ABSTRACT
Although much is known regarding the physiology, ecology and life history of Macrocystis pyrifera, there is little accessible information for establishing robust and reliable culturing practices to support aquaculture and habitat restoration. Naturally occurring kelp forests formed by M. pyrifera support productive coastal ecosystems, and because it is one of the fastest growing macroalgal species, its high biomass production, and high affinity for nutrients and significant polysaccharide content makes it a species of considerable interest for aquaculture. This species has undergone substantial decline throughout its biogeographic range and is threatened by local and global stressors. Here, we synthesize the current knowledge on culturing of M. pyrifera and discuss approaches to stock collection, preservation of diversity and applications for experimental studies. It is crucial to preserve the current genetic diversity of this species immediately and long-term culture storage approaches such as germplasm banking and cryopreservation provide the tools to allow this. A concerted effort is also needed to better understand the physiological attributes of M. pyrifera in order to select strains for aquaculture and restorative applications that may provide resilience to future environmental stressors. Finally, attention must be given to developing effective in situ restoration approaches whereby large-scale stock production can be optimized and out-planting strategies developed to ensure restoration success.
Introduction
Farming of marine macroalgae has a long history but has seen a significant increase in scale and advancement in research and infrastructure since the middle of the twentieth century. In 2018, the global production of macroalgae reached more than 32.4 million tonnes per annum (FAO, Citation2020a), with 97.1% coming from farmed systems (Chopin & Tacon, Citation2021). 45.3% of that production came from cultivated kelp species of the order Laminariales (FAO, Citation2020a). The main focus of macroalgal production is on polysaccharide products followed by direct human consumption (Naylor et al., Citation2021). However, in recent years, and in response to environmental challenges, there has been a shift in research focus to better understand culturing practices for other uses, such as multitrophic aquaculture, preserving and buffering ecosystem function, maintaining biodiversity and restoration (Layton et al., Citation2020; Ling, Johnson, Frusher, & Ridgway, Citation2009; Terawaki, Hasegawa, Arai, & Ohno, Citation2001).
Of all macroalgae, kelps (Laminariales) are generally the largest and exhibit some of the highest productive yields (Bolton, Anderson, Smit, & Rothman, Citation2012; Reed, Rassweiler, & Arkema, Citation2008). They are found throughout temperate regions and provide key services to nearshore and coastal systems through their role as ecosystem engineers (Bolton et al., Citation2012; Graham, Vásquez, & Buschmann, Citation2007). Globally, 38% of ecoregions have exhibited kelp forest decline over the past 50 years (Krumhansl et al., Citation2016). Multiple drivers, such as increased ocean temperature (Filbee-Dexter, Feehan, & Scheibling, Citation2016; Johnson et al., Citation2011; Wernberg et al., Citation2013), sedimentation (Connell, Citation2003; Filbee-Dexter & Wernberg, Citation2018), eutrophication (Filbee-Dexter & Wernberg, Citation2018), overgrazing (Kriegisch, Reeves, Johnson, & Ling, Citation2019; Ling et al., Citation2009), invasion (Blamey & Branch, Citation2012) and overfishing (Andrew & O’Neill, Citation2000; Ling et al., Citation2009; Mabin, Johnson, & Wright, Citation2019) are to blame for continued decline.
The giant kelp, Macrocystis pyrifera, forms and maintains the largest ecosystems of all macroalgae, creating forests that modify the physical and chemical environment, providing key habitat, food resources and services to a plethora of species (Graham et al., Citation2007). M. pyrifera holds significant value as a direct harvest resource, predominantly for the extraction of the polysaccharide alginate (McHugh, Citation2003; Ortiz et al., Citation2009), abalone feed (Correa et al., Citation2016), as an additive for liquid fertilizer production (Gutierrez et al., Citation2006) and for its role in supporting cultural, recreational and commercially important fisheries (Jones, Lawron, & Shachak, Citation1997; Wernberg, Krumhansl, Filbee-Dexter, & Pedersen, Citation2019). Historically, the majority of harvests have been taken from wild populations (Buschmann et al., Citation2004; Purcell-Meyerink, Packer, Wheeler, & Hayes, Citation2021), with Chile and Peru being the main producers (i.e., 33 979 and 32 794 tonnes, respectively, in 2019; Cai et al.,). Like many other kelp species, wild M. pyrifera forests have undergone significant global decline (Hay, Citation1990; Johnson et al., Citation2011; Krumhansl et al., Citation2016; Filbee-Dexter & Wernberg, Citation2018; Tait et al., 2021) and as a result, many of the services they provide have declined or been lost.
As attention shifts more towards the culturing of M. pyrifera for both harvest and bioremediation and restoration purposes, it has become clear that there is a lack of comprehensive information regarding culturing practices for this important species. Many studies have investigated the life history of M. pyrifera and by contrasting these bodies of work it is apparent that the conditions needed for successful growth are variable from one region to another (Gutierrez et al., Citation2006; Leal, Roleda, Fernández, Nitschke, & Hurd, Citation2021; Lüning, Citation1981; Macchiavello, Araya, & Bulboa, Citation2010; Neushul, Citation1963; Schiel & Foster, Citation2015; Westermeier, Patiño, Piel, Maier, & Mueller, Citation2006), making it difficult to establish robust and reliable culturing practices. Approaches to culturing a particular species are also highly dependent on the purpose of culturing. This critical assessment aims to synthesize the current methods and conditions used when culturing M. pyrifera, with a specific focus on the needs and application of the cultured product. This assessment will discuss methods for stock collection, storage and grow-out, with reference to experimental, aquacultural and restorative applications.
Life history and distribution
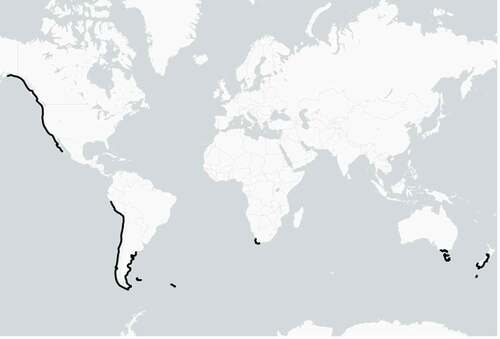
M. pyrifera is found in temperate waters along the coasts of Southern Africa, east and west South America, Tasmania and South Australia, central and southern New Zealand (including the sub Antarctic Islands) and the west coast of North America and Canada (). It requires rocky substrata for attachment and can be found in both sheltered and exposed environments (Graham et al., Citation2007; Schiel & Foster, Citation2015).
Figure 1. Global distribution of Macrocystis pyrifera (modified from Graham et al., Citation2007).
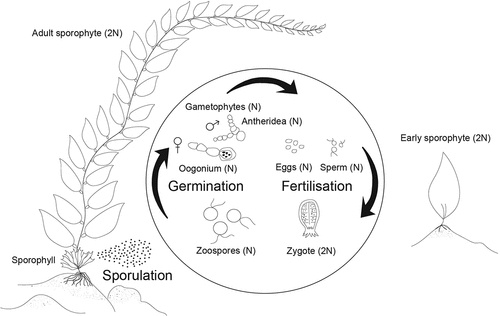
Reproduction of M. pyrifera can occur year round (Brown, Nyman, Keogh, & Chin, Citation1997; Reed, Ebeling, Anderson, & Anghera, Citation1996) but the abundance of reproductive tissue (sorus) and density of zoospores (hereafter spores) within sori varies seasonally (Buschmann et al., Citation2004; Leal et al., Citation2021; Neushul, Citation1963; Reed et al., Citation1996). Like other Laminariales, M. pyrifera has a biphasic life cycle, alternating between a microscopic haploid stage and a macroscopic diploid stage (Schiel & Foster, Citation2015). The main reproductive tissue is located on the sporophyll at the base of the sporophyte, above the holdfast, although spore bearing tissue can also be found in the blades and the apical scimitar of some individuals (Leal, Hurd, & Roleda, Citation2014; Leal et al., Citation2021; Neushul, Citation1963).
In the wild, spore reproduction occurs throughout the year, with the highest production coinciding with low-temperature months, such as early winter and late spring/early summer (Anderson & North, Citation1967; Reed et al., Citation1996), and storms (Reed et al., Citation2006). Mature sori release spores that swim freely in the water column and are transported by currents with a typical dispersal range of <1 km. Spore settlement occurs within hours to a few days post release (Devinny & Volse, Citation1978; Reed, Amsler, & Ebeling, Citation1992) and is triggered by the available nutrients (i.e., by following a concentration gradient) (Amsler & Neushul, Citation1989), presence of other biota (Reed et al., Citation1996), and light condition (Reed et al., Citation1992). Germination can occur immediately after spore release and is typically complete within 48 hrs under laboratory conditions (Anderson & Hunt, Citation1988; Garman, Pillai, Goff, & Cherr, Citation1994), or a few days post release in the field (Devinny & Volse, Citation1978; Reed et al., Citation1992; Santelices, Citation1990). After germination, germlings start to grow by increasing cell number and size, forming male and female gametophytes (Devinny & Volse, Citation1978; Reed et al., Citation1992; Schiel & Foster, Citation2015). In optimal laboratory conditions, gametophytes of both sexes can become fertile in approximately two weeks (Lüning & Neushul, Citation1978). The fertilization process takes place when eggs of female gametophytes produce a pheromone called lamoxirene, which attracts the sperm of male gametophytes (Maier, Hertweck, & Boland, Citation2001). The effective distance for hormone attraction is reported to be within 1 mm (Boland, Marner, Jaenicke, Muller, & Folster, Citation1983). If successful fertilization occurs, then female gametophytes turn into embryonic sporophytes.
Once established, the growth of sporophytes can be rapid. Sporophyte growth can result in a doubling of the length every month from an initial length of 1–2 cm (Neushul, Citation1963). Growth rates are variable across season and by region (North, Citation1976a; Wheeler & North, Citation1981; González-Fragoso, Ibarra-Obando, & North, Citation1991; Hernández-Carmona, Citation1996; Brown et al., Citation1997; Graham et al., Citation2007; Macchiavello et al., Citation2010), for example, greatest growth rates occur during the summer in Alaska and British Columbia, while populations in California and New Zealand displayed greatest growth during the winter and spring (North, Citation1976a; Wheeler & North, Citation1981; Brown et al., Citation1997; Graham et al., Citation2007). This variation in growth is driven by resource availability, namely, light and nutrients (Desmond, Pritchard, & Hepburn, Citation2015; Graham et al., Citation2007; Schiel & Foster, Citation2015; Stephens & Hepburn, Citation2014). M. pyrifera populations have a relatively high turnover rate due to their large size, which makes them vulnerable to physical forces, but individuals have been shown to persist for up to 7 years (Graham et al., Citation2007).
Stock collection
In the following section stock refers to the culture of M. pyrifera from a known source that may be used for multiple applications as outlined below.
Seasonality and timing
M. pyrifera can be reproductive year-round, but there appear to be seasonal trends when spore production is highest, and depending on location, there may be periods where no sorus tissue is present (Brown et al., Citation1997; Buschmann et al., Citation2004; Hay, Citation1990; Leal et al., Citation2021; Neushul, Citation1963; Reed et al., Citation1996). To ensure the greatest chance of successful spore release, collection should be carried out when spore production is highest. Highest sporophyll counts and spore densities are typically observed during the austral summer and autumn period in the Southern Hemisphere (Buschmann, Moreno, Vásquez, & Hernández-González, Citation2006; Hay, Citation1990; Henríquez et al., Citation2011), while populations along the west coast of the United States show highest spore densities during the winter and spring (Reed et al., Citation1996).
Collection and transportation
Mature sorus tissue can be removed by cutting it away from the sporophyll without removing the entire individual. If possible, immediate sporulation in the field is the best method to obtain the highest spore density (Suebsanguan, Strain, Morris, & Swearer, Citation2021) but often this is not practical. If it is not possible to sporulate immediately, then spore tissue can be transported either semi-dry, packed in damp paper cloth which maintains higher spore densities when compared to wet transportation (Bak, Mols-Mortensen, & Gregersen, Citation2018; Kim, Yarish, Hwang, Park, & Kim, Citation2017; Suebsanguan et al., Citation2021), or in a plastic zip-lock bag or similar sealed container filled with seawater and kept in the dark at ~4°C (Barrento, Camus, Sousa-Pinto, & Buschmann, Citation2016; Gutierrez et al., Citation2006; Westermeier et al., Citation2006). Generally, longer transportation time (>24 hrs) leads to lower spore yield so facilities and equipment should be well prepared and sterilized to be ready to receive reproductive material (North, Citation1976a; Devinny & Leventhal, Citation1979; Gutierrez et al., Citation2006; Kim et al., Citation2017; Suebsanguan et al., Citation2021) .
Sporulation
Pre-treatment
After transporting the fresh mature sorus to the laboratory or processing facility, it is important to clean the tissue to reduce the likelihood of culture contamination. Typically, cleaning is performed by gently rinsing the sorus tissue with filtered (0.2 µm pore diameter) and/or sterilized (autoclaved) seawater and wiping off any visible fouling. Tissue can also be rinsed using freshwater, freshwater with chlorine (5 ml of chlorine l–1 water) or iodine without any noted negative effects on spore quality (Alsuwaiyan et al., Citation2019; Camus & Buschmann, Citation2017; Plá & Alveal, Citation2012). After cleaning, the sorus tissue should undergo a desiccation period to enhance spore release (Leal et al., Citation2014). Most studies store sorus tissue in damp paper cloth for between 1 and 24 hrs in the dark at temperatures between 4°C and 15°C (Camus & Buschmann, Citation2017; Gutierrez et al., Citation2006; Leal et al., Citation2014; Plá & Alveal, Citation2012). After the desiccation period, sporulation can take place. It should be noted that sporulation can take place without a desiccation period; however, this approach is more time consuming, has a greater risk of contamination and produces more mucilage (Neushul, Citation1963; Sanbonsuga & Neushul, Citation1978).
Medium
For sporulation, a range of culture media are reported, the most common being filtered, sterilized and/or nutrient enriched seawater (i.e., Provasoli, Alsuwaiyan et al., Citation2019). Artificial seawater is sometimes used; however, the advantages or disadvantages of this approach are not clear (Amsler & Neushul, Citation1989, Citation1990). A general guide should be that the medium used should minimize the chance of infection or contamination and therefore both filtration and sterilization either using UV or autoclave are recommended. Very few studies report using germanium oxide or antibiotics to control contamination at the time of sporulation, and it is advised that if used this should be precise in concentration and duration to reduce negative effect on growth (Kawai, Motomura, & Okuda, Citation2005; Markham & Hagmeier, Citation1982; Shea & Chopin, Citation2007).
Sporulation time, temperature, and light intensity
The desiccated sorus tissue should be submerged in the medium and left for a period of time (“sporulation time”) to allow release. Of the studies that report sporulation time, most range between 15 minutes to 1 hr (Camus & Buschmann, Citation2017; Gutierrez et al., Citation2006; Leal et al., Citation2014; Macchiavello et al., Citation2010; Neushul, Citation1963; Plá & Alveal, Citation2012). Many studies do not state the temperature used for sporulation, of those that do, most range between 10°C and 18°C (Alsuwaiyan et al., Citation2019; Camus & Buschmann, Citation2017; Gutierrez et al., Citation2006; Leal et al., Citation2014; Macchiavello et al., Citation2010; Neushul, Citation1963; Plá & Alveal, Citation2012). The lowest temperature reported was 0°C (James, Stull, & North, Citation1990) and the highest 19.8°C (Hollarsmith, Buschmann, Camus, & Grosholz, Citation2020). Little information is available regarding the potential effects of sporulation temperature on developmental parameters, and this is an area that requires further research given its potential ecological importance. Light intensity is variable across studies, ranging from complete darkness to dim irradiance (Alsuwaiyan et al., Citation2019). Most studies did not mention irradiance levels during sporulation (Alsuwaiyan et al., Citation2019). Care must, however, be taken to avoid potential photo damage during this time (Graham, Citation1996), and it is therefore recommended that sporulation be conducted under dim light conditions.
Spore density
The number of spores per unit area of sorus tissue is variable between populations (Buschmann et al., Citation2004), season (Buschmann et al., Citation2004; Neushul, Citation1963) and tissue types (Leal et al., Citation2014, Citation2021; Neushul, Citation1963); therefore, the number of spores produced during sporulation will also vary. Spore density can be assessed post sporulation by taking an aliquot of the medium and quantifying spore density under magnification using a haemocytometer. Studies report densities in the range of 40,000–5,000,000 spores ml–1 (Barrento et al., Citation2016; Buschmann et al., Citation2006, Citation2004; Camus & Buschmann, Citation2017; Cie & Edwards, Citation2008; Deysher & Dean, Citation1984; Leal et al., Citation2014; Macchiavello et al., Citation2010; Muñoz, Hernández-González, Buschmann, Graham, & Vásquez, Citation2004; Westermeier et al., Citation2006). Spore density can be diluted by adding an additional medium to the sporulation vessel in order to achieve the desired concentration, which will be highly dependent on the application of the cultured stock and will be discussed further below.
Stock management
It is important to have a clear understanding of the intended application of cultured M. pyrifera as this will dictate how much tissue should be collected, how sporulation should be undertaken and most importantly what happens to stock post sporulation. In this section, we discuss long-term storage techniques that allow for the preservation of genetic diversity and culture stocks, short-term storage and culturing methods for experimental applications, and finally grow-out approaches for aquacultural and restoration purposes.
Preserving diversity
The need for long-term storage of M. pyrifera is multifaceted. Given the decline of M. pyrifera forests worldwide, a loss of genetic diversity has more than likely occurred. Continued pressure on these systems means that a concerted effort is needed to preserve current genetic strains for future uses (Barrento et al., Citation2016), whatever they may be. In situ protection measures, such as marine protected areas, do not offer the insurance needed to preserve genetic diversity as these areas remain vulnerable to the effects of global stressors (Williams, Citation2001). Ex situ preservation methods are therefore the only way to safeguard and preserve diversity (Coleman et al., Citation2020; Wade et al., Citation2020). Large-scale ex situ preservation of terrestrial plants and crop species has occurred in climate-controlled seed banks since the early 20th century in order to ensure food security and preserve biodiversity (Wade et al., Citation2020). Long-term storage of macroalgae, however, lags significantly behind terrestrial examples, but two main approaches are slowly becoming utilized.
Germplasm banking
Germplasm banking is defined as the preservation of biological, animal or plant, for the purposes of delayed breeding and propagation. For macroalgae, germplasm banking involves holding male and female gametophytes, often separately, in a state of dormancy by placing them under reduced temperature, light and nutrient conditions (Barrento et al., Citation2016). For most kelps, including M. pyrifera, the gametophytic (haploid) stage responds best to long-term storage conditions (Barrento et al., Citation2016; Carney & Edwards, Citation2010; Westermeier et al., Citation2006). Only a small number of studies have reported storing M. pyrifera under germplasm conditions (Barrento et al., Citation2016; Lewis & Neushul, Citation1994; Westermeier et al., Citation2006; Xu et al., Citation2015).
The key factors found to affect the survivorship of M. pyrifera gametophytes under germplasm conditions are temperature, light, nutrient concentration and cell density. Ideally, germplasm storage temperature should be at the low end of the naturally occurring temperature range for the region where stock originates. Temperature conditions vary between 8°C and 15°C which is considered very close to the optimal growth range for M. pyrifera (i.e., 12–17°C, Lüning & Neushul, Citation1978) and so the temperature for germplasm should be lower than 12°C. Importantly, light levels must be kept low, <5 µmol m–2 s–1 is most common, and light:dark photoperiods between 10 L:14D – 16 L:8D are recommended (Barrento et al., Citation2016; Westermeier et al., Citation2006; Xu et al., Citation2015). The removal of blue wavelength light from the light source (e.g., using red light LEDs) provides further insurance to avoid gametophytic sexual development (Lüning & Neushul, Citation1978).
Nutrient availability is particularly important for controlling vegetative growth and maintaining germplasm cultures at manageable densities. Cultures should be held in nutrient enriched, filtered, autoclaved seawater with the preferred medium being Provasoli (Barrento et al., Citation2016; Carney & Edwards, Citation2010; Wade et al., Citation2020; Westermeier et al., Citation2006). If cultures are maintained at low densities, the medium can be changed approximately twice per year as long as evaporation is kept to a minimum (Barrento et al., Citation2016; Westermeier et al., Citation2006). To completely prevent any chance of reproduction, it is advised that male and female gametophytes are held separately once distinguishable. The 96-well plates are commonly used to store gametophytes as they offer highly independent storage replication at low densities. Studies that place M. pyrifera gametophytes in long-term storage have shown viability of stock after more than 5 years post preservation (Barrento et al., Citation2016; Lewis & Neushul, Citation1994; Westermeier et al., Citation2006; Xu et al., Citation2015).
Cryopreservation
Cryopreservation is a second approach, which is gaining attention for its potential as a long-term storage option for algal cultures (Choi, Nam, & Kuwano, Citation2013; Kuwano, Kono, Jo, Shin, & Saga, Citation2004; Vigneron, Arbault, & Kaas, Citation1997; Zhang et al., Citation2007; Zhang, Cong, Qu, Luo, & Yang, Citation2008). Cryopreservation was first used to preserve human sperm in the 1960ʹs (Sherman, Citation1973) and has since been used in both agricultural and horticultural practices to preserve biological material (Taylor & Fletcher, Citation1998). The process, in general, requires the staged cooling of the subject to very low temperatures where biological cell degradation effectively ceases (Grout, Citation1995). Typically, the samples are stored under solid carbon dioxide or liquid nitrogen conditions to achieve temperatures of – 80 to – 196°C. As with germplasm banking, the gametophytic stage of M. pyrifera responds best to cryopreservation (Piel, Avila, & Alcapán, Citation2015).
The process of preservation requires gametophytes to be suspended in a cryoprotectant solution made up of dimethyl sulphoxide (DMSO), glycerol, sucrose, dextrose and sorbitol (Piel et al., Citation2015; Zhang et al., Citation2007, Citation2008) before it is cooled. The cooling step is separated into two minor steps to avoid the damage to cell walls (Piel et al., Citation2015; Zhang et al., Citation2007, Citation2008). Briefly, gametophyte suspensions are pre-frozen either at –18°C to –20°C for 30 min. After 30 min pre-frozen samples are placed in liquid nitrogen (Piel et al., Citation2015; Zhang et al., Citation2007). Only one published study considers the cryopreservation of M. pyrifera (Piel et al., Citation2015). Four weeks after being thawed and returned to optimal growing conditions 29% of female gametophytes were still immature and in a vegetative state, 9% released eggs and 9% developed into embryonic sporophytes. From other species, it appears that survival rate and viability of preserved tissue is species dependent (Ginsburger-Vogel, Arbault, & Pérez, Citation1992; Kono, Kuwano, & Saga, Citation1998; Kuwano et al., Citation2004; Piel et al., Citation2015; Zhang et al., Citation2007, Citation2008) and is significantly influenced by the rate of freezing and thawing process (Zhang et al., Citation2007). Therefore, more research is needed to optimize this approach to enable the successful long-term storage of M. pyrifera.
Both germplasm banking and cryopreservation allow for the long-term storage of M. pyrifera, which is essential for preserving the genetic diversity of this species. Government, research agencies and other parties interested in the values surrounding kelp forests should make a concerted effort to establish long-term storage facilities to preserve this species. Not only does storage safeguard genetic diversity but it also forms the basis for breeding programs and allows for timely propagation of stock for experimental, aquacultural and restorative applications.
Farming and applications
Culturing methods for laboratory experimentation
Laboratory experimentation is important to understand the physiological function of M. pyrifera and knowledge gained often provides perspective on ecological trends or guide approaches for other applications, such as aquaculture or restoration. Typically, these types of experiments focus on the early life stages from the spore through to the juvenile sporophyte (Barrento et al., Citation2016; Carney & Edwards, Citation2010; Hollarsmith et al., Citation2020; Leal, Hurd, Fernández, & Roleda, Citation2017; Westermeier et al., Citation2006). This tends to be because of the importance of these early life stages in future development (Schiel & Foster, Citation2015) as well as the difficulty of maintaining large adult M. pyrifera in culture. This section will specifically assess the culturing of early life stages for experimental purposes.
Experimentation may begin from as early as the sporulation stage when sorus tissue is exposed to different experimental conditions, for these types of experiments, sporulation can take place as advised above while manipulating the factors of interest. After sporulation takes place and before settlement occurs it is important to achieve the desired concentration of spores for any ongoing experiment, this will vary depending on the needs of the experiment. Although a wide range of spore densities have been applied in other studies (Barrento et al., Citation2016; Buschmann et al., Citation2006, Citation2004; Camus & Buschmann, Citation2017; Cie & Edwards, Citation2008; Deysher & Dean, Citation1984; Leal et al., Citation2014; Macchiavello et al., Citation2010; Muñoz et al., Citation2004; Westermeier et al., Citation2006), the optimal range of settled spores for maximum sporophyte production is roughly 50 spore mm–2 (Reed, Neushul, & Ebeling, Citation1991).
Once the desired concentration of spores is achieved, the spore solution can be seeded to suitable culture vessels depending on experimental purposes. Most studies utilize glass or plastic vessels, usually vials, flasks or Petri dishes (Barrento et al., Citation2016; Carney & Edwards, Citation2010; Cie & Edwards, Citation2008; Deysher & Dean, Citation1984; Hollarsmith et al., Citation2020; Morris et al., Citation2016; Westermeier et al., Citation2006). When selecting vessels, ensure they are of appropriate size, consider that settlement is greatest on horizontal as opposed to vertical surfaces (Reed et al., Citation1992) and if the experiment requires observation of development, the vessel has a flat, optically clear and uniform base. Observation is best achieved using an inverted microscope with a magnification range of 4x–80x depending on developmental stage (Carney & Edwards, Citation2010; Hollarsmith et al., Citation2020; Leal et al., Citation2017; Morris et al., Citation2016; Muñoz et al., Citation2004).
Spore settlement generally ranges from 30 min to 1 h in high light i.e., <50 µmol m–2 s–1 or 1–2 hrs in darkness if the medium is not constantly agitated (Amsler & Neushul, Citation1989, Citation1990). This will allow for the settlement of the majority of viable spores. After 12–24 hrs, the medium can be gently decanted and replaced to discard any unsettled spores. Experimental treatments can commence from this point if not already initiated from the sporulation stage. If it is desirable that development occurs in a free-floating state, i.e., not attached to the bottom of the vessel, then aeration can be applied through an airstone or through mechanical mixing using a shaker table or stirring device. Depending on experimental conditions, the development from spore to juvenile sporophyte may progress over 2 to 4 weeks (Camus & Buschmann, Citation2017; Carney & Edwards, Citation2010; Hollarsmith et al., Citation2020; Neushul, Citation1963). If conditions are not suitable for development, then development may cease at a particular life stage and remain in a vegetative state or perish (Lüning & Neushul, Citation1978; Westermeier et al., Citation2006).
When performing experiments that seek to quantify population characteristics rather than those at the individual level, it is important to utilize sori from many individual sporophytes (>30) as the response of M. pyrifera at an individual level can be highly variable due to plastic responses, genetic diversity and/or local adaptations (i.e., Fernández, Navarro, Camus, Torres, & Buschmann, Citation2021). It should be noted that each life stage will respond differently to experimental conditions, and this should be considered when designing experiments.
Breeding
The purpose of breeding programs is generally to enhance the performance of the macroalgal culture (e.g., to increase yield, breed stress tolerance and increase reproduction). This can be achieved by 1) cross-breeding males and females that exhibit desirable traits to create a hybrid generation, these are usually identified from physiological experiments, 2) creating a combination between hybridization and simple phenotypic mass selection or 3) more advanced gene editing of particular individuals to promote certain characteristics (Goecke, Klemetsdal, & Ergon, Citation2020).
For M. pyriferabreeding strategies are relatively underdeveloped with most approaches focusing on hybridization across one generation (Camus et al., Citation2021; Murúa, Patiño, Müller, & Westermeier, Citation2021; Raimondi, Reed, Gaylord, & Washburn, Citation2004; Westermeier, Patiño, Müller, & Müller, Citation2010, Citation2011). However, for other species that have a longer history in commercial aquaculture, such as Saccharina spp. and Undaria spp.,Pyropia spp., more advanced breeding programs have been developed and have observed success in increasing productivity, disease resistance, heat tolerance, seasonal duration, and chemical contents (Goecke et al., Citation2020; Hu et al., Citation2021; Hwang, Yotsukura, Pang, Su, & Shan, Citation2019; Wang, Yao, Zhang, & Duan, Citation2020). It is likely that as M. pyrifera is incorporated into greater commercial production, similar breeding programs will be undertaken, but this is currently an area for further research. The selection of particular traits will also benefit restoration efforts, which is discussed in more detail below.
Farming
Macroalgal aquaculture is rapidly growing, driven by the demands of the food, pharmaceutical, agricultural and aquacultural industries (Ferdouse, Holdt, Smith, Murúa, & Yang, Citation2018; Wernberg et al., Citation2019; Naylor et al., Citation2021). M. pyrifera, being one of the fastest growing macroalgal species with high biomass production (Reed et al., Citation2008), high affinity for nutrients (Correa et al., Citation2016; Purcell-Meyerink et al., Citation2021) and polysaccharide content (Ortiz et al., Citation2009), holds significant value as a desirable culture species.
The history of M. pyrifera farming provides many examples of the challenges faced by this industry. Over time, reliable protocols for mass production have been developed and are now being implemented (Buschmann et al., Citation2004; Westermeier et al., Citation2006; Gutierrez et al., Citation2006; Macchiavello et al., Citation2010; Camus et al., Citation2018b). Typically, culture and production rely on sourcing spores directly either from wild populations or from gametophytes, which are held under controlled culture conditions. The latter approach allows the selection of specific traits (i.e., high-yield sporophytes) through cross-breeding specific gametophytes (Westermeier et al., Citation2010, Citation2011; Camus, Faugeron, & Buschmann, Citation2018a; Buschmann et al., Citation2020; Camus et al., Citation2021; Murúa et al., Citation2021). The set-up and requirement for growing kelp is discussed in two separate phases: hatchery and grow-out.
Hatchery requirements
The purpose of this section is to provide a general overview of the essential capabilities a hatchery must support. Several very detailed guides on the requirements for macroalgal hatcheries have been written (Andersen, Citation2005; Redmond, Green, Yarish, Kim, & Neefus, Citation2014). Although we focus on M. pyrifera, the requirements will scale well to many other laminarian kelps. Depending on the scale of aquacultural application, hatchery requirements will vary; however, approaches to producing and maintaining stock will be comparable regardless of scale. As with all culturing practices, sterility is essential. All water should be filtered and sterilized to the requirements laid out above, and air sources should also be filtered using a 0.2 µm (polytetrafluoroethylene) inline air filter.
Seeding stock onto seed lines is the most common and by far the most efficient means for large-scale aquaculture production (Camus et al., Citation2018b). These seeded lines should be maintained in the hatchery until juvenile sporophytes of desirable size are present before being relocated to an aquaculture setup in a natural environment where the grow-out phase will take place. Preferable seed lines are synthetic twines such as nylon and vinylon ~1–2 mm thick (Camus & Buschmann, Citation2017; Gutierrez et al., Citation2006); this should be wrapped tightly around a sterilized PVC tube cut to an appropriate length for the application needed and the length should match the depth of the aquaria that will house the seed line as growth progresses (Camus & Buschmann, Citation2017; Gutierrez et al., Citation2006). Seed lines can be pre-prepared, wrapped in plastic wrap and stored in a refrigerator for later use (Redmond et al., Citation2014).
Seed stock will either be from wild sourced reproductive material or from gametophyte cultures already held in the hatchery under vegetative conditions. If wild sourced stock is used, then sporulation should take place as described above whereby spores are released into a glass vessel after the pretreatment phase, and a density of ~40 000 spores ml–1 is desirable (Camus & Buschmann, Citation2017; Gutierrez et al., Citation2006). Spore solution is added to aquarium or culture containers, previously filled with filtered seawater, holding the PVC tubes wrapped with seed line (Camus & Buschmann, Citation2017; Redmond et al., Citation2014). These should be left for 24 hrs before removing the seed line wrapped tubes and placing them into culture aquaria tanks filled with Provasoli enriched seawater held at a constant temperature. If gametophyte stock cultures are used, then these should be vegetatively propagated in order to obtain a high density of gametophytes. This is done under similar culture conditions described in the germplasm banking section above. This may take weeks to months depending on the initial density of gametophytes held in the culture (Barrento et al., Citation2016; Westermeier et al., Citation2006). Once a dense culture is present, it can be transferred to an electric blender or a tissue disruptor and fragmented until a dark brown solution results. This solution can then be sprayed directly onto the seed line using a pressure sprayer, left for 30 min – 1 hr, before being placed in culture aquaria tanks. Seawater changes should be carried out weekly to refresh nutrient concentrations (Redmond et al., Citation2014). Care should be taken to avoid overstocking aquaria with seed line PVC tubes as this can create shading, limit water movement and deplete nutrients, meaning water will need to be changed more frequently. Camus and Buschmann (Citation2017) detail the optimal production conditions for rapid growth at a temperature of 12°C, a photon flux density of 12 µmol m–2 s–1, a photo period of 16 L:8D and aeration of 414 l h–1. Under these conditions they were able to produce 4–5 mm long juvenile sporophytes after 45 days post sporulation. During the hatchery phase, environmental conditions should be monitored daily to ensure optimal conditions for growth.
Another applicable seeding approach is to attach free-floating sporophytes directly onto seed lines. This method, which has been described in detail elsewhere (see Camus, Infante, & Buschmann, Citation2018b; Westermeier et al., Citation2006), is less common than direct seeding due to its labour-intensive nature (Camus et al., Citation2018b). Briefly, embryonic sporophytes are produced in floating cultures, by providing constant water motion in the culture vessel, until a desired size of 4–10 cm is reached. These are then extracted from the culture and sown directly onto seed lines either using adhesives or simply by inserting the holdfast between the weaves of the seed lines. Individuals are typically spaced 10–30 cm apart for a grow-out.
Grow-out requirements
The grow-out phase should typically be carried out in a natural environment where nutrient and light availability are not limiting, where water motion is present but not detrimental and where temperature ranges are well within the thermal limits of M. pyrifera (Fain & Murray, Citation1982; Deysher & Dean, Citation1984; Harrison & Hurd, Citation2001; Camus & Buschmann, Citation2017; Camus et al., 2018; North et al., Citation1986). A wide range of approaches have been trialled using varying infrastructures, ranging from shallow-water grid arrays to offshore deep-water suspension lines (Buschmann et al., Citation2014; Bak et al., Citation2018; Bak, Gregersen, & Infante, Citation2020; Camus et al., Citation2018b). Grow-out facilities are usually located in shallow coastal environments, <30 m deep with strong current flow, nitrogen levels of no less than 5–8 µMol and high light availability. Site selection is a critical decision as high-temperature pulses (normally not detected by observing average values, salinities and nutrient drops and pest organism (epibionts, grazer and eventually pathogens) can determine the viability of the farming activity (Camus et al., Citation2018b).
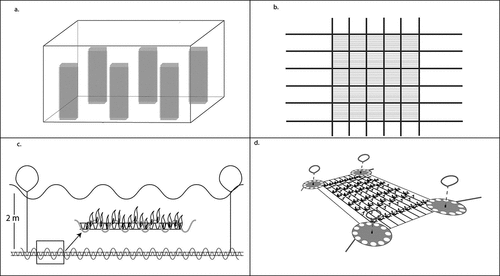
Grid arrays are commonly employed as grow-out structures (Bak et al., Citation2020) as they provide the greatest area coverage and are rigid and maintain a stable desired depth (). These arrays involve a series of mooring blocks or steel anchors that fix the grid to the seabed. From these moorings, ropes are suspended in a cross-linked fashion creating a semirigid grid (Camus et al., Citation2018b). In each grid, backbone lines can be strung from one side to the other, parallel to one another, and it is on these backbone lines that the seed line will be deployed. Seed line (either direct seeded or free-floating) can be deployed by feeding one end of the backbone line through the PVC seed line tube, tying the end of the seed line to the backbone line, attaching it to the grid and then moving the PVC seed line tube along the backbone line, letting it coil around the backbone as it moves along, before tying it off once it reaches the other end ().
Setting the depth of the grid array is important (Camus et al., Citation2018b) and should be based on knowledge of light, nutrient, temperature, and water motion characteristics at the chosen site. The depth of the array can be manipulated by attaching surface or subsurface buoys to the grid to provide floatation while altering the length of the mooring ropes that suspend the grid. Manipulation using buoys initially attached to the grid is essential to maintain buoyancy and as pneumatocysts develop, buoys can be removed and weights added to offset the floatation of the developing kelp.
Seasonality dictates growth and for M. pyrifera grow-out will typically be most successful if commenced in autumn/winter with a spring/summer harvest (Buschmann et al., Citation2004, Citation2014; Camus et al., Citation2018b). This will, however, depend on local conditions and in situ physiological understanding (Gerard, Citation1982; Varela et al., Citation2018).
Restoration
Significant declines in kelp forest habitat have been recorded across the globe over the past 50–100 years (Desmond et al., Citation2015; Friedlander et al., Citation2018; Krumhansl et al., Citation2016; Layton et al., Citation2020; Wernberg et al., Citation2019), included in that loss have been large swaths of M. pyrifera (Hay, Citation1990; Johnson et al., Citation2011; Butler, Lucieer, Wotherspoon, & Johnson, Citation2020; Layton et al., Citation2020, Tail et al., 2021). The driving factors behind kelp forest decline are relatively well understood and include increasing sea surface temperature (Filbee-Dexter et al., Citation2016; Johnson et al., Citation2011; Wernberg et al., Citation2013), light limitation (Cie & Edwards, Citation2008; Desmond et al., Citation2015; Deysher & Dean, Citation1984; Fain & Murray, Citation1982; Navarro, Mansilla, & Palacios, Citation2007), increased storm frequency (Buschmann et al., Citation2004), sedimentation (Connell, Citation2003; Filbee-Dexter & Wernberg, Citation2018), food web alterations through overfishing (Friedlander et al., Citation2018; Krumhansl et al., Citation2016), eutrophication (Filbee-Dexter & Wernberg, Citation2018) and direct harvest (Buschmann et al., Citation2014). This decade, 2021–2030, is the UN Decade on Ecosystem Restoration with a goal to restore 350 million hectares of degraded ecosystems (FAO, Citation2020b). Arguably, the revitalization and restoration of vast areas of lost kelp forest would deliver some of the greatest benefits in terms of fisheries productivity, ecosystem services and socio-ecological outcomes of any ecosystem type.
Early kelp forest restoration efforts began sometime before 1970 and have shown mixed results over the last five decades (Campbell, Marzinelli, Coleman, Verge, & Steinberg, Citation2014; Carney, Waaland, Klinger, & Ewing, Citation2005; Fredriksen et al., Citation2020; Mcleod et al., Citation2018; Mearns, Hanan, & Harris, Citation1997; North, Citation1976; Westermeier et al., Citation2014; Wood et al., Citation2019). Approaches have included encouraging natural recruitment through the placement of artificial substrate, to directly seeding habitat with microscopic life stages, to transplantation of juvenile and adult individuals. Many of these approaches have been extremely time, labour and financially demanding, often making them prohibitively expensive to maintain. As approaches advance, a move to aquacultural practices (Alleway et al., Citation2019; Froehlich, Gentry, & Halpern, Citation2017; Giangrande, Gravina, Rossi, Longo, & Pierri, Citation2021) is emerging which allows large quantities of stock to be produced under laboratory conditions with known genetic and physiological characteristics. This stock can then be out-planted into the receiving environment when conditions are appropriate. Out-planting approaches vary, and their success is highly dependent on the site conditions. This area of research is currently underdeveloped but promising results have been seen using seed line attached directly to or above the substrate (Giangrande et al., Citation2021; Kraufvelin & Díaz, Citation2015), seeded tiles attached to the seafloor (Layton et al., Citation2020; Shelamoff et al., Citation2020) and an approach termed “green gravel” which is gravel seeded in the laboratory and distributed directly onto the seafloor (Coleman et al., Citation2020; Fredriksen et al., Citation2020). All of these approaches out-plant when M. pyrifera are at the juvenile sporophyte stage.
Conclusion
This critical analysis summarizes the current state of knowledge regarding the culture of M. pyrifera and details the necessary steps for obtaining and preserving stock as well as propagation for experimental, aquacultural and restorative purposes. Current threats to M. pyrifera and the value this species holds from an aquacultural perspective mean that a concerted effort is required to standardize preservation and propagation methods. It is essential to safeguard this iconic species from the current local and global stressors and to lock-in the many benefits it offers for sustainable aquaculture.
Figure 3. Culture infrastructure for the production of Macrocystis pyrifera. Seed line in hatchery aquaria (a), layout of grow-out grid (modified from Camus et al., Citation2018b) (b), seed line attachment to main backbone line (modified from Gutierrez et al., Citation2006) (c), low angle view of grid array (modified from Bak et al., Citation2020) (d).
Authors’ contribution
CDH and MJD proposed the idea behind this study. DML and MJD wrote most parts of the manuscript as well as produced all the figures. However, all authors contributed critically to the draft with valuable inputs and experience. All authors read and gave final approval for publication.
Acknowledgments
This study was funded by New Zealand Ministry of Business, Innovation and Employment to MJD and CDH (grant number UOOX1908 - “Cultivating resilient marine forests to rebuild productive coastal ecosystems”). DML was funded by a postgraduate scholarship from University of Otago, New Zealand.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alleway, H. K., Gillies, C. L., Bishop, M. J., Gentry, R. R., Theuerkauf, S. J., & Jones, R. (2019). The ecosystem services of marine aquaculture: Valuing benefits to people and nature. BioScience, 69, 59–68.
- Alsuwaiyan, N. A., Mohring, M. B., Cambridge, M., Coleman, M. A., Kendrick, G. A., & Wernberg, T. (2019). A review of protocols for the experimental release of kelp (Laminariales) zoospores. Ecology and Evolution, 9, 8387–8398.
- Amsler, C. D., & Neushul, M. (1989). Chemotactic effects of nutrients on spores of the kelps Macrocystis pyrifera and Pterygophora California. Marine Biology, 102, 557–564.
- Amsler, C. D., & Neushul, M. (1990). Nutrient stimulation of spore settlement in the kelps Pterygophora californica and Macrocystis pyrifera. Marine Biology, 107, 297–304.
- Andersen, R. A. (2005). Algal culturing techniques (1st ed.). Elsevier: Elsevier Academic Press.
- Anderson, E. K., & North, W. J. (1967). Zoospore release rates in giant kelp Macrocystis. Bull. South.Calif. Acad. Sci, 66, 223–232.
- Anderson, B. S., & Hunt, J. W. (1988). Bioassay methods for evaluating the toxicity of heavy metals, biocides and sewage effluent using microscopic stages of giant kelp Macrocystis pyrifera (Agardh): A preliminary report. Marine Environmental Research, 26, 113–134.
- Andrew, N. L., & O’Neill, A. L. (2000). Large-scale patterns in habitat structure on subtidal rocky reefs in New South Wales. Marine & Freshwater Research, 51, 255.
- Bak, U. G., Mols-Mortensen, A., & Gregersen, O. (2018). Production method and cost of commercial-scale offshore cultivation of kelp in the Faroe Islands using multiple partial harvesting. Algal Research, 33, 36–47.
- Bak, U. G., Gregersen, Ó., & Infante, J. (2020). Technical challenges for offshore cultivation of kelp species: Lessons learned and future directions. Botanica Marina, 63, 341–353.
- Barrento, S., Camus, C., Sousa-Pinto, I., & Buschmann, A. H. (2016). Germplasm banking of the giant kelp: Our biological insurance in a changing environment. Algal Research, 13, 134–140.
- Blamey, L. K., & Branch, G. M. (2012). Regime shift of a kelp-forest benthic community induced by an “invasion” of the rock lobster Jasus lalandii. Experimental Marine Biology and Ecology, 420–421, 33–47.
- Boland, W., Marner, F.-J., Jaenicke, L., Muller, D. G., & Folster, E. (1983). Comparative receptor study in gamete chemotaxis of the seaweeds Ectocarpus siliculosus and Cutleria multjida: An approach to interspecific communication of algal gametes. Eur. J. Biochem, 134, 97–103.
- Bolton, J. J., Anderson, R. J., Smit, A. J., & Rothman, M. D. (2012). South African kelp moving eastwards: The discovery of Ecklonia maxima (Osbeck) Papenfuss at De Hoop nature reserve on the south coast of South Africa. African Journal of Marine Science, 34, 147–151.
- Brown, M. T., Nyman, M. A., Keogh, J. A., & Chin, N. K. M. (1997). Seasonal growth of the giant kelp Macrocystis pyrifera in New Zealand. Marine Biology, 129, 417–424.
- Buschmann, A. H., Väsquez, J. A., Osorio, P., Reyes, E., Filún, L., Hernández-González, M. C., & Vega, A. (2004). The effect of water movement, temperature and salinity on abundance and reproductive patterns of Macrocystis spp. (Phaeophyta) at different latitudes in Chile. Marine Biology, 145, 849–862.
- Buschmann, A. H., Moreno, C., Vásquez, J. A., & Hernández-González, M. C. (2006). Reproduction strategies of Macrocystis pyrifera (Phaeophyta) in southern Chile: The importance of population dynamics. Applied Phycology, 18. doi:10.1007/s10811-006-9063-5
- Buschmann, A. H., Prescott, S., Potin, P., Faugeron, S., Vásquez, J. A., Camus, C., & Varela, D. A. (2014). The status of kelp exploitation and marine agronomy, with emphasis on Macrocystis pyrifera, in Chile. In Advances in botanical research (pp. 161–188). Academic Press. doi.10.1016/B978-0-12-408062-1.00006-8
- Buschmann, A. H., Villegas, K., Pereda, S. V., Camus, C., Kappes, J. L., Altamirano, R., & Hernández-González, M. C. (2020). Enhancing yield on Macrocystis pyrifera (Ochrophyta): The effect of gametophytic developmental strategy. Algal Research, 52, 102124.
- Butler, C. L., Lucieer, V. L., Wotherspoon, S. J., & Johnson, C. R. (2020). Multi-decadal decline in cover of giant kelp Macrocystis pyrifera at the southern limit of its Australian range. Marine Ecology Progress Series, 653, 1–18.
- Cai, J., Lovatelli, A., Aguilar-Manjarrez, J., Cornish, L., Dabbadie, L., Desrochers, A., Diffey, S., Garrido Gamarro, E., Geehan, J., Hurtado, A., Lucente, D., Mair, G., Miao, W., Potin, P., Przybyla, C., Reantaso, M., Roubach, R., Tauati, M. & Yuan, X.(2021). Seaweeds and microalgae: An overview for unlocking their potential in global aquaculture development. FAO Fisheries and Aquaculture Circular No. 1229. Rome, FAO: . doi:10.4060/cb5670en
- Campbell, A. H., Marzinelli, E. M., Coleman, M. A., Verge, A., & Steinberg, P. D. (2014). Towards restoration of missing underwater forests. PLoS ONE, 9, e84106.
- Camus, C., & Buschmann, A. H. (2017). Macrocystis pyrifera aquafarming: Production optimization of rope-seeded juvenile sporophytes. Aquaculture, 468, 107–114.
- Camus, C., Faugeron, S., & Buschmann, A. H. (2018a). Assessment of genetic and phenotypic diversity of the giant kelp, Macrocystis pyrifera, to support breeding programs. Algal Research, 30, 101–112.
- Camus, C., Infante, J., & Buschmann, A. H. (2018b). Overview of 3 year precommercial seafarming of Macrocystis pyrifera along the Chilean coast. Reviews in Aquaculture, 10, 543–559.
- Camus, C., Solas, M., Martínez, C., Vargas, J., Garcés, C., & Gil-Kodaka, P. (2021). Mates matter: Gametophyte kinship recognition and inbreeding in the giant kelp, macrocystis pyrifera (Laminariales, Phaeophyceae). Journal of Phycology, 57, 711–725.
- Carney, L. T., Waaland, J. R., Klinger, T., & Ewing, K. (2005). Restoration of the bull kelp Nereocystis luetkeana in nearshore rocky habitats. Marine Ecology Progress Series, 302, 49–61.
- Carney, L. T., & Edwards, M. S. (2010). Role of nutrient fluctuations and delayed development in gametophyte reproduction by Macrocystis pyrifera (Phaeophyceae) in Southern California. Phycology, 46. doi:10.1111/j.1529-8817.2010.00882.x
- Choi, Y. H., Nam, T. J., & Kuwano, K. (2013). Cryopreservation of gametophytic thalli of Porphyra yezoensis (Rhodophyceae) by one-step fast cooling. Applied Phycology, 25. doi:10.1007/s10811-012-9887-0
- Chopin, T., & Tacon, A. G. J. (2021). Importance of seaweeds and extractive species in global aquaculture production. Reviews in Fisheries Science and Aquaculture, 29, 139–148.
- Cie, D. K., & Edwards, M. S. (2008). The effects of high irradiance on the settlement competency and viability of kelp zoospores. Phycology, 44. doi:10.1111/j.1529-8817.2008.00464.x
- Coleman, M. A., Wood, G., Filbee-Dexter, K., Minne, A. J. P., Goold, H. D., Vergés, A., … Wernberg, T. (2020). Restore or redefine: Future trajectories for restoration. Frontiers in Marine Science, 7. doi:10.3389/fmars.2020.00237
- Connell, S. D. (2003). Negative effects overpower the positive of kelp to exclude invertebrates from the understorey community. Oecologia, 137, 97–103.
- Correa, T., Gutiérrez, A., Flores, R., Buschmann, A. H., Cornejo, P., & Bucarey, C. (2016). Production and economic assessment of giant kelp Macrocystis pyrifera cultivation for abalone feed in the south of Chile. Aquaculture Research, 47, 698–707.
- Desmond, M. J., Pritchard, D. W., & Hepburn, C. D. (2015). Light limitation within southern New Zealand kelp forest communities. PLoS ONE, 10, e0123676.
- Devinny, J. S., & Volse, L. A. (1978). Effects of sediments on the development of Macrocystis pyrifera gametophytes. Marine Biology, 48, 343–348.
- Devinny, J. S., & Leventhal, J. (1979). New methods for mass culture of Macrocystis pyrifera sporophytes. Aquaculture, 17, 241–250.
- Deysher, L. E., & Dean, T. A. (1984). Critical irradiance levels and the interactive effects of quantum irradiance and dose on gametogenesis in the giant kelp, Macrocystis pyrifera. Phycology, 20, 520–524.
- Fain, S. R., & Murray, S. N. (1982). Effects of light and temperature on net photosynthesis and dark respiration of gametophytes and embryonic sporophytes of Macrocystis pyrifera. Phycology, 18, 92–98.
- FAO. (2018). The global status of seaweed production, trade and utilization. GLOBEFISH Research Programme Vol. 124 Rome: IGO.
- FAO. (2020a). The state of world fisheries and aquaculture 2020. Sustainability in action. Rome. doi:10.4060/ca9229en
- FAO. (2020b). The UN decade on ecosystem restoration 2021-2030. Retrieved from: www.unep.org.
- Fernández, P. A., Navarro, J. M., Camus, C., Torres, R., & Buschmann, A. H. (2021). Effect of environmental history on the habitat-forming kelp Macrocystis pyrifera responses to ocean acidification and warming: A physiological and molecular approach. Scientific Reports, 11. doi:10.1038/s41598-021-82094-7
- Filbee-Dexter, K., Feehan, C. J., & Scheibling, R. E. (2016). Large-scale degradation of a kelp ecosystem in an ocean warming hotspot. Marine Ecology Progress Series, 543, 141–152.
- Filbee-Dexter, K., & Wernberg, T. (2018). Rise of Turfs: A new battlefront for globally declining kelp forests. BioScience, 68, 64–76.
- Fredriksen, S., Filbee-Dexter, K., Norderhaug, K. M., Steen, H., Bodvin, T., Coleman, M. A., … Wernberg, T. (2020). Green gravel: A novel restoration tool to combat kelp forest decline. Scientific Reports, 10. doi:10.1038/s41598-020-60553-x
- Friedlander, A. M., Ballesteros, E., Bell, T. W., Giddens, J., Henning, B., Hüne, M., … Bernardi, G. (2018). Marine biodiversity at the end of the world: Cape Horn and Diego Ramírez islands. PLOS ONE, 13, e0189930.
- Froehlich, H. E., Gentry, R. R., & Halpern, B. S. (2017). Conservation aquaculture: Shifting the narrative and paradigm of aquaculture’s role in resource management. Biological Conservation, 215, 162–168.
- Garman, G. D., Pillai, M. C., Goff, L. J., & Cherr, G. N. (1994). Nuclear events during early development in gametophytes of Macrocystis pyrifera, and the temporal effects of a marine contaminant. Marine Biology, 121, 355–362.
- Gerard, V. A. (1982). In situ rates of nitrate uptake by giant kelp, Macrocystis Pyrifera (L.) C. Agardh: Tissue differences, environmental effects, and predictions of nitrogen-limited growth. Journal of Experimental Marine Biology and Ecology, 62, 211–224.
- Giangrande, A., Gravina, M. F., Rossi, S., Longo, C., & Pierri, C. (2021). Aquaculture and restoration: Perspectives from Mediterranean sea experiences. Water (Switzerland), 13. doi:10.3390/w13070991
- Ginsburger-Vogel, T., Arbault, S., & Pérez, R. (1992). Ultrastructural study of the effect of freezingthawing on the gametophyte of the brown alga Undaria pinnatifida. Aquaculture, 106, 171–181.
- Goecke, F., Klemetsdal, G., & Ergon, Å. (2020). Cultivar development of kelps for commercial cultivation—past lessons and future prospects. Frontiers in Marine Science, 8. doi:10.3389/fmars.2020.00110
- González-Fragoso, J., Ibarra-Obando, S. E., & North, W. J. (1991). Frond elongation rates of shallow waterMacrocystis pyrifera (L.) Ag. In northern Baja California, Mexico. Applied Phycology, 3. doi:10.1007/bf00026093
- Graham, M. H. (1996). Effect of high irradiance on recruitment of the giant kelp Macrocystis (Phaeophyta) in shallow water. Phycology, 32, 903–906.
- Graham, M. H., Vásquez, J. A., & Buschmann, A. H. (2007). Global ecology of the giant kelp Macrocystis: From ecotypes to ecosystems. Oceanography and Marine Biology: An Annual Review, 45, 39–88.
- Grout, B. W. W. (1995). Introduction to the in vitro preservation of plant cells, tissues and organs. In Genetic preservation of plant cells in vitro. doi:10.1007/978-3-642-78661-7_1
- Gutierrez, A., Correa, T., Muñoz, V., Santibañez, A., Marcos, R., Cáceres, C., et al. (2006). Farming of the giant kelp Macrocystis pyrifera in southern Chile for development of novel food products. In Eighteenth international seaweed symposium (pp. 259–267). Dordrecht: Springer Netherlands. doi:10.1007/978-1-4020-5670-3_5
- Harrison, P. J., & Hurd, C. L. (2001). Nutrient physiology of seaweeds: Application of concepts to aquaculture. Cahiers de Biologie Marine, 42.
- Hay, C. H. (1990). The distribution of Macrocystis (Phaeophyta: Laminariales) as a biological indicator of cool sea surface temperature, with special reference to New Zealand waters. Journal of the Royal Society of New Zealand, 20, 313–336.
- Henríquez, L. A., Buschmann, A. H., Maldonado, M. A., Graham, M. H., Hernández-González, M. C., Pereda, S. V., & Bobadilla, M. I. (2011). Grazing on giant kelp microscopic phases and the recruitment success of annual populations of Macrocystis pyrifera (laminariales, phaeophyta) in southern Chile. Phycology, 47. doi:10.1111/j.1529-8817.2010.00955.x
- Hernández-Carmona, G. (1996). Frond elongation rates of Macrocystis pyrifera (L.) AG. at Bahía Tortugas, Baja California Sur, Mexico. Ciencias Marinas, 22, 57–72.
- Hollarsmith, J. A., Buschmann, A. H., Camus, C., & Grosholz, E. D. (2020). Varying reproductive success under ocean warming and acidification across giant kelp (Macrocystis pyrifera) populations. Experimental Marine Biology and Ecology, 522, 151247.
- Hu, Z. M., Shan, T. F., Zhang, J., Zhang, Q. S., Critchley, A. T., Choi, H. G., & Duan, D.-L. (2021). Kelp aquaculture in China: A retrospective and future prospects. Reviews in Aquaculture, 13, 1324–1351.
- Hwang, E. K., Yotsukura, N., Pang, S. J., Su, L., & Shan, T. F. (2019). Seaweed breeding programs and progress in Eastern Asian countries. Phycologia, 58, 484–495.
- James, D. E., Stull, J. K., & North, W. J. (1990). Toxicity of sewage-contaminated sediment cores to Macrocystis pyrifera (Laminariales, Phaeophyta) gametophytes determined by digital image analysis. Hydrobiologia, 204, 483–489.
- Johnson, C. R., Banks, S. C., Barrett, N. S., Cazassus, F., Dunstan, P. K., Edgar, G. J., & Taw, N. (2011). Climate change cascades: Shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. Journal of Experimental Marine Biology and Ecology, 400, 17–32.
- Jones, C. G., Lawron, J. H., & Shachak, M. (1997). Positive and negative effects of organisms as physical ecosystem engineers. Ecology, 78, 1946–1957.
- Kawai, H., Motomura, T., & Okuda, K. (2005). Isolation and purification techniques for macroalgae. In Algal Culturing Techniques 133 . doi:10.1016/b978-012088426-1/50010-x
- Kim, J. K., Yarish, C., Hwang, E. K., Park, M., & Kim, Y. (2017). Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. Algae, 32, 1–13.
- Kono, S., Kuwano, K., & Saga, N. (1998). Cryopreservation of Eisenia bicyclis (Laminariales, Phaeophyta) in liquid nitrogen. Marine Biotechnology, 6 220–223 .
- Kraufvelin, P., & Díaz, E. R. (2015). Sediment macrofauna communities at a small mussel farm in the northern Baltic proper. Boreal Environment Research, 20 378–390 .
- Kriegisch, N., Reeves, S. E., Johnson, C. R., & Ling, S. D. (2019). Top-down sea urchin overgrazing overwhelms bottom-up stimulation of kelp beds despite sediment enhancement. Experimental Marine Biology and Ecology, 514–515, 48–58.
- Krumhansl, K. A., Okamoto, D. K., Rassweiler, A., Novak, M., Bolton, J. J., Cavanaugh, K. C., & Byrnes, J. E. (2016). Global patterns of kelp forest change over the past half-century. Proceedings of the National Academy of Sciences of the United States of America 113, 13785–13790.
- Kuwano, K., Kono, S., Jo, Y. H., Shin, J. A., & Saga, N. (2004). Cryopreservation of the gametophytic cells of Laminariales (Phaeophyta) in liquid nitrogen. Phycology, 40. doi:10.1111/j.1529-8817.2004.03121.x
- Layton, C., Coleman, M. A., Marzinelli, E. M., Steinberg, P. D., Swearer, S. E., Vergés, A., & Johnson, C. R. (2020). Kelp forest restoration in Australia. Frontiers in Marine Science, 7. doi:10.3389/fmars.2020.00074
- Leal, P. P., Hurd, C. L., & Roleda, M. Y. (2014). Meiospores produced in sori of nonsporophyllous laminae of Macrocystis pyrifera (Laminariales, Phaeophyceae) may enhance reproductive output. Phycology, 50, 400–405.
- Leal, P. P., Hurd, C. L., Fernández, P. A., & Roleda, M. Y. (2017). Ocean acidification and kelp development: reduced pH has no negative effects on meiospore germination and gametophyte development of Macrocystis pyrifera and Undaria pinnatifida. Phycology, 53. doi:10.1111/jpy.12518
- Leal, P. P., Roleda, M. Y., Fernández, P. A., Nitschke, U., & Hurd, C. L. (2021). Reproductive phenology and morphology of Macrocystis pyrifera (Laminariales, Ochrophyta) from southern New Zealand in relation to wave exposure 1. Phycology, 57, 1619–1635.
- Lewis, R. J., & Neushul, M. (1994). Northern and Southern hemisphere hybrids of Macrocystis (Phaeophyceae). Phycology, 30. doi:10.1111/j.0022-3646.1994.00346.x
- Ling, S. D., Johnson, C. R., Frusher, S. D., & Ridgway, K. R. (2009). Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Ecology, 106. Available at www.pnas.org/cgi/content/full/
- Lüning, K., & Neushul, M. (1978). Light and temperature demands for growth and reproduction of laminarian gametophytes in southern and central California. Marine Biology, 45, 297–309.
- Lüning, K. (1981). Photobiology of seaweeds: Ecophysiological aspects. In T. Levrig (Ed.), International seaweed symposium (Xth) (pp. 35–56). Berlin, Boston: De Gruyter. doi:10.1515/9783110865271-005
- Mabin, C. J. T., Johnson, C. R., & Wright, J. T. (2019). Physiological response to temperature, light, and nitrates in the giant kelp Macrocystis pyrifera from Tasmania, Australia. Marine Ecology Progress Series, 614, 1–19.
- Macchiavello, J., Araya, E., & Bulboa, C. (2010). Production of Macrocystis pyrifera (Laminariales; Phaeophyceae) in northern Chile on spore-based culture. Journal of Applied Phycology, 22, 691–697.
- Maier, I., Hertweck, C., & Boland, W. (2001). Stereochemical specificity of lamoxirene, the sperm-releasing pheromone in kelp (Laminariales, Phaeophyceae). Biological Bulletin, 201, 121–125.
- Markham, J. W., & Hagmeier, E. (1982). Observations on the effects of germanium dioxide on the growth of macro-algae and diatoms. Phycologia, 21, 125–130.
- McHugh, D. (2003). A guide to the seaweed industry: FAO fisheries technical paper No. 441.
- Mcleod, I. M., Boström-Einarsson, L., Johnson, C. R., Kendrick, G., Layton, C., Rogers, A. A., et al. (2018). The role of restoration in conserving matters of national environmental significance in marine and coastal environments.98-101.
- Mearns, A. J., Hanan, D. A., & Harris, L. (1997). Recovery of kelp forest off Palos verdes.
- Morris, M. M., Haggerty, J. M., Papudeshi, B. N., Vega, A. A., Edwards, M. S., & Dinsdale, E. A. (2016). Nearshore pelagic microbial community abundance affects recruitment success of giant kelp, Macrocystis pyrifera. Frontiers in Microbiology, 7. doi:10.3389/fmicb.2016.01800
- Muñoz, V., Hernández-González, M. C., Buschmann, A. H., Graham, M. H., & Vásquez, J. A. (2004). Variability in per capita oogonia and sporophyte production from giant kelp gametophytes (Macrocystis pyrifera, Phaeophyceae). Revista Chilena de Historia Natural, 77. doi:10.4067/S0716-078X2004000400007
- Murúa, P., Patiño, D. J., Müller, D. G., & Westermeier, R. (2021). Sexual compatibility in giant kelp gametophytes: Inter-cultivar hybridization is average between parents but excels under harsher conditions. Journal of Applied Phycology, 33, 3261–3275.
- Navarro, N. P., Mansilla, A., & Palacios, M. (2007). UVB effects on early developmental stages of commercially important macroalgae in southern Chile. Applied Phycology, 447–456. UVB, doi:10.1007/s10811-007-9276-2
- Naylor, R. L., Hardy, R. W., Buschmann, A. H., Bush, S. R., Cao, L., Klinger, D. H. … Troell, M. (2021). A 20-year retrospective review of global aquaculture. Nature, 591, 551–563. doi:10.1038/s41586-021-03308-6
- Neushul, M. (1963). Studies on the giant kelp, Macrocystis. II. Reproduction. American Journal of Botany, 50. doi:10.1002/j.1537-2197.1963.tb07203.x
- North, W. J. (1976). Aquacultural techniques for creating and restoring beds of giant kelp, Macrocystis spp. Journal of the Fisheries Research Board of Canada, 33, 1015–1023.
- North, W. J., Jackson, G. A., & Manley, S. L. (1986). Macrocystis and its environment, knowns and unknowns. Aquatic Botany, 26, 9–26. doi:10.1016/0304-3770(86)90003-3
- Ortiz, J., Uquiche, E., Robert, P., Romero, N., Quitral, V., & Llantén, C. (2009). Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. European Journal of Lipid Science and Technology, 111, 320–327.
- Piel, M. I., Avila, M., & Alcapán, A. (2015). Cryopreservation of early stages of Macrocystis pyrifera gametophytes (Laminariales, Ochrophyta) under controlled laboratory conditions. Revista de Biologia Marina Y Oceanografia, 50, 157–162.
- Plá, P. C., & Alveal, K. (2012). Development of Macrocystis pyrifera from spores and gametes on artificial substrate. Algal production in a surface culture. Latin American Journal of Aquatic Research, 40. doi:10.3856/vol40-issue2-fulltext-5
- Purcell-Meyerink, D., Packer, M. A., Wheeler, T. T., & Hayes, M. (2021). Aquaculture production of the brown seaweeds Laminaria digitata and Macrocystis pyrifera: Applications in food and pharmaceuticals. Molecules, 26, 1306.
- Raimondi, P. T., Reed, D. C., Gaylord, B., & Washburn, L. (2004). Effects of self-fertilization in the giant kelp, Macrocystis pyrifera. Ecology, 85, 3267–3276.
- Redmond, S., Green, L., Yarish, C., Kim, J., & Neefus, C. (2014). New England seaweed culture handbook-nursery systems. Groton Available at: http://s.uconn.edu/seaweedplaylist.
- Reed, D. C., Neushul, M., & Ebeling, A. W. (1991). Role of settlement density on gametophyte growth and reproduction in the kelps Pterygophora californica and Macrocystis pyrifera (Phaeophyceae). Phycology, 27, 361–366.
- Reed, D. C., Amsler, C. D., & Ebeling, A. W. (1992). Dispersal in kelps : Factors affecting spore swimming and competency. Ecology, 73, 1577–1585. Accessed September 27, 2021. https://www.jstor.org/stable/1940011
- Reed, D. C., Ebeling, A. W., Anderson, T. W., & Anghera, M. (1996). Differential reproductive responses to fluctuating resources in two seaweeds with different reproductive strategies. Ecology, 77, 300–316.
- Reed, D. C., Kinlan, B. P., Raimondi, P. T., Washburn, L., Gaylord, B., & Drake, P. T. (2006). A Metapopulation perspective on the patch dynamics of giant kelp in Southern California. In Marine metapopulations (pp. 353–386). Elsevier: Academic Press.
- Reed, D. C., Rassweiler, A., & Arkema, K. K. (2008). Biomass rather than growth rate determines variation in net primary production by giant kelp. Ecology, 89, 2493–2505.
- Sanbonsuga, Y., & Neushul, M. (1978). Hybridization of macrocystis (Phaeophyta) with other float-bearing kelps 1, 2. Journal of Phycology, 14, 214–224.
- Santelices, B. (1990). Patterns of reproduction, dispersal and recruitment in seaweeds. Oceanography and Marine Biology Annual Review, 28 177–276.
- Schiel, D. R., & Foster, M. S. (2015). The biology and ecology of giant kelp forests (University of California Press). doi:10.2216/5501br01
- Shea, R., & Chopin, T. (2007). Effects of germanium dioxide, an inhibitor of diatom growth, on the microscopic laboratory cultivation stage of the kelp, Laminaria saccharina. Applied Phycology, 19, 27–32.
- Shelamoff, V., Layton, C., Tatsumi, M., Cameron, M. J., Wright, J. T., Edgar, G. J., & Johnson, C. R. (2020). High kelp density attracts fishes except for recruiting cryptobenthic species. Marine Environmental Research, 161, 105127.
- Sherman, J. K. (1973). Synopsis of the use of frozen human semen since 1964: State of the art of human semen banking. Fertility and Sterility, 24, 397–412.
- Stephens, T. A., & Hepburn, C. D. (2014). Mass-transfer gradients across kelp beds influence Macrocystis pyrifera growth over small spatial scales. Marine Ecology Progress Series, 515, 97–109.
- Suebsanguan, S., Strain, E. M. A., Morris, R. L., & Swearer, S. E. (2021). Optimizing the initial cultivation stages of kelp Ecklonia radiata for restoration. Restoration Ecology, 29, 1–9.
- Taylor, R., & Fletcher, R. L. (1998). Cryopreservation of eukaryotic algae - A review of methodologies. Applied Phycology, 10. doi:10.1023/A:1008094622412
- Terawaki, T., Hasegawa, H., Arai, S., & Ohno, M. (2001). Management-free techniques for restoration of Eisenia and Ecklonia beds along the central Pacific coast of Japan. Applied Phycology, 13. doi:10.1023/A:1008135515037
- Varela, D. A., Hernríquez, L. A., Fernández, P. A., Leal, P., Hernández-González, M. C., Figueroa, F. L., & Buschmann, A. H. (2018). Photosynthesis and nitrogen uptake of the giant kelp Macrocystis pyrifera (Ochrophyta) grown close to salmon farms. Marine Environmental Research, 135, 93–102.
- Vigneron, T., Arbault, S., & Kaas, R. (1997). Cryopreservation of gametophytes of Laminaria digitata (L) lamouroux by encapsulation dehydration. In Cryo-Letters 93–98 .
- Wade, R., Augyte, S., Harden, M., Nuzhdin, S., Yarish, C., & Alberto, F. (2020). Macroalgal germplasm banking for conservation, food security, and industry. PLoS Biology, 18, e3000641.
- Wang, X., Yao, J., Zhang, J., & Duan, D. (2020). Status of genetic studies and breeding of Saccharina japonica in China. Journal of Oceanology and Limnology, 38, 1064–1079.
- Wernberg, T., Smale, D. A., Tuya, F., Thomsen, M. S., Langlois, T. J., de Bettignies, T., & Rousseaux, C. S. (2013). An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nature Climate Change, 3, 78–82.
- Wernberg, T., Krumhansl, K., Filbee-Dexter, K., & Pedersen, M. F. (2019). Status and trends for the world’s kelp forests. Second. : Academic Press. doi.10.1016/B978-0-12-805052-1.00003-6
- Westermeier, R., Patiño, D., Piel, M. I., Maier, I., & Mueller, D. G. (2006). A new approach to kelp mariculture in Chile: Production of free-floating sporophyte seedlings from gametophyte cultures of Lessonia trabeculata and Macrocystis pyrifera. Aquaculture Research, 37(2), 164–171.
- Westermeier, R., Patiño, D. J., Müller, H., & Müller, D. G. (2010). Towards domestication of giant kelp (Macrocystis pyrifera) in Chile: Selection of haploid parent genotypes, outbreeding, and heterosis. Applied Phycology, 22, 357–361.
- Westermeier, R., Patiño, D. J., Murúa, P., & Müller, D. G. (2011). Macrocystis mariculture in Chile: Growth performance of heterosis genotype constructs under field conditions. Applied Phycology, 23, 819–825.
- Westermeier, R., Murúa, P., Patiño, D. J., Muñoz, L., Atero, C., & Müller, D. G. (2014). Repopulation techniques for Macrocystis integrifolia (Phaeophyceae: Laminariales) in Atacama, Chile. Journal of Applied Phycology, 26, 511–518.
- Wheeler, P. A., & North, W. J. (1981). Nitrogen supply, tissue composition and frond growth rates for Macrocystis pyrifera off the coast of Southern California. Marine Biology, 64, 59–69.
- Williams, S. L. (2001). Reduced genetic diversity in eelgrass transplantations affects both population growth and individual fitness. Ecological Applications, 11, 1472–1488.
- Wood, G., Marzinelli, E. M., Coleman, M. A., Campbell, A. H., Santini, N. S., Kajlich, L., … Vergés, A. (2019). Restoring subtidal marine macrophytes in the Anthropocene: Trajectories and future-proofing. Marine & Freshwater Research, 70, 936.
- Xu, D., Ye, N., Cao, S., Wang, Y., Wang, D., Fan, X., … Mao, Y. (2015). Variation in morphology and PSII photosynthetic characteristics of Macrocystis pyrifera during development from gametophyte to juvenile sporophyte. Aquaculture Research, 46, 1699–1706.
- Zhang, Q. S., Cong, Y. Z., Qu, S. C., Luo, S. J., Li, X. J., & Tang, X. X. (2007). A simple and highly efficient method for the cryopreservation of Laminaria japonica (Phaeophyceae) germplasm. European Journal of Phycology, 42, 209–213.
- Zhang, Q., Cong, Y., Qu, S., Luo, S., & Yang, G. (2008). Cryopreservation of gametophytes of Laminaria japonica (Phaeophyta) using encapsulation-dehydration with two-step cooling method. Ocean University of China, 7, 65–71.