Abstract
Background: Tenofovir disoproxil fumarate (TDF) can induce proximal renal tubulopathy (PRT) and necessitate changes in treatment regimen. This prospective study aimed to compare tubular function recovery following early switching versus late switching of TDF in human immunodeficiency virus (HIV)-infected patients with TDF-induced PRT.
Methods: For this prospective study, conducted during 2017–2019, we enrolled HIV-1-infected, virologically suppressed adults undergoing TDF-containing combination antiretroviral therapy. Patients were separated into a late-switching group (LSG) and an early-switching group (ESG). The LSG included patients having an estimated glomerular filtration rate (eGFR) decrease ≥25% from the pretreatment level or Fanconi syndrome. The ESG included patients having ≥2 of the following indicators of PRT: fractional excretion of phosphate (FEUP) ≥10%, low tubular maximum reabsorption of phosphate (TmP)/GFR, or uricosuria; fractional uric acid excretion ≥10%; urine protein–creatinine index (UPCI) ≥500 mg/g creatinine, normoglycemic glycosuria, or decrease in eGFR of 15%–24%. Recovery of proximal tubular function at 6 and 12 months after TDF discontinuation was assessed. Complete recovery was defined as normalization of all abnormal tubular markers.
Results: Thirty-three HIV-infected patients were enrolled (70% male). Except for tubular function markers, baseline characteristics were not significantly different between the two groups. The proportion of patients having complete recovery was significantly higher in the ESG (p = 0.007, log-rank test). FEUP improved significantly in the ESG after TDF discontinuation; improvements of eGFR and UPCI were greater in the LSG. An eGFR change of 10% from baseline was the only independent predictor of failure to achieve complete recovery after switching. After median follow-up of 2.25 years post-trial, sustained recovery of eGFR within 5% of pre-TDF eGFR was achieved only in the ESG.
Conclusions: Early-switching of TDF in HIV patients with PRT may allow complete recovery of proximal renal tubular function.
Introduction
Tenofovir disoproxil fumarate (TDF) is recommended as part of first-line combination antiretroviral therapy for treatment-naïve patients with human immunodeficiency virus (HIV) infection. However, it can cause renal toxicity, which may range from asymptomatic proximal renal tubulopathy (PRT) detected by laboratory investigations (phosphaturia, hypophosphatemia, uricosuria, proteinuria, glycosuria) to clinically evident acute kidney injury that may necessitate dialysis and, rarely, progress to end-stage renal disease.Citation1–3 The pathogenesis is postulated to be proximal tubular accumulation of TDF, which is toxic to tubular mitochondria.Citation4 Reported recovery of proximal renal tubular function after discontinuation of TDF varies between studies.Citation5–8 One retrospective study revealed that ≤61% of proximal renal tubules recovered completely.Citation8 Determinants of recovery include the duration of TDF treatment, the estimated glomerular filtration rate (eGFR) at the time of discontinuation, and the severity of proximal tubular dysfunction before discontinuation of TDF.Citation3,Citation7 Thus, early detection of PRT and prompt discontinuation of TDF may result in earlier recovery.
This prospective interventional study aimed to compare tubular function recovery in HIV-infected patients with TDF-induced PRT receiving early switching versus late switching.
Methods
Study design and setting
This open-label, interventional, prospective study was conducted King Chulalongkorn Memorial Hospital, Bangkok, from October 2017 to February 2019.
Patient inclusion and exclusion criteria
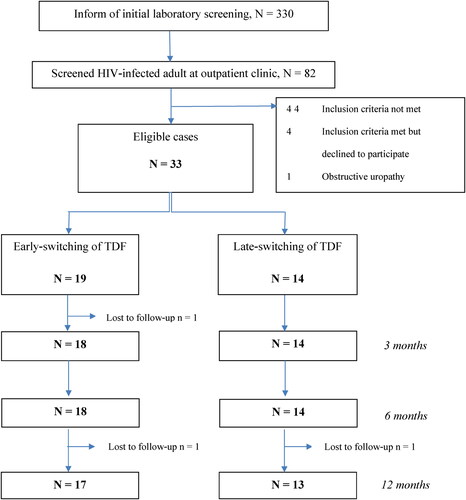
Patients were recruited from the outpatient clinics. The inclusion criteria were: 1) age ≥18 years; 2) virologically suppressed HIV infection; 3) on TDF-containing regimen; and 4) evidence of PRT. PRT was diagnosed if patients had any two of the following tubular abnormalities: 1) phosphaturia; 2) uricosuria; 3) normoglycemic glycosuria; 4) high urinary β2 microglobulin (β2M) level; 5) proteinuria; 6) eGFR decrease of ≥15% from pre-TDF eGFR. The exclusion criteria were: 1) concomitant kidney disease including previous diagnosis of tubulointerstitial or glomerular diseases other than TDF-induced tubulopathy before recruitment, or history of abnormal urinary sediments before initiation of TDF treatment; 2) use of nephrotoxic agents including radiocontrast media, non-steroid anti-inflammatory drugs, aminoglycosides, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, diuretics, acyclovir, amphotericin B, colistin, cidofovir, foscarnet, tacrolimus, or cyclosporin; or inhibitors of sodium-glucose co-transporter-2 or multidrug resistance protein in the 28 days before recruitment; 3) documented obstructive uropathy; or 4) pregnancy. The flow of case recruitment is shown in .
Grouping
The enrolled patients were separated into two groups: a late-switching group (LSG) and an early-switching group (ESG). The criteria for LSG inclusion were an eGFR decrease of ≥25% from pre-TDF eGFR or established Fanconi syndrome (characterized by abnormal proteinuria, phosphaturia, uricosuria, and normoglycemic glycosuria). The ESG comprised patients with PRT who did not meet the criteria for LSG inclusion. Both groups were assigned to discontinue TDF and switch to combination therapy with abacavir (ABC), lamivudine (3TC), and efavirenz, or to boosted lopinavir (LPV/r) and 3TC according to the HIV guidelines set by the Thai government in 2017.Citation9 The human leukocyte antigen-B*5701 (HLA-B*5701) test was performed in all participants before ABC initiation.
Outcomes
The primary outcome was the proportion achieving complete recovery of proximal tubule function at 12 months (± 1 month) after switching. The secondary outcomes were the proportion achieving virologic suppression, change in cluster of differentiation (CD)4 count, and tubular marker levels.
Definitions
‘Abnormal phosphaturia’ was defined as fractional excretion of urinary phosphate (FEUP) ≥10% or tubular maximum reabsorption of phosphate (TmP/GFR) less than the cutoff value.Citation10 We calculated TmP/GFR according to a formula adjusting for fractional tubular reabsorption of phosphate. The equation used for calculation of age- and sex-adjusted TmP/GFR cutoff was derived from the work of Payne and colleagues.Citation10 ‘Abnormal uricosuria’ was defined as fractional excretion of uric acid of ≥10%. ‘Abnormal proteinuria’ was defined as urinary reagent strips reading of ≥1+ or urine protein-creatinine index (UPCI) ≥500 mg/g. ‘Abnormal glycosuria’ was defined as a urine dipstick reading of ≥1+ with blood glucose <180 mg/dL (normoglycemic glycosuria). ‘Abnormal urinary β2M level’ was defined as urinary β2M ≥300 µg/L. ‘Complete recovery’ was defined as return of all markers of abnormal tubular function to their normal ranges and normalization of eGFR (i.e. to within 5% of pre-TDF eGFR). ‘Incomplete recovery’ was defined as failure to meet the criteria of complete recovery. Virologic suppression was defined as HIV RNA <50 copies/mL, per Thai national guidelines on HIV/AIDS treatment and prevention, 2017.Citation9
Measurements and sample collection
The pre-TDF and pre-switching serum creatinine concentrations were averaged from two or, preferably, three measurements obtained at least 1 month (but <1 year) apart. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for Asian populations. Spot samples of urine and blood were obtained and processed immediately. Supernatants of urine samples were used to measure the urinary level of β2M. Urine protein was measured using the benzethonium chloride method; the endpoint method was used to determine urine phosphate; uricase was used to determine urine uric acid; an enzymatic colorimetric assay was used for urine creatinine; a urine dipstick using the glucose oxidase/peroxidase reaction was used to measure urine glucose; and a turbidimetric method was used to determine β2M.Citation11,Citation12
Plasma HIV-1 RNA was measured using real-time reverse-transcription-quantitative polymerase chain reaction (RealTime HIV-1 Viral Load Kit; Abbott Molecular, Des Plaines, IL, USA); the lower limit of quantification of the kit is 40 copies/mL.
Ethical considerations
The study protocol was approved by the Ethics Committee of Chulalongkorn University, Bangkok, Thailand (No. 225/60). This study is registered at the Thai Clinical Trials Registry (TCTR20190307003).
Statistical analysis
Categorical data were expressed as frequencies and percentages and compared using the chi-square test. Fisher’s exact test was used when >20% of expected cell counts were <5. Repeated-measures ANOVA (for continuous variables) was used to analyze the changes in the markers of proximal tubule function within and between each group. For repeated-measures ANOVA, if the test of sphericity was not assumed, we used the Greenhouse–Geisser method to assess the time of follow-up and group effects. Pairwise comparisons at each time point for each tubular marker were calculated using repeated-measures ANOVA with Bonferroni’s correction for multiple comparisons. The Cox proportional hazards model was used to identify the factors associated with the complete-recovery rate; the hazard ratios (HR) and 95% confidence intervals (95% CI) were calculated. The Kaplan–Meier method was used to compare the time to complete recovery between the two groups. SPSS 24 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. P ≤ 0.05 (two-sided) was considered significant.
Results
The study population comprised 33 HIV-infected patients with TDF-induced PRT; there were 19 patients in the ESG and 14 in the LSG. All patients were residents of Thailand. Two ESG patients and one LSG patient were lost to follow-up at 12 months post-switching. The mean duration of follow-up was 11.3 months.
Age, sex, nadir and pre-switch CD4 counts, previous opportunistic infections, comorbidities, and duration of TDF exposure were not significantly different between the two groups (). The pre-switch urinary β2M level and UPCI were significantly higher, and eGFR significantly lower, in the LSG ().
Table 1 Baseline characteristics and pre-switch laboratory markers in the two groups
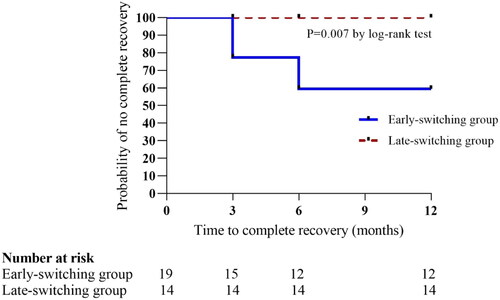
The proportion of cases who had complete recovery of all tubular markers was significantly higher in the ESG than in the LSG (p = 0.007; log-rank test; ). No patients in the LSG had a complete recovery within 1 year of follow-up. Of the 7/33 (21.2%) patients who had complete recovery, 5 (71.4%, 5/7) received the ABC-based regimen. Complete recovery of all tubular markers was seen at 3 months and 6 months after switching, but not thereafter. In the ESG, there was no significant difference in duration of TDF exposure between patients who attained complete recovery and those who did not (81.86 ± 32.37 months vs. 66.36 ± 33.71 months, p = 0.35). Age, bodyweight, previous opportunistic infections, diabetes mellitus, hypertension, pre-switch eGFR, percentage change in the eGFR, and levels of all tubular markers were not significantly different between patients who recovered completely and those who did not. Incomplete recovery was achieved by 7/17 (41.2%) patients in ESG vs. 10/13 (76.9%) patients in LSG (p = 0.07).
Figure 2 Kaplan–Meier survival curves comparing probability of no complete recovery between the two groups.
Survival analysis performed using Kaplan–Meier survival curves and log-rank test.
At a mean of 11.3 months post-switching, improvement in FEUP was significantly improved in the ESG compared with the LSG: the absolute reduction from pre-switch value was −7.8 (95% CI: −12.68 to −2.93) in the ESG vs. −13.4 (95% CI: −28.24 to 1.44) in the LSG, p = 0.02 (). shows the changes in each tubular marker.
Figure 3 Changes in tubular function markers from pre-switch levels to 3, 6, and 12 months after switching [mean ± SD (95% CI)]. (A) Estimated glomerular filtration rate (eGFR); (B) tubular maximum reabsorption of phosphate/glomerular filtration rate (TmP/GFR); (C) fractional excretion of urinary phosphate (FEUP); (D) fractional excretion of uric acid (FEUA); and (E) urinary protein-creatinine index (UPCI).
Repeated-measures ANOVA was used to calculate change for each urinary marker during the follow-up within each group. Pairwise comparisons at each time point were made using repeated-measures ANOVA with Bonferroni’s correction for multiple comparisons.
Striped light-gray box = early-switching group.
Dark-gray box = late-switching group.
Dashed line = cutoff of normal value.
*Significant.
![Figure 3 Changes in tubular function markers from pre-switch levels to 3, 6, and 12 months after switching [mean ± SD (95% CI)]. (A) Estimated glomerular filtration rate (eGFR); (B) tubular maximum reabsorption of phosphate/glomerular filtration rate (TmP/GFR); (C) fractional excretion of urinary phosphate (FEUP); (D) fractional excretion of uric acid (FEUA); and (E) urinary protein-creatinine index (UPCI).Repeated-measures ANOVA was used to calculate change for each urinary marker during the follow-up within each group. Pairwise comparisons at each time point were made using repeated-measures ANOVA with Bonferroni’s correction for multiple comparisons.Striped light-gray box = early-switching group.Dark-gray box = late-switching group.Dashed line = cutoff of normal value.*Significant.](/cms/asset/55b277c5-803f-4c0e-b6e4-97feb2820340/yhct_a_2113967_f0003_b.jpg)
Table 2 Changes in tubular markers, CD4, and lipid profile from the pre-switch value to 6 and 12 months after switching
An HIV load of 86 copies/mL and 2112 copies/mL were documented in two patients in the LSG treated with ABC/3TC/efavirenz (EFV) 6 months after TDF switching. In one of the patients, genetic-resistance testing showed a nucleoside reverse transcriptase inhibitor (NRTI)-resistant gene (M184V) and NNRTI-resistant genes (V106M, V179E, P225H). High-level resistance to 3TC, FTC, and EFV, and low-level resistance to ABC, were observed. The patient with HIV loads of 86 copies/mL continued the ABC/3TC/EFV regimen, while the patient with virologic failure was switched to zidovudine (AZT)/3TC/LPV/r. In both patients, undetectable HIV loads were achieved 6 months thereafter (12 months post TDF switching).
The CD4 percentage improved significantly in both groups: ESG (1.33%, 95% CI: 0.19 to 2.48, p = 0.03); and LSG (2.07%, 95% CI: 0.72 to 3.42, p = 0.006). The changes in CD4 percentage and numbers were not significantly different between the two groups at 6 months post-switching (p = 0.53 and p = 0.65, respectively).
The proportion of patients requiring lipid-lowering agents during the follow-up was comparable in the ESG and the LSG (41.18% [7/17] vs. 46.15% [6/13], p ≥ 0.99; ).
In Cox proportional hazards regression analysis, a high percentage change in the eGFR before switching was the only independent predictor of failure to achieve complete recovery, with HR = 1.052 (95% CI: 1.009 to 1.096, p = 0.02) per 1% decrease of eGFR. This corresponded to a 52% (range, 9%–96%) increase in the risk of not achieving complete recovery per each 10% decrease of eGFR.
At post-study median follow-up of 2.5 (interquartile range [IQR], 2.25, 2.67) years, data for eGFR were available for 18 and 14 patients in the ESG and LSG, respectively. Two previously lost to follow-up cases did show up later after the trial with available laboratory results. Complete recovery of the eGFR to within 5% of pre-TDF eGFR was achieved in seven patients in the ESG vs. none in the LSG (p = 0.01, log-rank test). The median change in eGFR from the pre-switch value to the final follow-up value was −5.87 (IQR, −17.52, +2) vs. −40 mL/min/m2 (IQR, −45.8, −32.01) in the ESG and LSG, respectively.
Discussion
In this HIV-infected, predominantly male, cohort with TDF-induced PRT, early switching from TDF therapy prompted complete recovery of renal tubular function. Improvement of phosphaturia was significantly better in patients with early switching. The percentage change in eGFR before switching was the only predictor of failure to achieve complete recovery after switching.
Jose and colleagues found longer duration of TDF treatment and pre-switch eGFR of <90 mL/min to be predictors of incomplete recovery or no recovery.Citation7 Consistent with our findings, Nishijima and coworkersCitation13 found that greater change in eGFR was associated with lower likelihood of recovery; they reported a nearly twofold increase in the risk of not having complete recovery for each 10% decrease in the eGFR. They also found the duration of TDF exposure to be related to the likelihood of recovery; however, this was not seen in our cohort.Citation13
The 2021 European AIDS Clinical Society (EACS) guidelines recommend switching from TDF if any of the following are present: 1) progressive decrease in eGFR to <90 mL/min without an identifiable cause; 2) hypophosphatemia, with evidence of phosphaturia and/or glycosuria; 3) osteopenia/osteoporosis, with evidence of phosphaturia; and 4) tubular proteinuria and/or glycosuria.Citation14 However, monitoring of proximal tubular function may not be possible in resource-limited settings. Calculation of TmP/GFR could substitute for measurement of serum phosphate level to identify renal phosphate loss. In our cohort, most patients in both groups had low TmP/GFR despite having normophosphatemia. Furthermore, high urinary β2M level was also observed in nearly all patients. Thus, close monitoring with markers such as TmP/GFR and urinary β2M can enable early switching in patients with TDF-induced PRT. Spot urine samples for urine chemistry tests, including urine phosphate and urine uric acid, showed good correlation with 24-h urine chemistry tests, thus enabling rapid screening for PRT that is possible in outpatient settings.Citation15–17
Long-term follow-up of major cardiovascular/cerebrovascular events should be conducted post-switching. In our cohort, worsening of the lipid profile occurred in both groups post-switching. Alternatives to boosted protease inhibitor-based antiretroviral therapy or combination with the ABC regimen should be considered in patients with high risk of cardiovascular disease.
Our study has some limitations. First, although the EACS guidelines suggest FEUP ≥20% in the absence of hypophosphatemia or FEUP ≥10% with normophosphatemia to be indicative of renal phosphate loss,Citation14 we used FEUP ≥10% as the cutoff regardless of the phosphate level; this improved sensitivity but may have reduced specificity. To increase the specificity for diagnosis of PRT, we used multiple tubular markers to define PRT. Only two ESG patients had FEUP of 10%–20% without hypophosphatemia and normal TmP/GFR; both patients achieved complete recovery. However, even after excluding these two patients, the primary endpoint of complete recovery remained significantly higher in the ESG (p = 0.025, log-rank test). Second, the follow-up period was relatively short; longer follow-up may demonstrate further tubular recovery. One previous study of a large cohort has demonstrated renal recovery occurring 5 years post-switching.Citation7 Third, the small sample size and non-randomized design of our study limits the generalizability of our findings.
In summary, screening for TDF-induced PRT using multiple tubular markers and prompt switching from TDF therapy can enable recovery of renal tubular function and prevent further damage to the kidney.
Author contributions
SP, PK, and OP developed the study concept. SP, NS, and PP were in charge of the data management. SP performed the statistical analysis and interpreted the findings. SP wrote the first draft of the manuscript. PL and OP supervised the writing process and were primarily responsible for the final content of the manuscript. SP, NS, PP, PK, and OP reviewed and approved the final manuscript.
Acknowledgments
We thank all the staff, laboratory technicians and statisticians at King Chulalongkorn Memorial Hospital (Bangkok, Thailand).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008;22(2):99–103.
- Mocroft A, Lundgren JD, Ross M, et al. Data collection on adverse events of Anti-HIV Drugs (D:A:D) Study. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV. 2016;3(1):e23–32–e32.
- Casado JL, del Rey JM, Bañón S, et al. Changes in kidney function and in the rate of tubular dysfunction after tenofovir withdrawal or continuation in HIV-infected patients. J Acquir Immune Defic Syndr. 2016;72(4):416–422.
- Kohler JJ, Hosseini SH, Hoying-Brandt A, et al. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab Invest. 2009;89(5):513–519.
- Wever K, van Agtmael MA, Carr A. Incomplete reversibility of tenofovir-related renal toxicity in HIV-infected men. J Acquir Immune Defic Syndr. 2010;55(1):78–81.
- Yoshino M, Yagura H, Kushida H, et al. Assessing recovery of renal function after tenofovir disoproxil fumarate discontinuation. J Infect Chemother. 2012;18(2):169–174.
- Jose S, Hamzah L, Campbell LJ, et al. Incomplete reversibility of estimated glomerular filtration rate decline following tenofovir disoproxil fumarate exposure. J Infect Dis. 2014;210(3):363–373.
- Bonjoch A, Echeverría P, Perez-Alvare N, et al. High rate of reversibility of renal damage in a cohort of HIV-infected patients receiving tenofovir-containing antiretroviral therapy. Antiviral Res. 2012;96(1):65–69.
- Thailand National Guidelines on HIV/AIDS Treatment and Prevention. 2017. http://www.thaiaidssociety.org/images/PDF/hiv_thai_guideline_2560.pdf
- Payne RB. Renal tubular reabsorption of phosphate, (TmP/GFR): indications and interpretation. Ann Clin Biochem. 1998;35(2):201–206.
- Henry JB, Richard AM, Matthew RP. Henry's Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia, PA: Elsevier/Saunders; 2011.
- Manual of Laboratory Investigations. https://chulalongkornhospital.go.th/kcmh/wp-content/uploads/2017/12/008-22-3-60.pdf. Accessed May 30, 2022.
- Nishijima T, Mutoh Y, Kawasaki Y, et al. Cumulative exposure of TDF is associated with kidney tubulopathy whether it is currently used or discontinued. AIDS. 2018;32(2):179–188.
- EACS guidelines v.11.0. Available at www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html
- Wahbeh AM, Ewais MH, Elsharif ME. Comparison of 24-hour urinary protein and protein-to-creatinine ratio in the assessment of proteinuria. Saudi J Kidney Dis Transpl. 2009;20(3):443–447.
- Gökçe Ç, Gökçe Ö, Baydinç C, et al. Use of Random Urine Samples to Estimate Total Urinary Calcium and Phosphate Excretion. Arch Intern Med. 1991;151(8):1587–1588.
- Choi ST, Moon SJ, Kang EJ. Random urinary uric acid/creatinine ratio is useful in the estimation of 24-hour urine uric acid excretion in patients with gout [abstract]. Arthritis Rheumatol. 2017;69(suppl 10).