Abstract
A rapidly changing landscape of antiretrovirals and their procurement at scale has permitted the evaluation of new optimised second-line antiretroviral therapy (ART) in low- and middle-income countries. D2EFT is an open-label randomised controlled non-inferiority phase IIIB/IV trial in people living with HIV-1 (PWH) whose first-line non-nucleoside reverse transcriptase inhibitor (NNRTI)-based ART is failing. At inception, it compared a standard of care of boosted darunavir with two nucleos(t)ide reverse transcriptase inhibitors (NRTIs) to the novel NRTI-sparing regimen of boosted darunavir with dolutegravir. Implemented in 2017, participating sites were across Africa, Asia and Latin America. Around the time of implementation, the World Health Organization updated its treatment guidelines and recommended scaling up tenofovir disoproxil fumarate-lamivudine-dolutegravir (TLD). This situation pushed D2EFT investigators to consider the impact of the roll-out of TLD on the D2EFT research question. The protocol team agreed it was important to study TLD in second-line when an NNRTI regimen was failing, and focused on options to expedite the work by studying the question within the existing trial and network. All key issues (statistical, programmatic and financial) were reviewed to assess the benefits and risks of adding a third arm to the ongoing study, as opposed to developing a new randomised clinical trial with the same control arm and within the same network. The development of a new trial was deemed to be longer than adding a third arm, and to create a challenging situation with two competing clinical trials at the same sites which would slow down recruitment and impair both trials. On the other hand, adding a third arm would be demanding in terms of operationalisation, increased sample size and statistical biases to control. The optimal strategy was deemed to be the addition of a third arm, arriving retrospectively at a simplified multi-arm multi-stage clinical trial design to achieve statistical validity. The D2EFT study maintains additional value in a quickly evolving second-line ART strategy allowed by the progress in global access to ART.
Introduction
With more effective and better-tolerated antiretrovirals available in generic fixed-dose pills, and ‘Treat all’ programs supported by the World Health Organization (WHO), evidence for a simplified switch to optimised second-line antiretroviral therapy (ART) in low- and middle-income countries (LMICs) was crucial.Citation1–3 People living with HIV (PWH) whose first-line regimen is failing should have equitable access to second-line ART, adapted to their first-line ART composition, irrespective of their setting and the HIV monitoring testing available.Citation4 D2EFT (Dolutegravir and Darunavir Evaluation in Adults Failing Therapy) is an international randomised open-label clinical trial which compares different second-line options to standard of care (SOC) when first-line non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimen is failing in HIV-1-infection (NCT03017872).
The D2EFT study was developed in 2016. It was originally designed to compare a dual therapy of darunavir boosted with ritonavir (DRV + r) and dolutegravir (DTG) to the WHO SOC, a boosted protease inhibitor (PI), darunavir in the case of D2EFT, plus a nucleos(t)ide reverse transcriptase inhibitors (NRTIs)-backbone.Citation5 During the initial stage of the trial, additional data on the efficacy of dolutegravir based regimens in both first- and second-line emerged. In 2018, the WHO recommended tenofovir disoproxil fumarate/lamivudine/dolutegravir (TDF/3TC/DTG, TLD) as a universal first-line regimenCitation6 and it started to be rolled out at the global level.Citation7 This situation prompted the D2EFT study team to reconsider the study design to inform the use of TLD in the setting of NNRTI-based regimen failure. After a period of consultation with stakeholders, funders and investigators, the D2EFT study was rapidly adapted by adding a third arm of TLD in preference to developing an additional clinical trial.
The aim of this article is to give an overview of the D2EFT protocol and to describe the transition from a two-arm comparative trial to a multiple-arm multiple-stage (MAMS) study. Furthermore, the challenges of such a transition along with the current status of D2EFT are outlined and discussed.
Original D2EFT trial
Background and objectives
The overarching goal of D2EFT was to gather high-quality evidence to advocate simpler switches to optimal second-line ART. The simplifications related to two levels of the process, promoting: i) task-shifting from HIV-specialists or physicians to other healthcare givers at Primary healthcare centres with ART robust enough to be used after an NNRTI-based regimen failed without a genotypic resistance testing, and ii) the combination of well-tolerated antiretrovirals in fixed-dose pills. At inception, the D2EFT design was based on the results of second-line ART trials studying lopinavir/ritonavir + raltegravir as alternative to SOC: SELECT,Citation8 SECOND-LINECitation9 and EARNEST.Citation10 The results of these studies supported a potential efficacy for a NRTI-free dual regimen of boosted darunavir 800 mg + ritonavir 100 mg + DTG 50 mg, once a day, in PWH whose NNRTI-based first-line regimen failed. The benefit of this regimen, based on two classes of antiretroviral with a high-barrier of resistance, and to which the PWH was naïve, is the potential non-requirement for genotypic resistance testing. The original idea was to compare this experimental arm to the SOC arm composed of DRV 800 mg + ritonavir 100 mg and two NRTIs as per WHO guidelines.Citation5
The primary objective of D2EFT (original and subsequent versions) is to compare the virological efficacy of the different regimens as defined below.
The eligibility criteria are presented in Supplemental material #1.
Study design and statistical analyses
D2EFT is a phase IIIB/IV, multinational, multicentre, randomised, open-label study in HIV-1 infected patients whose first-line NNRTI-based regimen had failed (defined here as two HIV plasma viral loads (pVL) >500 copies/millilitres (mL), at least 7 days apart). Originally participants were randomly allocated to either SOC or DTG + DRV + r in a 1:1 ratio. The original study aimed to recruit 610 participants from sites across Argentina, Chile, Colombia, India, Malaysia, Mexico, South Africa, Thailand and Zimbabwe. The primary endpoint was the proportion of participants in each arm whose HIV pVL was suppressed (<50 copies/mL) at 48 weeks, and subjects were to be followed for 96 weeks in total. Randomisation was stratified by sites, flagged for the availability of resistance testing, screening HIV pVL level and prior use of TDF.
The organisation of D2EFT working group: inclusive discussion process to develop a tailored protocol
The D2EFT governance structure is based on a Protocol steering committee (PSC) and a Community advisory board (CAB) (Supplemental material #2). The aim of this organisation was to ensure every stakeholder could be represented. The delegation to local or regional Site Coordination Centres enables efficient communication with research sites. The composition and organisation of the PSC and CAB empower members of the site research teams and site communities to raise issues from their own context. This process facilitates reaching consensus across the different stakeholders, it had also guaranteed a tailored original D2EFT protocol. These tailored study procedures aimed to be as close as possible to the guidelines for HIV management (beyond the experimental arm ART) across the various settings where D2EFT was to be implemented.
Indeed, the choice of NRTIs in the SOC arm raised two questions on the place of HIV genotyping and zidovudine (AZT) across the sites’ standard practice. Some sites where D2EFT would be implemented had access to genotype testing, and their national guidelines recommended HIV genotyping before any switch to second-line. This contrasted with practice at sites which followed WHO recommendations of a rotation of NRTIs in second-line.Citation5,Citation11 To overcome this difference across sites, and because one of the D2EFT goals was to promote a simple and effective switch to second-line when genotyping was not available, sites with access to HIV genotyping (N= 12/28) could base their choice of NTRI-backbone for participants randomised to SOC arm on the genotyping results. For those sites without access to HIV genotyping, the CAB and PSC discussed the NRTI options, their tolerance and availability, and the impact of the choice of NRTIs in the SOC arm on the validity of comparison with the literature, and its alignment with internationalCitation6 and local guidelines.Citation12–16 Following WHO recommendations, a rotation of NRTIs was maintained with the use of zidovudine in the second-line regimen when the first-line comprised tenofovir.
Additionally, DRV was chosen as the PI in the SOC arm with the prospect of a potential future accessible fixed-dose combination of boosted darunavir.Citation17–19 Study drugs supplied by the sponsor are DRV and DTG, other antiretroviral are locally sourced.
D2EFT original version roll-out and projections
The roll-out of D2EFT in its original version, from concept to first randomisation including raising funding and developing industry partnerships, took 25 months. Formal recruitment was forecast to begin in October 2017 with a projected accrual of 610 subjects (targeted sample size) completed by end of December 2018.
Rationale for the inclusion of an additional arm in the ongoing D2EFT trial
Evolution of WHO recommendations and DAWNING results
Since the end of 2016, the single-tablet regimen (STR) ‘TLD’ was being made widely available to countries accessing the ART generics program.Citation7,Citation20 The ‘generic accessible’ countries are countries where global generic manufacturers can register and supply a large proportion of that country’s ART; for this purpose, CHAI (Clinton Health Access Initiative) defines ‘generic accessible’ countries as those LMICs that are covered under voluntary licenses for generic TDF/Tenofovir alafenamide.Citation20,Citation21 This well-tolerated STR with a high barrier to resistance offered a promising option for first- and second-line. The goal of policy-makers and providers was to help LMICs switch their cohorts on NNRTI-based first-line to DTG.Citation6,Citation7,Citation11 Where pVL testing was accessible and if PWH had a detectable HIV pVL at the time of the switch, a rotation of NRTIs was recommended by WHO.Citation6,Citation11 Because some countries might not be able to check the HIV pVL before a switch from NNRTI and relied on clinical criteria of therapeutic success, a fair proportion of these PWH switching to TLD were likely to have some level of HIV replication when switching to TLD, meaning a non-rotation of the NRTI-backbone. A WHO working group started to reflect on the consequences of these switches in the context of HIV replication and aimed to support randomised clinical trials (RCTs) looking at switch to TLDCitation1. This changing landscape created an imperative to consider an upgrade of D2EFT with an additional arm, understanding that a boosted PI comparator would continue to be required in any second-line study in the medium term.
In addition, since D2EFT was designed and funded, data emerged from the DAWNING studyCitation22 which compared DTG + 2NRTIs with a lopinavir/ritonavir + 2NRTIs as second-line treatment. In DAWNING, all participants had resistance testing and required one active nucleoside, in contrast to D2EFT. The intervention arm of DTG + 2NRTIs showed superior efficacy and a favourable safety profile. Confirmation and extension of the results of DAWNING were desirable for several reasons: the consideration of DRV instead of lopinavir in the SOC arm, and the applicability of the result to PWH who may not access resistance testing (i.e. in people potentially with no active nucleosides in the DTG arm) was unknown.
D2EFT original protocol implementation and addition of TLD evaluation consideration
By the end of 2017, D2EFT version 1 implementation was well advanced, with screening of potential participants already underway at sites in Southern Africa. However, there was a brief window of opportunity available to consider options to leverage the investment in D2EFT to accelerate the evaluation of TLD in second-line. The key alternatives were to develop another study looking at the same group of PWH with the same SOC arm within the established infrastructure or not. At the time, there were no other RCTs tackling this research question in this specific population. Given the established framework in D2EFT for evaluation of second-line ART predominantly in LMICs, the possibility of modifying D2EFT to allow evaluation of DTG with a fixed NRTIs backbone of TDF plus 3TC or emtricitabine (FTC) was proposed to all stakeholders and funding agencies. Despite the challenges of adding an experimental arm to an ongoing clinical trial, it appeared to the PSC as an option to consider while acknowledging it was not an adaptative trial per se.Citation23–27 Incorporating an emerging therapy as a new randomisation arm in a clinical trial that was opened to recruitment was deemed to be desirable to ensure that the trial remains current, new treatments were evaluated as quickly as possible, and the time and cost for determining optimal therapies was minimised.
An alternative approach was to develop another second-line trial including DTG + 2NRTIs during D2EFT conduct. Estimated timelines were significantly longer than the new experimental arm proposal. Indeed, an adaptation of D2EFT study allowed the same control arm participants to serve as the basis for two novel comparisons rather than requiring duplication, and allowed the novel arms to be compared with each other (with reduced requirement for control subject). Time to reporting would be accelerated with primary endpoint results expected 18-24 months sooner when compared with planning an additional, new study from inception.
Adaptation of D2EFT with an additional experimental arm
Statistical considerations
The key issue when adding a new arm to an ongoing RCT was that treatment comparisons could only be made between contemporaneously randomised participants. This was to account for any changes in the patient population being randomised over time, especially as new recruiting sites were added contemporaneously with the addition of a third arm. Therefore, all new participants could not simply be pooled into the same treatment arm, as the comparisons would have been biased. The issue is illustrated in , where Stage 1 of the study refers to the initial design with two arms: Arm 1 (SOC) or Arm 2 (DTG + DRV + r), and Stage 2 refers to the addition of the Arm 3 (DTG + TDF + 3TC or FTC). For unbiased comparisons of the treatment arms, the comparison of Arm 3 with either Arm 1 or 2 could only include participants randomised during Stage 2. The comparison between Arms 1 and 2 could include participants randomised during Stages 1 and 2. The consequence of this staging, was the need to recruit more participants to Arm 3 than Arms 1 and 2, during Stage 2, to retain sufficient power across all treatment comparisons.
Figure 1. D2EFT study schematic and representation of the groups compared at stage 1 and 2.
ART: antiretroviral therapy; DRV/r: darunavir boosted with ritonavir; DTG: dolutegravir; NNRTI: non-nucleoside reverse transcriptase inhibitor; NRTI: nucleos(t)ide reverse transcriptase inhibitor; PWH-1: people living with HIV-1; SOC: standard of care; TDF/XTC: TDF (tenofovir disoproxil fumarate) + FTC (emtricitabine) or 3TC (lamivudine)
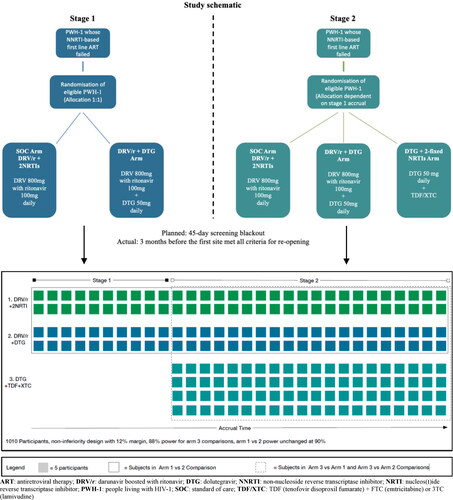
The primary endpoint is the comparison of proportions of participants in each arm whose pVL is <50 copies/mL at 48 weeks by intention-to-treat (ITT), completed by a per protocol analysis. Recent studies using once daily DRV + r combined with NRTI-backbone in ART experienced participants suggested that approximately 70% will achieve pVL <50 copies/mL at 48 weeks, with even greater suppression (82%) among those with no prior PI exposure.Citation28 Thus, we assumed 75% would be undetectable at week 48 for the calculation of the required sample size. Non-inferiority was defined as the lower 95% confidence limit on the difference in proportions with undetectable viral load lying above −12% (i.e. a non-inferiority margin of 12% - this delta value was selected on the basis of United States Food and Drug Administration guidance).Citation29
Under the null hypothesis of no difference between randomised treatment arms and a suppression rate of 75%, to have 90% power to demonstrate non-inferiority in the ITT analysis using a 12% margin would require 288 participants to be randomised into each of the two initial arms 1 and 2 (2-sided α = 5%) making a total of 576 participants. Adjusting for the anticipated low rates of loss to follow-up (approximately 5%) yielded a required sample size for the original two-arm comparison of 610 participants. Power for the third arm to be added in stage 2 depended on the number of subjects accrued in stage 1. At the time of the study redesign, 150 participants were expected to have been randomised to Arms 1 and 2 in Stage 1 (in total). Hence a little over 225 PWH would need to be randomised to each of Arms 1 and 2 in Stage 2, and 400 PWH to Arm 3 to give 90% power of showing non-inferiority to Arm 1. This gives a total revised sample size for the study of 1,010 participants. A series of sensitivity analyses were also performed to ensure we were confident that our sample size was robust to the assumptions made ().
Table 1. Sensitivity power calculations. The table gives the probability of the trial showing non-inferiority for pair-wise treatment arm comparisons, under differing scenarios, no adjustment of alpha-levels.
There are differing views among statisticians whether to adjust p-values and confidence intervals for multiple comparisons in multi-arm studies, and some argue that adjustments is not needed with a common control arm.Citation30 We also chose not to adjust for multiple comparisons to avoid inflating an already challenging sample size. Instead, we a priori identified two primary comparisons of each of the two simplified regimens (Arms 2 and 3) with Arm 1. The comparison of Arm 2 and 3 would be presented and interpreted as a secondary comparison. We accepted that this would affect the overall coverage of our confidence intervals, and results would be interpreted accordingly, but we thought this was the best balance of sample size target and power.
In addition to the adaptation below, the randomisation criteria and secondary objectives of the study will allow us to mitigate any other potential biases. First, randomisation was stratified by sites, screening HIV pVL level and prior use of TDF. Second, sites using genotype testing to adapt the NRTI-backbone of the SOC Arm were flagged. Finally, HIV drug resistance testing will be performed on mandatory stored blood sample at baseline (randomisation visit) with the outcomes to be analysed and discussed with the primary endpoint results.
Operational considerations of the implementation of a third arm
Adapting D2EFT to allow exploration of TLD in second-line provided a number of advantages. These included efficient use of established infrastructure, limited site and subject resources, an established site network and contractual frameworks. While contracts would need to be revised extensively, this still provided an accelerated timeline to implementation. Also, the capacity of skilled trial sites to undertake multiple trials was subject to saturation. Adaptation, rather than approaching sites for a later trial while they were implementing D2EFT, leveraged their existing availability and commitment.
A full scoping of the feasibility, resource requirements and cost, and probable timelines for the addition of a third arm to D2EFT required extensive consultations and dedicated resources.Citation31 Supplemental Material #3 lists the rationale of adding the third arm with the operational challenges associated with implementation, and considerations of all stakeholder’s perspectives.
The addition of Arm 3 prompted the Data safety monitoring board (DSMB) to add an early review of interim results to ensure that there was no evidence of poor response in Arm 3 due to the mandated nucleoside backbone used with no resistance testing.
Outcomes and lessons learnt
A swift transition with close collaboration
The D2EFT protocol version 2 development started in November 2017 and went along with the extension application to ongoing grants and the application to new grants. New funders granted the implementation of the third arm and the addition of 6 sites across Indonesia, Guinea and Mali. Two sites in Brazil and Nigeria were also to open. Funding was secured by March 2018, and all partners and site investigators approved the process.
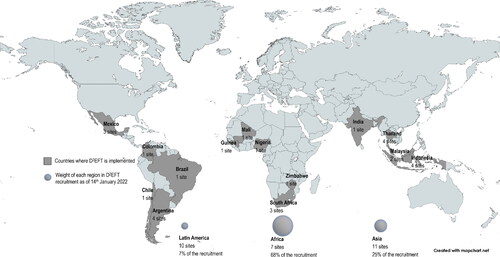
When version 1 recruitment was put on a hold as planned, 109 subjects were randomised in Arm 1 and 2; this was in accordance with the scenario described above assuming around 150 PWH would be randomised at Stage 1. Once the sponsor received the University of New South Wales ethics approval for version 2 in May 2018, local ethics submission and regulatory approval outcomes followed with timing being dependent on: whether the site had opened for version 1, time needed to translate the protocol or Participant’s information sheet and consent form only, time to collate documents needed for ethics committees, and time to organise face-to-face training with all sites. The first site was opened to version 2 in September 2018, and all 28 sites were opened by March 2020. shows the 14 countries where D2EFT is now implemented. The DSMB reviewed the early interim data in January 2020 and advised to continue without modification. As of January 2022, 831 participants were enrolled.
Figure 2. Countries where D2EFT study is implemented (N = 14), number of research sites per country (N = 28) and weight of each region in D2EFT recruitment as of 14th January 2022 (831 participants enrolled).
Choosing to add a third arm in an ongoing RCT was challenging but it took only 10 months (from the concept to the first site to open), including a three-month period of accrual suspension. Furthermore, it provided the opportunity to answer a pressing research question on the efficacy of TLD in second-line with recycling TDF when a NNRTI-based regimen had failed, while sustaining robust design and statistical power. This approach provided additional scientific value from the D2EFT findings in quickly evolving ART strategies. Also, this approach avoided wastage of resources and ethical issues regarding the participants if a substantial impact of the findings on PWH care was not retained. In the meantime, other studies were developed evaluating TLD in the same context: ARTIST which includes an induction period with DTG twice daily (NCT03991013),Citation32 NADIA which randomises for DTG or DRV and then AZT or TDFCitation33 and VISEND which includes a TLD arm where participants with a HIV pVL > 1,000 copies/mL could be randomised versus SOC (PACTR201904781300573).Citation34 Still, the distinguishing features of D2EFT among these current studies are: the larger population, the HIV pVL threshold of 50 copies/mL for the primary endpoint, and the composition of the regimens in the three arms.
Covid-19
The COVID-19 pandemic led to an inevitable pause of recruitment, for risk mitigation, with ten sites not resuming accrual. Adaptation of the participants’ schedule was aligned with the DSMB reports, and timely communications with research teams through the PSC and communities through the CAB. The goal was to enable a smooth transition of D2EFT study procedures to minimise the impact of COVID-19 while protecting participants, research teams and healthcare systems.Citation35 Research teams had to determine the benefit/risk ratio to continue enrolment in D2EFT, taking into consideration the safety of participants and healthcare givers, the accessibility of the services linked to the study, procurement, reallocation of infectious diseases healthcare givers and resources to control the COVID-19 outbreaks.Citation36,Citation37 The risk mitigation plan included options to enable teleconsultation for visits outside primary endpoint determination, and was submitted to the Ethics Committees to enable a rationalised resumption of accrual.
Completion of recruitment
Besides the delay of accrual induced by the pandemic, the widespread roll-out of TLD as first-line regimen was reducing the pool of potential participants in sites where D2EFT was implemented. Also, the delay of accrual led to budgetary pressure. The PSC elected to end recruitment in December 2021 to ensure that the results remain timely and relevant, after assessing that the sample size of 831 participants reached would provide approximately 80% statistical power for the three-arm comparison with the original 12% delta in the ITT analysis.
Conclusion
The addition of a third arm in D2EFT took 10 months. This timeline was considerably shorter than the 25 months needed to open the first site to the D2EFT original protocol. The established framework of an accruing clinical trial such as D2EFT can accelerate evaluation outcomes compared to planning a subsequent separate randomised trial, while reducing the number of control subjects needed compared with multiple trials. Adapting D2EFT also allows assessment of the place of resistance testing for TLD, and uniquely, the comparison of TLD with DTG + DRV + r. The MAMS adaption outlined minimises the risk associated with deferring the opening of D2EFT for revision to add a third arm, and accounts for the major scientific risks of additional arms in randomised trials including power and multiplicity of comparisons. The timeline of this adaptation was brief thanks to the support of existing and new stakeholders. With the end of recruitment in December 2021, the primary outcome will be disseminated in 2023. D2EFT results will offer a crucial exploration of the utility of resistance testing in second-line (TLD and NRTI rotation in SOC), and a path to potential further simplification of second-line ART with more affordable fixed-dose combinations of new antiretrovirals, in a public health approach to second-line ART provision.
Research ethics and patient consent
All subjects give their informed consent for inclusion before they participated in the D2EFT study. The study is conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of UNSW Sydney (HC17037) and by the Ethics Committee of each participating site.
Authors contribution
All authors contributed to the conception and design of the study, or the writing or review of the original protocol or subsequent ones. EP, SJ, ML and MP drafted the article. All authors revised the article critically for important intellectual content. All authors have given final approval for publication and take responsibility for the integrity of the data. ML and MP had full access to all data in the study and had final responsibility for decision to submit the article for publication.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of Unitaid, or any other agency.
Supplemental Material
Download Zip (464.7 KB)Acknowledgements
The authors gratefully acknowledge the contribution of the D2EFT Study Group (present representatives: see list below, and deceased: Prof. David Cooper, Prof. James Gita Hakim and Dr. Ernest Ekong), of the other academic collaborators (Dr. H. Clifford Lane; NIH), institutional collaborators (WHO and CHAI) and funding partners (Unitaid, NIH, NHMRC and ViiV Healthcare), and community representatives (chair: Leonardo Perelis) who support the D2EFT study; companies which donated darunavir and dolutegravir (ViiV Healthcare and Janssen Pharmaceutica); and the participation of the patients, healthcare givers and researchers in D2EFT project.
D2EFT Study group (alphabetical order for each sub-groups)
The Kirby Institute: Eamon Brown, Michelle Burns, Ai Caldis, Cate Carey, Megan Clewett, David Cooper, Sean Emery, Yuvaraj Ghodke, Simone Jacoby, Anthony Kelleher, Matthew Law, Margaret Lowe, Gail V. Matthews, Emmanuelle Papot, Mark N. Polizzotto, David Silk.
Protocol Steering Committee: Iskandar Azwa, Margaret Borok, Dannae Brown, Mohamed Cissé, Sounkalo Dao, Nnakelu Eriobu, Simone Jacoby, Richard Kaplan, Muhammad Karyana, Anthony Kelleher, Nagalingeswaran Kumarasamy, H. Clifford Lane, Matthew Law, Johnnie Lee, Marcelo H. Losso, Gail V. Matthews, Leonardo Perelis, Carmen Perez-Casas, Mark N. Polizzotto, Kiat Ruxrungtham, Jean Van Wyk, Melynda Watkins.
Country teams: Argentina (Patricia Burgoa, Marcelo H. Losso, Sergio Lupo, Luciana Peroni, Renzo Moretto, Ana Melisa Solari, Silvina Tavella, Maria Ines Vieni Deborah Vanina Villegas); Brazil (Kelly Gama, Beatriz Grinsztejn); Chile (Gladys Allendes, Marcelo Wolff); Colombia (Ana Julia Rojas, Otto Sussmann); Guinea (Mohamed Cisse, Thierno Mamadou Tounkara); India (Faith Beulah, Nagalingeswaran Kumarasamy, Poongulali Selvamuthu); Indonesia (Yusrina Adani, Dona Arlinda, Yufi Aulia Azmi, Usman Hadi, Sudirman Katu, Munawir Muhammad, Nur Herda Wati Nisa, Yanri Wijayanti Subronto, Evy Yunihastuti); Malaysia (Iskandar Azwa, Farhana Nadiah Abd Ghani, Chow Ting Soo, Margaret Tan); Mali (Sounkalo Dao, Yacouba Cissoko); Mexico (Jaime Andrade-Villanueva, Juan Luis Mosqueda Gómez, Fernando Amador Lara, Juan Sierra Madero, Sergio del Moral Ponce, Jorge Morales Varges); Nigeria (Maryam Al-Mujtaba, Peter Ekele, Nnakelu Eriobu); Thailand (Anchalee Avihingsanon, Ploenchan Chetchotisakd, Sivaporn Gatechompol, Suwimon Khusuwan, Weerawat Manosuthi, Supawadee Pongprapass, Kanitta Pussadee, Supeda Thongyen, Anchalee Tiyabut); South Africa (Desiree Van Amsterdam, Jaclyn Ann Bennet, Richard Kaplan, Lerato Mohapi, Suri Moonsamy, Mary Sihlangu); Zimbabwe (Margaret Borok, Ennie Chidziva-Chikuse).
Supplemental material
Supplemental material #1: D2EFT eligibility criteria
Supplemental material #2: D2EFT organigram
Supplemental material #3: Rationale to add a third arm balanced with expected implementation levers and challenges
Conflicts of interest
AA, AK, CPC, DA, EP, GVM, HCL, IA, KR, LP, MB, MC, MK, MW, NE, NK, RK, SD and SJ declare none. DB is a full-time employee of ViiV Healthcare and is a GSK shareholder. JL is an employee of Janssen. MHL received research funding to institution from ViiV. MNP received research funding to institution from ViiV, Gilead, Janssen, Bristol Myers Squibb. ML declared unrestricted research grants from Gilead Sciences, Janssen-Cilag and ViiV Healthcare.
Additional information
Funding
References
- World Health Organization. WHO Think-Tank Meeting on Optimizing Antiretroviral Therapy: Meeting Report. March 12, 2020. https://apps.who.int/iris/rest/bitstreams/1327637/retrieve. Accessed March 18, 2021.
- Vitoria M, Hill A, Ford N, et al. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: what are the issues? AIDS. 2018;32(12):1551–1561.
- World Health Organization. Treat All Implementation; 2019. https://apps.who.int/iris/bitstream/handle/10665/326035/WHO-CDS-HIV-19.20-eng.pdf?ua=1. Accessed March 18, 2021.
- World Health Organization. HIV Drug Resistance Report 2021. https://apps.who.int/iris/handle/10665/349340. Accessed March 18, 2021.
- World Health Organization. Guidelines Consolidated - Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection - Recommandations for a Public Health Approach. Page 31, Chapter 4: Clinical Guidelines: Antiretroviral Therapy, Table 4.11. WHO Definitions of Clinical, Immunological and Virological Failure for the Decision to Switch ART Regimens. 2nd ed.; 2016. https://apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdf?sequence=1. Accessed August 18, 2020.
- World Health Organization. Update of Recommendations on First- and Second-Line Antiretroviral Regimens; 2019. https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf?ua=1. Accessed August 18, 2020.
- World Health Organization. TLD Up-Take; 2018. http://www.who.int/hiv/pub/arv/treat-all-uptake/en/. Accessed August 18, 2020.
- La Rosa AM, Harrison LJ, Taiwo B, et al. Raltegravir in second-line antiretroviral therapy in resource-limited settings (SELECT): a randomised, phase 3, non-inferiority study. The Lancet HIV. 2016;3(6):e247-58–e258.
- Amin J, Boyd MA, Kumarasamy N, et al. Raltegravir non-inferior to nucleoside based regimens in second-line therapy with lopinavir/ritonavir over 96 weeks: a randomised open label study for the treatment of HIV-1 infection. PLoS ONE. 2015;10(2):e0118228.
- Hakim JG, Thompson J, Kityo C, et al. Lopinavir plus nucleoside reverse-transcriptase inhibitors, lopinavir plus raltegravir, or lopinavir monotherapy for second-line treatment of HIV (EARNEST): 144-week follow-up results from a randomised controlled trial. Lancet Infect Dis. 2018;18(1):47–57.
- World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach; 2021. https://www.who.int/publications/i/item/9789240031593. Accessed September 21, 2021.
- CSLS/MSAS. Normes et protocoles de prise en charge antiretrovirale du VIH et du SIDA; 2016. https://www.afro.who.int/publications. Accessed March 21, 2021.
- MASHM, Malaysia MoH. Malaysian consensus guidelines on antiretroviral therapy 2017; 2017. https://www.moh.gov.my/moh/resources/auto%20download%20images/589d71c4dd799.pdf. Accessed March 21, 2021.
- Meintjes G, Moorhouse MA, Carmona S, et al. Adult antiretroviral therapy guidelines 2017. South Afr J HIV Med. 2017;18(1):776–776.
- Censida/Secreteria de Salud. Guia de manejo antirretroviral de las personas con VIH; 2015. www.who.int/hiv/pub/guidelines/mexico_art.pdf. Accessed March 21, 2021.
- Ministerio de Salud, Santiago. Guida Clinica AUGE “Sindrome de la Immunodeficiencia adquirida VIH/SIDA”; 2013. http://www.repositoriodigital.minsal.cl/handle/2015/622?show=full. Access March 21, 2021
- Unitaid and the Clinton Health Access Initiative. Innovative Agreement Launches Affordable, Optimal Second-Line HIV Treatment in Low- and Middle-Income Countries. https://unitaid.org/news-blog/innovative-agreement-affordable-optimal-second-line-hiv-treatment/#en. Accessed December 8, 2021.
- MSF Access Campaign. Untangling The Web of Antiretroviral Price Reductions 18th edition. 2016. https://msfaccess.org/untangling-web-antiretroviral-price-reductions-18th-edition. Accessed November 1, 2021.
- World Health Organization. Application to Add Darunavir (DRV) to the Essential List of Medicines. https://www.who.int/selection_medicines/committees/expert/20/applications/Darunavir.pdf?ua=1. Accessed March 21, 2021.
- Clinton Health Access Initiative. CHAI HIV Market Report; 2020. https://www.clintonhealthaccess.org/the-state-of-the-hiv-market-in-low-and-middle-income-countries-3/. Accessed March 21, 2021.
- Clinton Health Access Initiative. 2021 HIV Market Report: The State of the HIV Market in Low- and Middle-Income Countries; 2021. https://www.clintonhealthaccess.org/2021-hiv-market-report-the-state-of-the-hiv-market-in-low-and-middle-income-countries/. Accessed December 8, 2021.
- Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis. 2019;19(3):253–264.
- Choodari-Oskooei B, Bratton DJ, Gannon MR, Meade AM, Sydes MR, Parmar MK. Adding new experimental arms to randomised clinical trials: Impact on error rates. Clin Trials. 2020;17(3):273–284.
- Bretz F, Gallo P, Maurer W. Adaptive designs: the Swiss Army knife among clinical trial designs? Clin Trials. 2017;14(5):417–424.
- Food and Drug Administration. Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research, eds. Adaptive Design Clinical Trials for Drugs and Biologics Guidance for Industry; 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/adaptive-design-clinical-trials-drugs-and-biologics-guidance-industry. Accessed March 21, 2021.
- Moore CL, Stohr W, Crook AM, et al. Multi-arm, multi-stage randomised controlled trials for evaluating therapeutic HIV cure interventions. Lancet Hiv. 2019;6(5):e334–e340.
- Parmar MK, Sydes MR, Cafferty FH, et al. Testing many treatments within a single protocol over 10 years at MRC clinical trials unit at UCL: multi-arm, multi-stage platform, umbrella and basket protocols. Clin Trials. 2017;14(5):451–461.
- Cahn P, Fourie J, Grinsztejn B, et al. Week 48 analysis of once-daily vs. twice-daily darunavir/ritonavir in treatment-experienced HIV-1-infected patients. Aids. 2011;25(7):929–939.
- Human Immunodeficiency Virus-1 Infection: Developing Antiretroviral Drugs for Treatment. Center for Drug Evaluation and Research; 2015.
- Cook R, Farewell V. Multiplicity considerations in the design and analysis of clinical trials. J R Stat Soc A. 1996;159(1):93–110.
- Schiavone F, Bathia R, Letchemanan K, et al. This is a platform alteration: a trial management perspective on the operational aspects of adaptive and platform and umbrella protocols. Trials. 2019;20(1):264.
- Keene CM, Griesel R, Zhao Y, et al. Virologic efficacy of tenofovir, lamivudine and dolutegravir as second-line in adults failing a tenofovir-based first-line regimen: a prospective cohort study. Aids. 2021;35(9):1423–1432.
- Paton NI, Musaazi J, Kityo C, et al. Dolutegravir or darunavir in combination with zidovudine or tenofovir to treat HIV. N Engl J Med. 2021;385(4):330–341. Jul 22
- Hill A, McCann K, Chirwa L, Qavi A, Mulenga L. A randomized prospective study evaluating virologic outcomes among patients switching with detectable and undetectable viral loads from efavirenz and nevirapine-based first line ART regimens to DTG regimens in Zambia presented at: AIDS conference; 2020; Virtual. http://programme.aids2020.org/Abstract/Abstract/10841
- Orkin AM, Gill PJ, Ghersi D, et al. Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances: the CONSERVE 2021 statement. JAMA 2021;326(3):257.
- Mgbako O, Miller EH, Santoro AF, et al. COVID-19, telemedicine, and patient empowerment in HIV care and research. AIDS Behav. 2020;24(7):1990–1993.
- World Health Organization. Disruption in HIV, Hepatitis and STI Services Due to COVID-19. http://covid.hivci.org/dashboard/covid_map.html?r=eyJrIjoiZmI4YmY4ZjktYjUwYi00MDY5LTk2MDEtZTE3YWJkZjYzYzAzIiwidCI6ImViNDJjZGViLWI3YjUtNGE2ZC1iYzFjLWQxZWJjYmZjNDgyZSJ9&pageName=ReportSectionedaf9deae210a0d50226-to-covid-19-november-2020.png?sfvrsn=d45dbe01_5. Accessed December 8, 2021.
