ABSTRACT
A simple method for preparing chelated calcium from black liquor produced in the paper industry was studied. Various factors influencing the chelation rate of Ca2+ were studied, including the system pH, reaction temperature, reaction time, reaction solution ratio. The optimum reaction conditions are as follows: the system pH, reaction temperature, reaction solution ratio (V(CaCl2):m (Black liquor)) and time were 5.6, 25°C, 1:20 and 60 min, respectively. The maximum Ca2+ chelation rate was 88.75%. The characteristics of chelated calcium fertilizer in black liquor were studied by Fourier transform infrared, scanning electron microscope . The results indicated that the CaCl2 could be chelated by the black liquor from paper-making industry, and showed a high chelating rate.
1. Introduction
Calcium ion is an important alkaline-earth metal ion in the biological system [Citation1]. It plays an important role in the proliferation and differentiation of animal osteoblasts and the composition of the plant cell wall [Citation2–Citation4]. In addition, calcium ions are difficult to absorb by plants or animals and must be transported into the cell in the form of chelating [Citation5,Citation6]. In order to improve the utilization absorption rate of calcium, chelating method was adopted. Ethylenediaminetetraacetic acid and amino acid are ideal carrier of Ca2+ due to their strong chelating ability [Citation7,Citation8]. However, due to the amount and cost of chelating agents, the range of their use is limited, especially in agriculture, where high costs do not allow them to be used in crops with low economic value. Therefore, it is necessary to find a widely used and cheap chelating agent to complete the calcium chelating, so as to promote the application in low production value field. Sodium lignosulfonate is a widely used chelating biological materials agent and its costs was low [Citation9]. In the paper industry, a large amount of sodium lignosulfonate will remain in the paper-making black liquor, which will be discharged into the environment along with the wastewater discharge, which will cause serious environmental pollution [Citation10,Citation11]. The application of sodium lignosulfonate in black liquor can’t only recover waste liquor, but also reduce pollutant emission effectively. In this study, the black liquor generated by the pulp and paper industry as raw material, through a simple chelating of CaCl2, we prepared one kind of chelated calcium. The chelating condition was investigated to explore the optimum chelating conditions for chelated calcium based on the black liquor.
2. Materials and methods
Anhydrous calcium chloride, NaOH and HCl were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The black liquor (pH = 5.6) was provided by Shandong Tranlin Group Co., Ltd. (Shandong, China). FTIR spectrum was obtained by NEXUS-470 series FTIR spectrometer (Thermo Nicolet, NEXUS). The pellets used for FTIR analysis were dried at 120°C and prepared by mixing powder of samples and KBr. The concentration of Ca2+ in solution was determined by calcium ion apparatus (Shanghai Haiheng Electrical and Mechanical Instrument Co., Ltd., CA-1) in a previous study [Citation12,Citation13]. The scanning electron microscope was employed to predict the characterization of the Black liquor and Black liquor-Ca (Hitachi SU8010 SEM 5.0 kV). The chelation rate is calculated as A = [1 − Y2/(Y1 + Y3)] × 100%, where, Y1 and Y2 are the free calcium ion content of original black liquid and reaction chelating liquid product, Y3 represents the added calcium content. A series of samples were prepared via the following procedure. Firstly, 500 mL black liquor was added to a 1000 mL four-necked flask equipped with a mechanical stirrer, thermometer and a nitrogen tube. Calcium ion electrode was used to determine the content of free calcium in the black liquor. The pH value was adjusted to the set value by 0.1 M HCl or 0.1 M NaOH. The black liquor was heated by a water bath at a set temperature under nitrogen atmosphere. After being purged with nitrogen for 10 min to remove the dissolved oxygen from the system, a certain amount of 0.67 M CaCl2 was added dropwise with dropping funnel and allowed to react at the set temperature. After the reaction was completed, the free calcium ion content in the reaction solution was determined by calcium ion electrode, and the reaction liquid was dried in a 105°C vacuum drying oven for use.
3. Results and discussion
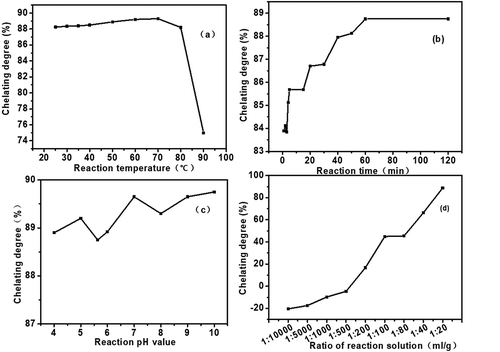
In order to find the appropriate chelating conditions of CaCl2 with the black liquor, an orthogonal experiment with four factors and four levels was conducted. ‘A’ stands for pH of the black liquor reaction system, which has four levels of 4, 6, 7, 10 ‘B’, ‘C’ and ‘D’ stand for V(CaCl2):m(Black liquor) (mL/g), reaction temperature and reaction time, respectively. According to the orthogonal array of L16(4)4 presented in , the result of the range analysis indicated that the influencing order of each factor on the Ca2+ chelating rate was B>D>C>A. The CaCl2 to black liquor ratio is the most important factor, the reaction temperature followed, and then reaction system pH, reaction time are the last. According to the results of the orthogonal experiment, the optimal combination A1B1C3D4 was obtained, which corresponded to the optimal synthesis conditions: reaction system pH = 5.6, V(CaCl2):m (Black liquor) = 1:20, 50°C reaction temperature and 60 min reaction time.
Table 1. The orthogonal L16(4)4 experiment of system pH, V(CaCl2):m(Black liquor) (mL/g), reaction temperature and reaction time (A: system pH; B: V(CaCl2):m(Black liquor); C: reaction temperature; D: reaction time).
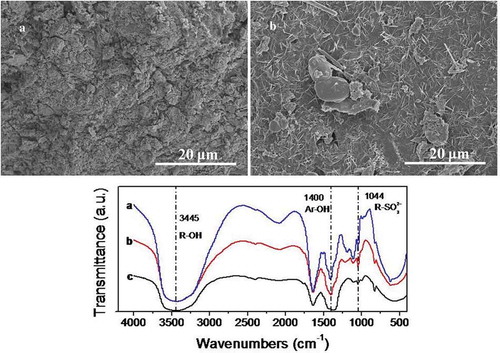
The FTIR spectra of sodium lignosulphonate, Black liquor and Black liquor-Ca are shown in . The predominant absorbance peaks at 3445, 1400 and 1044 cm−1 can be assigned to the R-OH, Ar-OH, R-SO32- stretching, respectively [Citation14]. It can be found that the three substances have similar absorption peaks, and the peak at 1400 cm−1 was markedly attenuated and almost disappeared, which showed that Ca2+ had been translated into Black liquor, and the main component of the black liquor is sodium lignosulphonate. SEM also indicated that the black liquor had a chelating reaction with Ca2+, and a large amount of crystal was attached to it ()).
Figure 1. SEM of (A) Black liquor, (B) Black liquor-Ca, and FI-IR spectra of (a) Sodium lignosulphonate, (b) Black liquor, (c) Black liquor-Ca.
In order to obtain the maximum chelating rate, the effect of a single factor on chelating rate was studied by changing the single factor based on the optimization results of orthogonal experiment. ) shows that the chelation rate of Ca2+ increased with the increase of reaction temperature from 25°C to 70°C, and then decreased with further increasing temperature to 90°C. This phenomenon is attributed to the fact that the decomposition rate of chelating degree is greater than that of chelating degree with the increase of temperature. The Ca2+ chelating rate increased with time, and the chelating rate didn’t change after 60 min ()), which was mainly due to the fact that the chelating sites are all occupied by Ca2+. ) indicates that the increase of pH was beneficial to the improvement of chelating rate, but the effect was not significant. Besides, it can be found that the volume ratio of CaCl2 to Black liquor has a significant effect on the Ca2+ chelating ((d)), and the maximum Ca2+ chelating rate was 88.75% when the ratio of CaCl2 to Black liquor was 1:20. However, there was a negative adsorption occurs when the ratio of CaCl2 to Black liquor was less than 1:500. High concentration of Ca2+ was helpful to promote the formation of Black liquor-Ca, which was mainly due to the balance between Ca2+ chelating and resolving balance in Black liquor.
4. Conclusion
In this paper, a simple method for the preparation of chelated calcium based on the black liquor generated by the pulp and paper industry was reported. The optimal conditions were 25°C reaction temperature, 60 min reaction time, the system pH = 5.6(Original)and V(CaCl2): m (Black liquor) = 1:20. The maximum Ca2+ chelating rate was 88.75%. The simple Ca2+ chelation method not only had solves the problem of black liquor pollution, but also greatly reduced the cost of preparation. So the Ca-black liquor has great application prospect in agriculture.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Medvedev S S, MarkovaI V. Role of calcium ions in plant growth and mechanism of IAA action. In: Macháčková I., Romanov G. A. (eds) Phytohormones in plant biotechnology and agriculture. Dordrecht, Netherlands: Springer. 2003; p. 157–169.
- Torvisco A, Anna Y. O’Brien, and Ruhlandt-Senge K. Advances in alkaline earth-nitrogen chemistry. Coord Chem Rev. 2011;255(11–12):1268–1292.
- Burgess J, Raven E. Calcium in biological systems. Adv Inorg Chem. 2009;18(1):29–124.
- R K S, Pyle AM. Lanthanide ions as probes for metal ions in the structure and catalytic mechanism of ribozymes. Met Ions Biol Syst. 2003;677(40):477–512.
- Y F L. Aarts M G M. The molecular mechanism of zinc and cadmium stress response in plants. Cell Mol Life Sci. 2012;69(19):3187–3206.
- Peng Z, Hou H, Zhang K, et al. Effect of calcium-binding peptide from Pacific cod (Gadus macrocephalus) bone on calcium bioavailability in rats. Food Chem. 2017;221:373–378.
- Ringwald J, Zingsem J, Zimmermann R, et al. First comparison of productivity and citrate donor load between the Trima version 4 (dual-stage filler) and the Trima Accel (single-stage filler) in the same donors. Vox Sang. 2010;85(4):267–275.
- Barri T, Holmer-Jensen J, Hermansen K, et al. Metabolic fingerprinting of high-fat plasma samples processed by centrifugation- and filtration-based protein precipitation delineates significant differences in metabolite information coverage. Anal Chim Acta. 2012;718:0–57.
- Oliveira FD, E C R, Frollini E, et al. Lignopolyurethanic materials based on oxypropylated sodium lignosulfonate and castor oil blends. Ind Crop Prod. 2015;72:77–86.
- Zhang X, Zhao Z, Ran G, et al. Synthesis of lignin-modified silica nanoparticles from black liquor of rice straw pulping. Powder Technol. 2013;246(9):664–668.
- Kang S, Li X, Fan J, et al. Hydrothermal conversion of lignin: a review. Renewable Sustainable Energy Rev. 2013;27(6):546–558.
- Craggs A, G J M. Thomas J D R. Calcium ion-selective electrode measurements in the presence of complexing ligands. Analyst. 1979;104(1243):961–972.
- N A A M, Popiel WJ. Calcium-selective electrode measurements of calcium molybdate solubilities in water. Anal Chem. 2002;46(13):2055–2056.
- Shimoaka T, Wakai C, Sakabe T, et al. Hydration structure of strongly bound water on the sulfonic acid group in a Nafion membrane studied by infrared spectroscopy and quantum chemical calculation. Phys Chem Chem Phys. 2015;17(14):8843–8849.