ABSTRACT
Kelp aquaculture is typically a two-stage process, with an indoor nursery phase and a grow-out phase at sea. For the successful development and implementation of commercial kelp aquaculture, production of viable seeded lines in the nursery is essential. This study investigated optimal nursery conditions of three kelp species native to Tasmania, Australia: Ecklonia radiata, Lessonia corrugata, and Macrocystis pyrifera. The interactive effects of temperature (12°C, 15°C, and 18°C) and light level (~30 µmol photons s−1 m−2, and ~ 60 µmol photons s−1 m−2) on sporophyte length, sporophyte density, and contamination of spools were examined over a 34-day period. The optimal temperature and light levels were 15°C and 30 µmol photons m−2 s−1 for E. radiata, 12°C and 60 µmol photons m−2 s−1 for L. corrugata, and 12°C and 30 or 60 µmol photons m−2 s−1 for M. pyrifera. Under these optimal conditions, the mean ± SEM sporophyte lengths after 34 days were 0.60 ± 0.02 mm, 1.04 ± 0.04 mm, and 0.60 ± 0.01 mm for E. radiata, L. corrugata, and M. pyrifera, respectively. The mean ± SEM sporophyte densities for each of these three species were 15.5 ± 6.2 sporophytes cm−1 of line, 10.8 ± 5.9 sporophytes cm−1 of line, and 19.3 ± 8.1 sporophytes cm−1 of line, respectively. Contamination increased with increasing temperature and was not significantly affected by light level. This work highlights the need for a species- and ecotype-specific approach in the nursery phase to ensure successful seaweed aquaculture outcomes in new regions of cultivation.
Introduction
Laminarian kelps (Phaeophyceae, Laminariales) have substantial global economic and ecological importance (Bennett et al., Citation2015; Chopin & Tacon, Citation2021; Steneck et al., Citation2002). Kelp aquaculture has developed significantly in recent years, with a wide array of products already on the market, and new kelp-based products constantly in development (Gutierrez et al., Citation2006; Holdt & Kraan, Citation2011). Whilst kelp aquaculture has historically been focused in Asia, it is gaining worldwide interest (Naylor et al., Citation2021). There are many benefits of seaweed farming when compared to terrestrial crop production, i.e., seaweeds do not need fertilizer, pesticides, or freshwater irrigation, and as such kelp aquaculture is now emerging in “non-traditional” regions of production, including Australia (Kelly, Citation2020).
Kelp mariculture is a two-stage process, with an indoor nursery (hatchery) phase, and an at sea grow-out phase. Optimizing the production of seeded lines bearing juvenile sporophytes in the nursery is essential for successful commercial kelp aquaculture (Camus & Buschmann, Citation2017; Hu et al., Citation2021; Peteiro, Bidegain, & Sánchez, Citation2019; Su, Pang, Shan, & Li, Citation2017). Culture conditions for gametophytes and early sporophytes of commercial kelp species non-native to Australia such as Saccharina japonica, S. latissima, and Undaria pinnatifida are well known, and optimal temperature, photon-flux density, light spectrum, photoperiod, and nutrient requirements have been elucidated (Camus & Buschmann, Citation2017; Nielsen, Kumar, Soler-Vila, Johnson, & Bruhn, Citation2016; Ratcliff, Soler-Vila, Hanniffy, Johnson, & Edwards, Citation2017; Su, Pang, Shan, & Li, Citation2017; Wang et al., Citation2010). As a result, several technical manuals have been produced to help people establish effective nursery culture (Edwards, O’Mahony, Connellan, Dring, & Werner, Citation2011; Flavin, Flavin, & Flahive, Citation2013; Forbord, Steinhovden, Rød, Handå, & Skjermo, Citation2018; Redmond, Green, Yarish, Kim, & Neefus, Citation2014; Rolin, Inkster, Laing, Hedges, & McEvoy, Citation2016). In Australia, to date, the research focus on kelps has been largely of an ecological nature (Layton & Johnson, Citation2021; Novaczek, Citation1984; Suebsanguan, Strain, Morris, & Swearer, Citation2021; Tatsumi et al., Citation2022; Tom Dieck, Citation1993), which provides a starting point but can be challenging to translate to aquaculture. Therefore, to support the development of the Australian kelp aquaculture industry, optimal conditions for gametophyte and early sporophyte development need to be determined.
There are three laminarian kelp species native to Tasmania, Ecklonia radiata (C.Agardh) J.Agardh, Lessonia corrugata A.H.S.Lucas, and Macrocystis pyrifera (L.) C.Agardh (Scott, Citation2017), and all are gaining commercial interest (Kelly, Citation2020). With respect to biogeographical genetic variation in southern Australia, E. radiata and M. pyrifera have relatively low genetic diversity compared to L. corrugata (Durrant, Barrett, Edgar, Coleman, & Burridge, Citation2015). E. radiata is subtidal and found in New Zealand, Madagascar, South Africa, and in southern Australia where it is the dominant habitat-forming kelp of the “Great Southern Reef” (Bennett et al., Citation2015; Wernberg et al., Citation2019). This relatively large geographical range suggests a broad tolerance of gametophytic and juvenile sporophytic life-stages to environmental conditions, including temperature and light level (Mabin, Gribben, Fischer, & Wright, Citation2013; Tom Dieck, Citation1993; Wernberg et al., Citation2019). M. pyrifera is more globally distributed and can be found from the low intertidal zone to a depth of 30 m in all cold-temperate waters in the Southern Hemisphere and along the west coast of North America (Schiel & Foster, Citation2015). Its distribution is largely determined by temperature, and its upper thermal tolerance for gametophytes is higher than that of sporophytes (Mabin, Johnson, & Wright, Citation2019; Schiel & Foster, Citation2015; Tom Dieck, Citation1993). L. corrugata is endemic to Tasmania and appears to have a relatively narrow temperature and irradiance range for optimal gametophyte growth (Paine et al., Citation2021; Tom Dieck, Citation1993).
In addition to establishing optimal culture conditions, management of contaminants is recognized as one of the major limitations to seaweed aquaculture development (Kim, Yarish, Hwang, Park, & Kim, Citation2017). Contamination and/or fouling by unwanted organisms, such as pathogens, diatoms and other algae, nematodes, and ciliates, that negatively impact growth in the nursery phase should be avoided or kept to a minimum (Bartsch, Citation2018; Forbord, Steinhovden, Rød, Handå, & Skjermo, Citation2018; Redmond, Green, Yarish, Kim, & Neefus, Citation2014). Measures to deal with contamination depend on the type and source of contaminants, and the nursery production method typically entails sterilizing glassware and culture media, disinfecting the sori prior to zoospore release, and using germanium dioxide or antibiotics to control diatom- and bacterial-growth, respectively (Bartsch, Citation2018; Forbord, Steinhovden, Rød, Handå, & Skjermo, Citation2018; Kerrison, Le, & Hughes, Citation2016; Redmond, Green, Yarish, Kim, & Neefus, Citation2014). However, despite these treatments, contamination often remains a challenge and is sometimes unavoidable, especially when scaling up to larger nursery volumes (e.g., Mooney-McAuley, Edwards, Champenois, & Gorman, Citation2016; Su, Pang, Shan, & Li, Citation2017). How the environmental conditions in nursery culture might affect levels of contaminants has not been tested systematically and is absent for Australian kelp species, but this information is needed to improve the effectiveness of kelp nurseries.
The purpose of this study was to optimize nursery conditions of three kelp species (E. radiata, L. corrugata, and M. pyrifera). We examined the interactive effects of temperature (12°C, 15°C, and 18°C) and light (~30 µmol photons s−1 m−2, and ~60 µmol photons s−1 m−2) on sporophyte length and density of spools inoculated with gametophytes. We simultaneously evaluated the effect of nursery conditions on contamination of the spools. The results provide information that will help optimize nursery conditions and assist in the development of a kelp aquaculture industry in southern Australia.
Materials and methods
Gametophyte culture establishment and spool preparation
Fertile sorus tissue from five individuals of E. radiata and L. corrugata was collected by divers from Apex Point (43.1033°S, 147.7077°E), with similar samples taken from M. pyrifera from Blackmans Bay (43.0122°S, 147.3312°E), Tasmania, Australia. Zoospores were released following the methods described in Forbord, Steinhovden, Rød, Handå, & Skjermo (Citation2018). In short, mature sorus tissue was dissected out of the main thallus with individuals treated separately. Sori were thoroughly wiped clean with a paper towel to remove any debris or epiphytes, then washed three times for 10 s in an iodine (Betadine®) solution (5 ml l−1) with successive rinsing using sterile seawater, before being kept overnight at 12°C in damp paper towel in the dark. Subsequently, zoospores were released in autoclaved seawater with F/2-medium (Guillard & Ryther, Citation1962) and kept in aerated (0.2 µm PTFE syringe filters) 3 l glass flasks at 12°C under red light conditions (~15 µmol photons m−2 s−1) and long-day photoperiod (16 h light:8 h dark) provided by LEDs (Fluval aquasky®). The zoospores were allowed to develop into gametophytes that were grown vegetatively for >3 months under these culture conditions, with the culture medium being changed biweekly (Bartsch, Citation2018).
Ten days before the start of the experiment, the gametophyte cultures from five individuals were mixed in equal densities, and the light spectrum changed to white light (~80 µmol photons m−2 s−1) to induce gametogenesis (Lüning & Dring, Citation1975). Gametogenesis was induced to reduce the time in the nursery and minimize potential differences in the transition rate of gametophytes to sporophytes between experimental treatments. The developing gametophyte solution was concentrated to 25 filaments per µl using a cell counting chamber (Sedgewick Rafter S50) with a 10-cm filter. This gametophyte culture was then evenly sprayed using a sterilized 1.25 l handheld pressure sprayer (Hozelock Ltd. Viton®) onto 10-cm PVC pipes (75 mm diameter) around which 10 m of polypropylene twine (~1 mm diameter) was wound, hereafter called “spools”. The spools were sterilized prior to spraying, by soaking in a 10% bleach solution overnight, after which they were thoroughly rinsed, dried in a laminar flow cabinet, and treated with UV light for 20 min.
Experimental design
The experiment sought to determine the interactive effects of light (30 and 60 µmol photons m−2 s−1) and temperature (12°C, 15°C, 18°C) on sporophyte development for E. radiata, L. corrugata, and M. pyrifera, with n = 3 replicates for each treatment. Both light and temperature treatments were chosen based on temperatures the species typically experience in nature in Tasmanian coastal waters and that have previously yielded relatively good results for M. pyrifera (Biancacci, Sanderson et al., Citation2022, Biancacci, Visch et al., Citation2022). In total, we examined 54 spools (3 species × 3 temperatures × 2 light × 3 replicates). Each seeded spool was treated as an independent replicate and carefully placed individually in 2 l transparent plastic jars filled with 1.5 l UV treated filtered seawater (0.22 µm) with F/2-medium and GeO2 (0.02 µmol l−1). To allow the gametophytes and microscopic juvenile sporophytes to adhere, there was no aeration in the jars for the first 3 days after spraying, after which the aeration was slowly increased until the jars were gently aerated. Each jar was supplied with air filtered with 0.2 µm syringe filters (25 mm diameter, PTFE).
Based on previous experience, the duration of the experiment was 34 days and carried out in a temperature-controlled room set at 11°C (Biancacci, Visch et al., Citation2022). The seawater temperature in the jars was controlled by randomly placing six (3 species × 2 light × 1 replicate) of the 2 l plastic jars into a tub (36 l; dimensions 34 cm wide, 47 cm long, 25 cm high) partially filled with freshwater that was thermally controlled to obtain the three temperature treatments (12°C, 15°C, and 18°C) using aquarium heaters (Aqua One®) regulated by a thermostat (Inkbird Tech Inc, ITC-308; see Supplementary fig. S1). Light was provided by LEDs (Clipsal©, 5000k, TPWPLED2) overhead and light level was controlled by wrapping half of the jars in white mesh to achieve the lowest light treatment (30 µmol photons m−2 s−1), the remaining jars were uncovered and received the highest light-level treatment (60 µmol photons m−2 s−1). Light levels were measured using a LI-COR Light Meter (LI-250A) with a spherical quantum sensor attached. Media and culture jars were changed twice per week, after which the jars were randomly placed back into the corresponding tub. All jars, lids, and culture media were UV sterilized in a laminar flow unit for 60 minutes prior to each media change.
Sporophyte length, density, and contamination measurements
At the end of the experiment, the length of each sporophyte (measured from holdfast to distal end of the thallus) and density of the sporophytes on the twine was measured using the ImageJ software. This was done by analysing images taken with the aid of a stereo microscope (Leica, M165 C) fitted with a camera, of a 10 cm section of twine carefully removed from the middle section of each spool. To assess contamination, each spool was randomly divided into thirds around its circumference, a photo from top to bottom was taken of each third, and a 1 cm column in the centre of each image given a contamination score (0–5; where zero is no contamination and five is 100% covered). This gave three scores per spool, which were averaged to represent the contamination score of the whole spool. A more detailed illustration of the sampling design can be found in Supplementary fig. S2.
Statistical analysis
All analyses were conducted using R software (R Core Team, Citation2018). The individual and interactive effect of temperature (fixed factor, three levels) and light level (fixed factor, two levels) on sporophyte length and density, and level of contamination were statistically analysed for each of the three species. Sporophyte length and density were analysed with two-factor analysis of variance (ANOVA) using the lm function. When significant, multiple comparisons were performed with a Student–Newman–Keuls (SNK) post hoc test (α = 0.05), using the SNK.test function of the agricolae package (version 1.2–8) (Mendiburu de Fy, Citation2020). Data were checked for violations of ANOVA assumptions (normality and homoscedasticity), and the best suited normalizing transformation was estimated using the bestNormalize package (Peterson, Citation2017), data was transformed where necessary. Contamination level was analysed with a non-parametric two-way ANOVA Scheirer–Ray–Hare test, using the scheirerRayHare function of the rcompanion package (version 2.4.15) (Mangiafico, Citation2022). When significant, pairwise comparisons were performed with a Dunn’s post-hoc test (α = 0.05), using the dunnTest function of the FSA package (version 0.9.3) (Ogle, Wheeler, & Dinno, Citation2022) with p value adjustment using the Bonferroni correction.
Results
Sporophyte length
The length of the sporophytes of L. corrugata was significantly affected by temperature, with a negative relationship between sporophyte length and increasing temperature (, ), and there was no effect of light level. Both E. radiata and M. pyrifera were interactively affected by temperature and light level. E. radiata sporophytes grew longer at 15°C and 18°C, compared to 12°C. The culture condition that resulted in the largest E. radiata sporophytes was 15°C and 30 µmol photons m−2 s−1. At 12°C, the 30 µmol photons m−2 s−1 treatment resulted in longer E. radiata sporophytes compared to 60 µmol m−2 s−1. M. pyrifera sporophytes were significantly longer at 12°C independent of light level and 15°C at 30 µmol photons m−2 s−1, compared to 15°C at 60 µmol photons m−2 s−1 and 18°C independent of light level. The maximum sporophyte length obtained after 34 days in the nursery was (mean ± SE, average n = 308) 0.60 ± 0.02 mm, 1.04 ± 0.04 mm, 0.60 ± 0.01 mm for E. radiata, L. corrugata, and M. pyrifera, respectively (Supplementary table S1).
Figure 1. Bar plots of (a) sporophyte length and (b) sporophyte density on the seeded twine. Letters above the bars indicate significant differences based on the SNK-test (p < 0.05). Error bars show SEM, with n = 3.
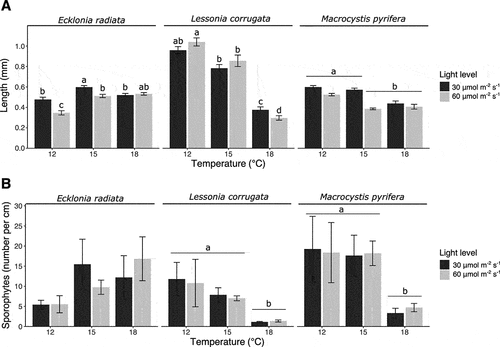
Table 1. Sporophyte length per species. Summary of two-way ANOVA of the mean sporophyte length (mm) exposed to three temperatures (12°C, 15°C, and 18°C) and two light levels (30 and 60 μmol photons m−2 s−1), followed by a Student–Newman–Keuls (SNK) post-hoc test.
Sporophyte density
Sporophyte density in the twine of both L. corrugata and M. pyrifera was significantly affected by temperature, with a negative relationship between sporophyte length and increasing temperature (, ). Light level had no significant effect on the sporophyte density of L. corrugata and M. pyrifera. The sporophyte density of E. radiata was not significantly affected by temperature and light level. The maximum sporophyte density per cm of line after 34 days in the nursery (mean ± SE, n = 3) was 16.8 ± 6.2, 11.8 ± 4.1, and 19.3 ± 8.1 for E. radiata, L. corrugata, and M. pyrifera, respectively (Supplementary table S1).
Table 2. Sporophyte density per species. Summary of two-way ANOVA for the main and interactive effect of the mean number of sporophytes per cm of line, followed by a Student–Newman–Keuls (SNK) post-hoc test.
Contamination score
There was a significant positive relationship between contamination score on the spools and temperature for all three species (, , Supplementary table S1). Pairwise comparisons using Dunn’s test indicated that the contamination scores at 12°C were significantly lower from those at 18°C for E. radiata and L. corrugata, but no other differences were statistically significant (Suplementary table S2). For M. pyrifera, we found significantly lower contamination scores at 12°C compared to 15°C and 18°C, and no significant difference between 15°C and 18°C. We were unable to provide a detailed taxonomic description of the contaminants, but cyanobacteria and Ectocarpus spp. appeared to be the most dominant.
Figure 2. Boxplots of contamination score (between 0–5) for three kelp species. One asterisk (*) indicates a p value smaller than 0.05. Two asterisks (**) indicate a p value smaller than 0.01 as per Dunn’s post-hoc test.
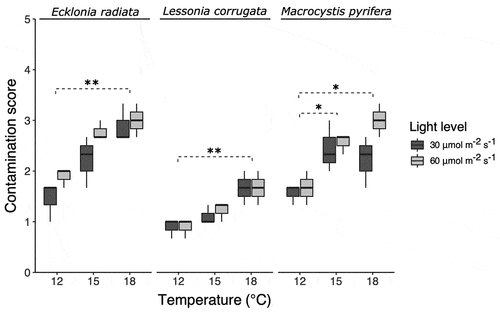
Table 3. Contamination score per species. Scheirer–Ray–Hare test results for the main and interactive effects of a two-way non-parametric ANOVA.
Discussion
Our findings suggest that understanding the effects of temperature and light levels are key to optimizing sporophyte growth of Australian kelp species (E. radiata, L. corrugata, and M. pyrifera). However, contaminants also need to be taken into account to ensure the best outcomes for nursery production. Temperature and light levels separately and interactively affect the growth and density of juvenile sporophytes, but contamination that accumulated over the 34-day culture period markedly affected the culture outcomes. For all three study species, temperature was a stronger driver for maximizing success in the nursery than light level. Growth maxima were at lower temperatures and light levels than previously reported for all three study species (Camus & Buschmann, Citation2017; Paine et al., Citation2021; tom Dieck, Citation1993; Wernberg et al., Citation2019). However, this lower temperature optima could be confounded by an increased level of contamination at higher temperatures, where more contamination leads to reduced growth, rather than a higher temperature per se.
For E. radiata, nursery conditions of 15°C and 30 µmol m−2 s−1 resulted in the longest sporophytes, relatively high sporophyte density in the twine, and a relatively low level of contamination. Our results suggest a thermal growth optimal for juvenile E. radiata sporophytes from Tasmanian of around 15°C, although a thermal response curve is needed to confirm this. In southern New Zealand (45°S) growth and productivity of mature E. radiata sporophytes was maximum in late winter/spring and correlated strongly with seawater temperature which is typically between 12°C and 14°C (Goodwin & Cornelisen, Citation2012; Miller, Hurd, & Wing, Citation2011). This contrasts with E. radiata sporophytes from lower latitudes (28°S–35°S), which have a higher optimal temperature (~24°C) for photosynthesis (Wernberg et al., Citation2019; Wernberg, de Bettignies, Joy, & Finnegan, Citation2016) that is a few degree Celsius higher than the thermal optimum for growth in most seaweeds (Hurd, Harrison, Bischof, & Lobban, Citation2014). Overall, temperature was found to be a stronger driver for maximizing the success of the nursery production for E. radiata compared to light level.
The optimal nursery condition for Lessonia corrugata was 12°C and 60 µmol m−2 s−1, with maximal growth and sporophyte density, and least contaminants in the spools. Similar to E. radiata, the optimal temperature was colder than expected based on the previously observed growth optimum between 15.7°C and 17.9°C (Paine et al., Citation2021) or other Lessonia species (Nelson, Citation2005; Oppliger et al., Citation2012; Tom Dieck, Citation1993). It is, however, within the range of its natural conditions in Tasmania. Furthermore, Paine et al. (Citation2021) found that L. corrugata gametophytes cultured from zoospores that were sampled in late autumn, grew maximally around 70 µmol photons m−2 s−1 which is broadly consistent with the highest experimental light treatment (60 µmol photons m−2 s−1) used in the present study. L. corrugata has a relatively high genetic diversity compared to the other two species in the study region (Durrant, Barrett, Edgar, Coleman, & Burridge, Citation2015), which provides opportunities to further explore the effect of high performing Tasmanian ecotypes with potentially improved growth at higher temperatures and irradiance.
M. pyrifera had a maximum sporophyte growth and density, and minimal contamination at 12°C and 30 or 60 µmol photons m−2 s−1. Our findings largely corroborate Camus & Buschmann (Citation2017) who found that the optimal nursery conditions for Chilian M. pyrifera were 12°C and 12 μmol photons m−2 s−1 and a long-day photoperiod (16 h light : 8 h dark). The lower optimal light level might be because gametophyte development can occur in a relatively low light environment (~5 μmol photons m−2 s−1), and a temperature between 11°C and 19°C (Deysher & Dean, Citation1984, Citation1986). Interestingly, even though Camus & Buschmann (Citation2017) seeded the spools with zoospores, they found 0.4 to 0.5 cm long sporophytes within 45 days. We found that the longest M. pyrifera sporophytes reached an average length of only 0.06 cm after 34 days, which suggests that contamination, different genotypes, or other potential differences in culture condition may have negatively affected sporophyte growth.
After 34 days in culture, contamination was observed in all experimental treatments, despite having undertaken significant preventive measures against epiphytic and epizoic contaminants, including cleaning the sorus tissue prior to zoospore release (Alsuwaiyan et al., Citation2019), filtering and UV-treatment of culture media necessary to support growth, filtering the air used in mixing of the culture medium, sterilizing the culture-ware, and working in a sterile laminar-flow cabinet for production and culture of both the free-floating gametophytes throughout the experiment. The source of the epiphytic and epizoic contamination was not identified, but we observed a trend of a lower contamination at the lowest light level (30 μmol photons m−2 s−1) compared with the high light-level treatment (60 μmol photons m−2 s−1). This partially corroborates with Su, Pang, Shan, & Li (Citation2017) who noted reduced contamination when the light level was limited to below 20–25 μmol photons m−2 s−1. This could, however, be confounded by the relatively low level of nutrients used in their study (8–11.8 μmol l−1 NO3 and 0.7–1.5 μmol l−1 PO4) compared to nutrient levels in the present study (F/2-medium: 882 μmol l−1 NO3 and 36.2 μmol l−1 PO4). In this study, we did not take any reactive measures to reduce or manage contaminants throughout the course of the experiment. Both proactive and reactive decontamination methods may be improved using a combination of treatments; mechanical removal of epiphytic contaminants (Alsuwaiyan et al., Citation2019; Su, Pang, Shan, & Li, Citation2017) may be used alongside chemical treatment (Guillard, Citation2005; Rød, Citation2012) and/or an adjustment of culture conditions (e.g., temperature, light level, or water motion) (Su, Pang, Shan, & Li, Citation2017) to discriminate against the contaminant and favour juveniles of the target kelp species.
Conclusions
Our results highlight the importance of species-specific culture conditions in the nursery phase of kelp aquaculture production systems. The results could furthermore be used to reduce contamination in juvenile sporophyte cultures and provide a framework to find growth optima for seaweeds in non-traditional regions of cultivation, such as the Tasmanian kelp species examined in this study. In addition to temperature and light level, future work could focus on the light quality (i.e., spectrum) (Lüning & Dring, Citation1975), nutrients (Fernández et al., Citation2020; Gao, Endo, Nagaki, & Agatsuma, Citation2016), hydrodynamics (Camus & Buschmann, Citation2017; Peteiro, Bidegain, & Sánchez, Citation2019), interactive effects of environmental variables (Camus & Buschmann, Citation2017; Gao, Endo, Nagaki, & Agatsuma, Citation2017; Mabin, Johnson, & Wright, Citation2019), and varying culture conditions following optimal culture conditions of each life-stage (i.e., zoospore, gametophyte, or juvenile sporophyte) throughout the nursery phase (Gerard, Citation1997; Su, Pang, Shan, & Li, Citation2017). Given the gametophyte stock-cultures of all the species used in the present study have been kept at ~12°C under laboratory conditions for >6 months, an additional concern may be the potential for drifting sex-ratios to negatively affect reproductive success (Ebbing et al., Citation2021) and a more narrow temperature optimum compared to in nature (Hurd, Harrison, Bischof, & Lobban, Citation2014). The potential beneficial effects of an acclimation period to the environmental conditions at-sea prior to deployment when using vegetatively propagated gametophytes in seaweed nurseries needs further examination (Fernández et al., Citation2020; Gauci, Bartsch, Martins, & Liesner, Citation2022). Finally, our study highlights the importance of optimized culture conditions in the nursery phase for a more efficient seaweed aquaculture production process.
Supplemental Material
Download MS Word (1.7 MB)Acknowledgements
We appreciate the support of the partners within the “Seaweed solutions for sustainable aquaculture CRC Project” (CRCPSIX000144), in particular Catriona Macleod and Alecia Bellgrove for their comments on the manuscript.
Disclosure statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Cost for sampling and analyses reports financial support was provided by Australian Government Fisheries Research and Development Corp. The corresponding author W. Visch is currently employed as a post-doctoral researcher in the Seaweed Solutions for Sustainable Aquaculture CRC-P project, in partnership with Tassal Group Ltd. and Spring Bay Seafood Pty Ltd.
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/26388081.2023.2174903
Additional information
Funding
References
- Alsuwaiyan, N. A., Mohring, M. B., Cambridge, M., Coleman, M. A., Kendrick, G. A., & Wernberg, T. (2019). A review of protocols for the experimental release of kelp (Laminariales) zoospores. Ecology and Evolution, 9, 8387–8398. doi:10.1002/ece3.5389
- Bartsch, I. (2018). Derivation of clonal stock cultures and hybridization of kelps. In B. Charrier, T. Wichard, & C. Reddy (Eds.), Protocols for Macroalgae research (pp. 61–78). New York, NY: CRC Press.
- Bennett, S., Wernberg, T., Connell, S. D., Hobday, A. J., Johnson, C. R., & Poloczanska, E. S. (2015). The ‘Great Southern Reef’: Social, ecological and economic value of Australia’s neglected kelp forests. Marine & Freshwater Research, 67, 47–56. doi:10.1071/MF15232
- Biancacci, C., Sanderson, J. C., Evans, B., Callahan, D. L., Francis, D. S., Skrzypczyk, V. M. … Bellgrove, A. (2022). Nutritional composition and heavy metal profiling of Australian kelps cultured in proximity to salmon and mussel farms. Algal Research, 64, 102672. doi:10.1016/j.algal.2022.102672
- Biancacci, C., Visch, W., Callahan, D. L., Farrington, G., Francis, D. S., Lamb, P. … Bellgrove, A. (2022). Optimisation of at-sea culture and harvest conditions for cultivated Macrocystis pyrifera: Yield, biofouling and biochemical composition of cultured biomass. Frontiers in Marine Science, 9, 9. doi:10.3389/fmars.2022.951538
- Camus, C., & Buschmann, A. H. (2017). Macrocystis pyrifera aquafarming: Production optimization of rope-seeded juvenile sporophytes. Aquaculture, 468, 107–114. doi:10.1016/j.aquaculture.2016.10.010
- Chopin, T., & Tacon, A. G. J. (2021). Importance of seaweeds and extractive species in global aquaculture production. Reviews in Fisheries Science & Aquaculture, 29, 139–148. doi:10.1080/23308249.2020.1810626
- Deysher, L. E., & Dean, T. A. (1984). Critical irradiance levels and the interactive effects of quantum irradiance and dose on gametogenesis in the giant kelp, Macrocystis pyrifera. Journal of Phycology, 20, 520–524. doi:10.1111/j.0022-3646.1984.00520.x
- Deysher, L. E., & Dean, T. A. (1986). In situ recruitment of sporophytes of the giant kelp, Macrocystis pyrifera (L.) C.A.Agardh: Effects of physical factors. Journal of Experimental Marine Biology and Ecology, 103, 41–63. doi:10.1016/0022-0981(86)90131-0
- Durrant, H. M., Barrett, N. S., Edgar, G. J., Coleman, M. A., & Burridge, C. P. (2015). Shallow phylogeographic histories of key species in a biodiversity hotspot. Phycologia, 54, 556–565. doi:10.2216/15-24.1
- Ebbing, A. P. J., Fivash, G. S., Martin, N. B., Pierik, R., Bouma, T. J., Kromkamp, J. C., & Timmermans, K. (2021). In-culture selection and the potential effects of changing sex ratios on the reproductive success of multiannual delayed gametophytes of Saccharina latissima and Alaria esculenta. Journal of Marine Science and Engineering, 9, 1250. doi:10.3390/jmse9111250
- Edwards, M., O’mahony, F., Connellan, I., Dring, M., & Werner, A. (2011). Aquaculture explained: Cultivating Laminaria digitata. Retrieved from https://bim.ie/wp-content/uploads/2021/02/BIM,Aquaculture,Explained,Issue,26,Cultivating,Laminaria,digitata.pdf
- Fernández, P. A., Gaitán-Espitia, J. D., Leal, P. P., Schmid, M., Revill, A. T., & Hurd, C. L. (2020). Nitrogen sufficiency enhances thermal tolerance in habitat-forming kelp: Implications for acclimation under thermal stress. Scientific Reports, 10, 1–12. doi:10.1038/s41598-020-60104-4
- Flavin, K., Flavin, N., & Flahive, B. (2013). Kelp farming manual. A guide to the processes, techniques and equipment for farming kelp in New England waters. Ocean Approved LLC. Retrieved from https://maineaqua.org/wp-content/uploads/2020/06/OceanApproved_KelpManualLowRez.pdf
- Forbord, S., Steinhovden, K. B., Rød, K. K., Handå, A., & Skjermo, J. (2018). Cultivation protocol for Saccharina latissima. In B. Charrier, T. Wichard, & C. Reddy (Eds.), Protocols for Macroalgae research (pp. 37–59). New York, NY, USA: CRC Press.
- Gao, X., Endo, H., Nagaki, M., & Agatsuma, Y. (2016). Growth and survival of juvenile sporophytes of the kelp Ecklonia cava in response to different nitrogen and temperature regimes. Fisheries Science, 82, 623–629. doi:10.1007/s12562-016-0998-4
- Gao, X., Endo, H., Nagaki, M., & Agatsuma, Y. (2017). Interactive effects of nutrient availability and temperature on growth and survival of different size classes of Saccharina japonica (Laminariales, Phaeophyceae). Phycologia, 56, 253–260. doi:10.2216/16-91.1
- Gauci, C., Bartsch, I., Martins, N., & Liesner, D. (2022). Cold thermal priming of Laminaria digitata (Laminariales, Phaeophyceae) gametophytes enhances gametogenesis and thermal performance of sporophytes. Frontiers in Marine Science, 9, 862923. doi:10.3389/fmars.2022.862923
- Gerard, V. A. (1997). Environmental stress during early development of kelp sporophytes (Laminaria saccharina): How long do effects persist? Journal of Applied Phycology, 9, 5–9. doi:10.1023/A:1007952030505
- Goodwin, E., & Cornelisen, C. (2012). Near-surface water temperatures in Doubtful Sound and response to natural and anthropogenic drivers. New Zealand Journal of Marine and Freshwater Research, 46, 411–429. doi:10.1080/00288330.2012.697071
- Guillard, R. R. (2005). Purification methods for microalgae. In R. Andersen (Ed.), Algal culturing techniques (pp. 117–132). San Diego, CA: Elsevier Academic Press.
- Guillard, R. R., & Ryther, J. H. (1962). Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Canadian Journal of Microbiology, 8, 229–239. doi:10.1139/m62-029
- Gutierrez, A., Correa, T., Munoz, V., Santibanez, A., Marcos, R., Cáceres, C., & Buschmann, A. H. (2006). Farming of the giant kelp Macrocystis pyrifera in southern Chile for development of novel food products. Journal of Applied Phycology, 18, 259–267. doi:10.1007/978-1-4020-5670-3_5
- Holdt, S., & Kraan, S. (2011). Bioactive compounds in seaweed: Functional food applications and legislation. Journal of Applied Phycology, 23, 543–597. doi:10.1007/s10811-010-9632-5
- Hurd, C. L., Harrison, P. J., Bischof, K., & Lobban, C. S. (2014). Seaweed ecology and physiology (2nd ed.). Cambridge, UK: Cambridge University Press.
- Hu, Z. M., Shan, T. F., Zhang, J., Zhang, Q. S., Critchley, A. T., Choi, H. G. … Duan, D. L. (2021). Kelp aquaculture in China: A retrospective and future prospects. Reviews in Aquaculture, 13, 1324–1351. doi:10.1111/raq.12524
- Kelly, J. (2020). Australian seaweed industry blueprint: A blueprint for growth. Publication No. 20-072. AgriFutures, Australia. https://www.agrifutures.com.au/wp-content/uploads/2020/09/20-072.pdf
- Kerrison, P. D., Le, H. N., & Hughes, A. D. (2016). Hatchery decontamination of Sargassum muticum juveniles and adults using a combination of sodium hypochlorite and potassium iodide. Journal of Applied Phycology, 28, 1169–1180. doi:10.1007/s10811-015-0672-8
- Kim, J. K., Yarish, C., Hwang, E. K., Park, M., & Kim, Y. (2017). Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. Algae, 32, 1–13. doi:10.4490/algae.2017.32.3.3
- Layton, C., & Johnson, C. R. (2021). Assessing the feasibility of restoring giant kelp forests in Tasmania. Report to the national environmental science program, marine biodiversity hub. Institute for Marine and Antarctic Studies, University of Tasmania. Retrieved from https://www.nespmarine.edu.au/system/files/Layton%20et%20al_E7_M5_Assessing%20the%20feasibility%20of%20restoring%20giant%20kelp%20forests%20in%20Tas.pdf
- Lüning, K., & Dring, M. (1975). Reproduction, growth and photosynthesis of gametophytes of Laminaria saccharina grown in blue and red light. Marine Biology, 29, 195–200. doi:10.1007/BF00391846
- Mabin, C. J., Gribben, P. E., Fischer, A., & Wright, J. T. (2013). Variation in the morphology, reproduction and development of the habitat-forming kelp Ecklonia radiata with changing temperature and nutrients. Marine Ecology Progress Series, 483, 117–131. doi:10.3354/meps10261
- Mabin, C. J., Johnson, C. R., & Wright, J. T. (2019). Physiological response to temperature, light, and nitrates in the giant kelp Macrocystis pyrifera from Tasmania, Australia. Marine Ecology Progress Series, 614, 1–19. doi:10.3354/meps12900
- Mangiafico, S. (2022). Rcompanion: Functions to support extension education program evaluation.
- Mendiburu de Fy, M. (2020). Agricolae: Statistical procedures for agricultural research.
- Miller, S. M., Hurd, C. L., & Wing, S. R. (2011). Variations in growth, erosion, productivity, and morphology of Ecklonia radiata (Alariaceae; Laminariales) along a fjord in southern New Zealand. Journal of Phycology, 47, 505–516. doi:10.1111/j.1529-8817.2011.00966.x
- Mooney-McAuley, K. M., Edwards, M. D., Champenois, J., & Gorman, E. (2016, June). Best Practice Guidelines for Seaweed Cultivation and Analysis, Public Output report of the EnAlgae project, Swansea, p. 36. doi:10.25607/OBP-792
- Naylor, R. L., Hardy, R. W., Buschmann, A. H., Bush, S. R., Cao, L., Klinger, D. H. … Troell, M. (2021). A 20-year retrospective review of global aquaculture. Nature, 591, 551–563. doi:10.1038/s41586-021-03308-6
- Nelson, W. (2005). Life history and growth in culture of the endemic New Zealand kelp Lessonia variegata J.Agardh in response to differing regimes of temperature, photoperiod and light. Journal of Applied Phycology, 17, 23–28. doi:10.1007/s10811-005-5521-8
- Nielsen, M. M., Kumar, J. P., Soler-Vila, A., Johnson, M. P., & Bruhn, A. (2016). Early stage growth responses of Saccharina latissima spores and gametophytes. Part 1: Inclusion of different phosphorus regimes. Journal of Applied Phycology, 28, 387–393. doi:10.1007/s10811-015-0547-z
- Novaczek, I. (1984). Response of gametophytes of Ecklonia radiata (Laminariales) to temperature in saturating light. Marine Biology, 82, 241–245. doi:10.1007/BF00392405
- Ogle, D. H. D., Wheeler, P., & Dinno, A. (2022). FSA: Fisheries Stock Analysis.
- Oppliger, L. V., Correa, J. A., Engelen, A. H., Tellier, F., Vieira, V., Faugeron, S. … Destombe, C. (2012). Temperature effects on gametophyte life-history traits and geographic distribution of two cryptic kelp species. Plos One, 7, e39289. doi:10.1371/journal.pone.0039289
- Paine, E. R., Schmid, M., Gaitán-Espitia, J. D., Castle, J., Jameson, I., Sanderson, J. C., & Hurd, C. L. (2021). Narrow range of temperature and irradiance supports optimal development of Lessonia corrugata (Ochrophyta) gametophytes: Implications for kelp aquaculture and responses to climate change. Journal of Applied Phycology, 33, 1721–1730. doi:10.1007/s10811-021-02382-7
- Peteiro, C., Bidegain, G., & Sánchez, N. (2019). Experimental evaluation of the effect of water velocity on the development of string-attached kelp seedlings (Laminariales) with implications for hatchery and nursery production. Algal Research, 44, 101678. doi:10.1016/j.algal.2019.101678
- Peterson, R. (2017). Estimating normalization transformations with bestNormalize.
- Ratcliff, J., Soler-Vila, A., Hanniffy, D., Johnson, M., & Edwards, M. (2017). Optimisation of kelp (Laminaria digitata) gametophyte growth and gametogenesis: Effects of photoperiod and culture media. Journal of Applied Phycology, 29, 1957–1966. doi:10.1007/s10811-017-1070-1
- Redmond, S., Green, L., Yarish, C., Kim, J., & Neefus, C. (2014). New England seaweed culture handbook-nursery systems. Connecticut Sea Grant CTSG-14-01. Retrieved from http://seagrant.uconn.edu/publications/aquaculture/handbook.pdf
- Rød, K. K. (2012). Sori disinfection in cultivation of saccharina latissima: Evaluation of chemical treatments against diatom contamination. Trondheim, Norway: NTNU, Department of Biology. https://ntnuopen.ntnu.no/ntnu-xmlui/handle/11250/245060
- Rolin, C., Inkster, R., Laing, J., Hedges, J., & McEvoy, L. (2016). Seaweed Cultivation Manual. Shetland Seaweed Growers Project 2014-16. NAFC Marine Centre. Retrieved from https://oceansalaska.org/wp-content/uploads/2021/06/Seaweed-Cultivation-Manual.pdf
- Schiel, D. R., & Foster, M. S. (2015). The biology and ecology of giant kelp forests. Oakland, CA: University of California Press.
- Scott, F. (2017). Marine plants of Tasmania. Hobart, TAS: Tasmanian Herbarium, Tasmanian Museum and Art Gallery.
- Steneck, R. S., Graham, M. H., Bourque, B. J., Corbett, D., Erlandson, J. M., Estes, J. A., & Tegner, M. J. (2002). Kelp forest ecosystems: Biodiversity, stability, resilience and future. In Environmental conservation (Vol. 29, vol. 04, pp. 436–459). Cambridge, UK: Cambridge University Press. doi:10.1017/S0376892902000322.
- Suebsanguan, S., Strain, E. M., Morris, R. L., & Swearer, S. E. (2021). Optimizing the initial cultivation stages of kelp Ecklonia radiata for restoration. Restoration Ecology, 29, e13388. doi:10.1111/rec.13388
- Su, L., Pang, S. J., Shan, T. F., & Li, X. (2017). Large-scale hatchery of the kelp Saccharina japonica: A case study experience at Lvshun in northern China. Journal of Applied Phycology, 29, 3003–3013. doi:10.1007/s10811-017-1154-y
- Tatsumi, M., Mabin, C. J., Layton, C., Shelamoff, V., Cameron, M. J., Johnson, C. R., & Wright, J. T. (2022). Density‐dependence and seasonal variation in reproductive output and sporophyte production in the kelp, Ecklonia radiata. Journal of Phycology, 58, 92–104. doi:10.1111/jpy.13214
- Team, R. C. (2018). R: A Language and Environment for Statistical Computing.
- Tom Dieck, I. (1993). Temperature tolerance and survival in darkness of kelp gametophytes (Laminariales, Phaeophyta): Ecological and biogeographical implications. Marine Ecology Progress Series, 100, 253–253. doi:10.3354/meps100253.
- Wang, W.-J., Sun, X.-T., Wang, G.-C., Xu, P., Wang, X.-Y., Lin, Z.-L., & Wang, F.-J. (2010). Effect of blue light on indoor seedling culture of Saccharina japonica (Phaeophyta). Journal of Applied Phycology, 22, 737–744. doi:10.1007/s10811-010-9514-x
- Wernberg, T., Coleman, M. A., Babcock, R. C., Bell, S. Y., Bolton, J. J., Connell, S. D. &, and Shears, N. T. (2019). Biology and ecology of the globally significant kelp Ecklonia radiata. Oceanography and Marine Biology: An annual review Eds: Gibson, R.N Atkinson, R. J. A. Gordon, J. D. M. (Vol. 57, pp. 265–324). Boca Raton, Florida, USA: CRC Press, Boca Raton, Florida, USA.
- Wernberg, T., de Bettignies, T., Joy, B. A., & Finnegan, P. M. (2016). Physiological responses of habitat‐forming seaweeds to increasing temperatures. Limnology and Oceanography, 61, 2180–2190. doi:10.1002/lno.10362
