?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Soil potential nitrification rate (PNR) and diversity of ammonia-oxidizing microbes were investigated in spiked soils with Cu and Sulfadiazine (SDZ). An obvious decrease of PNR was observed with the increase of Cu and SDZ concentrations in the soil. Real-time fluorescence quota PCR result showed that AOA and AOB were slightly stimulated at the gene level in both contaminated soils. Sequential analysis indicated that 200 mg kg−1 Cu could improve AOA diversity but reduce AOB diversity, but 5 mg kg−1 SDZ caused a decrease of both AOA and AOB diversity. Microbial community’s analysis also found that 200 mg kg−1 Cu and 5 mg kg−1 SDZ had different influence on the populations of AOA and AOB. It could be concluded that Cu and SDZ might have a different ecological effect mechanism on soil potential nitrification and ammonia-oxidizing microbial communities.
1. Introduction
Heavy metals and antibiotics were often added into the food or forage as addictive to promote the growth of livestock, but the poor absorption led to their large residual in feces and urine. Therefore, the large application of manures in agriculture led to lots of heavy metals and antibiotics to be released into soils and became pollutants [Citation1]. Besides, mining and sewage irrigation were also thought as the main reason causing the increase of heavy metals and antibiotics in agricultural soils. As the ultimate sink of pollutants, most pollutants were accumulated in soils because of its strong absorptivity. Now, heavy metals and antibiotics had often been detected in agricultural soils of the world, which contamination even reached a relatively serious level in some areas of China. An et al. found the concentrations of antibiotics in soils varied from 2.56 μg kg−1 of the sulfadimidine to 1590.16 μg kg−1 of the chlortetracycline in Shenyang [Citation2]. Meng et al. studied the pollution of heavy metals in topsoil after long-term sewage irrigation at Tianjin China, and found the concentrations of Cu, Zn, Pb and Cd were 41.17 mg kg−1, 276.41 mg kg−1, 41.56 mg kg−1 and 0.85 mg kg−1, respectively [Citation3].
Previous researches indicated that owing to their wonderful effect on promoting the growth and treating diseases, copper (Cu) and sulfonamides (SAs) were largely used in the livestock breeding industry, and they would finally enter into soil with agricultural application of manures, which might cause a serious ecological risk to soil [Citation4,Citation5]. For example, they were easy to be absorbed by vegetables or fruit growing on soils, which would directly endanger the health of humans. It was reported there was about 10 billion-kg grain per year being reduced by heavy metal pollution in China [Citation6,Citation7]. Now, more and more attention was paid on the soil pollution of Cu and SDZ. Previous researches indicated that heavy metals could decrease soil nitrification [Citation8]. Besides, some studies also found that addition of SDZ to the manure reduced microbial activity and then influenced the nitrogen cycle metabolism process [Citation9,Citation10]. As lying in the center of the global nitrogen cycle, soil nitrification was an essential process of nitrogen transformation. So, it was necessary to study the change of soil nitrification and their response mechanism with the pollution of heavy metals and antibiotics.
Soil microorganism was exceedingly important in the biogeochemical cycle and keeping the normal operation of soil ecosystems. With the increasingly serious soil pollution, the impact of pollutants on soil microbial communities had attracted the attention of the world. Many researches had shown it was very important to understand the process of soil degradation and explore the remediation methods of soil pollution by investigating the effects of pollutes on soil microbes [Citation11,Citation12]. Soil nitrification was often used as an index to evaluate the toxicity of pollutants to the microorganism in virtue of its sensibility to the change of environmental factors, especially to heavy metals and antibiotics contamination [Citation13,Citation14]. Kucharski et al. [Citation15] chose soil nitrification as an indicator to investigate the toxic effects of heavy metals Ni, Cu and Zn on soil microbial ecology. They found that soil nitrification was the most inhibited by Ni and less influence by Cu, while Zn had the least inhibition on soil nitrification. Moreover, previous studies also showed some sulfonamides had an inhibitory effect on soil nitrification [Citation16].
Recently, many advanced molecular biological technologies had been applied into the microbial ecology of soil pollution in order to study the toxic mechanism of pollutants on soil microbial communities [Citation17,Citation18]. And previous studies also indicated soil microbes were very sensitive to soil pollutants at the genetic level [Citation19,Citation20]. Currently, the fluorescence quantitative PCR, denaturing gel gradient electrophoresis, high-throughput sequencing and metagenome were commonly used genome techniques to analyze changes in microbial populations [Citation21,Citation22]. As the key enzyme that catalyzed the first reaction of ammonia oxidization, ammonia monooxygenase (AMO) was decoded by amoA gene, which was often chosen as molecular markers of ammonia-oxidizing microbes to investigate ammonia-oxidizing communities shift in polluted soils [Citation23]. Although more attention was paid on the ammonia oxidizing bacteria (AOB) formerly, the ammonia-oxidizing archaea (AOA) also played a key role in the process of nitrification as it was found that lots of Crenarchaeota had an ability to oxidize ammonia into nitrate or nitrite [Citation24,Citation25]. In the past, the effect of heavy metals and antibiotics on soil nitrification was investigated adequately, but the knowledge about molecular response mechanism of soil ammonia-oxidizing microbes and its relation to soil nitrification to the pollution of heavy metals and antibiotics were still lacking.
To analyze the toxic influence of Cu and SDZ pollution on soil microbial communities and their ecological function, batch experiments were conducted in the laboratory. Soil potential nitrification, abundance, diversity and community structure of AOB and AOA were chosen as indexes of soil microbial ecological systems with real-time fluorescence quantitative PCR and high-throughput sequencing. In this study, we addressed two issues in test soil: (1) the effect of Cu and SDZ on soil PNR; (2) response of ammonia-oxidizing microbes to Cu and SDZ, which provided a reliable theoretical basis for ecological risk assessment and remediation of the contaminated soil with heavy metals or antibiotics.
2. Materials and methods
2.1. Soil samples
The tested soil was gathered from Xinjiang, China, which had no history of contamination. Each topsoil sample (20 cm in depth) was the mixture of five samples obtained in a 30 × 30 m area. After air-drying and sieving with a 2 mm screen, the soil properties were analyzed. The maximum water holding capacity was measured by ring sampler, soil pH was determined by a pH meter at the soil to water ratio of 1:2.5, and organic matter was determined with K2Cr2O7 oxidation method. Soil texture was determined with a laser particle size analyzer (Rwase 2008, Rise, China).
The soil had a silty clay loam texture (sand 0.65%, silt 81.14% and clay 18.22%) with the maximum field water holding capacity (WHC) of 35.7%, a pH value of 7.77, the organic matter content of 18.6 mg kg−1, the cation exchange capacity of 13.65 cmol kg−1. Cu, Zn concentrations were 21.63 and 106.66 mg kg−1, respectively, and no SDZ was detected.
2.2. Experimental design
Short-term acute toxicity experiments were conducted in the laboratory. One hundred-gram dry soil samples were put into a 500 ml plastic jar. Before the experiment began, tested soil was adjusted to 20% of WHC and then equilibrated for 7 days at 25 ± 2°C for the recovery of soil microbial activities. Then, spreading pre-cultured soil with the stock solution of CuSO4 or SDZ to give a final soil concentration of 100 ~ 1000 mg kg−1 dry soil for Cu and 0.5 ~ 10 mg kg−1 dry soil for SDZ, respectively. Detailed contractions of SDZ and Cu in each treatment are listed in . Soil without the addition of Cu and SDZ was served as CK. Triplicates were conducted for each treatment. Then, all soil samples were adjusted to 75% of the maximum WHC and kept the same moisture content by adding deionized water periodically to compensate any water loss. After incubating another 7 days and 28 days in darkness at 25 ± 2°C, 10 g soil subsamples were gathered from each plastic jar for analysis of soil PNR and ammonia-oxidizing microbial diversity, respectively.
Table 1. Added and measured concentrations of Cu and SDZ.
2.3. Soil PNR
Soil potential nitrification was determined according to the ISO method described by Rusk et al. [Citation26]. A quantity of 0.1-g pre-incubated subsample was collected for the first extraction, a remaining subsample was treated with 0.044 M (NH4)2SO4 to give 100 mg N/kg and incubated another 14 days at 25 ± 2°C for the second extraction (0.1 g). The NO3–N concentration in soil was determined by the colorimetric method according to the introduction of a Nitrate nitrogen test kit (Cominbio Biotechnology Co. Ltd, Suzhou, China). Soil PNR was calculated with the following formula:
Where:
PNR was the potential nitrification rate, mg·kg−1·d−1; ω(NO3–N)1 and ω(NO3–N)2 was severally the content of NO3–N in the soil after adding (NH4)2SO4 0 days and 14 days, mg·kg−1; X was the days that continued to be incubated, day.
2.4. Isolation of soil DNA and PCR amplification
DNA was extracted from 0.25 g of each soil sample after incubation for 28 days with the PowerSoil DNA isolation Kit (TianGen Biotech, Beijing, China) according to the manufacturer’s instruction. The extracted DNA samples were stored at −80°C for further analysis.
For the amplification of bacterial amoA genes, following primers were used: amoA-1F (GGGGTTTCTACTGGTGGT) and amoA-2R (CCCCTCKGSAAAGCCTTCTTC), while archaeal amoA genes were amplified using the primers Arch-amoAF (STAATGGTCTGGCTTAGACG) and Arch-amoAR (GCGGCCATCCATCTGTATGT) [Citation27,Citation28]. The AOA amoA gene PCR conditions were as follows: an initial denaturation step at 95°C for 4 min, 30 cycles of denaturation at 94°C for 45 s, annealing at 53°C for 45 s, and extension at 72°C for 60 s, and 72°C for 15 min; the AOB amoA gene PCR conditions were as follows: an initial denaturation step at 94°C for 2 min, 35 cycles of denaturation at 94°C for 30 s, annealing at 51°C for 30 s, and extension at 72°C for 30 s, and 72°C for 10 min [Citation29]. The PCR amplification was carried out on the ABI 7500 Thermocycler (Applied Biosystems, California, USA) with the 20-μl reactions containing 10-μl SYBR Premix Ex Taq (TaKaRa, Dalian, China), 0.5 μM of each primer, 2 μl of DNA template and Milli-Q water to the final volume. The PCR products were checked by agarose gel electrophoresis and correct bands were excised. The excised PCR gels were then purified by using AxyPrep DNA Gel extraction kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s protocol. Then, the purified PCR amplicons were pooled from all the samples in an equimolar concentration for pyrosequencing.
2.5. Pyrosequencing and data analysis
The pyrosequencing work was accomplished on Illumina Hiseq/Miseq PE300 sequencing platform (Illumina, Inc., CA, USA). Obtained sequences were edited with the use of the Mothur 2.25.0 pipeline [Citation30]. Low-quality sequences (quality score <25, length <150 bp, ambiguous bases ≥1, homopolymer ≥6) were rejected and high-quality sequences were saved [Citation31]. Then, a total of 254,450 and 429,738 high-quality raw sequence reads were obtained for AOA and AOB, respectively. The obtained valid sequences were clustered into operational taxonomic units (OTUs) based on 97% sequence identity via the Qiime (quantitative insights into microbial ecology) pipeline for both AOA and AOB amoA genes. A total of 174 and 1204 OTUs were obtained for AOA and AOB, respectively. Then, the Coverage, Chaol and Shannon index were calculated based on the OTUs to describe the community’s richness and diversity. Besides, one sequence was randomly selected from each OTUs as a representative for that respective OTU, and the phylogenetic trees for AOA and AOB were constructed by MEGA 5.05 after using the BLAST to search the similar sequences as the respective OTUs in the NCBI.
2.6. Absolute quantification of amoA genes
Copy numbers of archaeal amoA and bacterial amoA genes were determined in triplicate for both samples by quantitative real-time PCR with the same primers and cycling protocols as those used for pyrosequencing. For the quantitative PCR standards, the plasmids containing each target gene were diluted by 10 times successively with the spanning of 108–101 and ten-fold serial dilutions were used as standard curves. The plasmids were extracted using a MiniBEST Plasmid Purification Kit (TaKaRa, Janpan) after the purified PCR products were cloned onto the pGEM-T Easy Vector (Promega, Madison, WI, USA) and transferred into Escherichia coli JM109 competent cells (Promega, Madison, WI, USA). Here, the linear correlation coefficients R2 of amoA-AOA and amoA-AOB were 0.9989 and 0.9991, respectively.
2.7. Analysis of Cu and SDZ in the soil
For Cu analysis, mainly referring to the method of Hu et al. [Citation32] with some modifications. Microwave digestion system (ETHOS 1, Italy, Milestone) was used to digest the samples. About 0.1 g of air-dried soil was digested with HCl, HNO3 and HF (8 ml, 3:9:4 v1/v2/v3). A two-stage digestion program was used, involving initial heating to 200°C over 10 min and digestion for 15 min at 200°C°C. Then, evaporating the cooling digestion solutions to near dryness and dissolving in 1.0 ml of 30% HNO3, followed by dilution to 50ml.with deionized water. Finally, determining Cu and other heavy metals concentrations in each solution by an inductively coupled plasma atomic emission spectrometer (Vista MPX, USA, Varian). The standard curve was got by diluting the material GBW07405 (National Research Center for Standards, China). Treating 30% HNO3 as reagent blanks and triplicates were conducted for each soil sample.
Towards the soil SDZ determine, the method of Zhou et al. [Citation33] was employed. A quantity of 1.0 g of soil samples was weighed into 100 ml conical flask and adding 10 ml 1% formic acid: methanol mixture (7:3, v1/v2). Then, 2 h mechanical shaking extraction and 10 min centrifugation with 4000 r/min was necessary. Extract samples again following these steps, and merge their supernatant. Finally, extracting solution was filtered 0.45 μm filtering film before HPLC analysis. For the standard curve, it was drawn by diluting the individual primary stock standard solution, which was prepared by dissolving the chromatographically pure sulfadiazine in methanol. Likewise, methanol without sulfadiazine was blank; CK and each analysis was repeated three times.
2.8. Statistics
The PNR values of soil with different treatments were calculated and analyzed by Excel2010, their Figures were accomplished using Oringin8.5. R program (Development Core Team, 2008) was adopted to conduct principal component analysis (PCA) and plot heatmaps. The phylogenetic tree was constructed by Mega5.05. Statistical analyses were performed using SPSS version 17.0. In order to describe the effective concentrations (EC20 and EC50), linear regression was performed with normalized data and fitted into a logistic response equation.
3. Results
3.1. Changes of PNR in polluted soils
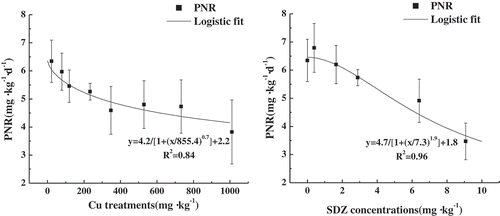
In this study, ranges of Cu and SDZ addition were 0–1000 mg kg−1 and 0–10 mg kg−1, respectively. As shown in , the PNR value was 6.34 mg NO3–N·kg−1·d−1 in the CK, and both Cu and SDZ addition had obvious inhibition on soil PNR.
For the Cu addition treatments, the PNR values were 5.97, 4.80 and 3.82 mg NO3–N·kg−1·d−1 in soils contaminated with 50, 500 and 1000 mg kg−1 Cu, respectively, all of which were obviously reduced compared with the CK. A dose-response curve was created for Cu and soil PNR. The corresponding Cu concentrations in soil at which the PNR was reduced by 20% (Cu EC20) and 50% (Cu EC50) were 156.63 and 1131.70 mg kg−1, respectively ().
Table 2. EC20 and EC50 values of different treatments with Cu and SDZ.
As shown in right), the PNR values decreased with the increase of SDZ concentrations, except for 0.5 mg kg−1 treatment, which were 6.79, 6.20, 5.70, 4.90 and 3.47 mg NO3–N·kg−1·d−1 in soils added with 0.5, 1, 3, 5 and 10 mg kg−1 SDZ, respectively. That was, the addition of 0.5 mg kg−1 SDZ had a slight promotion on soil nitrification ability, but it also presented an obvious inhibition on soil PNR when SDZ addition was over 1 mg kg−1. A dose-response curve was also created for SDZ and soil PNR. Corresponding SDZ concentrations in soil at which the PNR was reduced by 20% (Cu EC20) and 50% (Cu EC50) were 6.87 and 10.08 mg kg−1, respectively.
3.2. Effect of Cu and SDZ on gene abundance of AOA and AOB
According to the results of EC20 (Cu) and EC20 (SDZ), which could induce reduction by 20% of the measured PNR, three soil treatments: 200 mg kg−1 Cu, 5 mg kg−1 SDZ and CK were chosen for molecular analysis to explore the distinct response of ammonia-oxidizing microbes.
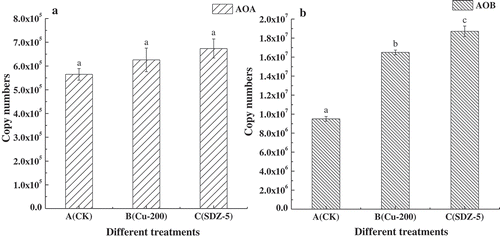
In the CK treatment, amoA-AOA copies (5.65 × 105 per soil gram) were one order of magnitude lower than the AOB amoA copies (9.50 × 106 per soil gram). Moreover, in comparing with CK, copies of AOA and AOB amoA genes had not reduced significantly in 200 mg kg−1 Cu and 5 mg kg−1 SDZ treated soils as expected (). On the contrary, amoA-AOB copies were significantly greater (P < 0.05) in 200 mg kg−1 Cu (1.65 × 107 per soil gram) and 5 mg kg−1 SDZ (1.87 × 107 per soil gram) treatments than in CK, and the copies of which were the highest in 5 mg kg−1 SDZ treatment. For amoA-AOA genes, the copies were obviously higher in 200 mg kg−1 Cu and 5 mg kg−1 SDZ treatment than in CK, although there was not a significant difference (P > 0.05) among these three treatments. It might indicate that both 200 mg kg−1 Cu and 5 mg kg−1 SDZ could not cause a significant reduction on populations of AOA and AOB.
3.3. Diversity of ammonia-oxidizing archaea
The amoA-AOA gene libraries of all three soil treatments were constructed by pyrosequencing. The coverage and alpha diversity indexes of the three libraries are listed in . For all soils of the selected three treatments, 56–70 OTUs were detected, and its maximum OTU was found in the treatment of 200 mg kg−1 Cu. As showed in , the coverage of all amoA-AOA gene libraries exceeded 0.99, so that the constructed library was large enough to reflect the archaeal diversity among the different treatments. Chaol and Shannon indexes of amoA-AOA genes in 200 mg kg−1 Cu treatments were 73.31 and 3.33, respectively, which were the highest values among the three treatments and indicated that AOA population of 200 mg kg−1 Cu treatments was the highest in richness and diversity of amoA-AOA gene, while treatment 5 mg kg−1 SDZ exhibited the lowest richness and diversity, which Chaol and Shannon indexes were 61.05 and 3.05, respectively.
Table 3. OTUs, Coverage and alpha diversity indexes of amoA genes with AOA and AOB under two treatments.
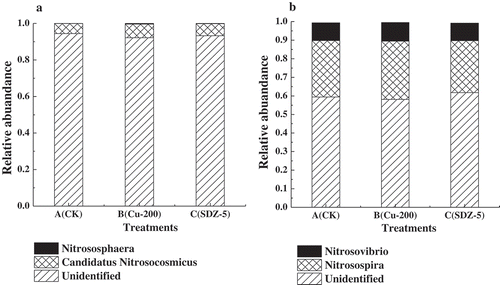
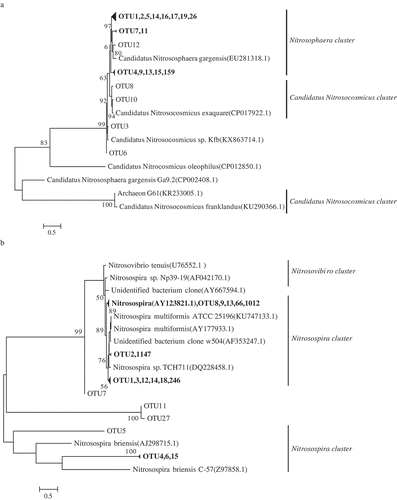
Principal component analysis (PCA) was conducted to analyze the variation of amoA-AOA genes libraries in soils treated with Cu and SDZ, respectively (). There was a delightful cluster for three treatments and the contribution rates of PC1 and PC2 with amoA-AOA were severally 78.05% and 16.62%. Besides, a phylogenetic tree was constructed (top20). As showed in , there were two main clusters with Candidatus Nitrosocosmicus and Nitrososphaera, other microorganism clusters were accounted for a very low abundance in all soil samples. And based on the phylogenetic tree, the distribution and relative abundance of phylogenetic AOA groups were obtained. As showed in , except for the maximum percentage of Unindentified, whose average relative abundance was 93.3% (92.2%-94.4%), Candidatus Nitrosocosmicus cluster with relative abundance 5.4%, 7.4% and 6.6% in each library was the predominant AOA group in CK, Cu 200 mg kg−1 and SDZ 5 mg kg−1 treatments, while Nitrososphaera cluster was the second dominated archaea cluster with very low abundance of 0.1%, 0.4% and 0.2%, respectively, which indicated Cu 200 mg kg−1 and SDZ 5 mg kg−1 treatments both could promote the relative abundance of Candidatus Nitrosocosmicus and Nitrososphaera cluster slightly.
Figure 3. Principal component analysis of A, B and C treatments at the OTUs (97% sequence similarity) (a) Ammonia-oxidizing archaea (b) Ammonia oxidizing bacterial. A treatment was CK, B treatment was added Cu 200 mg kg−1, C treatment was added SDZ 5 mg kg−1.
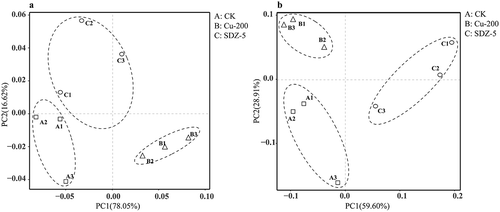
Figure 4. Phylogenetic tree constructed for (a) top20 amoA-AOA OTUs sequences and (b) top20 amoA-AOB OTUs sequences. The numbers (only those >50% were shown) on the branch nodes indicate the percentages of bootstrap support. Numbers in the brackets were the GenBank accession numbers of the strain in the NCBI.
3.4. Diversity shift of ammonia-oxidizing bacteria
The amoA-AOB gene libraries of all soil samples were constructed by pyrosequencing. The coverage and alpha diversity indexes of the three libraries are listed in . In the different samples, 290–399 OTUs were detected, and its maximum OTU was found in the treatment of 5 mg kg−1 SDZ. The coverage of all amoA-AOB gene libraries exceeded 0.96, indicated that the library was large enough to reflect the bacterial diversity among the different samples. The treatments CK and 5 mg kg−1 SDZ were severally found owning the highest diversity and richness of amoA-AOB gene, which Shannon and Chaol indexes were severally 3.94 and 451.86, while treatment of 200 mg kg−1 Cu exhibited the lowest richness and diversity, which Chaol and Shannon indexes were 327.75 and 3.51, respectively.
Principal component analysis (PCA) of three treatments was completed by R language program. As shown in , an obvious clustering for ammonia-oxidizing bacteria communities on the base of OTU (at a similar level of 97%) was found. The contribution rates of PC1 and PC2 with amoA-AOB were severally 59.6% and 28.91%. Besides, a phylogenetic tree was constructed (Top20). As showed in , there were two main clusters with Nitrosospira and Nitrosovibrio. And based on the phylogenetic tree, the distribution and relative abundance of phylogenetic AOB groups were obtained. As showed in ), except for the maximum percentage of Unindentified, whose average relative abundance was 59.73% (58.04%-61.76%), Nitrosospira cluster with relative abundance 30.34%, 31.35% and 27.94% in each library was the predominant AOB group in CK, Cu 200 mg kg−1 and SDZ 5 mg kg−1 treatments, while Nitrosovibrio cluster was the second dominated archaea with lower abundance of 9.57%, 10.10% and 9.46%, respectively, which indicated 200 mg kg−1 Cu could promote the relative abundance of two microorganism clusters, while 5 mg kg−1 SDZ could reduce the relative abundance of them.
4. Discussion
4.1. Effect of Cu and SDZ on PNR
In this study, we analyzed the response difference of soil PNR to Cu and SDZ. For Cu stress, a significant inhibition on soil PNR was found, and it could reduce the soil nitrification by 40.0% when the addition was 1000 mg kg−1 Cu. This result was similar to the report by Sun et al. [Citation34], which found the PNR of all tested soils decreased gradually with the increase of Cu concentrations. A possible explanation for this might be the case that heavy metal elements inhibited the replication and transcription of function gene amoA of AOB and AOA in the soil, as the DNA copies analysis showed a slight growth of nitrifying bacteria in contaminated soils with an obvious decrease of soil PNR [Citation35]. The logistic curve analysis indicated that Cu could cause a significant harm to soil nitrification at 156.63 mg kg−1 (EC20) and inhibit soil PNR by 50% at 1131.70 mg kg−1 (EC50), which toxicity to soil nitrification was some lower than the heavy metals Zn (EC50, 210 mg kg−1) by Rusk et al. [Citation26] and Cd (EC50, 350.8 mg kg−1) by Wang et al. [Citation11]. This could be mainly explained that soil nitrification was sensitive differently to different heavy metal besides soil environmental factors.
For the SDZ addition test, the response of soil PNR to SDZ was similar to the previous foundation that antibiotics only caused a significant inhibition on soil nitrification at relatively high concentration [Citation36]. However, Guo et al. [Citation37] showed that high concentrations of sulfanilamide antibiotics had a promotion on soil PNR in view of different soil properties and drug types. In this study, the spiking concentration of SDZ has not reached a high level as reported in previous studies. According to the results of EC20 and EC50, it still found a clear dose–response relationship between SDZ and soil PNRs. But these effective concentrations were not consistent with the reported other antibiotics [Citation38]. A possible explanation for this might be the case that the mechanisms of different antibiotics were different. Sulfonamide affected the soil nitrification by interfering with folate metabolism of nitrifying microorganism, while erythrocin and tetracycline antibiotics inhibiting the synthesis protein [Citation39].
Soil PNR had been widely used as an essential indicator to assess the contamination of soil systems [Citation40,Citation41]. The results of this study further proved the previous reports that soil nitrification was one of the most sensitive indicators for soil heavy metals pollution [Citation12], but it might not be true to the sulfonamides soil pollution.
4.2. The response difference of AOA and AOB to Cu and SDZ contamination in soils
Previous studies indicated that heavy metals inhibited soil nitrification by inhibiting the growth of ammonia-oxidizing microorganism. For example, amoA functional genes analysis indicated that trace metals could inhibit gene transcription, replication and growth of ammonia-oxidizing microorganism [Citation42,Citation43]. In the present study, we investigated the variation of AOA and AOB in soils after contaminated with Cu. The results indicated that 200 mg kg−1 Cu could significantly inhibit the soil PNR, but the gene abundance of ammonia-oxidizing microorganism did not reduce, which was different from the soil PNR. The result was consistent with He et al. [Citation44] that there was a different response to Cu stress for the abundance of amoA based on DNA and its PNR in flivo-aquic soil,but a consistent trend was found between soil PNR and their cDNA, which was transcribed by RNA of the amoA. A possible reason for this phenomenon was that only partial DNA of amoA being expressed successfully and amoA gene was more sensitive to heavy metals on RNA level than DNA [Citation8]. It had shown that transcription of amoA gene on RNA level was a better biological index to assess eco-toxicological effects of pollutes on soil microorganism [Citation45]. In addition, our results also suggested that AOB was more sensitive to Cu stress in the acute toxicity test in comparison with the AOA, which was agreed with previous studies showing that the abundance of amoA-AOB was significantly reduced with Cu stress while amoA-AOA abundance had no obvious decrease [Citation46]. It was probably because that archaeal membrane was less permeable to heavy metals ions than bacterial membrane due to its special chemical structure of membrane lipid [Citation47]. As for the diversity and community structure change, the results indicated that 200 mg kg−1 Cu treatment could increase AOA diversity and decrease AOB diversity compared to the CK. At the same time, the relative abundance of Candidatus Nitrosocosmicus and Nitrososphaera, which were predominant microbes of AOA, and Nitrosospira, dominate cluster of AOB, were all promoted with addition of 200 mg kg−1 Cu. Similarly, previous studies showed the diversity of the AOA community in low Cu dose group was higher than that in non-Cu-treated soil [Citation44]. Moreover, there was a good tolerance to heavy metals for Nitrosospira, which could be a reasonable explanation for the increase of Nitrosospira in the contaminated soil [Citation48].
For SDZ contamination, it indicated that 5 mg kg−1 SDZ could significantly increase the abundance of ammonia-oxidizing microorganism, and AOB had a more increase than AOA. But Chaol and Shannon indexes showed 5 mg kg−1 SDZ treatment could reduce their diversity. Similar results were also found in previous reports that antibiotic could reduce the diversity of ammonia-oxidizing microorganism in an acute toxicity test, but increase the abundance of amoA-AOB and amoA-AOA at different level [Citation49]. This might be ascribed to the selective susceptibility of the microorganism to drugs. It was reported that sulfonamides mainly inhibited the activity of the 30S ribosome subunit of gram-positive and negative bacteria, while archaea could develop resistance to them by changing their basic physiological and biochemical properties [Citation50,Citation51]. At the same time, previous studies also illustrated the long-term antibiotic stress could increase the diversity of ammonia-oxidizing microorganisms [Citation49]. So, it was suggested that antibiotics resistance gene could be a key factor for the microbial recovery with long-term antibiotic stress [Citation52]. Besides, microbial community structure analysis indicated 5 mg kg−1 SDZ promoted the abundance of Candidatus Nitrosocosmicus and Nitrososphaera, while Nitrosovibrio was reduced slightly. Some previous researches showed Nitrososphaera widely existed in soils, which did not play a key role in soil nitrification but also was sensitive for environmental pollution [Citation53,Citation54].
5. Conclusion
This study mainly presented a direct toxic effect of Cu or SDZ pollution on PNR and the related ammonia oxidizing microorganism, AOB and AOA. The results indicated that both Cu and SDZ had a significant negative effect on soil PNR, but promoted ammonia-oxidizing archaea and bacteria abundance on gene level. Sequential analysis illustrated that Cu increased the AOA diversity but reduced AOB’s diversity, while SDZ could reduce the diversity of both AOB and AOA. Moreover, community structure analysis suggested that both 200 mg kg−1 Cu and 5 mg kg−1 SDZ could promote the relative abundance of Candidatus Nitrosocosmicus and Nitrososphaera slightly, which were the predominated cluster of AOA, but for AOB’s prevalent microorganism, Nitrosospira and Nitrosovibrio, the promotion was only found in 200 mg kg−1 Cu treated soil while the inhibition happened in 5 mg kg−1 SDZ treatment.
The response of AOA and AOB to Cu and SDZ contamination in soils was analyzed. The results indicated that AOB was numerically dominant over AOA in the tested soil, and more sensitive response to Cu and SDZ stress than AOA. However, these results were conducted with short-time incubation. The long-term toxic effects of Cu and SDZ to ammonia oxidizers were not investigated, which might have a different result from the short-time incubation [Citation55]. In addition, only single pollution of Cu and SDZ was conducted in the present study. Actually, combined pollution of two or more pollutants was more common in soils [Citation56]. Therefore, further long-term field research and combined pollution study were essential to interpreter the response of ammonia-oxidizing microorganism in heavy metals and antibiotics polluted soils on the gene level.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wu LH, Pan X, Chen LK, et al.Occurrence and distribution of heavy metals and tetracyclines in agricultural soils after typical land use change in east China. Environ Sci Pollut Res. 2013;20:8342–8354.
- An J, Chen HW, Wei SH, et al. Antibiotic contamination in animal manure, soil, and sewage sludge in Shenyang, northeast China. Environ Earth Sci. 2015;74:5077–5086.
- Meng W, Wang Z, Hu B, et al. Heavy metals in soil and plants after long-term sewage irrigation at Tianjin China: a case study assessment. Agric Water Manage. 2016;171:153–161.
- Kaçar A, Koçyiğit A. Characterization of heavy metal and antibiotic resistant bacteria isolated from aliaga ship dismantling zone, eastern aegean sea, Turkey. Int J Environ Agric Res. 2013;7:895–902.
- Kong WD, Zhu YG, Fu BJ, et al. The veterinary antibiotic oxytetracycline and Cu influence functional diversity of the soil microbial community. Environ Pollut. 2006;143:129–137.
- Hu W, Zhang Y, Huang B, et al. Soil environmental quality in greenhouse vegetable production systems in eastern China: current status and management strategies. Chemosphere. 2017;170:183–195.
- Osma E, Serin M, Leblebici Z, et al. Heavy metals accumulation in some vegetables and soils in istanbul. Ekoloji. 2012;21:1–8.
- Huan HE, Shen TL, Dai JL, et al. The response of potential nitrification rate in fluvo-aquic soil to heavy metals Zn2+ and Cd2+. J Agro-Environ Sci. 2010;29:918–922.
- Hou L, Yin G, Liu M, et al. Effects of sulfamethazine on denitrification and the associated N2O release in estuarine and coastal sediments. Environ Sci Technol. 2015;49:326–333.
- Kotzerke A, Sharma S, Schauss K, et al. Alterations in soil microbial activity and N-transformation processes due to sulfadiazine loads in pig-manure. Environ Pollut. 2008;153:315–322.
- Wang Y, Wang X, Yang Y, et al. Influence of Cd Amendment on potential nitrification rate in suburban soil of Beijing. Asian J Ecotoxicol. 2014;9:367–374.
- Kene G, Ernst W, Stevep MG. Heavy metals and soil microbes. Soil Biol Biochem. 2009;41:2031–2037.
- Liu A, Fang D, Wang C, et al. Primary research on the recovery of soil nitrification and its key factors under the Cu stress. Ecol Environ Sci. 2014;23:1986–1990.
- Babich H, Stotzky G, Ehrlich HL. Environmental factors that influence the toxicity of heavy metal and gaseous pollutants to microorganisms. CRC Crit Rev Microbiol. 1980;8:99–145.
- Kucharski J, Wyrwał A, Boros E, et al. Nitrification process as an indicator of soil contamination with heavy metals. Inorganica Chim Acta. 2009;45:L107–L108.
- Toth JD, Feng Y, Dou Z. Veterinary antibiotics at environmentally relevant concentrations inhibit soil iron reduction and nitrification. Soil Biol Biochem. 2011;43:2470–2472.
- Srivastava PK, Vaish A, Dwivedi S, et al. Biological removal of arsenic pollution by soil fungi. SciTotal Environ. 2011;409:2430–2442.
- Stone D, Ritz K, Griffiths BG, et al. Selection of biological indicators appropriate for European soil monitoring. Appl Soil Ecol. 2016;97:12–22.
- Wessén E, Hallin S. Abundance of archaeal and bacterial ammonia oxidizers–possible bioindicator for soil monitoring. Ecol Indic. 2011;11:1696–1698.
- Feld L, Hjelmsø MH, Nielsen MS, et al. Pesticide side effects in an agricultural soil ecosystem as measured by amoA expression quantification and bacterial diversity changes. Plos One. 2015;10:e126080.
- Saito A, Ikeda S, Ezura H, et al. Microbial community analysis of the phytosphere using culture-independent methodologies. Microbes Environ. 2007;22:93–105.
- Woojim S, Joonhong P, Quensen JFI, et al. DNA-stable isotope probing integrated with metagenomics for retrieval of biphenyl dioxygenase genes from polychlorinated biphenyl-contaminated river sediment. Appl Environ Microbiol. 2009;75:5501.
- Kowalchuk GA, Stephen JR. Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol. 2001;55:485.
- Leininger S, Urich T, Schloter M, et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 2006;442:806.
- Wuchter C, Abbas B, Coolen MJ, et al. Archaeal nitrification in the ocean. Proc Natl Acad Sci U S A. 2006;103:12317–12322.
- Rusk JA, Hamon RE, Stevens DP, et al. Adaptation of soil biological nitrification to heavy metals. Environ Sci Technol. 2004;38:3092–3097.
- Xu Y, Yu W, Ma Q, et al. Responses of bacterial and archaeal ammonia oxidisers of an acidic luvisols soil to different nitrogen fertilization rates after 9 years. Biol Fertil Soils. 2012;48:827–837.
- Wang J, Zhang L, Lu Q, et al. Ammonia oxidizer abundance in paddy soil profile with different fertilizer regimes. Appl Soil Ecol. 2014;84:38–44.
- Liu H, Li J, Zhao Y, et al. Ammonia oxidizers and nitrite-oxidizing bacteria respond differently to long-term manure application in four paddy soils of south of China. SciTotal Environ. 2018;633:641–648.
- Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537.
- Huang L, Dong H, Wang S, et al. Diversity and abundance of ammonia-oxidizing archaea and bacteria in diverse Chinese paddy soils. Geomicrobiol J. 2014;31:12–22.
- Hu X, Zhang Y, Luo J, et al. Bioaccessibility and health risk of arsenic, mercury and other metals in urban street dusts from a mega-city, Nanjing, China. Environ Pollut. 2011;159:1215–1221.
- Aixia Z, Su X, Gao S, et al. Determination of four sulfa antibiotics in groundwater, soil and excreta samples using high performance liquid chromatography. Chin J Anal Chem. 2014;42:397–402.
- Sun JW, Huang YZ, Zhao LJ, et al. Effects of copper on nitrification rates in 17 kinds of typical soils in China. Asian J Ecotoxicol. 2008;3:513–520.
- Kapoor V, Li X, Elk M, et al. Impact of heavy metals on transcriptional and physiological activity of nitrifying bacteria. Environ Sci Technol. 2015;49:13454.
- Banerjee S, D Angelo E. Livestock antibiotic effects on nitrification, denitrification, and microbial community composition in soils. Open J Soil Sci. 2013;03:203–212.
- Guo B, Yao LX, Liu ZZ, et al. Effects of sulfonamide veterinary drugs on soil biochemical function and nitrogen. Soils. 2012;44:596–600.
- Louvet JN, Giammarino C, Potier O, et al. Adverse effects of erythromycin on the structure and chemistry of activated sludge. Environ Pollut. 2010;158:688–693.
- Alighardashi A, Pandolfi D, Potier O, et al. Acute sensitivity of activated sludge bacteria to erythromycin. J Hazard Mater. 2009;172:685–692.
- Zhou ZF, Liu YR, Sun GX, et al. Responses of soil ammonia oxidizers to a short-term severe mercury stress. J Environ Sci. 2015;38:8–13.
- Liu A, Cao H, Yang Y, et al. Combinational effects of sulfomethoxazole and copper on soil microbial community and function. Environ Sci Pollut Res. 2016;23:4235–4241.
- Song Y, Swedlund PJ, Singhal N, et al. Cadmium(II) speciation in complex aquatic systems: a study with ferrihydrite, bacteria, and an organic ligand. Environ Sci Technol. 2009;43:7430–7436.
- Ganguly S, Jana BB. Cadmium induced adaptive responses of certain biogeochemical cycling bacteria in an aquatic system. Water Res. 2002;36:1667–1676.
- He H, Liu H, Shen T, et al. Influence of Cu application on ammonia oxidizers in fluvo-aquic soil. Geofis Int. 2018;321:141–150.
- Ollivier J, Wanat N, Austruy A, et al. Abundance and diversity of ammonia-oxidizing prokaryotes in the root–rhizosphere complex of miscanthus × giganteus grown in heavy metal-contaminated soils. Microb Ecol. 2012;64:1038–1046.
- Li XF, Zhu YG, Cavagnaro TR, et al. Do ammonia-oxidizing archaea respond to soil Cu contamination similarly asammonia-oxidizing bacteria? Plant Soil. 2009;324:209–217.
- Kandler O, König H. Cell wall polymers in archaea (archaebacteria). Cell Mol Life Sci. 1998;54:305–308.
- Zhang HY, Zhai HY, Ji M, et al. Long-term effect of Cr(VI) on ammonia-oxidizing and nitrite-oxidizing bacteria in an activated sludge system. Desalin Water Treat. 2015;54:1981–1989.
- Sun J, Qian X, Gu J, et al. Effects of oxytetracycline on the abundance and community structure of nitrogen-fixing bacteria during cattle manure composting. Bioresour Technol. 2016;216:801–807.
- Hammesfahr U, Heuer H, Manzke B, et al. Impact of the antibiotic sulfadiazine and pig manure on the microbial community structure in agricultural soils. Soil Biol Biochem. 2008;40:1583–1591.
- Hilpert R, Winter J, Hammes W, et al. The sensitivity of archaebacteria to antibiotics. Zentralblatt Für Bakteriologie Und Hygiene Reihe C. 1981;2:11–20.
- Gonzalezmartinez A, Margareto A, Rodriguezsanchez A, et al. Linking the effect of antibiotics on partial-nitritation biofilters: performance, microbial communities and microbial activities. Front Microbiol. 2018;9:354.
- Zhou ZF, Wang MX, Liu WL, et al. A comparative study of ammonia-oxidizing archaea and bacteria in acidic and alkaline purple soils. Ann Microbiol. 2016;66:615–623.
- Li H, Weng BS, Huang FY, et al. pH regulates ammonia-oxidizing bacteria and archaea in paddy soils in Southern China. Appl Microbiol Biotechnol. 2015;99:6113–6123.
- Oliveira A, Pampulha ME. Effects of long-term heavy metal contamination on soil microbial characteristics. J Biosci Bioeng. 2006;102:157–161.
- Guo T, Lou C, Zhai W, et al. Increased occurrence of heavy metals, antibiotics and resistance genes in surface soil after long-term application of manure. SciTotal Environ. 2018;635:995–1003.