respectively (AOR 3.42 p < 0.001; AOR 2.48; p = 0.19). Providers who had experience transitioning patients from LA to oral ART had a higher odds of reporting that more of their patients would be appropriate candidates (AOR 1.64; p = 0.008) vs. those without experience.
Conclusion
A significant proportion of providers reported that many of their patients would be appropriate candidates for LA ART. To optimize roll-out after regulatory approval, it is important to support providers with tools to help identify patients who would most benefit from this option.
Keywords:
Background
To date, HIV treatment has been daily oral antiretroviral therapy (ART). The current standard of care for ART regimens is to combine three active drugs from two different classes.Citation1 These regimens draw upon six distinct classes of agents that have been approved for HIV treatment. To date, there are more than 30 such drugs available.Citation2
While an important feature of current ART, especially with single tablet regimens (STRs), is its ability to improve adherence, challenges remain in the treatment of HIV and the prevention of its transmission especially over the long-term.Citation3,Citation4 Suboptimal treatment adherence can result in failure to maintain virologic suppression and permit the development of antiretroviral (ARV) resistance through viral mutation.Citation5,Citation6
To help address the challenges of adherence with daily oral ART, long-acting (LA) injectable formulations of cabotegravir (CAB) and rilpivirine (RPV) are under study in Phase 3 clinical trials. Several Phase 3 studies (FLAIR, ATLAS) have shown that a two-drug regimen of CAB + RPV LA has similar antiviral efficacy to daily oral ART, in patients with long and short treatment history.Citation7–9 The ongoing ATLAS study, has demonstrated that monthly CAB + RPV LA is non-inferior to the daily oral Standard of Care (SoC); with 92.5% and 95.5% having HIV-1 RNA < 50 c/mL at 48 weeks, in the LA ART and 3-drug oral ART groups, respectively.Citation8 Furthermore, data from the Patient Reported Outcomes (PROs) showed high rates of satisfaction, acceptability, tolerability and preference for the LA regimen, compared to the daily oral treatment. Participants in the CAB + RPV LA arm demonstrated significantly greater improvement from baseline in treatment satisfaction compared to the daily oral ART at Weeks 24 and 44, with participants in the CAB + RPV LA arm reporting a 6.12-point improvement from baseline, compared to a 0.44 improvement for the oral ART arm (difference CAB + RPV.68 (4.37–6.98), p < 0.001). Furthermore, 97% of the participants in the CAB + RPV LA arm preferred the LA therapy compared to daily oral ART.Citation10
The ATLAS-2M Phase 3b study was conducted to understand whether the bi-monthly LA ART administration is safe and effective. Patients successfully treated from the ATLAS study with monthly CAB + RPV LA or daily oral ART, were eligible to enroll into ATLAS-2M.Citation11 Results from the ATLAS-2M study demonstrated the non-inferior antiviral activity and safety of CAB + RPV LA administered every two months compared to monthly LA ART, over a 48-week treatment period. Using the FDA Snapshot algorithm, at Week 48, showed that the two-month arm (9/522 [1.7%]) and one-month arm (5/523 [1.0%]) were similarly effective (adjusted difference: 0.8%, 95% confidence interval [CI]: −0.6, 2.2). The study also found that rates of virologic suppression (HIV-1 RNA <50 c/mL), a key secondary endpoint for ATLAS-2M, were similar, in both the every two-months arm (492/522 [94.3%]) and once monthly (489/523 [93.5%]) (adjusted difference: 0.8%, 95% CI: −2.1, 3.7).Citation12
While recent clinical trials have investigated the efficacy, safety, tolerability and included PROs, limited information exists on the clinical care management of CAB + RPV LA, including identifying the appropriate candidates for LA ART. In order to bridge the gaps around the opportunities and challenges for implementation, we conducted qualitative research in the US and Spain with participants and health care providers (HCPs) in Phase 2b (LATTE-2 trial) as well as Phase 3 (ATLAS; ATLAS-2M; FLAIR) studies to understand their thoughts and perspectives on the most appropriate patients for LA ART.Citation13 Patient and provider interviews within the LATTE-2 study, conducted in the US and Spain, found that the convenience of LA injections and emotional benefits, such as reduced potential for HIV disclosure and eliminating the “daily reminder of living with HIV” were important benefits of the LA regimen.Citation14 Further qualitative research within the Phase 3 ATLAS study found high levels of acceptability of LA ART and the opportunity for less frequent dosing addressed psycho-social adherence barriers, including stigma related to the daily reminder of living HIV and the associated constraints of daily therapy, including travel and needing to conceal medication. However, while clinicians understood the benefits of LA ART, they cautioned that patients needed to attend more frequent clinic visits as LA ART requires periodic intramuscular injections (every one or two months) administered by trained providers and that appointment adherence may be a challenge for some of their patients.
The goal of the current study is to understand the perceptions of HCPs regarding the appropriate candidates for LA ART which can inform the large-scale rollout of injectable therapy. The study is based on a structured survey amongst clinical care providers participating in the ATLAS-2M study. Findings from the survey will enable clinicians to identify patients they believe would be best-suited for injectable ART in real-world settings and routine care, outside of the realm of clinical trials.
Methods
Provider survey
A cross-sectional survey of HCPs participating in the ATLAS-2M trial settings was developed to understand the perceived barriers and opportunities for future real-world implementation of CAB + RPV as LA ART. The survey development was primarily based on the key themes raised by providers in prior formative research conducted within the Phase 2b LATTE-2 study. Additional insights were gleaned from the literature, advisory boards, and pilot-testing with investigators from the ATLAS-2M study, including investigators from the US, Spain, and South Africa. The survey was translated and back translated into seven languages.
The survey specifically targeted HCPs who were engaged in clinical care and included physician investigators, and sub-investigators (and other physicians), nurses, or clinical providers involved in ATLAS and/or ATLAS-2M trials. Specific providers included those who may be giving injections (e.g. phlebotomists), providing clinical oversight to patients during the trial (e.g. study coordinators) and/or those monitoring and managing patient safety. The survey was administered online between February and April, 2019.
HCPs were sent a letter via email from the trial sponsor (ViiV Healthcare) making them aware of the survey including its content and applications and requesting them to identify eligible responders from their sites. Eligibility criteria included providers participating in the ATLAS-2M study and engaged in clinical care with study participants. In parallel, investigators were encouraged from the study sponsor to participate in the survey, although no financial incentives were provided. Once the eligible HCPs were identified, the study team then sent an online survey link, using the Qualtrics survey platform, with information regarding the survey to obtain their informed consent and decision to participate. Providers were given two weeks to complete the survey. Those who did not initially respond were sent an email encouraging participation. The survey took approximately 15 minutes to complete.
Measures
The final online survey included 27 items (with four open-ended questions) and is divided into six sections: (1) background information on participating providers (country, role in the clinic, and participation in prior LA ART trials); (2) feasibility of LA ART implementation and roll-out; (3) clinical management of LA ART; (4) patient interest and clinic readiness for LA ART; (5) benefits, barriers and concerns related to LA ART; and (6) four additional open-ended questions on LA ART implementation post clinical trial and roll-out.
Statistical analyses
Descriptive analyses were performed on all the survey questions using frequency distributions (mean; median; standard deviation; range; and histograms). We examined the distribution of the response options related to the primary outcome of “appropriate candidates” for LA ART and identified potential covariates of interest such as: provider type, region, role in clinic, and barriers, benefits and concerns scores.
We conducted bivariate analyses of the data including chi-square tests of independence and developed multivariate multinomial logistic regression models to examine factors associated with the main study outcomes. Models were adjusted for clustering with countries with robust variance estimates. Multivariate models were developed using a backward stepwise selection approach to establish concise final models. Associations are expressed as odds ratios (ORs) with 95% confidence intervals (CI). Analyses were performed with STATA version 15.
Results
Provider eligibility & survey response rates
The ATLAS-2M study included a total of 1200 providers in 13 countries (Australia, Argentina, Canada, France, Germany, Italy, Mexico, Russia, South Africa, South Korean, Spain, Sweden and the US). As the inclusion criteria for the survey included HCPs who needed to be involved in direct patient care, many providers many did not meet the eligibility criteria and were thus not invited to participate in the survey. Of the 1200 providers, 449 met the eligibility criteria (as they participated in the ATLAS-2M study and were involved in direct patient care). Of the 429, 329 (73.3%) providers initiated the survey, 293 provided complete sociodemographic and background data, and 266 of those (80.0%) provided information on the “appropriate candidates” for LA ART and were ultimately included in multivariate analyses.
The overall geographic composition of the survey reflected the sites in the ATLAS-2M trial as well as the country-specific response rates to the survey. The proportion of HCPs who participated to the survey varied by country, ranging from <50% in Sweden (40.6%) and the United States (44.1%) to 100.0% participation in Australia, Mexico, Russia and Spain (). Overall, the response rate was 73.3% (329/449) in terms of survey initiation. One hundred percent (95/95) of the providers participated in the survey, hence Spain was the country with the single greatest participation. Conversely, in the US 44.1% of the providers responded to the survey (63/143), therefore fewer US HCPs were included.
Table 1 Provider participation, by country
Primary outcome
Appropriate Candidates: The primary outcome measure was derived directly from the survey question What proportion of your patients do you believe are appropriate candidates for CAB LA + RPV LA? The responses consisted of four ordinal options as follows: None/very few (0–10%); Few (11–25%); Some (25–50%); Many (more than 50%). The questions and response categories were informed by prior qualitative interviews with providers. For the modeling analysis this main outcome variable was grouped as: None/Few, 0–24% (0), Some, 25–50% (1), Many, >50% (2).The two lower categories (i.e. “None/Very Few” and “Few”) were combined based on the distribution of the observed data. In addition, patient characteristics related to appropriateness for LA ART were also recorded.
Covariates
Providers characteristics: Role in clinic, prior experience with LA ART, geographic region.
Barriers: The barriers score was calculated as the count of the number logistical barriers, the providers felt were encountered by patients, in adhering to their clinic appointments and included the following seven potential issues: 1. Travel burden (distance to clinic); 2. Time commitment (waiting time, duration of appointment); 3. Costs associated with visits (public transport, parking fees); 4. Burden associated with frequency of appointments (separate appointments for injection and clinical visit); 5. Travel for work or holiday; 6. Housing instability (moving around a lot or no home); and 7. Moved from the area.
Concerns: Providers indicated their level of concern (based on a 4-point Likert scale: very concerned (4), somewhat concerned (3), not very concerned (2), and not at all concerned (1) regarding the following clinical management issues when initiating a patient on LA ART: 1. The oral lead-in phase (before starting injections); 2. Risk of resistance (for patients not adherent to injection visits); 3. Patients not returning to clinic (for injection appointments); 4. Switching a patient from CAB + RPV LA to oral ART; 5. Drug interactions and comorbidities (e.g. TB, HCV); 6. Patients moving out of the area; and 7. Patients switching to a different provider.
Benefits: Providers indicated the level of importance (based on a 4-point Likert scale: very important (4), somewhat important (3), not very important (2), and not at all important (1) associated with the benefits of LA ART for patients: 1. Convenience; 2. Reduced side effects related to oral ART; 3. Reduced drug/food interactions; 4. Easier management of concomitant diseases (e.g. TB, Hepatitis B and C, diabetes); 5. Reduced stigma; 6. Other psychological/emotional benefits (e.g. eliminating daily reminder of living with HIV); 7. Privacy/confidentiality; 8. Lifestyle (e.g. no concern of pills seen in home, during travel); and 9. Increased contact with health care provider.
Composite scores: We developed composite scores for each of the three aggregate measures above which had the following distributions: barriers to adhere to clinic appointments score (median = 2; mean = 2.3; range 0–7), clinical concerns initiating patients on LA ART score (median = 18; mean = 17.2; range 0–28) and patient benefits score (median = 32; mean = 31.3; range 11–36) related to LA ART.
Provider characteristics
The majority of participating providers were from Europe (France, Italy, Germany, Russia, Spain, and Sweden) (61%), followed by approximately one-quarter (23%) from North America (US and Canada); and the remaining 18% came from East Asia and the Pacific (Australia and South Korea) (6%); Africa (South Africa) (5%); and Latin America (Argentina and Mexico) (4%) (). A little over half (59%) of the providers were physicians, followed by nurses or physician assistants (24%), research staff (such as study coordinators) (13%) and pharmacists (4%). Almost all of the providers had participated in previous LA ART trials (91%) with the majority participating in two trials (43%); one quarter (25%) in three trials and the remaining 13% of providers involved in four or more LA ART trials. A total of 43% of providers had experience of transitioning patients back from injection to oral ART giving them another aspect to consider when implementing large-scale LA ART.
Table 2 Provider characteristics
Appropriate candidates
The chart () below indicates that approximately half (47%) of health care providers believed that “some” patients (25–50%) in their clinics would be appropriate candidates for LA ART. Significantly, nearly one quarter (23%) of providers believed that “many” (more than 50%) are appropriate candidates and only 7% thought that “None/Very Few (0–10%)” would be appropriate candidates.
Providers were asked to identify the characteristics of patients who would be the “most appropriate” patients for LA ART. reports on the patient characteristics perceived as “very or somewhat appropriate” compared to “not appropriate.” The results from demonstrate that patient preference is the most important factor driving HCPs views around the “appropriate candidates.” Almost all (99%) of HCPs felt that patients’ choice would drive the uptake of LA ART. Interestingly, 96% thought that “young people” also are appropriate candidates even though LA requires regular adherence with clinic visits. HCPs felt overwhelmingly that CAB + RPV LA provided opportunities for traditionally “difficult-to-treat” populations, including non-adherent patients (93%); prior treatment failure (84%); active lifestyle including frequent travel (84%); etc. However, a few categories of patients including substance users (32%) and those with “unstable” lifestyles (33%) were considered as not appropriate candidates, relative to other populations.
Table 3 Patient characteristics related to appropriateness for LA ART
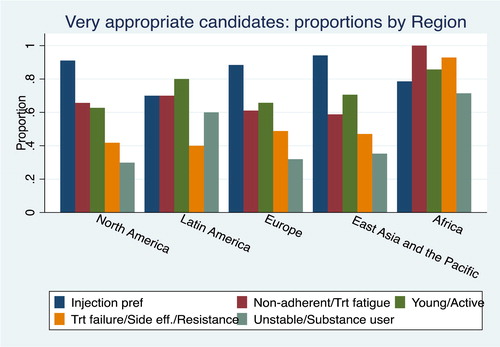
below shows differences based on region, regarding the patient groups who could benefit and are considered “very appropriate” for LA ART. For the North American, European and East Asia/Pacific based-providers, the most important consideration is injection preferences; in Latin America patients’ preference and the young/active were noted with highest priority; in Africa non-adherence with daily ART and treatment fatigue, treatment failure and resistance were considered the most important patients to benefit from LA ART. Importantly, the providers in the African region believed that almost all of the patient groups would be “very appropriate” candidates, with the exception of “substance users.”
Multivariate models
below describes factors associated with the proportion of patients deemed appropriate candidates for LA ART, comparing the outcomes of “some vs. few” and “many vs. few” candidates. Results of the multivariate multinomial model suggests that the African Region is an important predictor for “some” (AOR 2.29 p < 0.001) and “many” (AOR 19.54 p < 0.001) vs. “few” who are considered as appropriate candidates for LA ART compared to Europe. Likewise, a provider’s role in the clinic was an important factor, with nurses/PA and research staff and pharmacists, compared to physicians, reporting an increased likelihood that “some” (AOR = 2.51 95% CI 1.66–3.78 for nurse/PA; AOR = 2.65 95% CI 1.28–5.49 for research staff/pharmacists) and “many” patients in their clinics would be appropriate candidates (OR= 7.93 95% CI 4.72–13.21 for nurse/PA; AOR = 4.98 95% CI 1.37–18.10 for research staff/pharmacists). Similarly, the composite “benefits score,” indicated that greater benefit associated with LA ART was an important factor that “some” (AOR = 1.16; p < 0.001) and “many” (AOR = 1.29; p < 0.001) compared to “few” patients would be appropriate candidates, Lastly, provider experience with transitioning from LA to oral ART was found to be significantly related to a greater proportion of providers reporting that “some” (AOR 2.03; p = 0.007) and “many” (AOR 2.27; p = 0.004) compared to few candidates as appropriate for LA ART.
Table 4 Multivariate multinomial models of proportions of patients that are appropriate candidates for LA ART (N = 266)*
Based on the results from , we developed a multivariate ordinal proportional odds model examining the same outcome of proportion of appropriate candidates for LA ART. The ordinal logistic model is based on the outcome of the proportion of “appropriate candidates” operationalized on an ordinal scale (0, 1, 2). The principal assumption underlying the model is that the relationship between each pair of outcome groups is the same; the coefficients that describe the relationship between, for example, the lowest versus all higher categories of the response variable (“many/some” vs. “none”) are the same as those which describe the relationship between the next lowest category and all higher categories (“many/some” vs. “none”). This is also called the parallel regression assumption. The validity of the proportionally assumption was tested and upheld with the Brandt test and results of this model are shown in . Results from demonstrate that the African region remains highly significant as a higher proportion of these patients would be appropriate candidates for LA ART compared to all other regions (except Asia) (AOR 8.97; p < 0.001). Furthermore, nurses/PA and research staff/pharmacists had a higher odds of finding a greater number of appropriate candidates compared to physicians, respectively (AOR 3.42 p < 0.001; AOR 2.48; p = 0.19). Providers reporting a greater number of benefits of LA ART for patients also had a greater odds of finding an increased proportion of appropriate candidates (AOR 1.16; p < 0.001) for each unit increase in the benefits score. Lastly, those that had experience in transitioning patients from injection to oral were more likely to report a higher proportion of appropriate candidates in their clinic (AOR 1.64; p = 0.008).
Table 5 Multivariate proportional odds model for proportions of patients who are appropriate candidates (N = 266)
Discussion
The survey from health care providers participating in the ATLAS-2M trial is the first of its kind investigating the barriers and opportunities for large-scale rollout of LA ART across different geographies and settings. It provides an opportunity to glean information from providers regarding how to transition LA ART from clinical trial setting to a real-world implementation program. Our prior formative work focused on participants and providers in the US and Spain and included health care providers who were mainly physician investigators. The current study extends the scope of the research and includes a larger sample of health care professionals, including research staff, pharmacists, nurses and physician assistants, across 13 countries from multiple regions.
Understanding who may be the appropriate patients for LA ART in routine care from the perspective of health care providers is an important consideration as the research to date has been exclusively conducted within a clinical trial setting. Additionally, the clinical trial data have focused on the efficacy, safety, tolerability, and patient reported outcomes. Moreover, data from the ATLAS-2M study found that after switching to both long-acting arms (monthly and every-two-months), treatment satisfaction significantly improved for participants entering the trial from daily oral regimen, and participants favored the every-two-month regimen compared to the monthly regimen at Week 48 (HIVTSQs mean change from baseline was 4.86 points (95% CI: 4.02, 5.69) for the every-two-month arm and 3.12 points (95% CI: 2.29, 3.95) for the monthly arm.Citation12
Findings from the current study indicate that there are provider-specific differences in identifying the appropriate candidates based on several salient characteristics, including geographic location, role in clinic, and injection experience, among others. Providers in the African region reported greater odds of identifying appropriate candidates, despite potentially far greater implementation challenges in resource limited settings, such as providing the injections on a regular basis; maintaining the cold chain; and incorporating a clinic-based reminder system for patients. However, this is an important consideration as providers in the African region potentially need greater number of options in tackling the HIV epidemic. This finding related to the geographic variations points to the need to provide support to clinicians around identifying the appropriate candidates and ensuring a robust clinic management strategy.
Providers’ roles in the clinic and their experience administering injections were important predictors associated with the odds of identifying the appropriate candidates. We saw that nurses/physician assistants, pharmacists, and research staff had greater odds of reporting a greater proportion of appropriate candidates compared to physicians. The qualitative research conducted with providers in the US and Spain, in the Phase 2b and Phase 3 studies, were undertaken predominantly with physicians (most notably in Spain). Findings from the qualitative research suggest that the nurses (and not medical doctors) routinely administer the injections, and the same nurses often administer the injections to all the patients in the clinic. The reason for this practice is that as LA ART is an intramuscular injection and the proper administration is key to the successful implementation of the regimen. Therefore, it was important in the quantitative survey to explore the views of a wider group of providers regarding the appropriate candidates for LA ART. Overall, the body of evidence from the current quantitative study as well the prior formative interviews suggest that providers with more experience with long-acting therapy feel more comfortable with the regimen. Further research is needed to understand the role of the specific healthcare provider as well as the type and length of experience with the LA regimen. Lastly, as expected, providers who felt that there were more benefits to LA ART had a greater odds of identifying more patients as appropriate candidates.
While the study is the first of its kind to examine the “right” patients for LA ART, there are several considerations which limit the generalizability of the study. First, this is a cross-sectional study of providers within the context of a Phase 3 clinical trial. The providers participating in the survey have had more experience with the realities of administering and managing the process for LA in a clinic setting. Therefore, their views may be different and more positive than other infectious disease specialists outside of a clinical trial setting. In addition, some of the regions, especially the African region had a small number of providers who participated in the survey, and as such, findings may not be representative of the perceptions of infectious disease specialists in this region.
Conclusion
This study has demonstrated that there is a diverse group of patients that may be considered appropriate candidates for LA ART, from the perspective of the health care providers participating in the Phase 3 ATLAS-2M study. Ensuring that providers can identify the appropriate candidates and can modify their current practices to effectively integrate the LA treatment option will be one key factor for the successful implementation.
Acknowledgments
We wish to acknowledge the contributions of ViiV Healthcare and GSK who assisted with the site selection and identification of heath care providers included in the survey.
Additional information
Funding
Notes on contributors
Miranda Murray
Miranda Murray, Ph.D. is the CEO and founder of Healthy Analytics & Outcomes, a boutique consultancy specialising in health services research. She has 20 years of experienced in health research with a particular interest in infectious diseases. Most recently, she was the Head of the Global Health Outcomes division at ViiV Healthcare. She was instrumental in setting up the group and developing the health outcomes strategy for the Long-Acting Intramuscular Injection portfolio. She has extensive experience in academia, government, the UK National Health Service, and industry.
Deanna Kerrigan
Dr. Deanna Kerrigan is a Professor of Sociology and the Director of the Center on Health, Risk, and Society at American University. Her research focuses on social and structural factors affecting the health of underserved populations. She has led multiple studies of community-driven HIV prevention interventions among women in Latin America and sub-Saharan Africa. She was previously on the faculty of the Johns Hopkins University (JHU) Bloomberg School of Public Health for 15 years. At JHU, she served as the Co-Director of the Prevention Core of the NIH-funded JHU Center for AIDS Research (CFAR) and directed a USAID-sponsored global HIV prevention implementation science project, Research to Prevention (R2P).
Krischan J. Hudson
Krischan J. Hudson, PhD, MPH has 17 years of experience in academia and industry inclusive of phase 1-4 trials. Kris has been a Director of Clinical Development with ViiV for the past 5 years as a Clinical Investigation Lead and Manager of Early Phase Development. Previous positions held included clinical, operational lead, and portfolio management positions with Pfizer, and Amylin Pharmaceuticals, as well as academic research positions with the University of Virginia and the University of Miami. His career has encompassed multiple therapeutic indications including infectious diseases, diabetes, cardiovascular, obesity, and oncology indications. His research and focus on early drug development has led to important scientific advancements and contributed to successful marketing approvals for both small molecule and biologic products.
Nicola Walters
Nicola Walters is a clinical development manager at GSK. She has been in the role for the past four years and manages large-scale clinical trials on behalf of GSK, in the HIV area, including the LATTE2 trials as well as the ATLAS and ATLAS 2M studies.
Tahilin Sanchez Karver
Tahilin Sanchez Karver is a PhD candidate at the Johns Hopkins Bloomberg School of Public Health, where she is exploring the role of peer navigation on HIV treatment and care outcomes among female sex workers living with HIV in the Dominican Republic. She also provides research support to various projects including a longitudinal study exploring stigma, social cohesion and HIV outcomes among female sex workers living with HIV in Tanzania and the Dominican Republic; and a study seeking to understand the dynamics of switching to long-acting, injectable ART from fixed dose oral combination therapies among people living with HIV. She has worked domestically and internationally on topics related to women’s health, sexuality, and gender. Prior to her academic tenure at Hopkins, Tahilin collaborated on various projects, such as a pilot intervention to reduce gender-based violence in public buses in Mexico City, a study aiming at understanding the role of community level stigma on abortion practices, and a study evaluating access to contraception services across the United States.
Andrea Mantsios
Andrea Mantsios, PhD, MHS, is Founder and Principal Consultant of Public Health Innovation and Action (PHIA) based in New York, NY. She is a behavioral scientist by training working on research and programs focused on the role of social and structural factors that shape HIV risk behaviors as well as roll-out of biomedical approaches for optimal uptake and effectiveness. Her current work includes conducting research on long acting injectable (LAI) anti-retroviral therapy (ART) with clinical trial participants and providers on this new treatment modality to inform programmatic rollout. She also currently works on community-empowerment approaches to addressing HIV among female sex workers in Tanzania including research and programs aimed at addressing financial insecurity as part of a comprehensive approach to HIV prevention with sex workers. Previous work includes research on risks for HIV, tuberculosis, and syphilis among injection drug users in Tijuana, Mexico, and leading New York City’s jurisdictional efforts to scale up routine HIV screening in health care settings.
Noya Galai
Noya Galai, PhD, has over 30 years of experience in epidemiological and biostatistical research as a faculty member at the Johns Hopkins Bloomberg School of Public Health in Baltimore, MD, USA and in the department of statistics are the University of Haifa, Israel. Dr Galai also served for 10 years as head statistician at the Israel Ministry of Health as well as a consultant for a large HMO, focusing on quality of care studies. Her main research interests include the statistical methods utilized to study the natural history of HIV and substance use, risk factors among key populations and the evaluation of clinical and community-centered interventions. She has worked in the US and internationally in Europe, Asia and Africa. Dr Galai has also engaged in randomized clinical trials, both as PI of a data coordinating center for a multi-site trial of aspirin and pregnancy outcome as well as serving on the DSMB for an multi-national MS injectable treatment trial.
References
- Johnsen AT, Tholstrup D, Petersen MA, Pedersen L, Groenvold M. Health related quality of life in a nationally representative sample of haematological patients. Eur J Haematol. 2009;83(2):139–148.
- Gogtay JA, Malhotra G. Reformulation of existing antiretroviral drugs. Curr Opin HIV AIDS. 2013;8(6):550–555.
- Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929.
- Sullivan AK, Curtis H, Sabin CA, Johnson MA. Newly diagnosed HIV infections: review in UK and Ireland. BMJ. 2005;330(7503):1301–1302.
- Aldir I, Horta A, Serrado M. Single-tablet regimens in HIV: does it really make a difference? Curr Med Res Opin.. 2013;30(1):89–97.
- Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510.
- Swindells S, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020;382:1112–1123.
- Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020;382(12):1124–1135.
- Murray M, et al. Patient-reported outcomes on long-acting cabotegravir + rilpivirine as maintenance therapy: FLAIR 48-week results. Poster presented at: 10th IAS Conference; July 21–24, 2019; Mexico City, Mexico, MOPEB258.
- Murray M, et al. Patient views on long-acting HIV treatment: cabotegravir + rilpivirine as maintenance therapy (ATLAS 48-Week Results). Oral Presentation at: 10th IAS Conference; July 21–24, 2019; Mexico City, Mexico, MOAB0103.
- ATLAS-2M Press Release [cited August 31, 2019]. Available from: https://www.gsk.com/en-gb/media/press-releases/viiv-healthcare-reports-positive-phase-iii-study-results-of-investigational-long-acting-injectable-hiv-treatment-regimen-administered-every-two-months/.
- Overton ET, Richmond GJ, Rizzardini G, et al. Cabotegravir + rilpivirine every 2 months is noninferior to monthly: ATLAS-2M study. Conference on Retroviruses and Opportunistic Infections (CROI); 2020:8–11; Boston, Abstract 34.
- Mantsios A, Murray M, Sanchez Karver T, Davis W, Margolis D. "I feel empowered": women's perspectives on and experiences with long-acting antiretroviral therapy in the United States and Spain. Infectious Disease Week (IDWeek). Washington, DC; October 2, 2019.
- Kerrigan D, Mantsios A, Gorgolas M, et al. Experiences with long acting injectable ART: a qualitative study among PLHIV participating in a Phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One. 2018; 13(1):e0190487.