?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Bioactive peptides of marine macroalgae consist of various functional properties. Ulva lactuca and Sargassum crassifolium are economically important, abundant green and brown macroalgae species in Sri Lanka that are rich in bioactive and functional components. However, few studies have been conducted to identify these bioactive and functional components of seaweeds in the local context. The aim of this study was to determine the best method to generate bioactive peptides from crude water extracts of Ulva lactuca and S. crassifolium and determine the functional properties of their hydrolyzates. Crude proteins of U. lactuca (sample: deionized water, 1:4) and S. crassifolium (sample: deionized water, 1:3) were extracted with water, using previously developed method and then subjected to protein hydrolysis by using papain (65°C, pH 6.00–7.00), α-chymotrypsin (37°C, pH 7.00–8.00), elastase (25°C, pH 8.0–8.50), and protease (60°C, pH 6.50–8.50) enzymes (1:100) under their optimum pH and temperatures for 0, 3, 6, 9, 12, 24 hours followed by heat inactivation at 100°C for 15 min. The best enzyme treatment was selected by conducting 15% SDS PAGE. Accordingly, proteins treated with enzymes followed by heat inactivation at 100°C for 15 min were selected as the best after considering the SDS-PAGE images. These hydrolyzates were used to determine the antioxidant activity (DPPH scavenging activity assay) and metal chelating activity (ferrozine method). Apart from peptides generated by elastase, all protein hydrolyzates had strong antioxidant activity (p < 0.05), but hydrolyzates produced from both crude extracts using α-chymotrypsin exhibited low Fe2+ chelating activity (p < 0.05) while some showed metal releasing activity. Therefore, protein hydrolyzates produced from U. lactuca using papain, protease, and α-chymotrypsin, followed with heat inactivation showed strong antioxidant activity while having very low Fe2+ chelating activity than all protein hydrolyzates of S. crassifolium.
Highlights
Ulva lactuca and Sargassum crassifolium are promising marine algal resources found in Sri Lankan waters to produce bioactive peptides.
Enzymatic hydrolysis can be carried out with different enzymes as papain, α-chymotrypsin, protease, and elastase enzymes under their optimum temperature and pH conditions to produce bioactive peptides.
The crude peptides showed high DPPH radical scavenging activities but low Fe2+ chelating activity.
Introduction
Noncommunicable diseases (NCDs) and deaths from NCDs have increased in tandem with the world’s human population. NCDs cause 41 million deaths worldwide each year, with cardiovascular diseases (17.9 million), cancers (9.0 million), chronic respiratory diseases (3.9 million), and diabetes (1.6 million) accounting for the majority (WHO, Citation2021). Despite the existence of synthetic drugs for use in treating diseases, they can cause a variety of side effects in humans including skin rashes, cough, taste disturbances, and angioneurotic oedema (Harnedy & FitzGerald, Citation2013; Wijesekara & Kim, Citation2010). Furthermore, synthetic antioxidants such as butylated hydroxytoluence (BHT), butylated hydroxyanisole (BHA), and tert-butyllydroquinone (TBHQ) which are used in the food industry for food preservation, also pave the path to these chronic diseases (Admassu, Gasmalla, Yang, & Zhao, Citation2018; Cox, Turley, Rajauria, Abu-Ghannam, & Jaiswal, Citation2014). Thus researchers have focused their attention on developing functional foods and pharmaceuticals as well as natural antioxidant compounds from natural food-derived peptides as a result of focusing on human health and well-being beyond the nutritional benefits of foods, as a solution for the rapid increase in chronic diseases, and limitations in synthetic drugs (Admassu et al., Citation2018; Harnedy & FitzGerald, Citation2013; Lafarga, Acién-Fernández, & Garcia-Vaquero, Citation2020; tePimentel, Alves, Harnedy, FitzGerald, & Oliveira, Citation2019).
Marine organisms, including macroalgae, are valuable sources of nutritious food components as well as reservoirs of novel biologically active components, such as bioactive proteins, peptides, and amino acids, due to their extraordinary diversity (Harnedy & FitzGerald, Citation2011). Bioactive peptides are amino acid sequences ranging from 2 to 30 amino acids in length (Mills, Stanton, Hill, & Ross, Citation2011). They can be generated in vitro by hydrolysing proteins during food processing under heat, acid, or alkaline conditions, fermentation of proteolytic starter cultures, proteolysis by microorganisms or plant-derived enzymes, or enzymatic hydrolysis of food proteins by digestive enzymes (Bleakley & Hayes, Citation2017; Korhonen & Pihlanto, Citation2006; Pangestuti & Kim, Citation2015). Enzymatic hydrolysis is the most ideal approach for producing bioactive peptides due to the mild nature and predictability of the end product (Dhaval, Yadav, & Purwar, Citation2016). Bioactive peptides in seaweeds have various functional properties that provide health benefits to humans, including, antioxidant, antimicrobial, metal chelating, anticancer, ACE inhibitory activity, antihypertensive, anticoagulant, anti-diabetic, and anti-HIV, depending on their structural properties (Admassu et al., Citation2018; Bondu et al., Citation2015; Cian, Garzón, Ancona, Guerrero, & Drago, Citation2016; Harnedy & FitzGerald, Citation2011, Citation2013; Sarmadi & Ismail, Citation2010; Sun et al., Citation2019; tePimentel et al., Citation2019).
Sri Lanka’s coastal areas provide suitable habitats for the growth and distribution of a diverse range of algal species (Coppejans, Leliaert, Dargent, Gunasekara, & De Clerck, Citation2009). These algal species are rich in bioactive and functional compounds due to tropical climate conditions in Sri Lankan seas such as temperature fluctuations, extreme salinity variations, nutrient-deficient habitats, and low light intensity (Lakmal et al., Citation2014). Even though Sri Lanka is an island surrounded by a seaweed-rich sea ecosystem, the majority of seaweeds have yet to be investigated for their bioactive properties or potential industrial applications but few studies were done for several bioactive compounds (PiSanjeewa et al., Citation2019). Premakumara, Ratnasooriya, & Tillekeratne (Citation1996) isolated a non-steroidal contragestive agent from Geldiella acerosa while Lakmal et al. (Citation2014) reported the anticancer and antioxidant potential of methanolic extracts of several red, green, and brown macroalgae species. Maldeniya, Egodauyana, & Abeyrathne (Citation2020b) determined the antioxidant, antimicrobial, and metal chelating activity of crude protein extracts of S. crassifolium. Furthermore, Fernando et al. (Citation2018) antioxidant and anti-inflammatory activities of extracts of ten macroalgae produced by carbohydrase assisted extraction. However, there are no previous studies on the production of bioactive peptides from Sri Lankan macroalgae and analysis of functional properties of their protein hydrolyzate. Thus, the aim of this study is to determine the best enzyme hydrolysis method to generate bioactive peptides from crude water extracts of Sargassum crassifolium and Ulva lactuca and determine the functional properties of their hydrolyzates.
Materials and methodology
Sample collection, preparation, and identification
U. lacuta (6 kg) () and S. crassifolium (8 kg) () and were harvested from Matara Madiha beach (5.93677 N, 80.51471E) at the southern coast of Sri Lanka. The seaweed samples were immediately transported to the protein chemistry laboratory of Uva Wellassa University, Badulla in seawater-filled low-density polyethylene bags. They were then washed to remove sand and other debris. All the seaweed samples were identified according to Coppejans et al. (Citation2009) i.e., Sri Lankan seaweeds methodology and field guide to the dominant species. Abc Taxa (Vol. 6). Finally, they were stored at –21°C until they were utilized for crude protein extraction. All the enzymes and chemical reagents were supplied from Sigma-Aldrich Ltd. (USA).
Crude protein extraction
Crude protein extraction was undertaken with water extraction methods following the method of Maldeniya et al. (Citation2020b). In order to increase the efficiency of crude protein extraction, seaweed samples were dried in a hot air oven (Model: SOV140A) and ground into a fine powder. The two dried seaweed powders were suspended separately in 1% (w/v) deionized water. 100 g of dried U. lactuca seaweed powder was suspended in 400 ml deionized water (sample: solvent, 1:4) and 100 g of dried S. crassifolium powder was suspended in 300 ml deionized water (sample: solvent, 1:3) Three replicates were prepared for each seaweed species and kept in refrigerated condition overnight followed by centrifugation at 4°C for 20 minutes at 4,000 rpm (Model: Sorvall ST 40 R, Thermofisher Scientific Germany). The supernatants of two seaweed mixtures were collected separately and then deionized water was added to the sediments of two seaweed species according to the above-mentioned ratios and another extraction cycle was run for both seaweed mixtures separately. Finally, supernatants of each extraction cycle of each seaweed species combined separately and lyophilized using a freeze drier (Model: FD5512, ShinBioBase, Korea).
Enzyme hydrolysis
Enzymatic hydrolysis was carried out using a modified method of Abeyrathne, Lee, Jo, Nam, & Ahn (Citation2014), and Thilanja, Dissanayake, Kariyawasam, & Abeyrathne (Citation2020). Each seaweed species crude proteins were individually treated with enzymes after adjusting to their optimum pH as α-chymotrypsin (25°C, pH 7.00–8.00) (Sigma-Aldrich: USA), elastase (37°C, pH 8.00–8.50) (Sigma-Aldrich: USA), papain (65°C, pH 6.00–7.00) (Sigma-Aldrich: USA), and protease (60°C, pH 6.50–8.50) (Sigma-Aldrich: USA). The enzyme (2 mg) and crude protein (200 mg of the substrate) were then mixed in a 1:100 ratio and at 0, 3, 6, 9, 12, and 24 hours. At the end of the incubation, enzymes were deactivated by heating the samples at 100°C for 15 mins using a heat block (Model: DB-006E, GEMMYCO, USA). Levels of hydrolyzates were checked with 15% SDS-PAGE in order to identify the best enzyme treatment according to Sambrook & Russell (Citation2006) with some modifications.
Determination of functional properties of protein hydrolyzates
Antioxidant activity by DPPH radical scavenging assay
In order to find the antioxidant activity of selected hydrolyzates, the DPPH assay was conducted according to Maldeniya, Egodauyana & Abeyrathne (Citation2020b) with some modifications. 300 µl of DPPH solution (0.1 M in methanol) and 750 µl sample were mixed thoroughly and kept at room temperature for 30 min in the dark place. At the end of the incubation, period absorbance was measured at 518 nm using a spectrophotometer (Model: UV 2005, J.P. SELECTA, Spain). The scavenging effect was calculated by using the following equation,
As = Absorbance of the sample.
A1 = Absorbance of the pure DPPH.
Ao = Absorbance of the sample added to methanol
Fe2+ chelating activity
Fe2+ chelating activity of the best-selected hydrolyzates of two seaweed species was measured separately by using the Ferozine method according to Carter (Citation1971) with some modifications. First, 100 µl of hydrolyzate, 900 µl of distilled water, and 1 ml of 100 ppm FeSO4 were added to a falcon tube and mixed well by using the vortex machine (Model: ZX3). At the end of the mixing, the sample was incubated for 5 min at room temperature. Then 900 µl of 11.3% (w/v) Trichloroacetic acid (TCA) was added to the vortexed sample and centrifuged under 2,500 g for 10 min at 5°C (Model: Sorvall ST 40 R, Thermofisher Scientific, Germany). After centrifugation 1 ml of supernatant was collected and transferred into a disposable culture tube and then 1 ml of distilled water, 800 µl 0 f 10% ammonium acetate, and 200 µl of ferrion colour indicator (200 µl of ferrion colour indicator; 75 mg of ferriozin, 75 mg of neocuprion and 1 drop of 6 N HCl were mixed with 25 ml of distilled water) was added, vortexed and incubated for 5 min at room temperature. The absorbance was measured at 562 nm by using a UV spectrophotometer (Model: UV 2005, J.P. SELECTA, Spain). Fe2+ chelating activity was measured by using the following equation.
A1= Absorbance of the sample.
Ao= Absorbance of the blank.
Statistical analysis
One-way ANOVA was used with Complete Randomized Design to analyse data of functional properties. Data were analysed by using Minitab 17.0 statistical software and Microsoft Excel 2010 version. Data were expressed as means with standard deviations. Statistical significance was considered at p < 0.05. All treatments were replicated (n = 3).
Results
SDS-page and determination of best treatment for hydrolysis
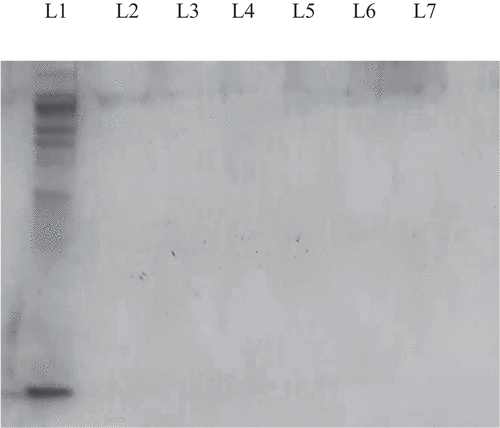
The best treatment method for the production of bioactive peptides from crude water extracts of U. lactuca and S. crassifolium was determined by using SDS-PAGE (). Each seaweed species’ crude protein can be seen as an opaque line (Lane 1). The enzyme-treated crude extracts were indicated from lane 2 to lane 7 where the opaque lines have disappeared. Thus, the absence of protein bands from lane 2 to lane 7 in each of the aforementioned gel images indicated that all proteins have been completely hydrolysed. Furthermore, using papain, α-chymotrypsin, elastase, and protease enzymes, crude proteins of U. lactuca and S. crassifolium can be hydrolysed even with heat inactivation at 100°C for 15 min. As a result, the optimal protein hydrolysing time lag for each treatment of both seaweed species was selected as immediate heat inactivation at 100°C for 15 min after enzyme addition.
Figure 3. 15% SDS-PAGE gel patterns for protein hydrolyzates of Sargassum crassifolium crude protein using a water extraction method. Lane 1 = Crude protein. Lane 2 to Lane 7 = protein hydrolyzates of S. crassifolium as a results of elastase enzyme treatment at 37°C for 0, 3, 6, 9, 12, 24 hours respectively followed by heat inactivation at 100°C for 15 min.

Figure 4. 15% SDS-PAGE gel patterns for protein hydrolyzates of Ulva lactuca crude protein using a water extraction method. Lane 1 = Crude protein. Lane 2 to Lane 7 = protein hydrolyzates of U. lactuca as a result of elastase enzyme treatment at 37°C for 0, 3, 6, 9, 12, 24 hours respectively followed by heat inactivation at 100°C for 15 min.

Figure 5. 15% SDS-PAGE gel patterns for protein hydrolyzates of Sargassum crassifolium crude protein using a water extraction method. Lane 1 = Crude protein. Lane 2 to Lane 7 = protein hydrolyzates of Sargassum crassifolium as a result of protease enzyme treatment at 37°C for 0, 3, 6, 9, 12, 24 hours respectively followed by heat inactivation at 100°C for 15 min. a,b,c Means with different superscript letters differ significantly (p < 0.05).
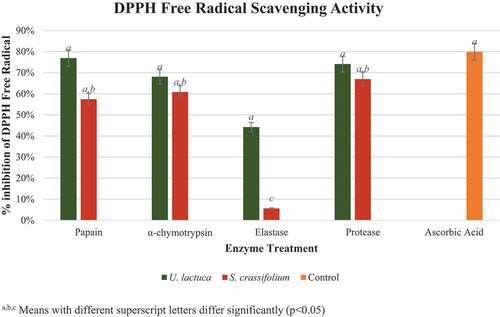
DPPH free radical scavenging activity assay
The mean percentage inhibitions of DPPH free radical scavenging activity of U. lactuca and S. crassifolium protein hydrolyzates are demonstrated in . According to the findings, all U. lactuca and S. crassifolium hydrolyzates possess antioxidant activity. Ascorbic acid served as the control (79.91 ± 0.69%) DPPH free radical scavenging activity while all the hydrolyzates of U. lactuca and S. crassifolium demonstrated more than 50% radical scavenging activity (p < 0.05) other than hydrolyzate which was produced by using elastase enzyme. According to the graph (), the hydrolyzates of U. lactuca produced in the presence of papain (76.89 ± 0.04%), protease (74.09 ± 0.02%), and α-chymotrypsin (68.08 ± 0.03%) exhibited higher free radical scavenging activity than the hydrolyzate produced by elastase enzyme (44.20 ± 0.17%). However, this result differed in S. crassifolium protein hydrolyzates as elastase (5.64 ± 0.01%), papain (57.45 ± 0.09%), α-chymotrypsin (60.84 ± 0.06%), protease (66.98 ± 0.03%). When comparing the values obtained from two seaweeds, U. lactuca crude proteins which were incorporated with papain have the strongest DPPH free radical scavenging activity.
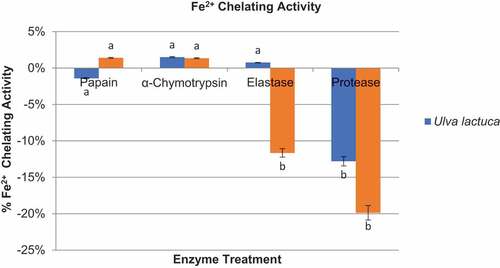
Fe2+ chelating activity
As demonstrated in , the Fe2+ chelating activity of all U. lactuca and S. crassifolium protein hydrolyzates was significantly lower than their antioxidant activity (p < 0.05). Despite its low Fe2+ chelating activity, U. lactuca hydrolyzate produced in the presence of α-chymotrypsin enzyme had significantly higher Fe2+ chelating activity (1.50 ± 0.001%) than any other U. lactuca and S. crassifolium hydrolyzates produced. Despite the low Fe2+ chelating activity of some protein hydrolyzates of U. lactuca and S. crassifolium, demonstrated substantial metal releasing activity. When metal releasing activity of protein hydrolyzates of U. lactuca and S. crassifolium was compared, the protein hydrolyzate of S. crassifolium produced in the presence of protease enzyme had the highest metal releasing activity (19.87 ± 0.02%) compared to other protein hydrolyzates of both seaweed species.
Discussion
Production of bioactive peptides using the seaweeds
Bioactive peptides from macroalgae have a chain length of 2–20 amino acids which acts like hormones released from the parent compound (protein), and which have beneficial properties for human health (Agyei & Danquah, Citation2011; Mills et al., Citation2011; Sathya, MubarakAli, MohamedSaalis, & Kim, Citation2021). Protein can be hydrolysed into peptides with different amino acid sequences and peptide lengths using different enzymes depending on their specificity, which influences the bioactivities of protein hydrolyzates (Bougatef et al., Citation2010; Zhang, Cao, Sun, Sun, & Xu, Citation2019). In this research papain, α-chymotrypsin, elastase, and protease enzymes are capable of generating protein hydrolyzates with antioxidant and metal chelating activity, under their optimum temperature and pH conditions for 0, 3, 6, 9, 12, 24 hours followed by heat inactivation at 100°C for 15 min, from crude proteins of U. lactuca and S. crassifolium. Heo, Lee, Song, and Jeon (Citation2003) state that, enzymatic extracts of Sargassum sp. (S. fulvellum, S. horneri, S. coreanum, and S. thunbergii) which produced from Protamex, Kojizyme, Neutrase, Flavourzyme and Alcalase under 12 h incubation period, shows antioxidant activity whereas according to Bondu et al., Citation2015) U. lactuca protein hydrolyzates which were produced by using trypsin and chymotrypsin at 30°C for 24 h followed by heat inactivation at 80°C for 20 min, posses with antioxidant and antihypertensive activities. Since enzymes have different cleavage specificities, hydrolysis with different enzyme preparations can lead to different hydrolyzates and peptide profiles (tePimentel et al., Citation2019). Furthermore, for generating protein hydrolyzates with desirable functional qualities, optimizing digestion conditions such as substrate concentration, E/S, and temperature, as well as pH for the enzymatic hydrolysis reaction, is critical (Tavares et al., Citation2011).
The best hydrolysis treatment was determined by SDS-PAGE. According to previous research, peptides with molecular weights < 2 kDa do not show up on a 15% SDS-PAGE (Abeyrathne et al., Citation2014). Hence, even with a heat inactivation at 100°C for 15 min, papain, α-chymotrypsin, elastase, and protease enzymes are capable of generating peptides with a low molecular weight from crude extract with the heat treatment given. Furthermore, considering the time efficiency to produce these protein hydrolyzates with bioactive properties 0 hour incubation period followed with heat inactivation at 100°C for 15 min was selected as the best enzyme treatment method for each seaweed species.
Functional properties of Ulva lactuca and Sargassum crassifolium protein hydrolyzates
Antioxidant activity
Antioxidants are compounds that prevent other molecules from oxidizing and protect cells from the damage of free radicals (Atta, Mohamed, & Silaev, Citation2017). The antioxidant activity of protein hydrolyzates of U. lactuca and S. crassifolium was evaluated in this study. According to the above results of the DPPH scavenging activity assay, protein hydrolyzates which were produced by U. lactuca and S. crassifolium showed strong antioxidant activity except for protein hydrolyzates which were produced using elastase enzyme. Crude proteins from U. lactuca and S. crassifolium extracted with water exhibited 47.102 ± 0.4% and 30.85 ± 1.87% antioxidant activity, respectively (Maldeniya, Egodauyana, & Abeyrathne, Citation2020a, b). When antioxidant activity of protein hydrolyzates of U. lactuca and S. crassifolium were compared to their crude proteins, all protein hydrolyzates of both seaweed species had higher antioxidant activity than their crude proteins (p < 0.05), with the exception of S. crassifolium protein hydrolyzate, which was produced using elastase enzyme (5.64 ± 0.01%). Since the low molecular weight of the peptides (about 340 Da) and free amino acids generated during the hydrolysis process, hydrolyzates may have a stronger radical scavenging ability (Cian, Martínez-Augustin, & Drago, Citation2012). According to several studies, low molecular weight hydrolyzates have a better DPPH radical scavenging ability than high molecular weight hydrolyzates (Wang et al., Citation2010). Additionally, recent studies suggest that phenolic content in the hydrolyzate, in addition to peptides and free amino acids released during the hydrolysis process, may be responsible for the hydrolyzate’s high antioxidant activity (Cian et al., Citation2012; Habeebullah et al., Citation2021). Therefore, the presence of low molecular weight peptides and free amino acids which are released during the hydrolysis process, and the phenolic content in the hydrolyzates may be the reason for the high DPPH radical scavenging activity of hydrolyzates of both seaweed species than their crude proteins.
In this study, protein hydrolyzate of U. lactuca which was produced by α-chymotrypsin showed good antioxidant activity (68.08 ± 0.03%). Also, Bondu et al. (Citation2015) state that U. lactuca protein hydrolyzate produces by using α-chymotrypsin showed better antioxidant activity with low molecular weight fraction (<10 kDa). Furthermore, protein hydrolyzates of U. lactuca produced by using papain, protease, chymotrypsin showed strong antioxidant activity, except for protein hydrolyzate which was produced from elastase enzyme. But according to Habeebullah et al. (Citation2021) protein hydrolyzates of U. lactuca which were produced by using flavourzyme, alcalase and neutrase showed low antioxidant activity. However, when this comes to protein hydrolyzates of S. crassifolium, protein hydrolyzates of S. horneri produced by flavozyme, alcalase and neutrase showed high antioxidant activity (Park, Shahidi, & Jeon, Citation2004) than the protein hydrolyzates of S. crassifolium which were produced during our study. The biological activity of the released peptides is influenced by the nature of the parent protein, pre-hydrolysis treatments, and the specificity of the enzymes used in the reaction (Ozuna, Paniagua-Martínez, Castaño-Tostado, Ozimek, & Amaya-Llano, Citation2015). Moreover, hydrolysis with various enzyme preparations can result in different hydrolyzates and peptide profiles, since enzymes have different cleavage specificities (tePimentel et al., Citation2019). Thus, this is the reason for variation in antioxidant activity of protein hydrolyzates which were produced by different enzyme treatments.
Fe2+ chelating activity
Metals can form reactive oxygen species (ROS) and can degrade biomolecules (Cian et al., Citation2016). Chelating peptides can be employed to reduce pro-oxidant effects by chelating metal ions. U. lactuca and S. crassifolium crude proteins displayed Fe2+ chelating activity of 46.68 ± 1.68% and 15.63 ± 1.17%, respectively (Maldeniya et al., Citation2020a, b). When the Fe2+ chelating activity of crude proteins from each seaweed species was compared to the Fe2+ chelating activity of their protein hydrolyzates, the protein hydrolyzates had lower Fe2+ chelating activity than their crude proteins. According to Zhu, Glahn, Yeung, and Miller (Citation2006), the Fe2+ chelating activity of protein hydrolyzates is due to the particular structure of peptides and amino acid side chain groups. Also, Fe2+ chelating activity is facilitated by side-chain residues of amino acid residues inside peptide structures such as cysteine, serine, histidine, aspartate, and glutamate. Because of their structures, these amino acid residues can form a compound with peptides via electrostatic interactions or H-bond coordination. Additionally, the N- and C-termini of peptides aid in metal chelating ability (Walters, Esfandi & Tsopmo, Citation2018).
Despite the low Fe2+ chelating activity of U. lactuca protein hydrolyzates which were produced in our study, by papain, chymotrypsin, protease, and elastase, protein hydrolyzates of U. lactuca which were produced by flavourzyme, alcalase, and neutrase showed high Fe2+ chelating activity (Habeebullah et al., Citation2021). Hydrolysis with various enzyme preparations can result in different hydrolyzates and peptide profiles since enzymes have different cleavage specificities, which is the reason for this variation (tePimentel et al., Citation2019). Moreover, the protein content of seaweed varies depending on the season, temperature, and place of harvest (Joubert & Fleurence, Citation2008). The relative composition of specific proteins can also fluctuate, influencing the quantities of amino acids and as a result, the yield and composition of desirable peptides (Stengel, Connan, & Popper, Citation2011). The absence of certain amino acid residues within the peptide structures of protein hydrolyzates from U. lactuca and S. crassifolium could be the cause of the hydrolyzates’ low Fe2+ chelating activity
In conclusion, protein hydrolyzates of U. lactuca and S. crassifolium which were produced during this research can be used as the natural antioxidant in food and pharmaceutical industry but the further studies are recommended.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abeyrathne, E. D. N. S., Lee, H. Y., Jo, C., Nam, K. C., & Ahn, D. U. (2014). Enzymatic hydrolysis of ovalbumin and the functional properties of the hydrolysates. Poultry Science, 93, 2678–2686.
- Admassu, H., Gasmalla, M. A. A., Yang, R., & Zhao, W. (2018). Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and anti-diabetic properties. Journal of Food Science, 83, 6–16.
- Agyei, D., & Danquah, M. K. (2011). Industrial-scale manufacturing of pharmaceutical-grade bioactive peptides. Biotechnology Advances, 29, 272–277.
- Atta, E. M., Mohamed, N. H., & Silaev, A. A. M. A. (2017). Antioxidants: An overview on the natural and synthetic types. European Chemical Bulletin, 6, 365–375.
- Bleakley, S., & Hayes, M. (2017). Algal proteins: Extraction, application, and challenges concerning production. Foods, 6, 33.
- Bondu, S., Bonnet, C., Gaubert, J., Deslandes, É., Turgeon, S. L., & Beaulieu, L. (2015). Bioassay-guided fractionation approach for determination of protein precursors of proteolytic bioactive metabolites from macroalgae. Journal of Applied Phycology, 27, 2059–2074.
- Bougatef, A., Nedjar-Arroume, N., Manni, L., Ravallec, R., Barkia, A., Guillochon, D., & Nasri, M. (2010). Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chemistry, 118, 559–565.
- Carter, P. (1971). Spectrophotometric determination of serum iron at the sub microgram level with a new reagent (ferrozine). Analytical Biochemistry, 40, 450–458.
- Cian, R. E., Garzón, A. G., Ancona, D. B., Guerrero, L. C., & Drago, S. R. (2016). Chelating properties of peptides from red seaweed Pyropia columbina and its effect on iron bio-accessibility. Plant Foods for Human Nutrition, 71, 96–101.
- Cian, R. E., Martínez-Augustin, O., & Drago, S. R. (2012). Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Research International, 49, 364–372.
- Coppejans, E., Leliaert, F., Dargent, O., Gunasekara, R., & De Clerck, O. (2009). Sri Lankan seaweeds: Methodologies and field guide to the dominant species. Abc Taxa (Vol. 6). Brussels: Belgian Development Cooperation.
- Cox, S., Turley, G. H., Rajauria, G., Abu-Ghannam, N., & Jaiswal, A. K. (2014). Antioxidant potential and antimicrobial efficacy of seaweed (Himanthalia elongata) extract in model food systems. Journal of Applied Phycology, 26, 1823–1831.
- Dhaval, A., Yadav, N., & Purwar, S. (2016). Potential applications of food-derived bioactive peptides in management of health. International Journal of Peptide Research and Therapeutics, 22, 377–398.
- Fernando, S. I. P., Sanjeewa, A. K. K., Samarakoon, K. W., Lee, W. W., Kim, H. S., Ranasinghe, P., … Jeon, Y. J. (2018). Antioxidant and anti-inflammatory functionality of ten Sri Lankan seaweed extracts obtained by carbohydrase assisted extraction. Food Science and Biotechnology, 27, 1761–1769.
- Habeebullah, S. F. K., Alagarsamy, S., Sattari, Z., Al‐Haddad, S., Fakhraldeen, S., Al‐Ghunaim, A., & Al‐Yamani, F. (2021). Effect of enzymatic hydrolysis on the antioxidant activity of red and green seaweeds and characterization of the active extracts. Journal of the American Oil Chemists’ Society, 98, 185–200.
- Harnedy, P. A., & FitzGerald, R. J. (2011). Bioactive proteins, peptides, and amino acids from macroalgae (1): Macroalgae: Bioactive agent source. Journal of Phycology, 47, 218–232.
- Harnedy, P. A., & FitzGerald, R. J. (2013). In vitro assessment of the cardioprotective, anti-diabetic and antioxidant potential of Palmaria palmata protein hydrolysates. Journal of Applied Phycology, 25, 1793–1803.
- Heo, S. J., Lee, G. W., Song, C. B., & Jeon, Y. J. (2003). Antioxidant activity of enzymatic extracts from brown seaweeds. Algae (Korean Phycological Society), 18, 71–81.
- Joubert, Y., & Fleurence, J. (2008). Simultaneous extraction of proteins and DNA by an enzymatic treatment of the cell wall of Palmaria palmata (Rhodophyta). Journal of Applied Phycology, 20, 55–61.
- Korhonen, H., & Pihlanto, A. (2006). Bioactive peptides: Production and functionality. International Dairy Journal, 16, 945–960.
- Lafarga, T., Acién-Fernández, F. G., & Garcia-Vaquero, M. (2020). Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Research, 48, 101909.
- Lakmal, H. H. C., Samarakoon, K. W., Lee, W., Lee, J. H., Abeytunga, D. T. U., Lee, H. S., & Jeon, Y. J. (2014). Anticancer and antioxidant effects of selected Sri Lankan marine algae. Journal of the National Science Foundation of Sri Lanka, 42, 315.
- Maldeniya, M. U. S., Egodauyana, K. P. U. T., & Abeyrathne, E. D. N. S. (2020a). Comparison of antioxidant properties of different crude extracts from Ulva lactuca. Sri Lanka Journal of Animal Production, 12, 11–21.
- Maldeniya, M. U. S., Egodauyana, K. P. U. T., & Abeyrathne, E. D. N. S. (2020b). Extraction of crude protein from Sargassum crassifolium, harvested from south coast of Sri Lanka and determination of functional properties of the crude extracts. Journal of Technology and Value Addition, 2, 39–64.
- Mills, S., Stanton, C., Hill, C., & Ross, R. P. (2011). New developments and applications of bacteriocins and peptides in foods. Annual Review of Food Science and Technology, 2, 299–329.
- Ozuna, C., Paniagua-Martínez, I., Castaño-Tostado, E., Ozimek, L., & Amaya-Llano, S. L. (2015). Innovative applications of high-intensity ultrasound in the development of functional food ingredients: Production of protein hydrolysates and bioactive peptides. Food Research International, 77, 685–696.
- Pangestuti, R., & Kim, S. K. (2015). Seaweed proteins, peptides, and amino acids. In Tiwari, B. K., & Troy, D. (Eds.), Seaweed sustainability: Food and non-food applications (pp. 125–140). Massachusetts, USA: Academic Press.
- Park, P. J., Shahidi, F., & Jeon, Y. J. (2004). Antioxidant activities of enzymatic extracts from an edible seaweed Sargassum horneri using ESR spectrometry. Journal of Food Lipids, 11, 15–27.
- PiSanjeewa, K. A., Jayawardena, T. U., Kim, H. S., Kim, S. Y., Fernando, I. S., Wang, L., … Jeon, Y. J. (2019). Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-κB signal pathway. Carbohydrate Polymers, 224, 115195.
- Premakumara, G. A., Ratnasooriya, W. D., & Tillekeratne, L. M. (1996). Isolation of a non-steroidal contragestative agent from Sri Lankan marine red alga, Gelidiella acerosa. Contraception, 54, 379–383.
- Sambrook, J., & Russell, D. W. (2006). SDS-polyacrylamide gel electrophoresis of proteins. CSH Protoc, 2006, db–prot4540.
- Sarmadi, B. H., & Ismail, A. (2010). Antioxidative peptides from food proteins: A review. Peptides, 31, 1949–1956.
- Sathya, R., MubarakAli, D., MohamedSaalis, J., & Kim, J. W. (2021). A systemic review on microalgal peptides: Bioprocess and sustainable applications. Sustainability, 13, 3262.
- Stengel, D. B., Connan, S., & Popper, Z. A. (2011). Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnology Advances, 29, 483–501.
- Sun, S., Xu, X., Sun, X., Zhang, X., Chen, X., & Xu, N. (2019). Preparation and identification of ACE inhibitory peptides from the marine macroalga Ulva intestinalis. Marine Drugs, 17, 179.
- Tavares, T. G., Contreras, M. M., Amorim, M., Martín-Álvarez, P. J., Pintado, M. E., Recio, I., & Malcata, F. X. (2011). Optimisation, by response surface methodology, of degree of hydrolysis and antioxidant and ACE-inhibitory activities of whey protein hydrolysates obtained with cardoon extract. International Dairy Journal, 21, 926–933.
- tePimentel, F. B., Alves, R. C., Harnedy, P. A., FitzGerald, R. J., & Oliveira, M. B. P. P. (2019). Macroalgal-derived protein hydrolysates and bioactive peptides: Enzymatic release and potential health enhancing properties. Trends in Food Science & Technology, 93, 106–124.
- Thilanja, G. P. D. D. S., Dissanayake, K. S. M., Kariyawasam, M. G. T. R., & Abeyrathne, E. D. N. S. (2020). Extraction of crude collagen from yellowfin Tuna (Thunnus albacares) skin and determination of the functional properties of its hydrolysates. Journal of Technology and Value Addition Research, 2, 21–35.
- Walters, M. E., Esfandi, R., & Tsopmo, A. (2018). Potential of food hydrolyzed proteins and peptides to chelate iron or calcium and enhance their absorption. Foods (Basel, Switzerland)), 7(10), 172.
- Wang, T., Jónsdóttir, R., Kristinsson, H. G., Hreggvidsson, G. O., Jónsson, J. Ó., Thorkelsson, G., & Ólafsdóttir, G. (2010). Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT-Food Science and Technology, 43, 1387–1393.
- WHO. (2021), Noncommunicable diseases.World Health Organization. Retrieved June 5, 2021, from https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases
- Wijesekara, I., & Kim, S. K. (2010). Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Marine Drugs, 8, 1080–1093.
- Zhang, X., Cao, D., Sun, X., Sun, S., & Xu, N. (2019). Preparation and identification of antioxidant peptides from protein hydrolysate of marine alga Gracilariopsis lemaneiformis. Journal of Applied Phycology, 31, 2585–2596.
- Zhu, L., Glahn, R. P., Yeung, C. K., & Miller, D. D. (2006). Iron uptake by Caco-2 cells from NaFeEDTA and FeSO4: Effects of ascorbic acid, pH, and a Fe (II) chelating agent. Journal of Agricultural and Food Chemistry, 54, 7924–7928.