Abstract
Background
SARS-CoV-2 infection among People Living With HIV (PLWH) is not well-described.
Objective
To study COVID-19 symptoms and SARS-CoV-2 PCR-based swab testing among participants of the Multicenter AIDS Cohort Study (MACS) and Women’s Interagency HIV Study (WIHS).
Methods
A telephone survey was collected April-June 30, 2020. Symptom and testing prevalence were explored. Multivariable logistic regression was used to examine the factors associated with SARS-CoV-2 positivity.
Results
The survey was completed by 3411 participants, including 2078 (61%) PLWH and 1333 HIV-seronegative (SN) participants from across the US. Thirteen percent (n = 441) were tested for SARS-CoV-2 infection (13.4% of PLWH vs 12.2% of SN). Among those tested, positivity was higher in PLWH than SN (11.2% vs 6.1%, p = 0.08). Reasons for not being tested included testing not being available (30% of participants) and not knowing where to get tested (16% of participants). Most symptoms reported since January 2020 were similar in PLWH and SN, including headache (23% vs. 24%), myalgias (19% vs 18%), shortness of breath (14% vs 13%), chills (12% vs 10%), fever (6% vs 6%) and loss of taste or smell (6% vs 7%). Among PLWH who tested positive for SARS-CoV-2 DNA, the most common symptoms were headache (71%), myalgia (68%), cough (68%) and chills (65%). In multivariable analysis among those tested, the odds of SARS-CoV-2 positivity were higher among PLWH than SN (aOR = 2.22 95%CI = 01.01–4.85, p = 0.046) and among those living with others versus living alone (aOR = 2.95 95%CI = 1.18–7.40).
Conclusion
Prevalence and type of COVID-19 symptoms were similar in PLWH and SN. SARS-CoV-2 infection may be elevated among PLWH.
Introduction
Infections with the novel SARS-CoV-2 virus were first reported to the World Health Organization (WHO) on 31 December 2019, with SARS-Cov-2 being identified as the causative agent resulting in severe viral pneumonia. By August 1, 2020, there were >17.8 million confirmed cases of COronaVIrus Disease-2019 (COVID-19) due to SARS-Cov-2 infection and >685,000 deaths reported worldwide, including >4.7 million confirmed cases in the US.Citation1 However, the epidemiology of COVID-19 in the US population and people with chronic health conditions is not well understood, especially given limited screening capabilities at the time of this study. Screening questions have focused on common COVID-19 symptoms, including fever, cough, loss of taste or smell, and shortness of breath, but the occurrence of these symptoms varies in persons with COVID-19Citation2–5 with up to 40–45% of cases reportedly asymptomatic.Citation6,Citation7 Several COVID-19 case seriesCitation8,Citation9 and other US data from the CDC report that people hospitalized for COVID-19 are disproportionately older,Citation10 male, more likely to have comorbidities such as cardiovascular disease, hypertension, cancer, diabetes, sickle cell disease, and obesity,Citation11 and more likely to be Black or Latinx Americans.Citation12–15
People living with HIV (PLWH) are thought to possibly be at higher risk for acquiring SARS-CoV-2 and severe COVID-19 manifestations because of relative immunosuppression, higher rates of comorbidity, and higher social vulnerabilities, such as unstable or crowded housing and use of public transportation. However, initial studies suggest that PLWH have similar prevalence of SARS-CoV-2 positivity, severe COVID-19 disease,Citation16–18 and COVID-19 associated mortalityCitation17 compared to HIV-seronegative persons (SN). SARS-CoV-2 prevalence has varied geographically in the US, including an outbreak in New York City in spring of 2020Citation19 when our study was performed.
SARS-CoV-2 infection and COVID-19 disease risk among PLWH are not well-described, and there are few data to guide prevention and treatment recommendations. The US Department of Health and Human Services stated on 1 August 2020: “People with HIV who have COVID-19 have an excellent prognosis, and they should be clinically managed the same as persons in the general population with COVID-19”Citation20 but also noted that “until more is known, additional caution for all persons with HIV, especially those with advanced HIV or poorly controlled HIV, is warranted.”Citation20 It remains unclear whether HIV infection itself and/or the high prevalence of comorbid conditions among PLWH, including cardiovascularCitation21 and pulmonary disease,Citation22 increase risk of worse COVID-19 severity. The United Nations therefore advises that “As in the general population, older people living with HIV or people living with HIV with heart or lung problems may be at a higher risk of becoming infected with the virus and of suffering more serious symptoms.”Citation23
We sought to understand the type and occurrence of COVID-19 symptoms and prevalence of SARS-CoV-2 PCR-based testing and positivity among PLWH and SN adults who comprise a large longitudinal cohort of older women and men.Citation24 These sociodemographic attributes are under-represented in current case series of PLWH and COVID-19 patients.
Methods
The MACS/WIHS Combined Cohort Study (MWCCS) is a merger of two long-standing large multicenter US cohorts of PLWH and SN people, namely the Multicenter AIDS Cohort Study (MACS) and the Women’s Interagency HIV Study (WIHS).Citation24 COVID-19 related surveys were reviewed and approved by each MACS and WIHS local IRB. All MACS and WIHS participants (called MWCCS participants hereafter) were eligible. An interviewer-administered telephone survey was conducted between 8 April and 30 June 2020, with most interviews taking place in May 2020. The survey was offered in English and Spanish. Verbal consent was collected prior to the start of the telephone interview by trained study staff. Participants were compensated for their time.
The survey was developed by MWCCS investigators to study COVID-19 symptoms, SARS-CoV-2 testing, and psychosocial effects of the pandemic (the latter are not included in this report). This survey is in the public domain and available at https://statepi.jhsph.edu/mwccs/data-collection-forms/(see V100/COVID). Interviewers asked about 14 individual COVID-19 symptoms, and their duration since January 2020. The recall period was the previous four months for most participants (range, three to six months depending on survey completion date). The survey also queried whether participants had been tested for SARS-CoV-2, test results, and possible risk factors for SARS-CoV-2 infection, including social distancing measures and household density.
Documented SARS-CoV-2 testing and diagnosis were verified through medical records when possible. At the time of this analysis, acquisition of medical records for confirmation of self-reported positive result is ongoing since the MWCCS is not a clinic-based cohort and thus requires request and retrieval of medical records from an array of facilities and healthcare providers. SARS-CoV-2 testing and results are therefore self-reported by participants, with confirmation among a subset. SARS-COV-2 antibody testing was not broadly available or utilized at the time this survey was administered, so all self-reported test results are assumed to be PCR-based tests for presence of SARS-CoV-2 DNA in biospecimens collected via nasopharyngeal or oropharyngeal swabs.
In addition, we collected self-report of hospitalization due to “COVID-19 disease or difficulty breathing or a respiratory infection.” This was done since SARS-CoV-2 diagnostic testing was not widely available in many places early in the pandemic when this survey was administered.
Statistical methods
Baseline participant characteristics were carried forward from the last in-person MACS and WIHS visit (2018–2019) and presented stratified both by HIV status and sex, as the men and women enrolled into MACS and WIHS differ sociodemographically. Social distancing measures reported by the participant and the locally mandated social distancing policies at each study site at the time of the survey were described. Prevalence of COVID-19 symptoms and SARS-CoV-2 test positivity were explored overall and stratified by HIV serostatus. Differences in categorical variables were tested using Chi-square analyses (or Fischer’s exact test when values in subgroups were <5). Predictors of SARS-CoV-2 positivity were explored using logistic regression models. Odds ratios (OR) and 95% Confidence Intervals (95% CI) are reported. The following covariates were included in the analyses: sociodemographic factors (age, race, study site, number of people in household), biologic factors, body mass index, plasma HIV RNA (viral load), CD4 cell count, antiretroviral therapy use), and COVID-19 symptoms. Multivariate models included covariates that were significant in univariate analyses. Significance was evaluated using 2-tailed tests, at p < 0.05. SAS version 9.4 was used for all analyses. Funder had no role in the design, collection or analysis of data.
Results
MWCCS participant characteristics
A total of 4123 eligible MWCCS participants were contacted. Of these, 82.7% (N = 3411) completed the survey, 13.9% (N = 573) could not be reached, and 3.4% (N = 139) declined to participate. Participants completing the survey included 61% (N = 2078) PLWH and 39% (N = 1333) SN MWCCS participants from diverse regions of the US (). Most participants completed the survey in May (median date = 11 May 2020, IQR = 1–22 May 2020). The median age was 57 years (range 26–94 years) and, among PLWH at their most recent in person visit (2018–19), the median CD4+ T lymphocyte cell count was 682 cells/mm3; 74% had undetectable HIV viral loads load, i.e. <20 copies/mL.
Table 1 Prevalence of COVID-19 symptoms since the U.S. outbreak started, by HIV serostatus^ (administered April–June 2020 and representing past ∼4 months) in the MWCCS
Participants included men (46%) and women (54%) who were Black non-Hispanic (48%), White non-Hispanic (36%) Hispanic (13%), and other races (3%); 24% currently smoked. Most (87%) participants lived in their own house, 10% lived in a parent’s or other person’s house. One-third of participants lived alone (33%).
Safety measures, including social distancing
We explored social distancing behaviors as context for exposure to SARS-CoV-2. Most participants reported practicing multiple social distancing measures including staying home as much as possible (97%) and maintaining physical distance of at least 6 feet (98%) between themselves and others. These practices were similarly common among PLWH and SN (). Another 8% reported self-isolating, which included self-isolating due to symptoms or having a positive SARS-CoV-2 test (2.5% of participants), due to exposure to an infected person (2.2%), due to being unsure of their infection status (5.4%). Most participants (75%) reported taking “other steps” in addition to those asked about in our survey. The most common ‘other steps’ included mask use (62%), glove use (22%), and cleaning of hands and surfaces (34%). There were 456 participants (13%) who reported not making changes to daily life and routine, and this was more commonly reported among PLWH than SN (15% vs 11%, p = 0.003); these participants were primarily living in Illinois (29%), Maryland (18%), and New York, DC, and Mississippi (each 10%).
Table 2 Cumulative prevalence of SARS-CoV-2 infection via PCR-based testing at time of survey completion (April–June 2020), by HIV status^ among MWCCS participants
A summary of local social distancing policies in effect at the time of survey collection is provided in for context. During this time, every site was under local social distancing mandates including sheltering in place directives when survey data collection began and ended ().
Table 3 Reasons not tested for SARS-CoV-2 infection among those not tested by HIV serostatus^
COVID-19 symptoms in spring 2020 in the MWCCS
Despite social distancing, many (53%) MWCCS participants self-reported having at least one of the symptoms queried in the survey since the COVID-19 outbreak began (); 20% had at least one symptom while taking the survey. Despite current symptom prevalence being similar in PLWH and SN (20% vs 21%, p = 0.43), PLWH were less likely to report at least one symptom since the outbreak began compared to SN (51% vs. 57%, p = 0.002).
The most common symptoms reported were similar in PLWH and SN, including headache (23% vs. 24%), myalgias (19% vs 18%), shortness of breath (14% vs 13%), chills (12% vs 10%), fever (6% vs 6%) and loss of taste or smell (6% vs 7%). Symptom profiles were similar in PLWH and SN (), except for runny nose, cough and sore throat, for which cumulative prevalence was slightly but significantly lower in PLWH.
Among 41 MWCCS participants (31 PLWH and 10 SN) who reported they tested positive for SARS-CoV-2 (), the most frequently reported symptoms were headache (71%), myalgia (68%), cough (68%) and chills (65%). Over half (55%) reported loss of taste or smell. Median duration of symptoms was greater than one week for cough (14 days, IQR 7–21) and loss of taste or smell (9 days, IQR 7–12), and a week for myalgia and sore throat. Myalgia (68% vs 100%, p = 0.039) and having a fever (35% vs 80%, p = 0.027) were less common among PLWH than SN ().
Table 4 Predictors of SARS-CoV-2 positivity (PCR-based test) among 433 MWCCS participants tested at the time of this survey April–June 2020
SARS-CoV-2 positivity (PCR-based testing) in the MWCCS
In total, 441 participants (12.9%) reported having been tested for SARS-CoV-2 infection, and this was similar by HIV serostatus (). Of the 41 self-reported positive SARS-CoV-2 tests, records were received for 12 (all confirming positive status) by time of this publication. While similar proportions of PLWH and SN were tested, PLWH had a higher prevalence of positive tests than SN (11.2% vs 6.1%, p = 0.08). Of the 31 PLWH who reported a positive test, 29 (94%) were receiving antiretroviral therapy at the time of the interview, 15 (63%) were virologically suppressed; and median CD4 cell count at last study visit was 673 cells/mm3 (IQR 536–871). Among PLWH, the prevalence of SARS-CoV-2 positivity did not differ by race/ethnicity, but was higher among PLWH than SN for each race/ethnic group (, p = 0.008), including Black (11.1% vs 4.7%), Hispanic (13.7% vs 11.8%), and White (11.4% vs 5.5%) participants.
Figure 1 Prevalence of SARS-CoV-2 positivity among MWCCS participants tested by study site and HIV serostatus∼
∼Number tested at each site is shown below each bars, by HIV status. There were 41 SARS-CoV-2 positive cases, this included 16 cases in New York (11 PLWH and 5 SN), 2 PLWH cases in Pennsylvania, 4 cases in Georgia (3 PLWH and 1 SN), 1 PLWH case in Florida, 7 PLWH cases in Illinois, 4 cases in Maryland (2 PLWH and 2 SN), 6 cases in California (4 PLWH and 2 SN), and 1 PLWH case in Washington DC. Fischer’s Exact test was used for the p-value for the difference in percent positive by site.
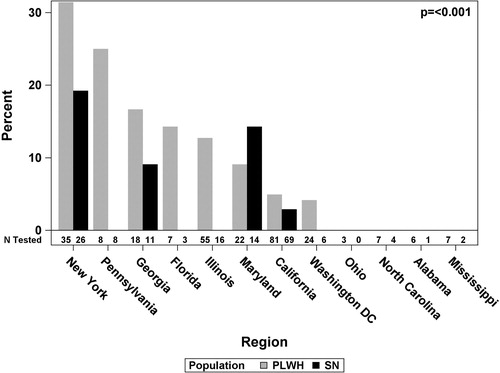
Prevalence of SARS-CoV-2 test positivity also varied across study sites, (p < 0.001, ) as expected, given geographic differences in the epidemic during this time period, but was consistently higher among PLWH than SN at every site, except Maryland (). Among PLWH tested for SARS-CoV-2, the highest positivity was in New York City (26%), followed by Georgia (13.8%), Florida (10.0%) and Illinois (9.8%). No positive cases were reported in North Carolina, Alabama or Mississippi, but COVID-19 cases had not yet spiked in the Southern US and testing was limited in these locations during the time period of our survey.
Given limitations in access to SARS-CoV-2 testing at the time of survey administration, we asked participants about hospitalization since January 2020 for “COVID-19, difficulty breathing, or respiratory infection” (). PLWH and SN reported similar prevalences of COVID/respiratory-related hospitalization since January 2020 (1.8% vs 1.6%, p = 0.58). Only 33 (56%) of 59 hospitalized participants had been tested for SARS-CoV-2, of whom 12 (36%) were positive. Given testing limitations we also asked participants about reason(s) that they had not been tested (). Among participants who did not get tested for SARS-CoV-2, most (82.2%) reported “not having any symptoms” as the reason for not testing. However, several other reasons for not being tested were reported, including: “testing not available” (29.5%), “not knowing where to get tested” (16.2%) and being “told by a healthcare provider to self-isolate instead of testing” (3.4%). Barriers to testing included lack of transportation (4.8%), concerns about the cost of testing (3.5%), and needing to take time off work to get tested (1.3%). Some participants reported other reasons for not testing, including fear of getting tested (1.9%), mistrust in the available tests (1.4%), and a belief that they previously had been infected with SARS-CoV-2 (0.5%). Reasons for not being tested were similar by HIV serostatus ().
Predictors of SARS-CoV-2 positivity
Predictors of SARS-CoV-2 positivity are shown in . Data suggested higher odds of a self-reported positive test among PLWH than SN (OR = 1.92, 95%CI = 0.92–4.03, p = 0.08, ). Participants in the Northeast (New York City) had eight times the odds of positivity compared to those in the West (California, OR = 8.53 95%CI = 3.15–23.11). People who shared their residence were also more likely to report a positive SARS-CoV-2 test compared to those who lived alone (OR = 2.87, 95%CI = 1.18–7.0, p = 0.036). Odds of positivity did not increase further with increasing number of people per household, as odds were similarly increased among those living with one to two people (OR = 3.0, 95%CI = 0.81–10.9) or three or more people (OR = 2.9, 95%CI = 0.15–15.6) compared to those living alone. Age and race/ethnicity were not risk factors for SARS-CoV-2 positivity among persons tested. After adjusting for region and people in the household, PLWH remained at higher odds of SARS-CoV-2 positivity compared to SN persons (aOR = 2.22 95%CI = 1.01–4.85, p = 0.046). Living with at least one other person (aOR = 2.95 95%CI = 1.18–7.4) also remained an independent predictor of positivity.
A variety of symptoms were associated with higher odds of SARS-CoV-2 positivity, and these associations were similar by HIV serostatus (Supplemental Table 5) and sex. The symptom with the strongest association for SARS-CoV-2 positivity was loss of taste or smell (aOR = 10.5, 95%CI = 4.9–22.4). Muscle aches, chills, and fever each were associated with a five to eight-fold increase in odds of SARS-CoV-2 positivity (). Odds of SARS-CoV-2 positivity was greatly elevated among those who had both fever and shortness of breath (aOR = 26.3 95%CI = 5.0–138.3).
Discussion
Among a cohort of 3411 men and women including PLWH and SN individuals residing in diverse US locations who were surveyed between April and June 2020, 9.3% (41 of 441) of those tested reported having a positive SARS-CoV-2 test and over 50% of all participants reported having at least one symptom since the COVID-19 outbreak began. SARS-CoV-2 positivity appered higher among PLWH compared to SN participants, however this difference was marginally significant and based upon a limited number of individuals tested. While 83% of participants reported not having been tested for SARS-CoV-2 because they had not experienced symptoms, other participants reported wishing to test but not knowing where to get tested, being told to self-isolate instead of testing, or having barriers to testing (transportation, needing to take time off work, testing being hard to get); reports of testing barriers and symptoms in untested individuals, suggests actual infection rates may be higher.
Our finding that positive SARS-CoV-2 tests may be higher among PLWH than SN participants differs from several other reports suggesting that PLWH are not at increased risk for SARS-CoV-2 positivityCitation18,Citation25 or over-represented among COVID-19 cases thus far during the pandemic.Citation26 The Veterans Aging Cohort Study (VACS) found similiar SARS-CoV-2 positivity in their PLWH and SN participants (6–8% of whom had been tested, compared to the 12–14% of MWCCS participants tested). SARS-CoV-2 positivity was slightly lower in VACS PLWH than MWCCS PLWH (9.7% vs 11.2%), and higher in VACS SN than MWCCS SN (10.1% vs 6.1%). Higher SARS-CoV-2 positivity among PLWH than SN in our study could be explained by several factors other than HIV itself such as higher prevalence of co‐morbidities, differences in social distancing measures, or other confounding factors that influence infection among PLWH. Several barriers to testing were also reported and some participants who were infected may not have been tested, or the subset of participants tested may not have been representative.
The type, prevalence, and duration of self-reported COVID-19 symptoms were similar among PLWH and SN in the MWCCS overall, and among those with a history of a SARS-CoV-2 positive test, although myalgias and fever appeared less common among PLWH with SARS-CoV-2 positivity. Symptoms reported by SARS-CoV-2 positive PLWH in our study were similar to those generally reported for COVID-19, with high prevalence of fever, myalgia, and cough;Citation27,Citation28 however, prevalence of headache (71%), and loss of smell or taste (55%) in our study was higher than some others.Citation27,Citation28
In contrast to the marked racial/ethnic disparities in the overall US epidemic, we observed no racial or ethnic differences in the distribution of SARS-CoV-2 positivity. This may be due to the higher age of our participants, or the similar socioeconomic characteristics represented across MWCCS racial groups. A large proportion of Black participants in the MWCCS are female (80%) and there may be unmeasured confounding factors.
Regional differences in the prevalence of infection in the MWCCS reflect the epidemiology of SARS-CoV-2 infection in the US during the spring of 2020. The odds of having a positive SARS-CoV-2 test were substantially higher among participants who lived in the Northeast (New York) than those who lived in the West or South, reflective of the outbreak in New York during the time this survey was administered.
Strengths of these data are many. They were collected in a large sample of US men and women LWH and SN individuals from geographic locales across the US during the first six months of the SARS CoV-2 pandemic. MWCCS adults represent those at risk for SARS-CoV-2 and severe COVID-19 disease due to their diverse sociodemographic backgrounds, older age, multimorbidity, as well as HIV- and other infectious disease-related factors. We were able to implement telephone-based survey interviews across 13 MWCCS sites within a short period and with high response rates during this challenging time. As with any survey-based study design, there were limitations. Notably, given the nature of the COVID-19 outbreak and “stay in place” mandates across the US, we relied on self-reported symptoms and test results. However, we were able to perform medical record abstraction to confirm a subset (29%) of SARS-CoV-2 positivity, and we continue to seek records to confirm remaining test results. MWCCS clinical research sites either are comprised of all women (WIHS) or all men (MACS), therefore, the geographic distribution of participants was different by sex and the effect of sex on SARS-CoV-2 positivity could not be analyzed. We collected data on 3411 participants, 441 of whom had been tested for SARS-CoV-2 infection, but with 41 positive tests we had limited power to explore multivariate risk factors for positivity. Moreover, 13.9% of MWCCS participants could not be reached. It is possible that some of these men and women were hospitalized or had COVID-19 at this time.
Conclusion
Our report represents a first real-time, longitudinal effort to estimate SARS-CoV-2 prevalence and symptoms associated with COVID-19, as well as testing patterns, among a well-characterized, diverse group of adults who are at increased COVID-19 risk due to older age, race and ethnicity, HIV, and chronic disease morbidities. It describes the prevalence of COVID-19 symptoms among those tested for SARS-CoV-2, but also overall among the entire cohort during a period when many people with symptoms were not undergoing testing. This study informs our understanding of SARS-CoV-2 among PLWH and our findings indicate that COVID-19 symptoms are similar by HIV serostatus. We will continue to administer the telephone survey in MWCCS participants to understand the epidemiology and clinical characteristics of SARS-CoV-2 as it unfolds.
Author contributions
All authors contributed to the drating or revising of the manuscript and final approval of the submitted version. All authors contributed to the design of work (GD, DG, SK, CR, FJP, PCT, AAA) and/or acquisition of data (DG, SK, MLA, CR, AS, FJP, PCT, RD, MCK, CL, CR, ALF, JM, AAA) and/or analysis of data (GD, GS).
Supplemental Material
Download MS Word (46.9 KB)Acknowedgement
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR). Dr. Lahiri is also supported by NIH/NIAID K23AI124913.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Disclosure statement
Palella FJ has been a consultant and/or has provided lectures for the following: Gilead Sciences, Janssen Pharmaceuticals, ViiV Healthcare and Merck. Sharma A has received grant funding from Gilead Sciences, Inc. PCT has received grant support from Merck. Aadimora AA has received consulting fees from Viiv, and Gilead and her institution has received funding from Gilead for her research. All other authors have no conflicts to report.
Additional information
Notes on contributors
Gypsyamber D’Souza
Gypsyamber D’Souza, PhD, is a Professor of Epidemiology at the Johns Hopkins Bloomberg School of Public Health, Baltimore MD, USA. She is mPI of MWCCS datacenter.
Gayle Springer
Gayle Springer, MLA, is a senior research data manager at the Johns Hopkins Bloomberg School of Public Health, Baltimore MD, USA. She is the lead data center manager for MWCCS data.
Deborah Gustafson
Deborah Gustafson, PhD, is Professor of Neurology at the State of New York Downstate Health Sciences University. She is mPI of the Brooklyn MWCCS site.
Seble Kassaye
Seble Kassaye, MD, is an Associate Professor of Medicine at Georgetown University, Washington DC, USA. She is mPI of the Washington D.C. MWCCS site.
Maria L. Alcaide
Maria L. Alcaide, MD, is an Associate Professor of Medicine at the University of Miami Miller School of Medicine, Miami, Fl, USA. She is mPI of the Miami MWCCS site.
Catalina Ramirez
Catalina Ramirez, MPH, is a Research Director at the University of North Carolina at Chapel Hill, Chapel Hill, NC, USA. She is the project director of the Chapel Hill MWCCS site.
Anjali Sharma
Anjali Sharma, MD, is an Associate Professor of Medicine at the Albert Einstein College of Medicine, Bronx, NY, USA. She is mPI of the Bronx MWCCS site.
Frank J. Palella
Frank J. Palella, MD, is a Professor of Medicine at Northwestern University Feinberg School of Medicine, Chicago, IL, USA. He is a coinvestigator of the Chicago Northwestern MWCCS site.
Phyllis C. Tien
Phyllis C. Tien, MD, is a Professor of Medicine at the University of California- San Francisco, San Francisco, CA, USA. She is mPI of the San Francisco MWCCS site and MWCCS EC co-chair.
Roger Detels
Roger Detels, MD, is a Professor of Epidemiology at the Fielding School of Public Health, UCLA, Los Angele, USA. He is mPI of the Los Angeles MWCCS site.
Mirjam-Colette Kempf
Mirjam-Colette Kempf, PhDm is a Professor of Medicine at the University of Alabama at Birmingham, Birmingham, AL, USA. She is mPI of the Birmingham MWCCS site.
Cecile D. Lahiri
Cecile D. Lahiri, MD, is an Assistant Professor of Medicine at the Emory University School of Medicine, Atlanta, GA, USA. She is a coinvestigator of the Atlanta MWCCS site.
Charles R. Rinaldo
Charles R. Rinaldo, PhD, is a Professor of Infection Disease and Microbiology at the University of Pittsburgh, Pittsburgh, PA, USA. He is mPI of the Pittsburgh MWCCS site.
Audrey L. French
Audrey L. French, MD, is Director of HIV Inpatient Services at the CORE Center/Stroger Hospital of Cook County, Chicago IL, USA. She is mPI of the Chicago Cook County MWCCS site.
Joseph B. Margolick
Joseph B. Margolick, MD14 Professor of Molecular Microbiology and Immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore MD, USA. He is mPI of the Baltimore MWCCS site.
Ada A. Adimora
Ada A. Adimora, MD, is a Professor of Epidemiology at the UNC Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA. She is mPI of the Chapel Hill MWCCS site.
References
- WHO. WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int. Accessed August 3, 2020.
- Ge H, Wang X, Yuan X, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020;39(6):1011–1019.
- Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;55(5):105955.
- Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis. 2020;7(2):91–96.
- Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012–2022.
- CDC. COVID-19 pandemic planning scenarios. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html. Published 2020. Accessed August 6, 2020.
- Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362–367.
- Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020; 369:m1996.
- Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985.
- CDC. COVID-19 risk: older adults. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html. Published 2020. Accessed August 6, 2020.
- CDC. COVID-19 risk: people with certain medical conditions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Published 2020. Accessed August 6, 2020.
- Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464.
- Gold JAW, Wong KK, Szablewski CM, et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 - Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(18):545–550.
- Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543.
- Hsu HE, Ashe EM, Silverstein M, et al. Race/ethnicity, underlying medical conditions, homelessness, and hospitalization status of adult patients with COVID-19 at an Urban Safety-Net Medical Center - Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(27):864–869.
- Park L, Rentsch C, Sigel K, Rodriguez-Barradas M. COVID-19 in the largest US HIV cohort. AIDS 2020 Virtual Meeting Abstract.
- Sigel K, Swartz T, Golden E, et al. Covid-19 and people with HIV infection: outcomes for hospitalized patients in New York City [Epub Ahead of Print]. Clin Infect Dis. 2020;ciaa880. https://doi.org/10.1093/cid/ciaa880.
- Kanwugu ON, Adadi P. HIV/SARS-CoV-2 coinfection: a global perspective [Epub Ahead of Print]. J Med Virol. 2020. https://doi.org/10.1002/jmv.26321.
- CDCMMWR. Preliminary estimate of excess mortality during the COVID-19 outbreak — New York City, March 11–May 2, 2020. MMWR Morb Mortal Wkly Rep 2020;69(19):603–605.
- Interim guidance for COVID-19 and persons with HIV COVID-19 and persons with HIV (Interim Guidance). AIDSinfo. https://aidsinfo.nih.gov/guidelines/html/8/covid-19-and-persons-with-hiv–interim-guidance-/554/interim-guidance-for-covid-19-and-persons-with-hiv. Accessed June 19, 2020.
- Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160(7):458–467.
- Gingo MR, Balasubramani GK, Rice TB, et al. Pulmonary symptoms and diagnoses are associated with HIV in the MACS and WIHS cohorts. BMC Pulm Med. 2014;14:75.
- UNAIDS. Covid-19 and HIV. What people living with HIV need to know about HIV and COVID-19. https://www.unaids.org/en/covid19. Accessed July 19, 2020.
- MACS/WIHS combined cohort study. MACS/WIHS combined cohort study. https://mwccs.org/. Published 2020. Accessed July 28, 2020.
- No link between HIV status and coronavirus outcomes in large US study. aidsmap.com. https://www.aidsmap.com/news/jul-2020/no-link-between-hiv-status-and-coronavirus-outcomes-large-us-study. Accessed July 13, 2020.
- Masukume G, Mapanga W, Grinberg S, van Zyl DS. COVID-19 and HIV co-infection an emerging consensus [Epub Ahead of Print]. J Med Virol. https://doi.org/10.1002/jmv.26270.
- Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):759–765.
- Stawicki SP, Jeanmonod R, Miller AC, et al. The 2019-2020 novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic: a Joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group Consensus Paper . J Glob Infect Dis. 2020;12(2):47–93.
