ABSTRACT
The hydrofluoric acid modified biochar (HF-BC) was obtained, aiming to improve its performance for activating persulfate (PS) to make acid orange 7 (AO7) degraded in water solution. Results showed that the surface area, micropore volume, carboxyl and phenolic hydroxyl group content of HF-BC increased by 171%, 172%, 23.8% and 50%, respectively, compared to unmodified biochar (BC). The decolorization of AO7 in HF-BC/PS and BC/PS systemwas much more rapid and efficient than that in HF-BC, BC and PS alone system. For the HF-BC/PS system, the AO7 was removal of 99.8%, much higher than that of BC/PS system, probably because of its better surface characteristics. Moreover, for the HF-BC/PS system, it was the higher PS concentration and HF-BC dosage that caused greater AO7 degradation rates, and lower pH was favorable for the degradation. Our results provided a novel method to design biochar as an activator for persulfate-based remediation of dye wastewater.
Abbreviations: HF-BC, hydrofluoric acid modified biochar; BC, unmodified biochar; AO7, Acid orange 7; PS, persulfate
1. Introduction
In recent years, the dyes wastewater pollution, generated from the textile, printing, paper, dyeing as well as food-stuff industry discharge, has become an extrusive environmental issue and drawn more and more concern [Citation1–Citation3]. Azo dyes account for nearly 50% among global production of commercial dyes, the molecular structure of which includes the chromophore of –N=N– unit, making it difficult to degrade with biological treatment methods [Citation4–Citation6]. The conventional treatment processes like flocculation and adsorption are inadequate for treatment of azo dye wastewater with high concentration and chroma. Moreover, they also cause solid waste, which creates additional environmental problems that require further treatment [Citation7,Citation8]. Therefore, it is urgent to find an appropriate and efficient way to remove azo dye from wastewater.
With the development of advanced oxidation processes (AOPs), the organic pollutants with toxicity and bio-refractory features in wastewater can be degraded [Citation9]. Activation persulfate (PS) oxidation has been viewed as a burgeoning AOP for degradation of the organic pollutants [Citation10]. Although PS has limited capacity to make organics oxidized in an independent manner, it could be activated by transition metal ions (mainly iron), heat or UV-light to produce free radicals SO4−• [Citation11–Citation15], with oxidation-reducted potential ranging from +2.5 to +3.1 V vs. NHE [Citation16], making a great majority of the organics oxidized and decomposed in water [Citation17].
As a stable carbon-rich by-product, biochar (BC) is derived from biomass pyrolysis under the conditions of limited oxygen and low temperature. It attracted more and more attention owing to multi-functionality including soil improvement, waste management, climate change, energy production and pollution remediation [Citation18]. Furthermore, most studies showed that biochar was an extremely effective and cost-efficient sorbent in removal of contaminant [Citation19,Citation20]; moreover, biochar like other carbon materials could also use as a catalyst to activate H2O2 or PS for organic pollutant degradation [Citation21–Citation25]. Fang, et al. pointed out that persulfate was activated by biochar for production of sulfate radicals to make polychlorinated biphenyls degraded [Citation24]. Huang et al. also showed that biochar could influence hydroxyl radical generation and sulfamethazine degradation [Citation26]. Kemmoua, et al. reported that persulfate activation seems to occur on biochar surface through interaction with the surface functional groups [Citation25]. All of them pointed that the catalytic effect and mechanism depend on biochar characteristics including specific surface area and functional group. And that biochar with high specific surface area and abundant functional group is considered as a good catalyst.
In order to design high-performance biochar for catalyzing PS, rice-hull was used as a biomass material to prepare biochar sampler with or without HF solution pretreatment, which is used for the removal of mineral matter from biomass beneficial to the development of porous structure for biochar production [Citation27–Citation29]. Azo-dye acid orange 7 (AO7) usually serves as a kind of model compound used for dyes degradation [Citation4,Citation9,Citation30,Citation31]. AO7 degradation into an aqueous solution by using those two biochars as persulfate activators were studied in this paper. The aims of this research included: (1) to check the improvement of AO7 degradation using modified biochar as persulfate activator and its mechanism; (2) to testify the factors that influence modified biochar activate PS for AO7 degradation in aqueous solution.
2. Materials and methods
2.1. Materials
Purchase of materials was made from Sinopharm Chemical Reagent Company, such as sodium thiosulfate (Na2S2O3) and AO7 (4-(2-hydroxy-1-naphthylazo) benzenesulfonic acid). All chemicals adopted in this study are analytical grade.
Collection of rice hull was made from a rice manufactory in the suburb of Bengbu, China, air-dried to make the water content below 5% and then ground to <0.15 mm. Biochar was produced in a laboratory-scale pyrolysis system under an N2 atmosphere with a heating rate of 15°C min−1 to reach the temperatures at 500°C and the heating time was kept for 2 h [Citation32]. Residues in the solid state left within the reactor were referred to as the biochar. In order to prepare HF-modified biochar, 50 g of rice hull powder was mixed with 1000 mL of HF solution (2 mol L−1) in a plastic container. After stirred uniformly, the container was under ambient conditions (25°C) for 24 h and then the treated rice hull was separated and washed with deionized water until the F – cannot be detected, after that it was dried at the temperature of 80°C to a constant weight. The dried rice hull was used to produce HF-modified biochar. What’s more, for simplicity, biochar with and without HF pretreatment was referred to as BC and HF-BC, respectively.
2.2. Degradation experiments
Experiments had been performed in batches within 500 mL beaker and shaken by using the thermal oscillator tank, with the agitator rotation at the speed of 150 r min−1 under the temperature of 25°C. AO7 solution (200 mL, 20 mg L−1), PS as well as biochar were placed in a simultaneous manner into a beaker at the beginning of every experiment. Through using 5 mL injection syringe for analysis, withdrawal of those samples was performed within preset time intervals from the beaker. And the molar ratio of PS/AO7 was 100: 1, with 5.0 g L−1 of biochar dosage. The reaction time was 24 h.
Effect of initial pH from 3.5 to 10.5 adjusted by 0.1 mol L−1 NaOH or 0.1 mol L−1 H2SO4, on the decolourization of AO7 was studied. Moreover, effects of biochar dosage (1 to 10 g L−1) and PS concentration (molar ratio of PS/AO7 ranging from 50/1 to 700/1) on the decolourization of AO7 were also studied.
2.3. Analysis
The monitoring of AO7 concentration was performed through measurement of the maximum absorbance at λ = 484 nm by using an ultraviolet spectrophotometer (UV1800, Shimadzu, Japan). With application of an element analyzer, total C and H content of biochar was analyzed (Vario ELIII, Elementar, Germany). In addition, certain surface area as well as distribution of biochar pore size was also determined by utilizing the BET-N2 SA analyzer (JW-BK222, China). Acidic groups of biochar surfaces were characterized via the Boehm titration [Citation33]. Fourier transform infrared (FTIR) spectroscopy was used to make a determination of the distributions of surface functional group of biochar (IR Prestige 21 FTIR, Shimadzu). The morphology as well as surface atomic composition of biochar was analyzed by adoption of a scan electron microscope with energy-dispersive spectra (SEM-EDS, Zeiss EVO LS-185, England).
3. Results and discussion
3.1. Characterization of the biochar and the HF modified biochar
The physical-chemical properties of both BC and HF-BC are shown in . The pH of BC was alkaline, the similar results were observed in many former researches [Citation34,Citation35]. Conversely, hydrofluoric acid treatment led to the pH value of HF-BC was reduced to 6.21. Compared to BC, the yield of HF-BC was reduced by 36.4%, however, the total C and H content of HF-BC increased by 72.9% and 24.7%, respectively. The H/C of HF-BC was much lower than that of BC indicated that the stability of HF-BC improved compared to BC [Citation32]. Moreover, both the surface area (SA) and micropore volume (MV) of HF-BC were also increased by up to 2.7 times except that the average pore diameter (APD) was reduced about 20%, compared to BC. Phenolic hydroxyl of the two biochar was low. Compared to BC, the carboxy groups of HF-BC increased by 23.8%, on the contrary, the lactonic group contents reduced by 45.8%.
Table 1. Selected properties of BC and HF-BC.
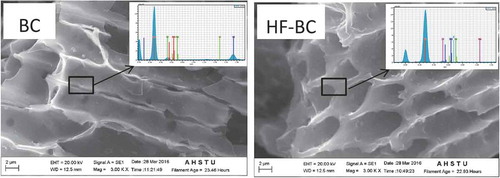
The SEM images of BC and HF-BC were shown in . The rice hull cell morphology was still clearly observed after pyrolysis and it clearly showed that biochar had a porous structure. The quantitative surface chemical compositions were presented in . The C content of HF-BC was higher than that of BC, indicating accordance with the result based on element analysis (). Moreover, the content of Si and O in HF-BC was obviously lower, indicating that the differences between the two biochars were probably due to the decrease of SiO2 with HF pretreatment.
Table 2. The surface atomic composition of BC and HF-BC.
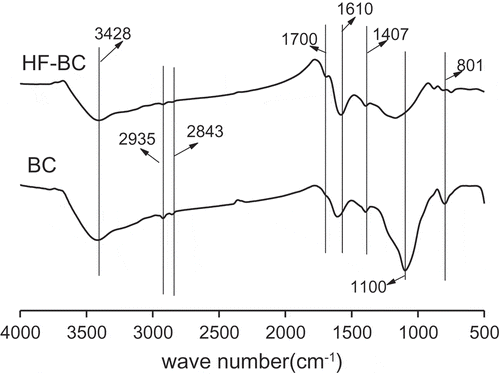
It was the FTIR spectra that clearly demonstrated changes in surface functional groups of biochars with/without HF modifications (). And its absorption peak at 3428 cm−1 represents the O-H stretching vibration, and 2935 cm−1, 2843 cm−1 and 1407 cm−1 assigned to –CH2 groups [Citation36], respectively, and there was no obvious difference between the two biochars. Nevertheless, the peak at 1700 cm−1 resulted from the carboxyl C = O stretching vibration [Citation37,Citation38] obviously appeared in HF-BC in accord with the Boehm titration analysis results (). The C = C peaks of 1610 [Citation39]intensities remained nearly unaltered. Relatively, the intensities of the Si−O−Si peaks at 1100 cm−1 and 800 cm−1 [Citation27,Citation40] obviously decreased of HF-BC, indicating that removal of Si of HF pretreatment in accord with the SEM-EDS analysis results ().
3.2. Decolorization of AO7 under different systems
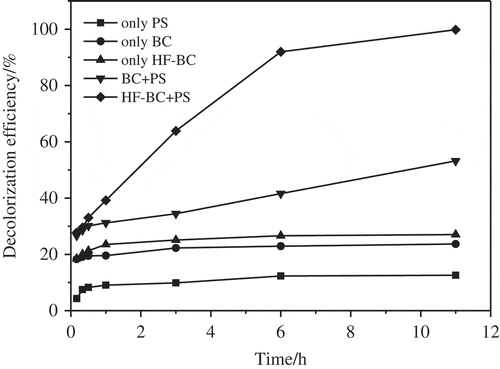
AO7, as a representative azo dye, is either absorbed by biochar or oxidized by PS as presented in . When the initial concentration of AO7 was 20 mg• L−1, 23.7% to 27.0% of AO7 was adsorbed by the sole biochar (BC or HF-BC) and about12.6% AO7 was oxidized by the sole PS within 11 h. Biochar together with PS can induce decolorization of AO7 in a more significant manner with its removal rate ranging from 53.2% to 99.8%. Hence, an important synergistic effect was seen in the combined system of BC/PS and HF-BC/PS. The outcomes were similar to other studies [Citation41,Citation42]. Compared to the BC, HF-BC showed a good catalyst for PS decomposition activation and the AO7 degradation induction at ambient temperature () probably due to its high surface area and micropore volume (). Good development of surface area may make it very convenient for the existence of substantial un-saturated carbon atoms as well as the functional group, which results in great concentration of the unpaired electron and active site [Citation26]. Moreover, the higher surface area stood for more chances for S2O82- ions to become capable of interacting with active spots of biochars in this study. Furthermore, HF-BC has much more carboxyl and phenolic hydroxyl groups (, ) that were beneficial for activating S2O82- to produce more SO4−• [Citation22,Citation43] led to more AO7 degradation. Some studies reported that the long-lasting free radical was found within biochars to make persulfates activated for contaminant degradation [Citation23,Citation24]. Whether the kinds or/and quantity of the persistent free radicals changing in both unmodified biochar and HF-modified biochar in this study need to explore in the future. All of which offered a new idea that the designated biochar could be explored and produced with great catalysis ability.
3.3. Factors influencing activated persulfate on degradation of AO7
3.3.1. Effect of PS concentration
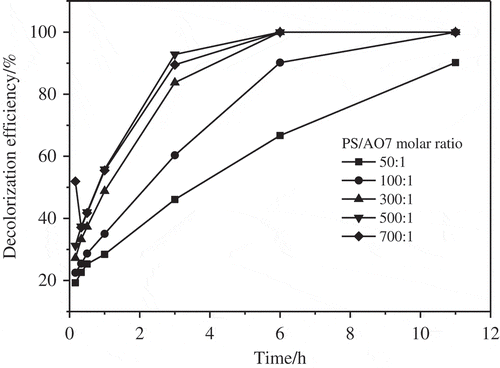
shows that the increase in the concentration of PS may accelerate AO7 degradation in the combined system of HF-BC/PS. 46.1–92.8% of AO7 was degraded in 3 h, with the molar ratio of PS/AO7 increasing from 50/1 to 700/1. After 11 h, almost 100% of AO7 was degraded under different PS/AO7 molar ratios except for the ratio of 50/1 with 90.2% removal. Nevertheless, no obvious raise was found for the removal of AO7 as the oxidant increased, when the ratio of PS/AO7 was >300: 1. These similar outcomes had been revealed in prior researches [Citation44,Citation45]. It was found that a slightly slow increment was seen in the degradation rate of organics, with the concentration of PS rising above a certain numerical value. According to the report, SO4−• that was produced by PS catalysis could react with superfluous PS for the formation of SO42− [Citation46].
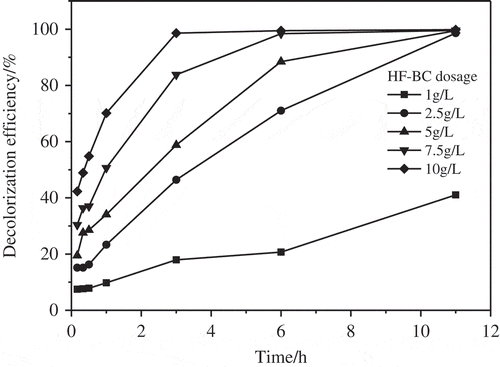
3.3.2. Effect of HF-BC dosage
demonstrates the effect of HF-BC dosage on AO7 decolorization. Within 3 h, 18.0% to 98.6% of AO7 was degraded by the HF-BC/PS-combined system with the HF-BC dosage of 1 g L−1 to 10 g L−1, respectively. It was clear that with the increment in HF-BC dosage, a quicker AO7 bleaching rate got enhanced in this HF-BC/PS combined system. As for its reason, higher HF-BC surface would increase the number of active spots for the purposes of adsorption and catalytic reaction.
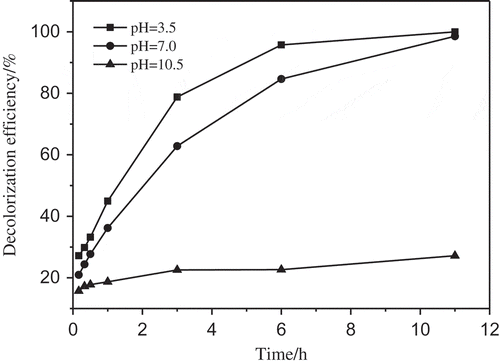
3.3.3. Effect of initial pH
Additionally, study on the initial pH effectiveness was performed at the HF-BC dosage of 5.0 g/L, PS/AO7 molar ratio 100/1. As the aqueous phase pH raised from 3.5 to 10.5, the decolorization efficiency of AO7 was obviously decreased from 100% to 27.2% (). Under the conditions of acidic and neutral pH, SO4−• was the primary radical in the solution. When the solution pH was under alkaline, SO4−• react with OH- to generate •OH, and the lifetime of the •OH is shorter than that of SO4−• led to low decolorization efficiency of AO7 [Citation15,Citation45]. Moreover, the pHPZC of HF-BC in our researches was about 5.5, when the solution pH <5.5, the surface of HF-BC was positive charge which was beneficial to attract S2O82- ion (negative charge) on its surface where persulfate activation seems to occur on through interaction with the surface functional groups generating radicals to degrade AO7 [Citation25]; in contrast, when the solution pH > 5.5, the surface of HF-BC was negative charge and with pH increased, the negative charge strength enhanced, which repelled S2O82- ions easily led to the free radicals decrease from HF-BC surface and decolorization efficiency of AO7 was obviously declined. Therefore, the pH of the aqueous solution had great influence in organic degradation and lower pH was favorable for organic degradation in the combined system of HF-BC/PS.
4. Conclusions
In summary, in contrast with the process of simple use of PS or biochar, the process of combined use of PS and biochar played a more effective role in removing AO7. The HF-BC had obviously advantage for catalyzing PS to degrade the AO7 indicated that it was a good way for exploration and production of specified biochar featured with great catalysis ability by HF pretreatment. For the HF-BC/PS system, with the increase of PS concentration and the HF-BC dosage, an improvement was seen in the degradation efficiency. The pH of an aqueous solution had an important impact on organic degradation. Moreover, lower pH was favorable for its degradation. HF-BC can be easily gained through this easy-to-operate combined system, which demonstrates that HF-BC/PS has a potential practical application. It is important to note that this research only adopted a biomass of rice hull; tests on various biomass feedstocks ought to be carried out and further study should be made in the future in terms of HF-pretreatment effectiveness and the persistence of catalytic ability.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Carneiro PA, Umbuzeiro GA, Oliveira DP, et al. Assessment of water contamination caused by a mutagenic textile effluent/dyehouse effluent bearing disperse dyes. J Hazard Mater. 2010;174:694–699.
- Nguyen TD, Phan NH, Do MH, et al. Magnetic Fe2MO4 (M: fe, Mn) activated carbons: fabrication, characterization and heterogeneous fenton oxidation of methyl orange. J Hazard Mater. 2011;185:653–661.
- Wang Y, Wang JF, Zou HM, et al. Heterogeneous activation of hydrogen peroxide using γ-Al2O2 supported bimetallic Fe, Mn for the degradation of reactive black 5. RSC Adv. 2016;6:15394–15401.
- Liu H, Li G, Qu J, et al. Degradation of azo dye acid orange 7 in water by FeO/granular activated carbon system in the presence of ultrasound. J Hazard Mater. 2007;144:180–186.
- Stylidi M, Kondarides DI, Verykios XE. Visible light-induced photocatalytic degradation of acid orange 7 in aqueous TiO2, suspensions. Appl Catal B-Environ. 2004;47:189–201.
- Zhang SJ, Yu HQ, Li QR. Radiolytic degradation of acid orange 7: a mechanistic study. Chemosphere. 2005;61:1003–1011.
- Azam A, Hamid A. Effects of gap size and UV dosage on decolorization of C.I. acid orange 7 by UV/H2O2 process. J Hazard Mater. 2006;133:167–171.
- Pendyal B, Johns MM, Marshall WE, et al. Removal of sugar colorants by granular activated carbons made from binders and agricultural by-products. Bioresour Technol. 1999;69:45–51.
- Yang SY, Wang P, Yang X, et al. Degradation efficiencies of azo dye acid orange 7 by the interaction of heat, UV and anions with common oxidants: persulfate, peroxymonosulfate and hydrogen peroxide. J Hazard Mater. 2010;179:552–558.
- Yang SY, Wang P, Yang X, et al. A novel advanced oxidation process to degrade organic pollutants in wastewater: microwave activated persulfate oxidation. J Environ Sci. 2009;21:1175–1180.
- Huang KC, Couttenye RA, Hoag GE. Kinetics of heat-assisted persulfate oxidation of methyl tertbutyl ether (MTBE). Chemosphere. 2002;49:413–420.
- Waldemer RH, Tratnyek PG, Johnson RL, et al. Oxidation of chlorinated ethenes by heat-activated persulfate: kinetics and products. Environ Sci Technol. 2007;41:1010–1015.
- Oh SY, Kim HW, Park JM, et al. Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J Hazard Mater. 2009;168:346–351.
- Lau TK, Wei C, Graham NJD. The aqueous degradation of butylated hydroxyanisole by UV/S2O82−: study of reaction mechanisms via dimerization and mineralization. Environ Sci Technol. 2007;41:613–619.
- Matzek LW, Carter KE. Activated persulfate for organic chemical degradation: a review. Chemosphere. 2016;151:178–188.
- Neta P, Huie RE, Ross AB. Rate constants for reactions of inorganic radicals in aqueous solution. J Phys Chem Ref Data. 1988;17:1027–1284.
- Buxton GV, Greenstock CL, Helman WP, et al. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J Phys Chem Ref Data. 1988;17:51–886.
- Lehmann J, Joseph S. Biochar for environmental management: science and technology. London: Earthscan; 2009.
- Cao X, Ma L, Gao B, et al. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol. 2009;43:3285–3291.
- Liu WJ, Zeng FX, Jiang H, et al. Preparation of high adsorption capacity biochars from waste biomass. Bioresour Technol. 2011;102:8247–8252.
- Duan XG, Sun HQ, Wang SB. Metal-Free carbocatalysis in advanced oxidation reactions. Acc Chem Res. 2018;51:678–687.
- Yan J, Lu H, Gao W, et al. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour Technol. 2015;175:269–274.
- Fang G, Gao J, Liu C, et al. Key role of persistent free radicals in hydrogen peroxide activation by biochar: implications to organic contaminant degradation. Environ Sci Technol. 2014;48:1902–1910.
- Fang G, Liu C, Gao J, et al. Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ Sci Technol. 2015;49:5645–5653.
- Kemmoua L, Frontistis Z, Vakros J, et al. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: factors affecting the activation and degradation processes. CatalToday. 2018;313:128–133.
- Huang D, Wang Y, Zhang C, et al. Influence of morphological and chemical features of biochar on hydrogen peroxide activation: implications on sulfamethazine degradation. RSC Adv. 2016;6:73186–73196.
- Zhang X, Zhang S, Yang H, et al. Effects of hydrofluoric acid pre-deashing of rice husk on physicochemical properties and CO2 adsorption performance of nitrogen-enriched biochar. Energy. 2015;91:903–910.
- Das P, Ganesh A, Wangikar P. Influence of pretreatment for deashing of sugarcane bagasse on pyrolysis products. Biomass Bioenergy. 2004;27:445–457.
- Martin SM, Kookana RS, Van ZL, et al. Marked changes in herbicide sorption-desorption upon ageing of biochars in soil. J Hazard Mater. 2012;231–232:70–78.
- Davies LC, Pedro IS, Novais JM, et al. Aerobic degradation of acid orange 7 in a vertical-flow constructed wetland. Water Res. 2006;40:2055–2063.
- Daneshvar N, Rasoulifard MH, Khataee AR, et al. Removal of C.I. acid orange 7 from aqueous solution by UV irradiation in the presence of ZnO nanopowder. J Hazard Mater. 2007;143:95–101.
- Li FY, Cao XD, Zhao L, et al. Effects of mineral additives on biochar formation: carbon retention, stability, and properties. Environ Sci Technol. 2014;48:11211−11217.
- Liang C, Huang CF, Mohanty N, et al. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere. 2008;73:1540–1543.
- Lehmann L, Rillig MC, Thies J, et al. Biochar effects on soil biota – A review. Soil Biol Biochem. 2011;43:1812–1836.
- Sun Y, Gao B, Yao Y, et al. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem Eng J. 2014;240:574–578.
- Xiao X, Chen B, Zhu L. Transformation, morphology, and dissolution of silicon and carbon in rice straw-derived biochars under different pyrolytic temperatures. Environ Sci Technol. 2014;48:3411–3419.
- Koch A, Krzton A, Finqueneisel G, et al. A study of carbonaceous char oxidation in air by semi-quantitative FTIR spectroscopy. Fuel. 1998;77:563−569.
- Pradhan BK, Sandle NK. Effect of different oxidizing agent treatments on the surface properties of activated carbons. Carbon. 1998;37:1323−1332.
- Chen Z, Chen B, Chiou CT. Fast and slow rates of naphthalene sorption to biochars produced at different temperatures. Environ Sci Technol. 2012;46:11104−11111.
- Guo J, Chen B. Insights on the molecular mechanism for the recalcitrance of biochars: interactive effects of carbon and silicon components. Environ Sci Technol. 2014;48:9103–9112.
- Zhang J, Shao X, Shi C, et al. Decolorization of acid orange 7 with peroxymonosulfate oxidation catalyzed by granular activated carbon. Chem Eng J. 2013;232:259–265.
- Yang S, Yang X, Shao X, et al. Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J Hazard Mater. 2011;186:659–666.
- Yan J, Zhu L, Luo Z, et al. Oxidative decomposition of organic pollutants by using persulfate with ferrous hydroxide colloids as efficient heterogeneous activator. Sep Purif Technol. 2013;106:8–14.
- Chu W, Lau TK, Fung SC. Effects of combined and sequential addition of dual oxidants (H2O2/S2O82−) on the aqueous carbofuran photodegradation. J Agric Food Chem. 2006;54:10047–10052.
- Hori H, Yamamoto A, Hayakawa E, et al. Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant. Environ Sci Technol. 2005;39:2383–2388.
- Yu XY, Bao ZC, Barker JR. Free radical reactions involving Cl•, Cl2•−, and SO4•− in the 248 nm photolysis of aqueous solutions containing S2O82− and Cl−. J Phys Chem. 2004;108:295–308.