Abstract
This study aimed to investigate the role of sevoflurane on anti-oxidant effects in sepsis-induced multiple organ failure. Western blot and quantitative RT–PCR were used to measure the expression of Nrf2 and its downstream genes. The DCFH-DA fluorescence probe assay was performed to assess the intracellular levels in HUVECs and LMVECs. Electrophoretic mobility shift assay was used to assess the DNA binding activities of Nrf2. Better survival, reduced expression of inflammatory cytokines, and improved pathological infiltration in the lungs and kidneys were observed in septic rats treated with sevoflurane. SOD activity increased and Nrf2–ARE signaling pathway was activated following sevoflurane treatment. The protective effect was lost in sepsis-induced acute lung injury (ALI) following inhibition of Nrf2 by intratracheal delivery of siRNA-Nrf2. In vitro experiments, we found the sevoflurane could suppress ROS production after stimulation with LPS and the application of sevoflurane augments the DNA-binding activity of Nrf2 in HUVECs and LMVECs. However, knockdown of Nrf2 by siRNA-Nrf2, the Protein kinase C (PKC) inhibitor, and the Nrf2 inhibitor ML385 could partially recover ROS production, inhibit the activity of SOD, and repress the expression of anti-oxidant genes following sevoflurane treatment. Sevoflurane could have protective effects in ALI and septic multiple organ disorders (MODs).
Introduction
Multiple organ failure in sepsis has a high mortality rate and poor prognosis and sepsis-induced acute lung injury is the most important complication that leads to admission to the intensive care unit and death (Angus et al. Citation2001; Mayr et al. Citation2014). Further investigation is needed to determine the underlying mechanisms of excessive inflammation and tissue damage in septic-induced target organ injuries (Angus et al. Citation2001). Therefore sepsis is considered as a public health concern in worldwide. The severe infection and secondary release of proinflammatory cytokines in septic patients would result in systemic inflammatory response syndrome (SIRS) and multiple organ failure, which are a rather high risk of death (Angus et al. Citation2001; Gustot Citation2011; Singer et al. Citation2016).
Several important biological mechanisms related to sepsis have been reported, including regulation of cytokine production, infiltration of neutrophils, oxidative stress, mitochondrial injury, microvascular endothelial cell dysfunction, and microcirculation disturbance (Hawiger et al. Citation2015). Redox homeostasis is disrupted during sepsis and MODS. The oxidative stress response is uncontrolled in various pathological conditions including sepsis-induced organ failure, where the deficiency in antioxidant leads to inadequate protection against reactive oxygen species (ROS) and lipid peroxidation and cytokine cascade (Guo and Ward Citation2007; Victor et al. Citation2009; Galley Citation2011; Hawiger et al. Citation2015). In sepsis, some factors including activation of neutrophils, ischemia, and reperfusion contribute to excessive production of ROS, which subsequently lead to oxidative stress injury and mitochondrial damage (Victor et al. Citation2009; Galley Citation2011). These alterations result in the release of superoxides, destruction of microvascular endothelial cells, and change in membrane permeability, further promoting the infiltration of inflammatory cells and cytokine production. Inhibition of oxidative stress response and recovery of redox homeostasis should be considered as a vital intervention strategy.
In sepsis, Nrf2–ARE is an important signaling pathway serving to prevent oxidative damage. Nrf2 expression is controlled by its upstream regulator Keap-1 via ubiquitination and degradation (Zenkov et al. Citation2013; Suzuki and Yamamoto Citation2015). The increased stability and nuclear translocation of the transcription factor Nrf2 occurs via post-translational regulation during oxidative stress. Phosphorylation and subsequent dissociation of the Keap-1/Nrf2 complex leads to Nrf2 stabilization (Zenkov et al. Citation2013; Niture et al. Citation2014; Suzuki and Yamamoto Citation2015; Tebay et al. Citation2015). Then Nrf2 enters the nucleus and binds to antioxidant response elements, thereby promoting the expression of ARE-driven antioxidative enzymes. Some protein kinases, including protein kinase C, GSK-3β, and p38MAPK, are proved to be involved in the regulation of Keap1/Nrf2/ARE signaling (Niture et al. Citation2014).
Some drugs have proven to be effective inducers of keap-1/Nrf2/ARE signaling, including sulforaphane and α-lipoic acid (Petersen Shay et al. Citation2008; Keum Citation2011). Lee et al found that sevoflurane increased the expression of Nrf2 in rat models of cerebral ischemia, and had neuroprotective effects dependent on the PKC pathway (Lee et al. Citation2015). However, the antioxidant effects of sevoflurane in other diseases models, such as inflammatory diseases and sepsis-induced MODS, is currently unknown, although the disturbance in anti-oxidant defenses and oxidative stress injury are known to be important to the uncontrolled inflammation and tissue damage.
In this study, we found that intratracheal inhalation of sevoflurane improves MODS in septic rats dependent on activation of Nrf2/ARE signaling, including in acute kidney and lung injury. The activation of Nrf2/ARE signaling inhibited inflammatory infiltration and release of proinflammatory cytokines via improvement of antioxidant defenses and maintenance of redox homeostasis. This study provides a novel intervention strategy for sepsis-induced MODS and demonstrates that sevoflurane has therapeutic value in sepsis-induced MODS.
Materials and methods
Septic models
We used 8- to 10-week-old, male, Sprague Dawley rats (n = 15) in our study. SD rats were purchased from the Experimental Animals Center of Nanjing Medical University. All animals were kept under standard housing environment, 12-h light/dark cycle in a temperature controlled (22 ± 2°C), and fed a chow diet. The experimental protocol performed in the study was approved by the Institutional Animal Care and Ethics Committee of the Second People's Hospital of Yuhang District. Cecal ligation and puncture performed in our study were used to establish the septic rat models according to protocols previous described (Erbas and Taskiran Citation2014). The rats were anesthetized intraperitoneally by ketamine hydrochloride at a dose of 75 mg/kg (Tocris Biosecience, Bristol, England) in combination with xylazine (Rompun®) (10 mg/kg) and then the junction between the intestine and the cecum was exposed after 3–4 cm midline laparotomy. The bottom of the cecum at the site of the ileo-cecal valve was then ligated tightly with a 3.0 silk suture and punctured three times with a 22-gauge needle in the position of the ligature. Eventually, cecum was then gently squeezed to the abdominal cavity and the laparotomy incision was closed with 4-0 polyglactin 910 sutures. In the control group, under aseptic conditions, only laparotomy was performed but the cecum was neither ligated nor punctured. The septic rats received intratracheal inhalation of 5% sevoflurane in 97% oxygen via tracheal intubation connection with anesthesia machine (R620, Woruide Ltd., Shenzhen, China) for 30 min for seven days. The control group only received the inhalation of 97% oxygen.
Intratracheal injection of viral vectors containing Nrf2-siRNA
The intratracheal injection of adenovirus vectors dissolved in the medium was performed 3 days before the first performance of sevoflurane delivery according to protocols described in a previous study (Chen et al. Citation2014). To generate a mouse model of airway epithelial cells-derived Nrf2 knockdown, Ad-siRNA-Nrf2 or Ad-Scramble (approximately 109PFU) was intratracheally injected into the rats via tracheal intubation after anesthesia with chloral hydrate (400 mg/kg, intraperitoneally (i.p.)). To improve the knockdown efficiency, a second administration of the adenovirus was performed 12 h later.
RNA isolation and quantitative real-time PCR
Total RNA of the lung and kidney tissue and liver tissues as well as the alveolar macrophages was extracted using the TRIzol™ Reagent. Reverse transcription was performed using 1 μg of total RNA with the Reverse Transcription System (Promega, Madison, WI, USA). Gene expression analysis was performed by quantitative real-time PCR (qPCR) following reverse transcription according to standard protocols. The sequences of primers used for mRNA expression analysis by RT-qPCR are shown in . qPCR was performed on QuantStudio 5 (Applied Biosystems, USA) using SYBR green PCR master mix (Applied Biosystems, Carlsbad, CA). The qPCR data were analyzed using the 2-ΔΔCt method. The relative expression of target genes was normalized to the expression of GAPDH mRNA, and represented as fold change.
Table 1. Primers used in RT-PCR.
Evaluation of lung infiltration
Lung tissues were dissected from septic or sham rats, and were immediately fixed in buffered 4% paraformaldehyde solution for 24 h, embedded in paraffin, and sectioned at a thickness of 4 μm. The histological examination was conducted in a blinded fashion after staining with hematoxylin and eosin.
Serum biochemical analysis
Blood samples from rats were collected from the caudal vein at 1, 2, 4, 6, 8, 10, 12, and 14 days after CLP surgery. Serum was obtained and prepared by centrifugation at 12,000 g for 3 min. We analyzed the levels of blood urea nitrogen (BUN) or serum creatinine (Scr) using commercial kit reagents (AppliedBio assay kits; Hercules, CA, USA) and an auto-analyzer (QuikLab, USA). Each sample was run in duplicate.
Detection of SOD activities
SOD activity was detected using a Superoxide Dismutase Activity Assay Kit (ab65354, Abcam, Cambridge, British) according to the standard instruction. A total of 10 mg fresh, non-fixed lung and kidney tissues or cultured cells were immediately frozen in liquid nitrogen following washing, and then stored at −80°C in a refrigerator. Homogenization was performed in ice-cold 0.1M Tris/HCl, pH 7.4 containing 0.5% Triton X-100, 5 mM β-ME, 0.1 mg/ml PMSF. The supernatants were collected and transferred to a clean tube after Centrifuge at 14,000 x g for 5 min at +4°C. 20 µL of Enzyme Working Solution and 200 µL of WST working solution were added to each sample well, mixed and incubated at 37°C for 20 min, and measured the output by colorimetric method at OD 450 nm on chemiluminescence analyzer (Tohoku Electronic Industrial Co., Ltd., Japan). Each sample was assayed with two replicates.
Measurement of intracellular reactive oxygen species
The detection of intracellular levels of ROS was performed by microplate assays according to the instruction of DCFDA Cellular ROS Detection Kit (ab1113851, Abcam, Cambridge, British). HUVECs and LMVECs were harvested, seeded in a dark, clear-bottomed 96-well microplate with 25,000 cells per well, and allowed to adhere overnight. Then, the supernatant was removed and 100 µL/well of 1X Buffer and 100 µL/well of the diluted DCFDA Solution was added to stain cells. After 45 min at 37°C in the dark, DCFDA solution was removed, and fluorescence was measured immediately on a microplate reader at Ex/Em = 485/535 nm. The result is presented as the measured value after subtracting blank readings from all measurements and determining the fold change from assay control.
Cell culture
HUVECs and LMVECs cells (Fenghui Bio, Beijing, China) were purchased from Fenghui Biotech Ltd. and cultured in low-glucose Dulbecco's modified Eagle's medium (DMEM, Gibco Life Technologies, Rockville, MD) containing 100 units/ml of penicillin, 100 μg/ml of streptomycin, and 10% FBS in a humidified incubator at 37°C with 5% CO2. Then sevoflurane at a gradient concentration of 2, 5, 10, 15, 20, and 40 μM was added into the cultured medium containing LPS (1 EU/ml) and then the production of intracellular ROS levels in the cells were measured to find the minimum concentration of sevoflurane that could reduce the production of ROS.
Nuclear protein extraction and electrophoretic mobility shift assay
The DNA-binding of Nrf2 was detected by EMSA as previously described using nuclear extracts prepared by a nuclear extract kit (Active Motif, Rixensart, Belgium) from lung and kidney tissue. The sequences of the biotin-labeled oligonucleotide probe for EMSA were as follows: 5’-ACTGAGGGTGACTCAGCAAAATC-3’, 3’-TGACTCCCACTGAGTCGTTTTG-5’. The final concentration of probe was 1.5 pmol/L. The reaction mixtures were separated by 7% non-denaturing PAGE and then transferred onto a nitrocellulose membrane, and cross-linked for 10 min. After being blocked in blocking buffer for 1 h and incubated with strepto-avidin-conjugated horseradish peroxidase (1:300), the mixtures were subsequently washed five times. Peroxidase activity was detected using an ECL substrate system. Images were captured and quantified using the ImageJ software.
Western blot
Proteins from the lungs and kidney of mice with sepsis were prepared using lysis buffer. Samples containing equal amounts of proteins were resolved by SDS-PAGE and transferred to a PVDF membrane (Millipore). Membranes were blocked with 5% milk in Tris buffered saline (TBS) for 2 h at 37°C and incubated overnight at 4°C with the following primary antibodies: anti-Catalase/SOD1/TRX (Oxidative stress defense Western blot Cocktail, ab179843), anti-Nrf2 (ab137550), and anti-GAPDH (1:1000, sc-47778, Santa Cruz Biotechnology). Membranes were washed and incubated with the secondary antibody for 1 h at 25°C. The gray value of protein bands was determined using ECL Fuazon Fx (Vilber Lourmat).
Construction of Nrf2-siRNA
The Construction of an Adenovirus vector encoding Nrf2-siRNA was authorized to Shanghai Jikai gene chemical technology CO., Ltd (China, Shanghai). An adenovirus-mediated vector was used for silencing Nrf2 in HUVECs and LMVECs cells in vitro (at a concentration of 0.5 × 108∼108 PFU/ml). The negative control (NC) was constructed by using a scrambled sequence not capable of encoding the target gene.
Statistical analysis
All experiments were repeated at least five times, and the data are represented as mean ± standard error of the mean (SEM). Unpaired t-tests were used to determine the statistical differences between two groups in each analysis. An analysis of variance (ANOVA) for repeated experiments was used for multiple comparisons. p < .05 were considered to indicate statistical significance. All data were analyzed by using the log-rank test. All statistical analyses were performed with SPSS 13.0.
Results
Sevoflurane improves survival and reduces the expression of proinflammatory cytokines in septic rats
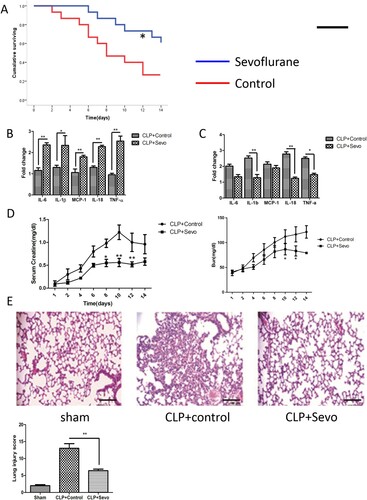
To study the organ protection of sevoflurane in sepsis-induced MODS, we examined the expression of inflammatory cytokines, serum creatine levels, and lung histopathology after administration of sevoflurane. Continuous inhalation of 5% sevoflurane for one week after CLP surgery significantly improved survival in septic rats. Survival curves are presented in (A). Mortality significantly decreased in groups treated with inhaled sevoflurane. Meanwhile, expression of inflammatory cytokines including IL-6, IL-1β, TNF-α, IL-18, MCP-1 was significantly reduced in the lungs and kidneys from septic rats treated with sevoflurane ((B,c)). Inflammatory infiltration in lung tissue and impaired renal function in septic rats were attenuated after administration of sevoflurane ((D,E)). These data demonstrate that intratracheal administration of sevoflurane inhibits inflammation due to cytokine production, ultimately improving organ function and reducing mortality in septic rats.
Figure 1. Inhaled sevoflurane improves survival and restricts lung inflammation. (A) Kaplan-Meier survival analysis according to the death rates of septic rats. The blue broken line represents the group treated with sevoflurane and red represented the control group. The asterisk above the blued line symbolized the significant difference compared to the controls (n = 15, p < .05). (B and C) The expression of proinflammation in lung (left, B) and kidney (right, C) tissue from septic rats measured by quantitative RT-PCR (n = 5). (D) The serum levels of Creatine and blood urea nitrogen (BUN) in septic rats by continuously dynamic measurement. The X-axis represented the measured time after CLP surgery. The difference in data at different points-in-time was analyzed by the t-test or ANOVA for repeated experiments (n = 15). (E) The representative histological figures and result of quantitative analysis of lung injury score (n = 5). Magnification X200. Black bar, 100 μm. The experiments were replicated three times. All data are represented as Mean ± SD. **p < .01, *p < .05.
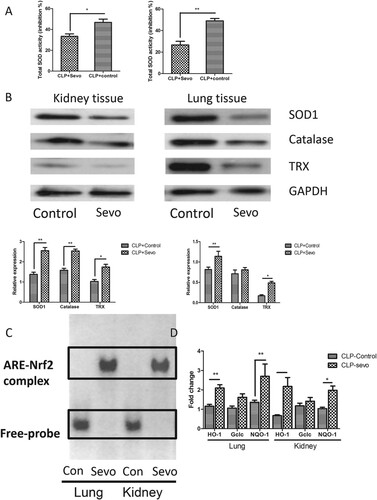
Sevoflurane increases superoxide dismutase expression and activates Nrf2–ARE signaling in septic rats
To explore the protective mechanisms of inhaled sevoflurane in septic models, we hypothesized that protective effects might be attributed to preventing oxidation stress injury. We measured the activities of superoxide dismutase and expression of proteins related to oxidative stress after treatment with inhaled sevoflurane. Significantly increased activity of SOD was observed in protein extracts from lung and kidney tissue ((A)). Expression of catalase, superoxide dismutase 1 and TRX significantly increased after administration of inhaled sevoflurane in septic rats ((B)). Sevoflurane promoted anti-oxidant production and activated SOD1, protecting the target tissue from oxidative stress injury. Reactive oxygen species causes mitochondrial function dysfunction and tissue damage via superoxide-derived free oxygen radicals. The sevoflurane-induced increase of anti-oxidant inhibited the ROS and free oxygen radical production, which recovered the redox balance and eliminated the oxidative stress injury. Furthermore, we examined the DNA binding activity of Nrf2 and measured the expression levels of free Nrf2 in nucleoprotein fraction in the target organ, including lung and kidney. We found increased DNA-binding activity of Nrf2 in lung and kidney from rats with inhaled administration of sevoflurane by an electrophoretic mobility shift assay ((C)), which suggested dissociation of Nrf2-Keap1 and subsequent nuclear translocation of free Nrf2, which upregulated the expression of various anti-oxidative genes by binding to antioxidant responsive elements (ARE), including HO-1, Gclc, and NQO-1. We next detected the expression of genes downstream of Nrf2–ARE signaling. We also found markedly increased expression in the lung and kidney from septic rats treated with sevoflurane ((D)). These data suggest that sevoflurane activates the Nrf2/ARE signaling pathway, resulting in anti-oxidative effects in septic rats.
Figure 2. Sevoflurane reduces oxidative stress-induced injury in lung and kidney via activation of Nrf2 signaling. (A) The total activity of superoxide dismutase measured by colorimetric method in lung and kidney from the septic rats with or without treatment with inhaled sevoflurane (n = 6). (B) The representative figures and quantitative analysis of immunoblot result reflect the expression of antioxidative defense species in lung and kidney from septic rats with or without inhalation of sevoflurane (n = 6). (C) The impact of sevoflurane on the ARE-binding activity of Nrf2 reflected by EMSA assays in the nuclear extract from lung and kidney tissue. (D) The expression of downstream genes of Nrf2/ARE signaling pathway in lung and kidney tissue measured by quantitative RT-PCR (n = 6). The experiments were replicated three times. All data are represented as Mean ± SD. **p < .01, *p < .05.
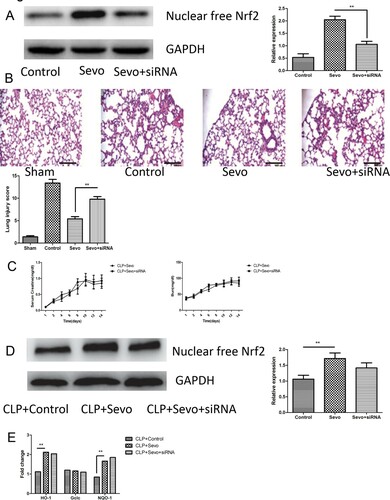
Intratracheal delivery of siRNA targeting Nrf2 prevents sevoflurane-mediated protection in acute lung injury
To verify the central role of Nrf2 and Nrf2/ARE activation in the protective effect of sevoflurane, we delivered adenovirus vectors containing Nrf2-siRNA to pulmonary alveoli twelve hours prior to sevoflurane inhalation in septic rats. We measured the expression of Nrf2 in lung tissue from rats treated with intratracheally injected Nrf2-siRNA. In these rats the expression of Nrf2 was partly diminished ((A)). We found that intratracheal delivery of Nrf2-siRNA would aggravated lung infiltration and tissue damage in those rats with inhalation of sevoflurane ((B)). Intriguingly, sevoflurane inhalation still improved renal function in those rats intratracheally treated with Nrf2-siRNA. We found that intratracheal injection of the Nrf2-siRNA could not have influence on the reduced serum creatinine and blood urea nitrogen (BUN) after administration of sevoflurane ((C)). Then we measured the activity of free Nrf2 in nuclear protein isolated from kidney tissue and downstream anti-oxidative genes ((D,E)). No difference in expression of free Nrf2 and HO-1, Gclc, and NQO-1 was observed ((E)). The intratracheal application of Nrf2-siRNA only silence the expression of Nrf2 in alveolar epithelial cells but fail to affect the expression of Nrf2 in kidney, therefore it have only adverse effect on the protective role of sevoflurane in lung injury. These data suggested that the sevoflurane attenuated inflammation, oxidative stress and tissue damage dependent on the Nrf2 signaling.
Figure 3. Nebulization of Nrf2-siRNA abrogates the protective effect of sevoflurane in acute lung injury. (A) The representative figures and quantitative analysis of immunoblotting reflect the expression of nuclear free Nrf2 in lung tissue from septic rats with or without administration of atomizing medium dissolved in adenovirus vector encoding Nrf2-siRNA after inhalation of sevoflurane (n = 6). (B) The representative histological figures and result of quantitative analysis of lung injury score in lung tissue from rats with or without administration of adenovirus vector encoding Nrf2-siRNA (n = 6). Magnification× 200. Black bar, 100 μm. (C) The serum levels of Creatine and blood urea nitrogen (BUN) were measured by continuous dynamic measurement in lung tissue from rats with or without intratracheal administration of adenovirus vector encoding Nrf2-siRNA (n = 6). (D) The representative figures and quantitative analysis of immunoblotting about expression of nuclear free Nrf2 in kidney tissue from septic rats treated with or Nrf2-siRNA after inhalation of sevoflurane (n = 6). (E) The expression of downstream genes of Nrf2/ARE signaling pathway in kidney tissue measured by quantitative RT-PCR from rats with or without intratracheal administration of adenovirus vector encoding Nrf2-siRNA (n = 6). The experiments were replicated three times. All data are represented as Mean ± SD. **p < .01, *p < .05.
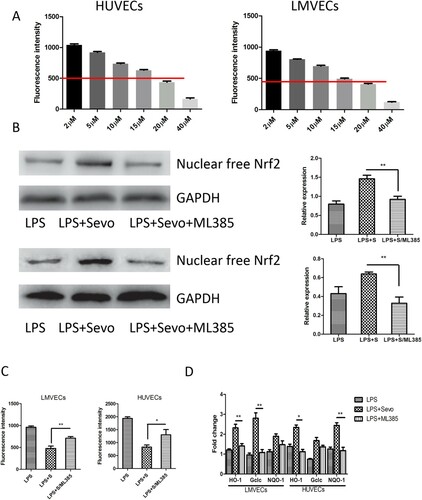
Sevoflurane attenuate LPS-induced intracellular oxidative stress response in HUVECs and LMVECs
It is have been proved that oxidative stress injury occurred in microvascular endothelial cells (HUVECs and LMVECs) following with LPS-stimulation (Fu et al. Citation2015; Li et al. Citation2016). Intracellular levels of ROS in HUVECs and LMVECs treated with sevoflurane were measured after stimulation with LPS (1 EU/ml). We determined the minimum effective concentration of sevoflurane capable of reducing intracellular ROS by 50% to be 20 μM ((A)). We measured the expression of endonuclear free Nrf2 and antioxidative genes (HO-1, Gclc, and NQO-1) in HUVECs and LMVECs treated with 20 μM sevoflurane. Increased expression of free nuclear Nrf2 and downstream genes was observed after administration of sevoflurane ((B,C)). A PKC inhibitor and a specific inhibitor for Nrf2 signaling, ML385, were used to block the Nrf2 signaling pathway and prevent PKC phosphorylation. The inhibitor of PKC ML385 partially recovered ROS production and repressed the expression of anti-oxidative genes following introduction of sevoflurane ((C,D)). Therefore, we verified the conclusion that sevoflurane has antioxidant pharmacologic action, and protects septic rats from sepsis-induced MODs via activation of Nrf2 dependent on protein kinase C.
Figure 4. Sevoflurane improve Protein kinase C-dependent oxidative stress responses in HUVECs and LMVECs stimulated with LPS. (A) The minimum concentration of sevoflurane necessary to inhibition of 50% ROS activity by intracellular ROS detection dependent on DCFDA probe at gradient concentration of sevoflurane. The cells per group were measured in five replicated wells and then calculate the average value. (B) The representative figures and quantitative analysis revealed the expression of nuclear free Nrf2 in LPS-stimulated HUVECs and LMVECs after treated by sevoflurane solely and combination with ML385 measured by immunoblot assay. (C) The ROS activity detected by intracellular ROS detection dependent on DCFDA probe in LPS-stimulated HUVECs and LMVECs after treated by sevoflurane solely and combination with ML385 measured by immunoblot assay. (D) The expression of downstream genes of Nrf2/ARE signaling pathway in kidney tissue measured by quantitative RT-PCR in LPS-stimulated HUVECs and LMVECs after treated by sevoflurane solely and combination with ML385 measured by immunoblot assay. The experiments were replicated three times. All data are represented as Mean ± SD. **p < .01, *p < .05.
Discussion
The pharmacology of sevoflurane is complex. In addition to anesthetic applications, potential neuroprotective effects of sevoflurane have also been reported (Adamczyk et al. Citation2010; Ye et al. Citation2012; Yeung et al. Citation2012), although inappropriate long-term exposure to sevoflurane leads to neurotoxicity (Shan et al. Citation2018; Xu et al. Citation2018) and impaired cognitive function, especially in aged and neonatal animal models. Some recent studies related to apoptosis and cell death in various brain injury models including transient cerebral ischemia have shown that exposure to sevoflurane at the molecular and cellular levels could produces anti-oxidative components and protects the neurocytes from oxidative stress injury-induced apoptosis (Lee et al. Citation2015; He et al. Citation2018).
In recent years, the pharmacological action of sevoflurane has received greater attention because of the potential value in combating oxidation and inflammation. In a pilot clinical study, Lavi et al. found reduced infarct size and greater resolution of ST-segments in acute anterior myocardial infarction after administration of sevoflurane (Lavi et al. Citation2014). The study reflects the potential myocardial protective effect in acute ischemia injury, which might be attributed to preventing inflammation and reducing injury/reperfusion injury (I/R injury) of endothelial cells. Inada et al proved that sevoflurane inhibits TNF-α-induced inflammation in airway epithelial cells (Watanabe et al. Citation2013). These studies demonstrate that sevoflurane has unknown pharmacological activities related to preventing inflammation and oxidation in various I/R injury models, including the lungs, heart, and brain.
In sepsis-induced organ dysfunction and tissue damage, oxidative stress-induced injury is a universal phenomenon. A wealth of research has shown that oxidative stress existed in patients with severe sepsis. Insufficient defenses against oxidation in septic patients leads to an imbalance in ROS production and antioxidative protection, which might be an important factor contributing to poor prognosis (Lorente et al. Citation2015). The persistent abnormal serum levels of total antioxidant capacity is strongly associated with poor prognosis and could be used as biomarker to outcome prediction in severe septic patients In another study, Chuang et al found that decreased serum levels of total antioxidant is significantly correlated with higher APACHEII scores and mortality (Chuang et al. Citation2006). These clinical studies demonstrate that low levels of antioxidant have an adverse influence on prognosis in septic patients. Mitochondrial injury in sepsis-induced oxidative stress might be the central mechanism involved in the intense inflammatory response and cellular apoptosis (Galley Citation2011; Quoilin et al. Citation2014; Mantzarlis et al. Citation2017). The peroxidation of the mitochondrial lipid cardiolipin, which is present in the inner mitochondrial membrane and is important for energy metabolism, leads to dissociation of cytochrome c and causes reduced ATP production, and even more ROS production.
Furthermore, mitochondrial DNA (mtDNA) is also a target for damage since it is close to the electron transport chain. This concurrent mitochondrial DNA damage also resulted in the loss of function of electron transport enzymes and overproduction of ROS, which is called a perpetual cycle of ROS production facilitated by ROS-induced ROS release. The destroyed and permeable mitochondrial membrane would lead to release of cytochrome C and other apoptosis-induced factors from inner mitochondrial space to cytoplasm, which ultimately cause the programmed cell death and apoptosis. In sepsis-induced acute lung injury, the disrupted redox homeostasis in local lung tissue have been reported previously. In lipopolysaccharide-induced sepsis, the inhibitor of NADPH oxidase (type2) can protect septic rats from endotoxemia and acute lung injury in a model of lung inflammation produced by the intratracheal (IT) instillation of LPS (Lee et al. Citation2014). Some studies demonstrated that exposure of animals or renal cells to lipopolysaccharide (LPS) induces inflammatory responses and free radical, including reactive oxygen species (ROS) and nitric oxide (NO), which lead to the acute kidney injury in vivo and cell death of renal cells in vitro. Antioxidants can protect against AKI caused by oxidative stress in murine models of endotoxemia. In a study about sepsis-induced acute kidney injury, the propofol could protect kidney from sepsis-induced AKI by increasing BMP-7 expression, decreasing inflammatory cytokines and inhibiting oxidative stress (Hsing et al. Citation2011). These studies show that the antioxidative effect of antioxidant defense and redress of imbalance between antioxidant and oxidant might be a vital factor leading to septic multiorgan failure. Therefore, it is rational that we considered inhibitors of oxidative stress response, and supplementation of antioxidants as a promising therapeutic approach in MODs induced by sepsis. The Keap-1/Nrf2/ARE signaling axis has been conventionally deemed as a classic signaling pathway. Activation of Nrf2 is characteristic of the dissociation of Nrf2 from Keap-1. Free Nrf2 enters the nucleus and binds to the AREs (antioxidant responsive element) in genes related to redox signaling. The process eventually upregulates the expression of antioxidant protein and enhanced the production of antioxidant. The regulation of Nrf2 stability, its transport into/from the nucleus, and binding to ARE involves protein kinases of different families: PKC (protein kinase C), JNK (c-Jun N-terminal protein kinase), PI3K (phosphoinositide 3 kinase), and ERK (extracellular signal-regulated kinase) (Niture et al. Citation2014; Tebay et al. Citation2015). Therefore, the Nrf2 have been considered a valuable pharmacological target for prevention and treatment of wide range of diseases associated with oxidative stress. Previous studies have demonstrated that methylene blue could upregulate the Nrf2/ARE signaling and reduce neural inflammation to protect neurons from tau-induced neurotoxicity (Stack et al. Citation2014). Another study found dihydromyricetin improves the ethanol-induced hepatic pathological change in alcoholic liver disease models via activation of Nrf2/ARE (Qiu et al. Citation2017). Meanwhile, sevoflurane also protect the HUVECs from injury induced by ox-LDL by activation of Nrf2/HO-1 signaling pathway through ERK and Akt (Luo et al. Citation2017). A recent study found that inhaled sevoflurane postconditioning improves neurological function in transient brain ischemia/reperfusion models, via activation of Nrf2 dependent on PKC signaling (Lee et al. Citation2015). Previous study also found that PKC phosphorylates serine residue S40 of Nrf2, which weakens its interaction with Keap1 and increases its stability, but it does not affect transport into the nucleus and binding to ARE. Sevoflurane also attenuated mitochondrial permeability transition pore opening after transient cerebral ischemia, which improved the mitochondrial function and long-term neurological sequelae. This study suggests that sevoflurane might be a potential therapeutic drug for preventing mitochondrial damage and activate Nrf2/ARE signaling because of satisfactory safety and widespread use in anesthesia. In another study exploring the role of ischaemia preconditioning in myocardial I/R injury, the inhibition of PKC by administration of PKC inhibitor Calphostin C, would significantly diminish the protective effect of ischaemia preconditioning in myocardial I/R injury (Okamura et al. Citation1999). These studies proved protein kinase C might be rather vital in the anti-oxidative and anti-apoptosis effects in various diseases models, especially in brain and myocardial ischeamia/reperfusion injury, which could be involved in the regulation of Nrf2 signaling. But there is no relative studies that show the sevoflurane could protect the septic animals from septis-induced organ failure via activation of Nrf2 and induction of anti-oxidative effect dependent on increased expression of downstream antioxidant.
In the present study, we found that inhaled sevoflurane improves septic MODs, including AKI and ALI in rat model. The protective effect from sevoflurane is dependent on the activation of Nrf2/ARE pathway. PKC is also indispensable to the activation of Nrf2 after treatment by sevoflurane, and the importance of PKC for Nrf2/ARE signaling have been proved in neurodegenerative disease and ischemia injury models. Our study demonstrated the indispensability of PKC in Nrf2 activation, and the therapeutic action of sevoflurane in sepsis. The PKC/Nrf2/ARE signaling in antioxidative biological process has therapeutic value, including in other diseases related to disturbance of redox chemistry and inflammation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Adamczyk S, Robin E, Simerabet M, Kipnis E, Tavernier B, Vallet B, Bordet R, Lebuffe G. 2010. Sevoflurane pre- and post-conditioning protect the brain via the mitochondrial K ATP channel. Br J Anaesth. 104(2):191–200. doi: https://doi.org/10.1093/bja/aep365
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 29(7):1303–1310. doi: https://doi.org/10.1097/00003246-200107000-00002
- Chen QX, Song SW, Chen QH, Zeng CL, Zheng X, Wang JL, Fang XM. 2014. Silencing airway epithelial cell-derived hepcidin exacerbates sepsis induced acute lung injury. Crit Care. 18(4):470. doi: https://doi.org/10.1186/s13054-014-0470-8
- Chuang CC, Shiesh SC, Chi CH, Tu YF, Hor LI, Shieh CC, Chen MF. 2006. Serum total antioxidant capacity reflects severity of illness in patients with severe sepsis. Crit Care. 10(1):R36. doi: https://doi.org/10.1186/cc4826
- Erbas O, Taskiran D. 2014. Sepsis-induced changes in behavioral stereotypy in rats; involvement of tumor necrosis factor-alpha, oxidative stress, and dopamine turnover. J Surg Res. 186(1):262–268. doi: https://doi.org/10.1016/j.jss.2013.08.001
- Fu Y, Hu X, Cao Y, Zhang Z, Zhang N. 2015. Saikosaponin a inhibits lipopolysaccharide-oxidative stress and inflammation in human umbilical vein endothelial cells via preventing TLR4 translocation into lipid rafts. Free Radic Biol Med. 89:777–785. doi: https://doi.org/10.1016/j.freeradbiomed.2015.10.407
- Galley HF. 2011. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 107(1):57–64. doi: https://doi.org/10.1093/bja/aer093
- Guo RF, Ward PA. 2007. Role of oxidants in lung injury during sepsis. Antioxid Redox Signal. 9(11):1991–2002. doi: https://doi.org/10.1089/ars.2007.1785
- Gustot T. 2011. Multiple organ failure in sepsis: prognosis and role of systemic inflammatory response. Curr Opin Crit Care. 17(2):153–159. doi: https://doi.org/10.1097/MCC.0b013e328344b446
- Hawiger J, Veach RA, Zienkiewicz J. 2015. New paradigms in sepsis: from prevention to protection of failing microcirculation. J Thromb Haemost. 13(10):1743–1756. doi: https://doi.org/10.1111/jth.13061
- He H, Liu W, Zhou Y, Liu Y, Weng P, Li Y, Fu H. 2018. Sevoflurane post-conditioning attenuates traumatic brain injury-induced neuronal apoptosis by promoting autophagy via the PI3K/AKT signaling pathway. Drug Des Devel Ther. 12:629–638. doi: https://doi.org/10.2147/DDDT.S158313
- Hsing CH, Chou W, Wang JJ, Chen HW, Yeh CH. 2011. Propofol increases bone morphogenetic protein-7 and decreases oxidative stress in sepsis-induced acute kidney injury. Nephrol Dial Transplant. 26(4):1162–1172. doi: https://doi.org/10.1093/ndt/gfq572
- Keum YS. 2011. Regulation of the Keap1/Nrf2 system by chemopreventive sulforaphane: implications of posttranslational modifications. Ann N Y Acad Sci. 1229:184–189. doi: https://doi.org/10.1111/j.1749-6632.2011.06092.x
- Lavi S, Bainbridge D, D'Alfonso S, Diamantouros P, Syed J, Jablonsky G, Lavi R. 2014. Sevoflurane in acute myocardial infarction: a pilot randomized study. Am Heart J. 168(5):776–783. doi: https://doi.org/10.1016/j.ahj.2014.07.009
- Lee H, Park YH, Jeon YT, Hwang JW, Lim YJ, Kim E, Park SY, Park HP. 2015. Sevoflurane post-conditioning increases nuclear factor erythroid 2-related factor and haemoxygenase-1 expression via protein kinase C pathway in a rat model of transient global cerebral ischaemia. Br J Anaesth. 114(2):307–318. doi: https://doi.org/10.1093/bja/aeu268
- Lee I, Dodia C, Chatterjee S, Feinstein SI, Fisher AB. 2014. Protection against LPS-induced acute lung injury by a mechanism-based inhibitor of NADPH oxidase (type 2). Am J Physiol Lung Cell Mol Physiol. 306(7):L635–L644. doi: https://doi.org/10.1152/ajplung.00374.2013
- Li C, Ma D, Chen M, Zhang L, Zhang L, Zhang J, Qu X, Wang C. 2016. Ulinastatin attenuates LPS-induced human endothelial cells oxidative damage through suppressing JNK/c-Jun signaling pathway. Biochem Biophys Res Commun. 474(3):572–578. doi: https://doi.org/10.1016/j.bbrc.2016.04.104
- Lorente L, Martin MM, Almeida T, Abreu-Gonzalez P, Ferreres J, Sole-Violan J, Labarta L, Diaz C, Jimenez A. 2015. Association between serum total antioxidant capacity and mortality in severe septic patients. J Crit Care. 30(1):217 e7–12. doi: https://doi.org/10.1016/j.jcrc.2014.09.012
- Luo Y, Lu S, Dong X, Xu L, Sun G, Sun X. 2017. Dihydromyricetin protects human umbilical vein endothelial cells from injury through ERK and Akt mediated Nrf2/HO-1 signaling pathway. Apoptosis. 22(8):1013–1024. doi: https://doi.org/10.1007/s10495-017-1381-3
- Mantzarlis K, Tsolaki V, Zakynthinos E. 2017. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential Therapies. Oxid Med Cell Longev. 2017:5985209. doi: https://doi.org/10.1155/2017/5985209
- Mayr FB, Yende S, Angus DC. 2014. Epidemiology of severe sepsis. Virulence. 5(1):4–11. doi: https://doi.org/10.4161/viru.27372
- Niture SK, Khatri R, Jaiswal AK. 2014. Regulation of Nrf2-an update. Free Radic Biol Med. 66:36–44. doi: https://doi.org/10.1016/j.freeradbiomed.2013.02.008
- Okamura T, Miura T, Iwamoto H, Shirakawa K, Kawamura S, Ikeda Y, Iwatate M, Matsuzaki M. 1999. Ischemic preconditioning attenuates apoptosis through protein kinase C in rat hearts. Am J Physiol. 277(5 Pt 2):H1997–H2001.
- Petersen Shay K, Moreau RF, Smith EJ, Hagen TM. 2008. Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life. 60(6):362–367. doi: https://doi.org/10.1002/iub.40
- Qiu P, Dong Y, Li B, Kang XJ, Gu C, Zhu T, Luo YY, Pang MX, Du WF, Ge WH. 2017. Dihydromyricetin modulates p62 and autophagy crosstalk with the keap-1/Nrf2 pathway to alleviate ethanol-induced hepatic injury. Toxicol Lett. 274:31–41. doi: https://doi.org/10.1016/j.toxlet.2017.04.009
- Quoilin C, Mouithys-Mickalad A, Lecart S, Fontaine-Aupart MP, Hoebeke M. 2014. Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochim Biophys Acta. 1837(10):1790–1800. doi: https://doi.org/10.1016/j.bbabio.2014.07.005
- Shan Y, Yang F, Tang Z, Bi C, Sun S, Zhang Y, Liu H. 2018. Dexmedetomidine ameliorates the neurotoxicity of sevoflurane on the immature brain through the BMP/SMAD signaling pathway. Front Neurosci. 12:964. doi: https://doi.org/10.3389/fnins.2018.00964
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 315(8):801–810. doi: https://doi.org/10.1001/jama.2016.0287
- Stack C, Jainuddin S, Elipenahli C, Gerges M, Starkova N, Starkov AA, Jove M, Portero-Otin M, Launay N, Pujol A, et al. 2014. Methylene blue upregulates Nrf2/ARE genes and prevents tau-related neurotoxicity. Hum Mol Genet. 23(14):3716–3732. doi: https://doi.org/10.1093/hmg/ddu080
- Suzuki T, Yamamoto M. 2015. Molecular basis of the keap1-Nrf2 system. Free Radic Biol Med. 88(Pt B):93–100. doi: https://doi.org/10.1016/j.freeradbiomed.2015.06.006
- Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, Hayes JD. 2015. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med. 88(Pt B):108–146. doi: https://doi.org/10.1016/j.freeradbiomed.2015.06.021
- Victor VM, Espulgues JV, Hernandez-Mijares A, Rocha M. 2009. Oxidative stress and mitochondrial dysfunction in sepsis: a potential therapy with mitochondria-targeted antioxidants. Infect Disord Drug Targets. 9(4):376–389. doi: https://doi.org/10.2174/187152609788922519
- Watanabe K, Iwahara C, Nakayama H, Iwabuchi K, Matsukawa T, Yokoyama K, Yamaguchi K, Kamiyama Y, Inada E. 2013. Sevoflurane suppresses tumour necrosis factor-alpha-induced inflammatory responses in small airway epithelial cells after anoxia/reoxygenation. Br J Anaesth. 110(4):637–645. doi: https://doi.org/10.1093/bja/aes469
- Xu H, Zhao B, She Y, Song X. 2018. Dexmedetomidine ameliorates lidocaine-induced spinal neurotoxicity via inhibiting glutamate release and the PKC pathway. Neurotoxicology. 69:77–83. doi: https://doi.org/10.1016/j.neuro.2018.09.004
- Ye R, Yang Q, Kong X, Li N, Zhang Y, Han J, Xiong L, Liu X, Zhao G. 2012. Sevoflurane preconditioning improves mitochondrial function and long-term neurologic sequelae after transient cerebral ischemia: role of mitochondrial permeability transition. Crit Care Med. 40(9):2685–2693. doi: https://doi.org/10.1097/CCM.0b013e318258fb90
- Yeung JH, Ong GJ, Davies RP, Gao F, Perkins GD. 2012. Factors affecting team leadership skills and their relationship with quality of cardiopulmonary resuscitation. Crit Care Med. 40(9):2617–2621. doi: https://doi.org/10.1097/CCM.0b013e3182591fda
- Zenkov NK, Menshchikova EB, Tkachev VO. 2013. Keap1/Nrf2/ARE redox-sensitive signaling system as a pharmacological target. Biochemistry (Mosc). 78(1):19–36. doi: https://doi.org/10.1134/S0006297913010033
