Abstract
Background: Hypersensitivity reaction (HSR) and hepatotoxicity are rare, but potentially serious side-effects of antiretroviral use.
Objective: To investigate discontinuations due to HSR, hepatotoxicity or other reasons among users of dolutegravir (DTG) vs. raltegravir (RAL) or elvitegravir (EVG) in the EuroSIDA cohort.
Methods: We compared individuals ≥18 years and starting combination antiretroviral therapy (ART, ≥3 drugs) with DTG vs. RAL or EVG, with or without abacavir (ABC), between January 16, 2014 and January 23, 2019. Discontinuations due to serious adverse events (SAEs) were independently reviewed.
Results: Altogether 4366 individuals started 5116 ART regimens including DTG, RAL, or EVG, contributing 9180 person-years of follow-up (PYFU), with median follow-up 1.6 (interquartile range 0.7–2.8) years per treatment episode. Of these, 3074 (60.1%) used DTG (1738 with ABC, 1336 without) and 2042 (39.9%) RAL or EVG (286 with ABC, 1756 without). 1261 (24.6%) INSTI episodes were discontinued, 649 of the DTG-containing regimens (discontinuation rate 115, 95% CI 106–124/1000 PYFU) and 612 RAL or EVG-containing regimens (173, CI 160–188/1000 PYFU). After independent review, there were five HSR discontinuations, two for DTG (one with and one without ABC, discontinuation rate 0.35, CI 0.04–1.28/1000 PYFU), and three for RAL or EVG without ABC (0.85, CI 0.18–2.48/1000 PYFU). There was one hepatotoxicity discontinuation on DTG with ABC (discontinuation rate 0.18, CI 0.00–0.99/1000 PYFU).
Conclusion: During 5 years of observations in the EuroSIDA cohort independently reviewed discontinuations due to HSR or hepatotoxicity were very rare, indicating a low rate of SAEs.
Introduction
Integrase strand-transfer inhibitors (INSTIs) have become key components of modern combination antiretroviral therapy (cART) to control HIV.Citation1,Citation2 Potent new second generation INSTIs such as dolutegravir (DTG), bictegravir, or cabotegavir, with high barriers to resistance, few drug–drug interactions and good tolerability, have recently been introduced. DTG is now widely recommended in both first- and second-line ART regimens,Citation3,Citation4 including in the widespread rollout of ART in low and middle income countries where resistance to some non-nucleoside reverse transcriptase inhibitors is increasing.Citation5–7
Although DTG is generally well-tolerated, some instances of rare serious adverse reactions, including hypersensitivity reaction (HSR), hepatotoxicity and severe skin rashes have been reported in early trials,Citation8–10 independent of HLA-B*5701-related HSRs to the nucleotide reverse transcriptase inhibitor (NRTI) abacavir (ABC)Citation11 that is frequently co-formulated with DTG.
In this post-authorization safety study, we monitored the incidence of serious adverse events, as adjudicated by an independent review panel, due to HSR, hepatotoxicity or severe skin rash, and discontinuations for any reason, among users of DTG compared to those taking raltegravir (RAL) or elvitegravir (EVG) over 5 years in the EuroSIDA study.
Methods
EuroSIDA is a multinational prospective observational cohort study which has systematically collected epidemiological, clinical, biological, and therapeutic data for more than 23,000 persons living with HIV in 35 European countries, Israel and Argentina since 1994 (https://www.chip.dk/Studies/EuroSIDA).Citation12
Individuals included were ≥18 years old and started or switched to a new cART regimen consisting of ≥3 anti-retroviral drugs (ARVs) including DTG, RAL, or EVG, with or without ABC. Baseline was the date of first INSTI use after January 16, 2014, when marketing authorization for DTG was granted by the European Medicines Agency; regimens with RAL or EVG prior to January 16, 2014 were not included. Individuals were divided into four groups, those using DTG with or without ABC, or another INSTI (RAL or EVG) with or without ABC, and followed until they discontinued the INSTI, their last clinic visit, or January 23, 2019. Individuals could be included in more than one treatment group over time.
HSR was defined as either (A) an event where the INSTI was discontinued due to hypersensitivity, anaphylactic reaction, allergic reaction or drug allergy related to the INSTI, or (B) where two or more events were reported from the following groups of symptoms: rash, fever, gastrointestinal symptoms (nausea, vomiting, diarrhea, abdominal pain), constitutional symptoms (lethargy, fatigue, malaise, myalgia, arthralgia, general ill feeling), respiratory symptoms (dyspnea, sore throat, cough, or chest X-ray changes), eosinophilia or hepatic dysfunction, and where there was a reasonable possibility of a causal relationship with INSTI use. Hepatotoxicity, as possible drug-induced liver injury (DILI), was defined as elevation of alanine aminotransferase (ALT) concentration ≥5-fold above the upper limit of normal (ULN, 40 U/L), or ALT ≥3-fold above ULN with simultaneous elevation of total bilirubin to ≥2-fold above ULN (ULN for bilirubin =1.2 mg/dL).Citation13 Severe skin rash was defined as a grade 3 or 4 skin rash according to the division of AIDS AE toxicity grading scale.Citation14
All discontinuations of DTG, RAL, or EVG and discontinuation reasons were recorded and reviewed by the Coordinating Center in Copenhagen. Any discontinuations where hypersensitivity (including allergic reaction, anaphylactic reaction or any drug allergy related to DTG or another INSTI) or liver toxicity were suspected or the discontinuation reason unknown, a case report form (CRF) was requested from the local site to provide additional information. There was a dedicated Endpoint Review Committee (ERC) of 12 independent clinicians. CRFs were reviewed by the ERC coordinator and another ERC member to identify any situations requiring full review. Suspected HSR, hepatotoxicity, or severe skin rash events were adjudicated by at least three members of the ERC who had to agree whether the reported event met the study diagnostic criteria for a confirmed or probable INSTI-related HSR, liver toxicity or rash. Disagreements among reviewers were adjudicated.
For analysis, discontinuations were stratified according to discontinuation reasons (verified serious adverse events, and other reasons, including treatment failure, toxicity, patient’s wish, physician’s decision, treatment simplification, other reasons, or unknown). Primary analysis was on-treatment. Rates of discontinuations were calculated as the number of discontinuations divided by person-years of follow-up (PYFU). Where there were <20 discontinuations, exact confidence intervals were used.
For multivariable modeling of discontinuation rates, we considered time-updated characteristics. Confounding and effect modifying factors that were significant in univariate analyses (p < 0.1) were included in multivariate models, as well as treatment group and whether the patients were antiretroviral naïve at starting the regimen. Excluded variables were added in turn to determine if their inclusion improved the fit of the model (defined as a significant reduction in the log-likelihood).
Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
During five years of observation from January 16, 2014, 4366 individuals started a new INSTI-containing regimen, of which 2711 started DTG (1545 with ABC, 1166 without) and 1655 started RAL or EVG (239 with ABC, 1416 without, Supplemental Table S1). Individuals starting an INSTI had a median age of 50.9 years (IQR: 43.9–56.2) and were mainly male (n = 3215; 73.6%) and of white ethnicity (n = 3587; 82.2%). Most individuals starting a new INSTI regimen had viral loads <400 copies/ml and ≥350 CD4 T-cells/µl. Fewer than 4% were ART-naïve and 82.2% were integrase inhibitor-naïve. In an analysis of INSTI use and discontinuations stratified by sex, women were younger at baseline, more women were of non-white or unknown ethnicity, 32.5% acquired HIV through intravenous drug use and 58.1% through heterosexual sex, with heterogeneity between treatment groups (see Supplemental Table S2). A larger proportion of women than men started their first INSTI regimen with unsuppressed viral load or low CD4 cell counts.
Individuals started 5116 INSTI-containing ARV regimens (note that some individuals took multiple regimens during the 5-year study period), of which 3074 were with DTG (1738 with ABC, 1336 without) and 2042 with RAL or EVG (286 with ABC, 1756 without), with altogether 9180 PYFU of INSTI use (median 1.6, IQR 0.7–2.8 years/person). Overall, 3760 individuals (86.1%) contributed only one INSTI episode, 494 (11.3%) used two different INSTI regimens and 112 (2.6%) had three or more INSTI episodes. One thousand two hundred and sixty-one INSTI use episodes (24.6%) were discontinued, of which 349 were for DTG with ABC, 300 for DTG without ABC, and 612 for RAL or EVG (98 with ABC and 514 without ABC) (). The discontinuation rate for any reason for DTG was 115 (95% CI 106–124)/1000 PYFU and for RAL or EVG was 173 (CI 160–188)/1000 PYFU (p < 0.0001).
Table 1. Discontinuations of DTG or other INSTIs, and reasons for discontinuation.
After independent review, there were five discontinuations due to HSR, two for DTG (one with and one without ABC, discontinuation incidence rate 0.35, CI 0.04–1.28/1000 PYFU), and three for RAL or EVG without ABC (discontinuation incidence rate 0.85, CI 0.18–2.48/1000 PYFU, ), none of which were fatal. One HSR occurred in an individual aged between 40 and 50 years and four were in individuals >50 years old, three were among men (one for DTG without ABC, and two for RAL or EVG without ABC) and two were among women (one for DTG with ABC and one for RAL or EVG without ABC). Two individuals with HSR had prior AIDS and two prior non-AIDS clinical conditions, three had hypertension, three were co-infected with hepatitis C virus and one with hepatitis B virus. Four of the HSR events included gastro-intestinal symptoms (nausea or vomiting), three individuals experienced skin rash, one reported a fever and one dyspnea. All except one of the events occurred more than 1 month after starting the INSTI. There was one hepatotoxicity discontinuation in a woman treated with DTG with ABC for >6 months (discontinuation incidence rate 0.18, CI 0.00–0.99/1000 PYFU). Alanine transaminase and aspartate transaminase levels were elevated to >10× the upper limit of normal (ULN, 40 U/L), while bilirubin was within the normal range; symptoms resolved without sequelae after discontinuation of DTG. There were no discontinuations for severe skin rash ().
Figure 1. Discontinuations of cART regimens with DTG (649 discontinuations during 5649 PYFU) or with other integrase inhibitors (RAL or EVG, 612 discontinuations during 3531 PYFU). Rates of discontinuations (with 95% confidence intervals) for independently reviewed hypersensitivity reaction (HSR), hepatotoxicity or severe skin rash events (a) or for other discontinuation reasons (b). Numbers of discontinuations and discontinuation incidence rates are shown on the right. Discontinuation rates were similar for RAL or EVG, except that of 30 discontinuations for treatment failure with RAL or EVG, 12 were for RAL (discontinuation rate 7.4 (95% CI 4.2–13.0)/1000 PYFU) and 18 for EVG (discontinuation rate 9.5 (6.0–15.0)/1000 PYFU). Similarly of 107 discontinuations for toxicity, 39 were for RAL (discontinuation rate 23.9 (95% CI 17.5–32.7)/1000 PYFU) and 68 for EVG (discontinuation rate 35.8 (28.2–45.4)/1000 PYFU), and of 64 discontinuations for treatment simplification, 57 were for RAL (discontinuation rate 34.9 (95% CI 27.0–45.3)/1000 PYFU) and 7 for EVG (discontinuation rate 3.7 (1.8–7.7)/1000 PYFU). (c) Adjusted discontinuations incidence rate ratios (IRRs) comparing discontinuations of RAL or EVG-containing regimens with DTG discontinuations (reference). Models were adjusted for participant’s characteristics at baseline (sex, ethnicity, risk group, region, ART naïve or INSTI naïve, prior use of PIs, NNRTIs or DTG, EVG or RAL, the total number of ARVs previously exposed to, and time since first ARV use) or time-updated variables (age, body mass index, smoking status, AIDS or non-AIDS clinical conditions, diabetes, hypertension, anemia, hepatitis B or C virus co-infection, as well as current and peak viral load, current and nadir CD4 counts, estimated glomerular filtration rate, ALT and AST levels and the proportion of follow-up time with low CD4 counts or high viral load). Factors that were significant in univariate analyses (p < 0.1) were included in multivariate models; excluded variables were added in turn to determine if their inclusion improved the fit of the model (Note: Due to low numbers of events, the aIRR for treatment failure was only adjusted for sex, CD4 cell counts at baseline and ARV treatment naïve). Abbreviations: ALT, alanine aminotransferase; ART, anti-retroviral therapy; AST, aspartate aminotransferase; DTG, dolutegravir; EVG, elvitegravir; HSR, hypersensitivity reaction; INSTI, integrase strand transfer inhibitor; IRR, incidence rate ratio; NNRTI, non- nucleotide reverse-transcriptase inhibitor; PI, protease inhibitor; PYFU, person-years of follow-up; RAL, raltegravir.
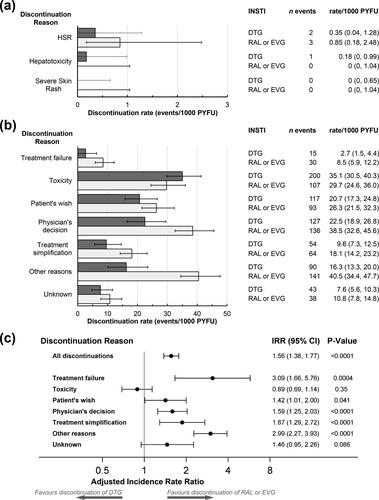
The most common discontinuation reasons were for toxicity (n = 307; 24.3%), physician’s decision (n = 263; 20.9%), and other reasons (n = 231; 18.3%, ). Discontinuation incidence rates and adjusted incidence rate ratios (aIRR) are shown in . Among those who discontinued, more discontinuations due to toxicity were reported for DTG compared to the other INSTIs, but discontinuation rates for toxicity were similar for RAL or EVG compared to DTG (aIRR for RAL or EVG vs. DTG 0.89, CI 0.69–1.14, p = 0.35). After adjustment, there were higher rates of discontinuation among users of RAL or EVG for treatment failure, patient’s wish, physician’s decision, treatment simplification and other reasons ().
In addition to the DTG episodes reported above, we also noted 492 episodes where DTG was used in mono- or two-drug ART after January 2014, with altogether 75 discontinuations (87 (CI 69–109) discontinuations per 1000 PYFU), none of which were due to HSR, hepatotoxicity or severe skin rash.
Discussion
In this 5-year post-authorization study, 4366 individuals started 5116 new cART regimens including an INSTI with >9000 PYFU, of which >60% were with DTG. Overall, there were 1261 discontinuations of INSTI regimens, with discontinuation rates higher among users of RAL or EVG compared to DTG. All discontinuation reasons were verified by the study sites, and, where relevant, sent for review by an independent ERC. Discontinuations due to serious adverse events were very rare, and included altogether five discontinuations due to HSR (two with DTG and three with RAL or EVG), one discontinuation due to INSTI-induced hepatotoxicity with DTG, and no discontinuations due to severe skin rash. Thus overall we observed fewer than one serious adverse event in >1800 person-years of DTG or in >1100 person-years of RAL or EVG use; the low number of serious events precluded detailed analyses. Our results are in keeping with estimates from clinical trials that suggested HSRs for DTG were seen in <1% of persons treated.Citation8–10 Likewise, there have been few reports of INSTI-related serious adverse events in a number of post-licensing observational studies,Citation15–18 although some instances of drug reaction with eosinophilia and systemic symptoms (DRESS) have been reported with RALCitation19 and in one person on DTG,Citation20 as have case reports of DTG-related hepatotoxicity.Citation21
While DTG is a common anchor agent in cART regimens, two-drug formulations with DTG are also increasingly common as part of drug simplification and NRTI-sparing formulations and also recommended in clinical guidelines.Citation4,Citation22 We noted a small number of episodes where DTG was used in mono- or two-drug ART, of which 15.2% were discontinued, indicating a lower rate of discontinuations compared to the three-drug DTG regimens. The low number of discontinuations precluded further analysis, though it should be noted that none were due to HSR, hepatotoxicity, or severe skin rash.
There were also altogether 1255 discontinuations of INSTIs due to other or unknown causes (i.e. not HSR or hepatotoxicity). In keeping with the high genetic barrier for DTG even in the presence of pre-existing INSTI resistance mutations,Citation8,Citation23 discontinuations due to treatment failure were more common among uses of RAL or EVG. While a lower proportion discontinued DTG, among those who discontinued, discontinuations due to toxicity were more common among those taking DTG compared to RAL or EVG. One possible reason was concerns about toxicity of the nervous system, as has also been reported elsewhere.Citation15,Citation24,Citation25 After adjustment, the IRR for toxicity discontinuation was nonsignificant in unadjusted and adjusted analyses. Other common reasons for discontinuation included patient’s wish, physician’s decision, and treatment simplification, in agreement with other studies of INSTI use.Citation15,Citation26
Strengths of the study include the observational design with participating clinical centers throughout the European region and Argentina, comprehensive ARV treatment records for participants and adjudication and verification of serious adverse events. There are also limitations. As this is a non-interventional observational study, treatment decisions were made by the treating physician according to standard practice, and therefore confounding by indication cannot be excluded. EuroSIDA does not routinely collect information on HLA-B*5701 genotype which carries a risk for ABC-related HSR. As per local guidelines, HLA-B*5701 genotype is checked at the clinics before prescribing ABC. All the HSRs reported in this study were adjudicated by the ERC as possible or definite INSTI-related HSRs. Observations started when DTG was licensed in January 2014, when some individuals were already using RAL- or EVG-based regimens, which were not included in this analysis. Discontinuation reasons may differ for RAL or EVG, but this study was not powered to assess these differences. We did not include use of bictegravir, which was licensed in 2018, and which may have led to some treatment switches. Although INSTI-containing ARV regimens were available throughout the European Region, use of DTG was rarer in Central East and East Europe. While we endeavored to collect discontinuation reasons whenever an INSTI-containing regimen was discontinued, some reasons were not available.
In conclusion, in this real-world observational cohort in Europe, discontinuation rates of INSTI-containing cART regimens were higher among users of RAL or EVG compared to DTG. After independent review, discontinuations due to HSR or hepatotoxicity in INSTI users were very rare, indicating a low rate of serious adverse events.
Author contributions
A.M., O.K., L.P., L.C., V.V., and D.R. conceived the study and planned the analysis. J.F.L. and L.P. managed and coordinated the project, data collection, and validation of events. L.S., A.P.-M., and A.R. performed statistical analysis and data interpretation and L.R. performed data checks. A.P.-M and A.M. produced the first draft of the manuscript. J.B., K.P., S.D.W., A.H., E.J., M.J., I.K., M.H.L., L.N.N., A.L.R., B.S., C.S., and I.Y. contributed to patient recruitment and data collection, and interpretation and presentation of results. A.M. supervised the project. All authors reviewed and approved the manuscript.
The multi-center study group, EuroSIDA (national coordinators in parenthesis)
Albania: (A Harxhi), University Hospital Center of Tirana, Tirana. Argentina: (M Losso), M Kundro, Hospital JM Ramos Mejia, Buenos Aires. Austria: (B Schmied), Otto Wagner Hospital, Vienna; R Zangerle, Medical University Innsbruck, Innsbruck. Belarus: (I Karpov), A Vassilenko, Belarusian State Medical University, Minsk, Belarus; VM Mitsura, Gomel State Medical University, Gomel; D Paduto, Regional AIDS Centre, Svetlogorsk. Belgium: (N Clumeck), S De Wit, M Delforge, Saint-Pierre Hospital, Brussels; E Florence, Institute of Tropical Medicine, Antwerp; L Vandekerckhove, University Ziekenhuis Gent, Gent. Bosnia-Herzegovina: (V Hadziosmanovic), Klinicki Centar Univerziteta Sarajevo, Sarajevo. Croatia: (J Begovac), University Hospital of Infectious Diseases, Zagreb. Czech Republic: (L Machala), D Jilich, Faculty Hospital Bulovka, Prague; D Sedlacek, Charles University Hospital, Plzen. Denmark: G Kronborg, T Benfield, Hvidovre Hospital, Copenhagen; J Gerstoft, T Katzenstein, Rigshospitalet, Copenhagen; C Pedersen, IS Johansen, Odense University Hospital, Odense; L Ostergaard, Skejby Hospital, Aarhus, L Wiese, NF Moller, Sjaellands Universitetshospital, Roskilde; L N Nielsen, Hillerod Hospital, Hillerod. Estonia: (K Zilmer), West-Tallinn Central Hospital, Tallinn; Jelena Smidt, Nakkusosakond Siseklinik, Kohtla-Järve. Finland: (I Aho), Helsinki University Hospital, Helsinki. France: (J-P Viard), Hôtel-Dieu, Paris; P-M Girard, Hospital Saint-Antoine, Paris; C Pradier, E Fontas, Hôpital de l'Archet, Nice; C Duvivier, Hôpital Necker-Enfants Malades, Paris. Germany: (J Rockstroh), Universitäts Klinik Bonn; G Behrens, Medizinische Hochschule Hannover; O Degen, University Medical Center Hamburg-Eppendorf, Infectious Diseases Unit, Hamburg; HJ Stellbrink, IPM Study Center, Hamburg; C Stephan, JW Goethe University Hospital, Frankfurt; J Bogner, Medizinische Poliklinik, Munich; G. Fätkenheuer, Universität Köln, Cologne. Georgia: (N Chkhartishvili) Infectious Diseases, AIDS & Clinical Immunology Research Center, Tbilisi. Greece: (H Sambatakou), Ippokration General Hospital, Athens; G Adamis, N Paissios, Athens General Hospital "G Gennimatas", Athens. Hungary: (J Szlávik), South-Pest Hospital Centre – National Institute for Infectology and Haematology, Budapest. Iceland: (M Gottfredsson), Landspitali University Hospital, Reykjavik. Ireland: (E Devitt), St. James's Hospital, Dublin. Israel: (L Tau), D Turner, M Burke, Ichilov Hospital, Tel Aviv; E Shahar, G Hassoun, Rambam Medical Center, Haifa; H Elinav, M Haouzi, Hadassah University Hospital, Jerusalem; D Elbirt, AIDS Center (Neve Or), Jerusalem. Italy: (A D’Arminio Monforte), Istituto Di Clinica Malattie Infettive e Tropicale, Milan; R Esposito, I Mazeu, C Mussini, Università Modena, Modena; F Mazzotta, A Gabbuti, Ospedale S Maria Annunziata, Firenze; A Lazzarin, A Castagna, N Gianotti, Ospedale San Raffaele, Milan; M Galli, A Ridolfo, Osp. L. Sacco, Milan. Lithuania: (V Uzdaviniene) Vilnius University Hospital Santaros Klinikos, Vilnius; R Matulionyte, Vilnius University, Faculty of Medicine, Department of Infectious Diseases and Dermatovenerology, Vilnius, Lithuania. Luxembourg: (T Staub), R Hemmer, Centre Hospitalier, Luxembourg. Montenegro: (S Dragas), M Stevanovic, Clinical Center of Montenegro, Podgorica. Netherlands: (P Reiss), Academisch Medisch Centrum bij de Universiteit van Amsterdam, Amsterdam. North Macedonia: (J Trajanovska), University Clinic for Infectious Diseases & Febrile Conditions, Mother Teresa 17, Skopje. Norway: (DH Reikvam), A Maeland, J Bruun, Oslo University Hospital, Ullevaal. Poland: (B Knysz), B Szetela, M Inglot, Medical University, Wroclaw; E Bakowska, Centrum Diagnostyki i Terapii AIDS, Warsaw; R Flisiak, A Grzeszczuk, Medical University, Bialystok; M Parczewski, K Maciejewska, B Aksak-Was, Medical University, Szczecin; M Beniowski, E Mularska, Osrodek Diagnostyki i Terapii AIDS, Chorzow; E Jablonowska, J Kamerys, K Wojcik, Wojewodzki Szpital Specjalistyczny, Lodz; I Mozer-Lisewska, B Rozplochowski, Poznan University of Medical Sciences, Poznan. Portugal: (A Zagalo), Hospital Santa Maria, Lisbon; K Mansinho, Hospital de Egas Moniz, Lisbon; F Maltez, Hospital Curry Cabral, Lisbon. Romania: (R Radoi), C Oprea, Carol Davila University of Medicine and Pharmacy Bucharest, Victor Babes Clinical Hospital for Infectious and Tropical Diseases, Bucharest. Russia: A Yakovlev, Medical Academy Botkin Hospital, St Petersburg; T Trofimora, Novgorod Centre for AIDS, Novgorod, I Khromova, Centre for HIV/AIDS & and Infectious Diseases, Kaliningrad; E Kuzovatova, Nizhny Novgorod Scientific and Research Institute of Epidemiology and Microbiology named after Academician I.N. Blokhina, Nizhny Novogrod; E Borodulina, E Vdoushkina, Samara State Medical University, Samara. Serbia: (J Ranin), The Institute for Infectious and Tropical Diseases, Belgrade. Slovenia: (J Tomazic), University Clinical Centre Ljubljana, Ljubljana. Spain: (JM Miro), M. Laguno, E. Martinez, F. Garcia, JL Blanco, M. Martinez-Rebollar, J. Mallolas, P Callau, J Rojas, A Inciarta, Hospital Clinic – IDIBAPS University of Barcelona, Barcelona; S Moreno, S. del Campo, Hospital Ramon y Cajal, Madrid; B Clotet, A Jou, R Paredes, J Puig, JM Llibre, JR Santos, Infectious Diseases Unit & IrsiCaixa AIDS Research Institute, Hospital germans Trias I Pujol, Badalona; P Domingo, M Gutierrez, G Mateo, MA Sambeat, Hospital Sant Pau, Barcelona; JM Laporte, Hospital Universitario de Alava, Vitoria-Gasteiz. Sweden: (V Svedhem), A Thalme, A Sonnerborg, Karolinska University Hospital, Stockholm; CJ Treutiger, Venhälsan-Sodersjukhuset, Stockholm; L Flamholc, Malmö University Hospital, Malmö. Switzerland: (K Kusejko), R Weber, University Hospital Zurich; M Cavassini, University Hospital Lausanne; A Calmy, University Hospital Geneva; H Furrer, University Hospital Bern; M Battegay, University Hospital Basel; P Schmid, Cantonal Hospital St. Gallen. Ukraine: A Kuznetsova, Kharkov State Medical University, Kharkov; J Mikhalik, Crimean Republican AIDS centre, Simferopol; M Sluzhynska, Lviv Regional HIV/AIDS Prevention and Control CTR, Lviv. United Kingdom: A Milinkovic, St. Stephen's Clinic, Chelsea and Westminster Hospital, London; AM Johnson, E Simons, S Edwards, Mortimer Market Centre, London; A Phillips, MA Johnson, A Mocroft, Royal Free and University College Medical School, London (Royal Free Campus); C Orkin, Royal London Hospital, London; A Winston, Imperial College School of Medicine at St. Mary's, London; A Clarke, Royal Sussex County Hospital, Brighton; C Leen, Western General Hospital, Edinburgh.
The following centers have previously contributed data to EuroSIDA: Medical University, Gdansk, Poland, Infectious Diseases Hospital, Sofia, Bulgaria, Hôpital de la Croix Rousse, Lyon, France, Hôpital de la Pitié-Salpétière, Paris, France, Unité INSERM, Bordeaux, France, Hôpital Edouard Herriot, Lyon, France, Bernhard Nocht Institut für Tropenmedizin, Hamburg, Germany, 1st I.K.A Hospital of Athens, Athens, Greece, Ospedale Riuniti, Divisione Malattie Infettive, Bergamo, Italy, Ospedale di Bolzano, Divisione Malattie Infettive, Bolzano, Italy, Ospedale Cotugno, III Divisione Malattie Infettive, Napoli, Italy, Dérer Hospital, Bratislava, Slovakia, Hospital Carlos III, Departamento de Enfermedades Infecciosas, Madrid, Spain, Kiev Centre for AIDS, Kiev, Ukraine, Luhansk State Medical University, Luhansk, Ukraine, Odessa Region AIDS Center, Odessa, Ukraine, St Petersburg AIDS Centre, St Petersburg, Russia, Infectology Centre of Latvia, Riga, Latvia, University di Roma la Sapienza, Rome, Italy, Istituto Nazionale Malattie Infettive Lazzaro Spallanzani, Rome, Italy.
EuroSIDA Steering Committee: I Karpov, M Losso, J Lundgren, J Rockstroh, I Aho, LD Rasmussen, V Svedhem, G Wandeler, C Pradier, N Chkhartishvili, R Matulionyte, C Oprea, JD Kowalska, J Begovac, JM Miró, G Guaraldi, R Paredes. Chair: G Wandeler. Co-Chair: R Paredes. Study lead: A Mocroft. EuroSIDA Coordinating Centre Staff: L Peters, JF Larsen, A Bojesen, B Neesgaard, M Sather, D Raben, EV Hansen, D Kristensen, AH Fischer, SK Jensen. Statistical Staff: A Mocroft, A Phillips, J Reekie, A Cozzi-Lepri, S Amele, A Pelchen-Matthews, A Roen, ES Tusch, W Bannister.
Supplemental Material
Download MS Word (58.3 KB)Acknowledgments
This work was previously presented at the HIV Glasgow 2020 Virtual Congress, October 5–8, 2020 (Abstract P049).
Disclosure statement
L.C. is an employee and shareholder of GlaxoSmithKline, and V.V. and L.R. are employees of ViiV Healthcare and shareholders of GlaxoSmithKline.
A.P.-M. reports personal fees from Gilead Sciences outside the submitted work; J.B. reports honoraria, consultancy fees or travel support from Gilead, ViiV and MSD, all outside the submitted work; O.K. reports personal fees from Gilead, ViiV and Merck, all outside the submitted work; A.M. reports honoraria, consultancy fees or travel support from Gilead, ViiV and Eiland and Bonnin, all outside the submitted work. The remaining authors have no conflicts of interest to disclose.
Additional information
Funding
References
- Trivedi J, Mahajan D, Jaffe RJ, Acharya A, Mitra D, Byrareddy SN. Recent advances in the development of integrase inhibitors for HIV treatment. Curr HIV/AIDS Rep. 2020;17(1):63–75.
- Brooks KM, Sherman EM, Egelund EF, et al. Integrase inhibitors: after 10 years of experience, is the best yet to come? Pharmacotherapy 2019;39(5):576–598.
- World Health Organization Geneva Switzerland. Update of Recommendations on First- and Second-Line Antiretroviral Regimens. https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf?ua=1. Updated July 2019. Accessed July 2020.
- EACS (European AIDS Clinical Society). EACS Guidelines Version 10.0. https://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html. Updated November 2019. Accessed January 2021.
- Vitoria M, Hill A, Ford N, et al. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: what are the issues? AIDS. 2018;32(12):1551–1561.
- Inzaule SC, Hamers RL, Doherty M, Shafer RW, Bertagnolio S, Rinke de Wit TF. Curbing the rise of HIV drug resistance in low-income and middle-income countries: the role of dolutegravir-containing regimens. Lancet Infect Dis. 2019;19(7):e246–e252.
- Phillips AN, Venter F, Havlir D, et al. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. Lancet HIV. 2019;6(2):e116–e127.
- Castagna A, Maggiolo F, Penco G, for the VIKING-3 Study Group, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis. 2014;210(3):354–362.
- Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–743.
- Curtis L, Nichols G, Stainsby C, et al. Dolutegravir: clinical and laboratory safety in integrase inhibitor-naive patients. HIV Clin Trials. 2014;15(5):199–208.
- Hughes CA, Foisy MM, Dewhurst N, et al. Abacavir hypersensitivity reaction: an update. Ann Pharmacother. 2008;42(3):387–396.
- Laut K, Kirk O, Rockstroh J, et al. The EuroSIDA study: 25 years of scientific achievements. HIV Med. 2020;21(2):71–83.
- European Association for the Study of the Liver. EASL Clinical practice guidelines: drug-induced liver injury. J Hepatol. 2019;70(6):1222–1261.
- U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2.1. https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. Updated July 2017. Accessed July 2020.
- Elzi L, Erb S, Furrer H, et al. Adverse events of raltegravir and dolutegravir. AIDS. 2017;31(13):1853–1858.
- Hongo H, Nagao T, Nakamura K, et al. Safety and effectiveness analysis of dolutegravir in patients with HIV-1: interim report of post-marketing surveillance in Japan. Adv Ther. 2021; 38(8):4480–4504.
- Llibre JM, Montoliu A, Miro JM, et al. Discontinuation of dolutegravir, elvitegravir/cobicistat and raltegravir because of toxicity in a prospective cohort. HIV Med. 2019;20(3):237–247.
- Penafiel J, de Lazzari E, Padilla M, et al. Tolerability of integrase inhibitors in a real-life setting. J Antimicrob Chemother. 2017;72(6):1752–1759.
- Scevola S, Tiraboschi JM, Podzamczer D. Nothing is perfect: the safety issues of integrase inhibitor regimens. Expert Opin Drug Saf. 2020;19(6):683–694.
- Martin C, Payen MC, De Wit S. Dolutegravir as a trigger for DRESS syndrome? Int J STD AIDS. 2018;29(10):1036–1038.
- Nhean S, Yoong D, Wong DK, Gough K, Tseng AL. Probable hepatotoxicity with dolutegravir: report of two cases and review of the literature. AIDS. 2019;33(7):1261–1263.
- Cento V, Perno CF. Two-drug regimens with dolutegravir plus rilpivirine or lamivudine in HIV-1 treatment-naïve, virologically-suppressed patients: latest evidence from the literature on their efficacy and safety. J Glob Antimicrob Resist. 2020;20:228–237.
- Lepik KJ, Harrigan PR, Yip B, et al. Emergent drug resistance with integrase strand transfer inhibitor-based regimens. AIDS. 2017;31(10):1425–1434.
- Brehm TT, Franz M, Hufner A, et al. Safety and efficacy of elvitegravir, dolutegravir, and raltegravir in a real-world cohort of treatment-naive and -experienced patients. Medicine. 2019;98(32):e16721.
- Hoffmann C, Welz T, Sabranski M, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. 2017;18(1):56–63.
- Greenberg L, Ryom L, Wandeler G, et al. Uptake and discontinuation of integrase inhibitors (INSTIs) in a large cohort setting. J Acquir Immune Defic Syndr. 2020;83(3):240–250.
