Abstract
Background: Technology-based directly observed therapy (DOT) is more cost-effective and efficient compared with in-person monitoring visits for medication adherence. While some evidence shows these technologies are feasible and acceptable, there is limited evidence collating information across medical conditions or in the context of HIV prevention, care, and treatment.
Objectives: We conducted a scoping review to understand the current evidence on the acceptability, feasibility, and efficacy of digital DOT to improve medication adherence and, specifically, to determine if digital DOT had been used to improve adherence for HIV prevention, care, and treatment
Methods: We searched the electronic databases PubMed, Embase, and the Web of Science in January 2021 for any published studies with terms related to digital technologies and DOT. We included peer-reviewed studies in any population, from any country, for any outcome, and excluded conference abstracts. We included three types of digital DOT interventions: synchronous DOT, asynchronous DOT, and automated DOT. We provide an assessment of the current evidence, gaps in literature, and opportunities for intervention development regarding the use digital DOT to improve antiretroviral therapy (ART) adherence, specifically in the field of HIV.
Results: We identified 28 studies that examined digital DOT. All studies found digital DOT to be acceptable and feasible. Patients using digital DOT had higher rates of treatment completion, observed doses, and adherence compared with in-person DOT, although data were limited on adherence. Only one study examined HIV prevention, and none examined ART adherence for HIV treatment.
Conclusions: Digital DOT is acceptable and feasible but has not been used to remotely monitor and support ART adherence for people living with HIV.
Introduction
Directly observed therapy (DOT), where a patient is observed while taking a medication, has been used across a range of health conditions to increase adherence to medications.Citation1–5 However, given the patient burden of repeated clinic visits needed for in-person DOT, digital technology such as video-conferencing software and mobile health (mHealth) applications (apps) can facilitate remote monitoring of medication adherence.Citation1–5 Technology-based DOT strategies have been shown to be more cost-effective and reduce provider and patient burden compared with regular monitoring visits for medication adherence.Citation6 Although some evidence suggests that these technologies are feasible, accessible, and effective for DOT, there is limited evidence collating information across medical conditions which could inform the development of new interventions to improve medication adherence. Specifically, limited evidence exists describing the use of digital DOT interventions in the context of HIV prevention, care, and treatment.
In the United States, the largest drop-offs in the HIV care continuum are from poor retention in care and viral suppression, whereby 50% of those diagnosed are not retained in care and 43% of those prescribed antiretroviral therapy (ART) do not achieve viral suppression.Citation7 Poor viral suppression can be largely attributed to suboptimal levels of ART adherence due to factors such as inability to consistently follow daily pill regimens due to housing instability or job insecurity, and/or competing demands in the form of co-morbid health conditions like mental health challenges.Citation8–12 Nearly two-thirds of new HIV diagnoses are attributed to people living with HIV (PLWH) who are aware of their HIV infection and either not in care or are receiving care but not virally suppressed, making ART adherence a top priority for curbing incidence.Citation13 ART adherence is strongly correlated with increased survival and improvement in quality of lifeCitation14,Citation15 and reductions in adherence have been associated with loss of virologic control, treatment failure, and onward transmission of drug-resistant virus.Citation16–18 Among PLWH, young adults, Black Americans, and sexual and gender minorities have some of the lowest rates of viral suppression and engagement in care.Citation7,Citation19 Similarly, oral antiretroviral pre-exposure prophylaxis (PrEP) is over 90% effective at preventing HIV in women and men,Citation20,Citation21 but low adherence and persistence has undermined the effective use of PrEP to prevent HIV particularly among adolescents and young adults and Black Americans.Citation22–27 Thus, improved strategies are needed to increase adherence to medications to prevent new HIV infections among those uninfected and improve virologic suppression among PLWH.
We conducted a scoping review to understand the current evidence related to the acceptability, feasibility, and efficacy (i.e. impact on medication adherence) of digital health technologies for DOT to monitor adherence in any population for any outcome, and to determine if digital DOT had been used to improve medication adherence for HIV prevention, care, and treatment. We conducted a scoping review instead of a systematic review to broadly synthesize evidence on the use of digital DOT to improve medication adherence and, specifically, to assess the scope of evidence in the field of HIV. Here, we provide an assessment of the current evidence, gaps in literature, and opportunities for intervention development regarding the use of digital DOT to improve medication adherence in general and specifically among PLWH and those at high risk for HIV acquisition.
Materials and methods
The scoping review was conducted according to the standard protocol for Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews checklist (PRISMA-ScR).Citation28 We included quantitative or qualitative studies of digital DOT technologies on adherence from any prior date until January 8, 2021. Digital DOT interventions were defined as interventions that allow a human or technology (e.g. a smartphone app) to digitally observe or view a participant consume a medication at the time of dosing. We included three types of digital DOT interventions: synchronous DOT, asynchronous DOT, and automated DOT. Synchronous DOT is where a patient and provider both simultaneously log onto a video-conferencing software and the provider observes medication ingestion by the patient in real time.Citation2–4 Asynchronous DOT involves a smartphone app or platform where a patient records a video of themselves taking the medication which is then uploaded for later review by a provider or researcher.Citation2–4 Automated DOT interventions use smartphone apps with artificial intelligence and facial recognition to confirm medication ingestion without the use of a provider. Citation1,Citation29 We did not include studies with ingestible sensors or reminders via text message as these constitute a different type of intervention that do not include observation by a provider or researcher. Therefore, the studies that met inclusion criteria were those that 1) included the use of a digital DOT technology, 2) focused on improving medication adherence for any medical condition, 3) were peer-reviewed publications, and 4) were published in English. We included interventions in any population and did not restrict by comparator group or study design. The outcomes of interest were medication adherence and the acceptability and feasibility of the intervention. Cost-effectiveness outcomes were not included. We did not restrict studies by type of reported statistics (e.g. odds ratio).
We reviewed published, peer-reviewed literature across a range of studies, from pilot and descriptive studies to randomized trials. The first search was conducted on November 23, 2020, with a second search on January 8, 2021, to confirm that we were able to identify key publications of interest. We searched the electonic databases PubMed, Embase, and the Web of Science, including published peer-reviewed literature and excluded conference abstracts. We used the following keywords: (digital* OR technolog* OR application* OR "app" OR "apps" OR smartphone* OR smart phone* OR mobile OR mhealth* OR telehealth OR tele-health OR telemedicine OR tele-medicine OR video* OR videoconferenc* OR telerehabilitation OR tele-rehabilitation OR remot*) AND ("directly observed therap*" OR "directly observed treatment*" OR "directly observed intervention*" OR "directly observed" OR "direct observation"). We also conducted a manual search of references of citations identified through the electronic searches, to identify any additional relevant citations.
Abstracts were imported from each electronic database and combined within Covidence online software, which automatically removes duplicates.Citation30 One co-author screened the abstracts and examined the full text of articles in relation to inclusion criteria. A second co-author then reviewed a random sample of 10% of the articles and flagged discrepancies which were reviewed again by the first co-author. If there were any discrepancies, an additional 10% of articles were reviewed and so on. A third co-author was consulted for any unresolved discrepancies. Additional studies that were abstracts, scoping reviews, or commentaries on the topic were only included in the introduction and discussion to provide information on newly conducted research or opinions in the field. Data charting was performed using a standard form that was created by the team through Covidence software and included variables of interest to extract. One reviewer extracted information from all studies including the citation, year, population, inclusion criteria, sample size, study design, medical condition (e.g. HIV, tuberculosis [TB]), outcome, intervention description, if incentives were provided, follow-up period, and findings. The same process for review of extracted data was used as was done for the inclusion process whereby a second co-author reviewed a random sample of 10% of the articles and flagged discrepancies. Extracted information was based on agreement between the two co-authors. We conducted a critical appraisal of study quality and potential biases for each study by examining the strength of the study design (e.g. pilot, programmatic data, randomized trial) and sample size. Given that most of the studies were not randomized trials and used different research designs and outcomes, we opted not to use a standardized critical appraisal tool. Study data were summarized overall and by medical condition (e.g. HIV) being examined to assess the overall evidence for medication adherence according to the type of medical condition and type of intervention (e.g. automated versus asynchronous DOT). Data are presented in table format by medical condition and type of intervention. A protocol was developed and distributed internally prior to conducting the review .
Results
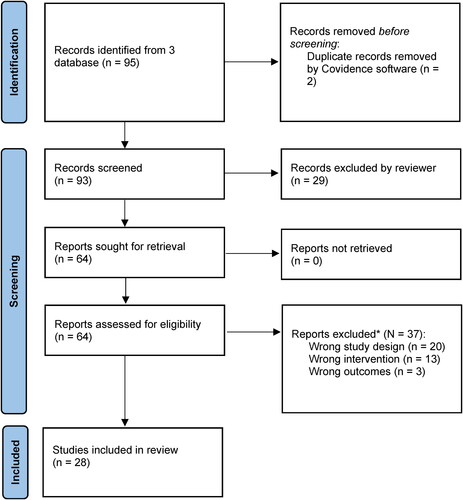
After exclusion of duplicate citations, a total of 93 studies were identified from the three databases (). The titles and abstracts were screened for relevance by one reviewer, and 64 were retained for full text review. Reasons for exclusion are reported in . A total of N = 28 studies were eligible for inclusion. These studies are summarized in , grouped by medical condition and type of intervention.
Table 1. Characteristics of 28 studies included in the scoping review.
Most studies examined medication adherence related to TB (n = 22, 79%; ). Of the remaining six studies each examined adherence to medications for different medical conditions or needs: schizophrenia, stroke, HIV prevention, asthma (inhaler use), dietary supplement use, and opioid use. Most publications were cohort studies (n = 19; 68%), five were single arm pilot studies (18%), three were randomized trials (11%), one was a non-randomized experimental study (4%), and one was a pilot study where patients were randomized to either immediate or delayed DOT with an mHealth app intervention (4%). Samples sizes ranged from 6 to 405. Nearly all studies (n = 24; 86%) recruited clinic patients. Studies were conducted in North America (n = 17, 61%; in the contiguous United States [n = 15; 54%], Puerto Rico, and Mexico), Asia (n = 5, 18%; in China [n = 2], Singapore, Taiwan, Vietnam), Europe (n = 3, 11%; in England, Norway, Belarus), Africa (n = 2, 7%; in Kenya, Uganda), and one study in Australia. Intervention types included synchronous DOT (n = 11; 39%), asynchronous DOT (n = 14; 50%), and automated DOT (n = 3; 11%).
Asynchronous DOT
Of the 14 asynchronous DOT studies, all found the tool to be acceptable and feasible to use ().Citation3,Citation31–42 Participants were satisfied with the intervention, would recommend it to a friend, would use it in the future and thought it was convenient, private, and easy to use. Both patients and providers found the app acceptable and thought it saved time and money compared to in-person DOT.Citation38,Citation39,Citation43 A study of inhaler use in children found that all but one child mentioned that daily recoding of their use of the inhaler was fun and enjoyable.Citation31 In five studies, patients stated that they preferred or would choose asynchronous DOT over in-person DOT.Citation32,Citation35,Citation38,Citation40,Citation41 Concerns with asynchronous DOT included technical difficulties, privacy concerns, and discomfort taking a video of oneself.
Table 2. Acceptability, feasibility, and adherence outcomes among 28 studies included in the scoping review.
Treatment completion and adherence were high across studies (82-93%). All studies that compared treatment completion between asynchronous DOT and in-person DOT found increased treatment completion with asynchronous DOT.Citation3,Citation37–39 A randomized trial of 226 patients reported that 70% of asynchronous DOT patients achieved 80% or more scheduled observations compared with 31% of in-person DOT patients.Citation37 Only one study explicitly compared adherence between asynchronous DOT and a control group that measured adherence through in-person DOT visits. The study found that medication adherence on DOT was comparable to that of in-person DOT (94% vs 98%, P = 0.17).Citation39
Synchronous DOT
Of the 11 studies that assessed synchronous DOT, all studies found synchronous DOT to be acceptable and feasible.Citation44–54 Patients and providers thought the tool was convenient, comfortable, easy to use, and ensured privacy. In all studies that compared synchronous DOT to in-person DOT, participants and providers preferred digital DOT due to increased convenience and ease of use.Citation46,Citation48,Citation53,Citation54 Synchronous DOT reduced time and costs for clinic visits and lead to fewer missed visits and higher treatment completion than in-person DOT.Citation44–52,Citation54 One cohort study found treatment completion (i.e. completing a course of TB treatment regardless of daily adherence) among patients using synchronous DOT was 88% (44/50) compared with 65% (196/302) among patients using in-person DOT (P < 0.001).Citation44 None of the studies compared adherence between synchronous DOT and a control arm.
Automated DOT
Of the two studies that assessed acceptability of automated DOT (aDOT), all found the intervention acceptable and feasible.Citation1,Citation29 Patients reported that the artificial intelligence (AI) platform improved the doctor-patient relationship,Citation29 high satisfaction, that they would recommend the app to a friend, that they would use it in the future, and that it helped with medication adherence.Citation1 Patients with little experience using a smartphone were able to successfully use aDOT.Citation29 Two studies assessed adherence measured using the AI platform versus adherence through mobile DOT or plasma drug concentration levels and found increases in adherence with aDOT. One study in patients who had experienced a stroke found that adherence based on plasma drug concentration levels was 100% (15 of 15) in patients using aDOT compared with 50% (6 of 12) in the control group. The second study found that mean adherence over 24 weeks was 90% (standard deviation [SD] = 24.9) for participants who were monitored using aDOT, compared with 72% (SD = 39.8) in those who were monitored in person by study staff (or a third party) for a difference of 18% (95% confidence interval [CI] = −2.0 to 37.7; P = 0.08).
Among all 28 studies, only one used digital DOT with an HIV prevention outcome.Citation1 Among young men who have sex with men in San Francisco, California, automated DOT was highly acceptable, 84% reported the app helped with taking pre-exposure prophylaxis (PrEP), and median PrEP adherence was 91%.Citation1
Discussion
We identified 28 studies that examined digital DOT interventions to improve adherence to medication for any health indication. All studies found digital DOT to be acceptable and feasible and noted that digital DOT provided more autonomy, convenience, and ease of use for patients and providers compared with in-person DOT.Citation1,Citation3,Citation29,Citation31–36,Citation38–42,Citation44,Citation46,Citation47,Citation49,Citation50,Citation54,Citation55 Patients using digital DOT had higher rates of treatment completion, observed doses, and adherence compared with in-person DOT. Citation29,Citation56 More research is needed from large randomized trials examining the impacts of digital DOT on adherence compared with other interventions and comparing different types of digital DOT. Evidence largely shows that digital DOT is acceptable, feasible, and has the potential to improve medication adherence.
Given the challenges with adherence to PrEP and ART, digital DOT has a high potential to improve HIV prevention, care, and treatment. However, we identified only one study that had an HIV prevention outcome.Citation1 The automated DOT approach used AI in a smartphone app and was found to be highly acceptable with high PrEP adherence.Citation1 This study highlights the potential benefit of using digital DOT technologies to increase adherence in the context of HIV prevention, care, and treatment. We did not identify any studies examining ART adherence for HIV; however, two excluded studies used mobile phone-based reminders to increase ART adherence.Citation57,Citation58 Our findings highlight the potential benefit of using digital DOT technologies to increase adherence in the context of HIV prevention and the gap in research focused on digital DOT to improve adherence to HIV treatment.
Technology-based interventions may be particularly appropriate to increase ART adherence among youth living with HIV (YLWH) given that youth are the largest group of consumers of technology and internet.Citation59–61 In addition, research has shown that younger age is associated with lower ART adherence and higher risk of virologic failure among PLWH.Citation62–66 A systematic review examining the HIV care continuum in YLWH found that only 54% of youth who initiate ART achieve viral suppression and an additional 43% are retained in care.Citation7 Suboptimal ART adherence and retention in care can increase risk of treatment failure, HIV transmission, and poor health outcomes. Thus, YLWH are a key population with whom to examine digital DOT interventions aimed at improving HIV clinical outcomes.
There are some limitations to our review. We reviewed only three databases and included studies in English. Therefore, there may have been studies that were not captured in our review that would have been using a more systematic review approach. While there were 28 studies that used digital DOT, there were few studies that were randomized trials and most had a small number of participants. Most studies focused on TB outcomes and were in the United States. More data are needed from regions outside of the United States and from large trials and those that examine adherence outcomes using digital DOT compared to other methods as well as studies that compare different types of digital DOT to each other. Additionally, while over 96% of individuals age 18–29 in the United States have a smartphone,Citation67 we acknowledge that there will be more marginalized groups who may not have smartphone access and this intervention may not be accessible to them. Further studies are also needed to review implementation outcomes with the scale up of digital DOT in healthcare settings and should also investigate digital DOT and other tools to assess adherence for non-clinic-based provision of long-acting injectables for HIV treatment and prevention.
Given the recent COVID-19 pandemic, remote-based research and the use of digital tools to support medication adherence are becoming increasingly important.Citation68 Digital DOT may offer a solution by which to remotely monitor and support medication adherence. However, there is a critical gap in literature on the use of these strategies to increase ART adherence among PLWH. It may be a particularly useful tool to promote ART adherence among groups experiencing inequities in HIV care such as youth, and people of color. Digital DOT may increase convenience and reduce patient and provider burden compared to in-person DOT. Compared to in-person DOT, several studies found improved treatment completion and adherence with digital DOT. However, randomized studies assessing adherence outcomes are limited. More data are needed from larger, experimental randomized trials, in settings with limited access to healthcare such as rural settings and comparing the different methods of digital DOT that now exist.
Author’s contributions
MCDS and PS conceptualize and designed the review. MCDS and AMB reviewed the literature and abstracted data. MCDS drafted the analysis and manuscript. PS, AMB and ASC provided insight on the analysis of data, reviewed the manuscript and participated in writing.
Disclosure statement
The authors report no conflict of interest
Additional information
Funding
References
- Liu AY, Laborde ND, Coleman K, et al. DOT diary: developing a novel mobile app using artificial intelligence and an electronic sexual diary to measure and support PrEP adherence among young men who have sex with men. AIDS Behav. 2021;25(4):1001–1012.
- MaCaraig M, Lobato MN, McGinnis Pilote K, Wegener D. A national survey on the use of electronic directly observed therapy for treatment of tuberculosis. J Public Heal Manag Pract. 2018;
- Garfein RS, Doshi RP. Synchronous and asynchronous video observed therapy (VOT) for tuberculosis treatment adherence monitoring and support. J Clin Tuberc Other Mycobact Dis. 2019;17:100098.
- Ngwatu BK, Nsengiyumva NP, Oxlade O, et al. The impact of digital health technologies on tuberculosis treatment: a systematic review. Eur Respir J. 2018;51(1):1701596.
- Parmar P, Mackie D, Varghese S, Cooper C. Use of telemedicine technologies in the management of infectious diseases: a review. Clin Infect Dis. 2015;
- Saberi P, Yuan P, John M, Sheon N, Johnson MO. A Pilot Study to Engage and Counsel HIV-Positive African American Youth Via Telehealth Technology. AIDS Patient Care STDS. 2013;27(9):529–532.
- Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS. 2014;
- Beer L, Skarbinski J. Adherence to antiretroviral therapy among HIV-Infected adults in the United States. AIDS Educ Prev. 2014;26(6):521–537.
- Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800.
- Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–187.
- Paolillo EW, Gongvatana A, Umlauf A, Letendre SL, Moore DJ. At-risk alcohol use is associated with antiretroviral treatment nonadherence among adults living with HIV/AIDS. Alcohol Clin Exp Res. 2017;41(8):1518–1525.
- Puskas CM, Forrest JI, Parashar S, et al. Women and vulnerability to HAART non-adherence: a literature review of treatment adherence by gender from 2000 to 2011. Curr HIV/AIDS Rep. 2011;8(4):277–287.
- Li Z, Purcell DW, Sansom SL, Hayes D, Hall HI. Vital signs: HIV transmission along the continuum of care—United States, 2016. MMWR Morb Mortal Wkly Rep. 2019;68(11):267–272.
- Chesney MA. The elusive gold standard. JAIDS J Acquir Immune Defic Syndr. 2006;43(Supplement 1):S149–S155.
- Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–1183.
- Little SJ, Holte S, Routy J-P, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347(6):385–394.
- Harrigan PR, Hogg RS, Dong WWY, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191(3):339–347.
- Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor–based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146(8):564.
- Crepaz N, Dong X, Hess KL, Bosh K. Brief report: racial and ethnic disparities in sustained viral suppression and transmission risk potential among persons aged 13-29 years living with diagnosed HIV infection, United States, 2016. J Acquir Immune Defic Syndr. 2020;83(4):334–339.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599.
- Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973–1983.
- Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375(22):2121–2132.
- Marrazzo JM, Ramjee G, Richardson BA, VOICE Study Team, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–518.
- Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–422.
- Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410.
- Centers for Disease Control. Pre-Exposure Prophylaxis (PrEP). https://www.cdc.gov/hiv/risk/prep/index.html. Accessed February 1, 2018.
- CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014: A Clinical Practice Guideline. Atlanta, Georgia; 2014. www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf.
- Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. 107326/M18-0850.
- Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke 2017;48(5):1416–1419.
- Veritas Health Innovation. Covidence systematic review sofware. Covidence. www.covidence.org %0A–0A. Published; 2019.
- Shields MD, ALQahtani F, Rivey MP, McElnay JC. Mobile direct observation of therapy (MDOT): a rapid systematic review and pilot study in children with asthma. PLoS ONE. 2018;13(2):e0190031.
- Holzman SB, Atre S, Sahasrabudhe T, et al. Use of smartphone-based video directly observed therapy (vDOT) in tuberculosis care: single-arm, prospective feasibility study. JMIR Form Res. 2019;3(3):e13411.
- Sekandi JN, Buregyeya E, Zalwango S, et al. Video directly observed therapy for supporting and monitoring adherence to tuberculosis treatment in Uganda: a pilot cohort study. ERJ Open Res. 2020;6(1):00175–2019–02019.
- Nguyen TA, Pham MT, Nguyen TL, et al. Video directly observed therapy to support adherence with treatment for tuberculosis in Vietnam: a prospective cohort study. Int J Infect Dis. 2017;65:85–89.
- Molton JS, Pang Y, Wang Z, et al. Prospective single-arm interventional pilot study to assess a smartphone-based system for measuring and supporting adherence to medication. BMJ Open. 2016;6(12):e014194.
- Godersky ME, Klein JW, Merrill JO, et al. Acceptability and feasibility of a mobile health application for video directly observed therapy of buprenorphine for opioid use disorders in an office-based setting. 2020.
- Story A, Aldridge RW, Smith CM, et al. Smartphone-enabled video-observed versus directly observed treatment for tuberculosis: a multicentre, analyst-blinded, randomised, controlled superiority trial. Lancet 2019;393(10177):1216–1224.
- Guo X, Yang Y, Takiff HE, et al. A comprehensive app that improves tuberculosis treatment management through video-observed therapy: usability study. JMIR Mhealth Uhealth. 2020;8(7):e17658.
- Holzman SB, Zenilman A, Shah M. Advancing patient-centered care in tuberculosis management: a mixed-methods appraisal of video directly observed therapy. Open Forum Infect Dis. 2018;5(4):ofy046.
- Garfein RS, Collins K, Muñoz F, et al. Feasibility of tuberculosis treatment monitoring by video directly observed therapy: a binational pilot study. Int J Tuberc Lung Dis. 2015;19(9):1057–1064.
- Hoffman JA, Cunningham JR, Suleh AJ, et al. Mobile direct observation treatment for tuberculosis patients: a technical feasibility pilot using mobile phones in Nairobi, Kenya. Am J Prev Med. 2010;39(1):78–80.
- Olano-Soler H, Thomas D, Joglar O, et al. Notes from the field: use of asynchronous video directly observed therapy for treatment of tuberculosis and latent tuberculosis infection in a long-term–care facility―Puerto Rico, 2016–2017. MMWR Morb Mortal Wkly Rep. 2017;66(50):1386–1387.
- Sinkou H, Hurevich H, Rusovich V, et al. Video-observed treatment for tuberculosis patients in Belarus: findings from the first programmatic experience. Eur Respir J. 2017;49(3):1602049.
- Lam CK, Pilote KMG, Haque A, Burzynski J, Chuck C, Macaraig M. Using video technology to increase treatment completion for patients with latent tuberculosis infection on 3-month isoniazid and rifapentine: an implementation study. J Med Internet Res. 2018;20(11):e287.
- Buchman T, Cabello C. A New Method to Directly Observe Tuberculosis Treatment: Skype Observed Therapy, a Patient-Centered Approach. 2017;
- Bendiksen R, Ovesen T, Asfeldt AM, Halvorsen DS, Gravningen K. Use of video directly observed treatment for tuberculosis in Northern Norway. Tidsskr Den nor Laegeforening. 2020;140(1)
- Donahue ML, Eberly MD, Rajnik M. Tele-TB: using telemedicine to increase access to directly observed therapy for latent tuberculosis infection. Mil Med. 2021;186(Suppl 1):25–31.
- Guo P, Qiao W, Sun Y, Liu F, Wang C. Telemedicine technologies and tuberculosis management: a randomized controlled trial. Telemed. e-Health 2020;26(9):1150–1156.
- Chen SH, Wang I, Hsu HL, et al. Advantage in privacy protection by using synchronous video observed treatment enhances treatment adherence among patients with latent tuberculosis infection. J Infect Public Health. 2020;13(9):1354–1359.
- DeMaio J, Schwartz L, Cooley P, Tice A. The application of telemedicine technology to a directly observed therapy program for tuberculosis: a pilot project. Clin Infect Dis. 2001;33(12):2082–2084.
- Chuck C, Robinson E, Macaraig M, Alexander M, Burzynski J. Enhancing management of tuberculosis treatment with video directly observed therapy in New York City. Int j Tuberc Lung Dis. 2016;20(5):588–593.
- Wade VA, Karnon J, Eliott JA, Hiller JE. Home videophones improve direct observation in tuberculosis treatment: a mixed methods evaluation. PLoS One. 2012;7(11):e50155.
- Mirsaeidi M, Farshidpour M, Banks-Tripp D, Hashmi S, Kujoth C, Schraufnagel D. Video directly observed therapy for treatment of tuberculosis is patient-oriented and cost-effective. Eur Respir J. 2015;46(3):871–874.
- Holzschuh EL, Province S, Johnson K, et al. Use of video directly observed therapy for treatment of latent tuberculosis infection—Johnson County, Kansas, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(14):387–389.
- Cole SR, Hudgens MG. Survival analysis in infectious disease research: describing events in time. AIDS. 2010;24(16):2423–2431.
- Bain EE, Shafner L, Walling DP, et al. Use of a novel artificial intelligence platform on mobile devices to assess dosing compliance in a phase 2 clinical trial in subjects with schizophrenia. JMIR Mhealth Uhealth. 2017;5(2):e18.
- Nachega JB, Skinner D, Jennings L, et al. Acceptability and feasibility of mHealth and community-based directly observed antiretroviral therapy to prevent mother-to-child HIV transmission in South African pregnant women under Option B+: an exploratory study. Patient Prefer Adherence. 2016;10:683–690.
- Himelhoch S, Kreyenbuhl J, Palmer-Bacon J, Chu M, Brown C, Potts W. Pilot feasibility study of Heart2HAART: a smartphone application to assist with adherence among substance users living with HIV. AIDS Care. 2017;29(7):898–904.
- Lee M, Lee H, Kim Y, et al. Mobile app-based health promotion programs: a systematic review of the literature. IJERPH. 2018;15(12):2838.
- Gold J, Lim MSC, Hocking JS, Keogh LA, Spelman T, Hellard ME. Determining the impact of text messaging for sexual health promotion to young people. Sex Transm Dis. 2011;
- Mulawa MI, LeGrand S, Hightow-Weidman LB. eHealth to enhance treatment adherence among youth living with HIV. Curr HIV/AIDS Rep. 2018;15(4):336–349.
- Hoots BE, Finlayson TJ, Wejnert C, Paz-Bailey G, NHBS Study Group Early linkage to hiv care and antiretroviral treatment among men who have sex with men—20 cities, United States, 2008 and 2011. PLoS One. 2015;10(7):e0132962.
- Silverberg MJ, Leyden W, Horberg MA, DeLorenze GN, Klein D, Quesenberry CP. Older age and the response to and tolerability of antiretroviral therapy. Arch Intern Med. 2007;167(7):684–691.
- Hadland SE, Milloy M-J, Kerr T, et al. Young age predicts poor antiretroviral adherence and viral load suppression among injection drug users. AIDS Patient Care STDS. 2012;26(5):274–280.
- Martinez J, Harper G, Carleton RA, et al. The impact of stigma on medication adherence among HIV-positive adolescent and young adult females and the moderating effects of coping and satisfaction with health care. AIDS Patient Care STDS. 2012;26(2):108–115.
- Marks G, Gardner LI, Rose CE, et al. Time above 1500 copies. AIDS. 2015;29(8):947–954.
- Demographics of Mobile Device Ownership and Adoption in the United States | Pew Research Center. https://www.pewresearch.org/internet/fact-sheet/mobile/. Accessed April 2, 2021.
- Saberi P. Research in the time of coronavirus: continuing ongoing studies in the midst of the COVID-19 pandemic. AIDS Behav. 2020;24(8):2232–2235.