Abstract
This study was conducted to investigate the efficacy of Tongguan Liyan Decoction on pharyngeal cancer-induced dysphagia (PCD) in elderly patients. Reverse transcription-polymerase chain reaction (RT–PCR) was performed to determine the expression level of microRNA-33a-3p (miR-33a-3p) in 22 patients with pharyngeal cancer aged 64.14 ± 5.06 years who had dysphagia caused by radiotherapy. The downstream target of miR-33a-3p was predicted using TargetScan and miRDB analysis software. The expression profiles of myf5 and sox11 in tumor tissues were determined using RT–PCR and western blot analysis. miR-33a-3p expression was downregulated in PCD tissues (P < 0.01) and targeted myf5 and sox11, whose expression levels were positively correlated with that of miR-33a-3p. In addition, Tongguan Liyan Decoction increased the viability and proliferation of muscle satellite cells, whereas an opposite effect was observed in tumor cells. Furthermore, in vivo experiment results demonstrated that Tongguan Liyan Decoction ameliorated the pathological conditions of tumor tissues in PCD rats (P < 0.001). Tongguan Liyan Decoction could upregulate the expression of miR-33a-3p and its downstream targets to ameliorate the pathological conditions of muscle satellite cells and inhibit the viability and proliferation of tumor cells, thereby improving PCD in elderly patients.
Introduction
Dysphagia, a common disease in the elderly population, causes weight loss, dehydration, and pneumonia; decreases the life span and quality; and may even result in death (Onoda et al. Citation2020; Picelli et al. Citation2020). In addition to the elderly population, dysphagia frequently occurs in patients with oral or pharyngeal cancer (Deantonio et al. Citation2013; Tomifuji et al. Citation2016; Ku et al. Citation2020). Radiotherapy or surgical resection usually results in muscle cell apoptosis or tissue atrophy in the pharynx, which may further contribute to the development of dysphagia (Tomifuji et al. Citation2016; Yuen et al. Citation2019).
Myogenic factor 5 (MYF5) is a major regulator of myogenicity and can interact with myocyte enhancer factor 2 transcription factor families to activate the transcription of muscle-specific genes (Cai et al. Citation2020). MYF5 is the first myogenic transcription factor expressed during mouse embryogenesis; this event marks the beginning of myogenic development in mammals (Ott et al. Citation1991). MYF5-deficient skeletal muscle exhibited a significant delay in regeneration after injury, and MYF5 deficiency led to reduced differentiation into myotubes. The primary reason for this was that MYF5 deficiency led to a delay in myoblast proliferation, indicating that MYF5 promoted myogenesis by promoting myoblast proliferation. In patients with dysphagia, normal proliferation of muscle cells is crucial for recovery (Panda et al. Citation2016). SOX11 mainly encodes a neural transcription factor and is widely expressed in the developmental nervous system, indicating its role in neurogenesis, neuron survival, and neurite growth (Wang et al. Citation2018). A number of studies have reported that SOX11 plays a key role in the progression of prostate cancer, mantle cell lymphoma, fibromatosis, gastric cancer, and other cancers (Wang et al. Citation2018).
As a classic traditional Chinese medicine preparation, Liyan Decoction is frequently used for the treatment of pharyngeal diseases (Wu et al. Citation2007; Lambros et al. Citation2015), with the addition or removal of components (Lambros et al. Citation2015; Zhang et al. Citation2016). For instance, Qingre Liyan Decoction, which is composed of Lonicera japonica, blackberry lily rhizome, Livistona chinensis, Astragalus membranaceus, Adenophora stricta, Radix Ophiopogonis, Mongolian snakegourd root, and Radix Scrophulariae, has the ability to inhibit bacteria and antagonize inflammation (Lambros et al. Citation2015). Yangyin Shengjin Liyan Decoction, which is composed of Radix Pseudostellariae, Radix Glehniae, Radix Scrophulariae, Mongolian snakegourd root, dried Radix Rehmanniae, and Lilium brownii, is primarily used in managing the inflammatory responses of the oral mucosa caused by radiotherapy for nasopharyngeal cancer (Li et al. Citation2019).
MicroRNAs (miRNAs) are a group of short non-coding RNAs (Chen et al. Citation2020; Zhang et al. Citation2020). To date, an increasing body of evidence has revealed their critical functions, including the regulation of cell proliferation, differentiation, and apoptosis; inflammatory responses; and development and progression of tumors (Yu et al. Citation2017; Chen et al. Citation2020; Zhang et al. Citation2020). According to available data, miR-33a-3p, an miRNA that regulates myocardial cell fibrosis, has the ability to regulate the differentiation and proliferation of tumor cells and myocardial cells (Yu et al. Citation2017).
In this study, Tongguan Liyan Decoction, which is composed of fructus aurantii immaturus, rhizoma Anemone altaica, ginger-processed Pinellia, ginseng, Platycodon grandiflorum, spina gleditsiae, Asarum sieboldii Miq., white muscardine silkworm, scorpio, and pseudo-ginseng, was used as the study drug to determine whether it regulates the expression of myf5 and sox11 by modulating miR-33a-3p expression to improve pharyngeal cancer-induced dysphagia (PCD) in elderly patients.
Materials and methods
Samples
A total of 22 patients with PCD aged 64.14 ± 5.06 years who were diagnosed based on computed tomography findings at our hospital and 10 age-matched healthy participants were enrolled in this study. For patients in the PCD group, samples were collected from tumor tissues or adjacent healthy tissues resected during surgery, while for healthy participants, samples were collected from mucosal tissues. This study was approved by the Ethics Board of Hunan Provincial People’s Hospital, and all clinical data, including age, sex, individual or family history, tumor site, differentiation, prognostic features, and follow-up data, were obtained from the patient database. All study participants provided written informed consent before participating in the study.
Tongguan Liyan Decoction
Tongguan Liyan Decoction is composed of the following ingredients: Asarum sieboldii Miq. 3 g, pseudo-ginseng 6 g, scorpio 6 g, ginseng 10 g, Platycodon grandiflorum 15 g, Fructus aurantii immaturus 15 g, white muscardine silkworm 12 g, ginger-processed Pinellia 12 g, spina gleditsiae 15 g, and rhizoma Anemone altaica 20 g. It was prepared using the water extraction and alcohol precipitation method, in which the content of crude drug was 5.0 g/mL.
Western blotting
Protein samples were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (20 μg/lane) at 80 V for 60 min and then transferred onto polyvinylidene fluoride membranes for 60 min at 80 V. The membranes were washed with phosphate-buffered saline (PBS) containing 0.2% Tween (PBST) and then blocked with 5% non-fat milk at room temperature for 1 h. Thereafter, proteins present on the membrane were incubated with the corresponding primary antibodies at 4°C overnight, followed by three washes with PBST (5 min/wash). The resulting immunoblots were incubated with the corresponding secondary antibodies for 1 h at room temperature for detection. The final immunoblots were developed into bands after incubation with the ECL reagent (Pierce) and were analyzed using ImageJ software.
Reverse transcription-polymerase chain reaction (RT–PCR)
Total RNA was extracted from the cells using the TRIzol kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. cDNA was then prepared using the cDNA Synthesis Kit (Bio-Rad Laboratories, Inc.) via reverse transcription at 42°C for 30 min. Reverse transcriptase was deactivated at 85°C for 15 s. qPCR was then performed using the SYBR-Green Master kit (Roche Diagnostics) and gene-specific primers. The PCR reaction mixture was composed of 10 µL of 2× SYBR-Green Master Mix, 1 µL of cDNA template, 1 µL of forward primer (10 μM), 1 µL of reverse primer (10 μM), and 7 µL of ddH2O. PCR was performed under the following conditions: initial denaturation at 95°C for 2 min, 40 cycles of denaturation at 95°C for 10 s and 60°C for 60 s, and annealing at 10°C for 2 min. The results were analyzed using the 2−ΔΔCt method.
The following primer sequences were used:
miR-33a-3p Forward: 5′-GGTTAGATCTTGCTCCAGCGGTTTG-3′
miR-33a-3p Reverse: 5′-GTA AAGCTTGCCCTCCTGTTTCCTG-3′
myf5 Forward: 5′-TATTACAGCCTGCCGGGACA-3′
myf5 Reverse: 5′-CTGCTGTTCTTTCGGGACCA-3′
sox11 Forward: 5′-CTCCGCACGAGACCCAG-3′
sox11 Reverse: 5′-GAAGCTGTAGTAGAGGCGGC-3′
GAPDH Forward: 5′-CAGTGCCAGCCTCGTCTCAT-3′
GAPDH Reverse: 5′-AGGGGCCATCCACAGTCTTC-3′
Dual-luciferase reporter gene assay
The TargetScan and miRDB databases were used to predict the downstream targets of miR-33a-3p. To verify the binding sites of myf5/sox11 in the miR-33a-3p promoter, we constructed pRL-SV40 vectors harboring the wild-type or mutant myf5/sox11 luciferase reporter genes, which were later transfected into muscle satellite cells (MSCs). After incubation for 48 h, the dual-luciferase reporter gene assay system (Promega Corporation, Fitchburg, WI, USA) was used to measure luciferase activity, which was later normalized. This experiment was conducted in triplicate.
Cell culture
Pharyngeal MSCs and cancer cells were considered as the cancer group and were extracted according to a previously described method (Qu-Petersen et al. Citation2002). Human skeletal MSCs (HSkMSCs) and the normal pharyngeal cell line NP69 were considered as the control group. HSkMSCs and NP69 cells were provided by the American Type Culture Collection (Rockville, MD, US). All cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) medium (Thermo Fisher Scientific, Waltham, USA) supplemented with 10% fetal bovine serum (FBS), 0.5% chick embryo extract, and 1% penicillin and streptomycin. Cells in the treatment group were treated with 50 μL/mL Tongguan Liyan Decoction and then cultured at 37°C in an atmosphere containing 5% CO2. Cell proliferation and viability were assessed using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and WST-1 assays (Yu et al. Citation2017; Xu et al. Citation2019).
Cell apoptosis
Cells in each group were resuspended in 100 μL of annexin-binding buffer and were incubated with 5 μL of propidium iodide (PI) and 5 μL of fluorescein isothiocyanate–annexin V (BD, Franklin, NJ, USA) for 20 min in the dark at room temperature. The volume was increased by adding annexin-binding buffer up to a volume of 400 μL, followed by the detection of fluorescent signals of PI and annexin V using flow cytometry (BeamCyte). Analysis was conducted using CytoSYS 1.0 software.
Construction of pcDNA3-FLAG3-myf5 and pcDNA3.1-sox11 plasmids
We obtained the nucleotide sequences of myf5 and sox11 from NCBI and provided them to GenScript Biotech (China) Co., Ltd. for synthesis. Fifty microliters of competent cells were collected into a 1.5-mL Eppendorf tube on ice, and a 5-μL mixture of plasmids expressing ampicillin was added. After 15 min, 1 mL of LB medium supplemented with ampicillin was added, followed by incubation at 37°C for 1 h on a shaker. Next, 200 μL of medium from the tubes was added to plates containing LB medium with ampicillin. Colonies were selected for subsequent monoclonal cultivation. Plasmids were then extracted from the colonies using the Qiagen kit (Germany), and 5 μg of plasmids was added to a cell medium containing 200 μL of Opti-MEM and 10 μL of transfection buffer.
Transfection of miR-33a-3p inhibitor
miR-33a-3p inhibitor (5′-GUGAUGCACUGUGGAAACAUUG-3′) was obtained from Biomics Biotechnologies Co., Ltd (Biomics Biotech, Nantong, China). Lipofectamine 2000 was used to transfect HSkMSCs and NP69 cells according to the manufacturer’s instructions, and miR-normal control inhibitor (miR-NC) was used as the control group.
Construction of rat PCD models
Sprague Dawley rats (Nanjing Laboratory Animal Technique Co., Ltd) aged 20–22 months and with a mean weight of 230 ± 10 g were housed at 20°C ± 25°C at a relative humidity of 45% ± 60% in the pathogen-free facility of Hunan Provincial People’s Hospital. PCD models were established by transplanting the patients’ tumor cells. Briefly, rats were anesthetized on a sterilized operation table using pentobarbital sodium, and the tumor cells were injected into the throat. After 2 weeks, obvious tumor nodules and swallowing difficulty indicated that the PCD models were successfully established. Twenty-four rats were categorized into the following groups: adipocyte transplantation control (FTC) group, treatment group, PCD group, and control group, with six rats in each group.
Hematoxylin and eosin (HE) staining
Tumor tissues collected from rats were embedded into paraffin and sectioned for HE staining. Briefly, paraffin sections were deparaffinized and cleaned with xylene and then treated sequentially with 100%, 90%, 80%, 70%, and 50% ethanol, with each treatment lasting 3 min. This was followed by washing with distilled water for 5 min. The cytoplasm and nucleus were then stained with hematoxylin and eosin, respectively, for 5 min. The stained sections were then dehydrated using gradient concentrations of ethanol, cleaned with xylene, and mounted in neutral balsam, followed by observation under a microscope.
Statistical analysis
Data were analyzed using GraphPad Prism version 8.0 (GraphPad Software, Inc.). Data were presented as the mean ± standard deviation, whereas between- and among-group differences were determined using unpaired Student t-test or one-way analysis of variance. P < 0.05 indicated statistical significance.
Results
miR-33a-3p expression is downregulated in elderly patients with PCD
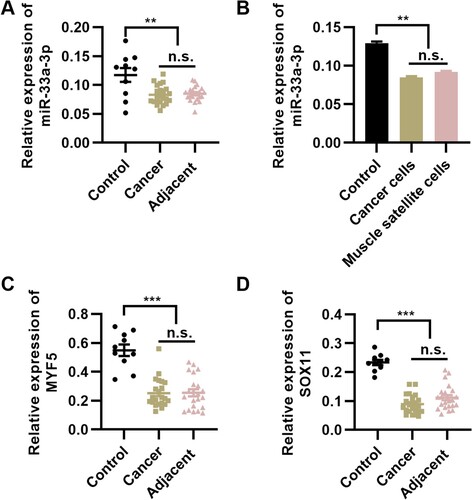
To verify the expression status of miR-33a-3p in elderly patients with PCD, we determined the mRNA expression level of miR-33a-3p in tumor and adjacent healthy tissues. Compared with that in the control group, miR-33a-3p expression was downregulated in both tumors and adjacent healthy tissues (Figure A, P < 0.01). To confirm this finding, we used NP69 cells and HSkMSCs as the control group and performed qPCR. We found a considerable decrease in the expression level of miR-33a-3p in tumor cells and HSkMSCs (Figure B, P < 0.01). To identify the potential downstream target genes of miR-33a-3p, we determined the expression levels of myf5 and sox11 in pharyngeal tumor tissues and adjacent healthy tissues in patients with PCD; the expression levels of myf5 and sox11 were downregulated in pharyngeal tumor tissues and adjacent healthy tissues (Figure C and D, P < 0.001). These results illustrate the potential association between the expressions of miR-33a-3p, myf5, and sox11 in pharyngeal tumor tissues and adjacent healthy tissues in patients with PCD. In adjacent tissues, the decreased expression levels of miR-33a-3p, myf5, and sox11 may be associated with the presence of potential lesions surrounding the tumor tissues.
Figure 1. miR-33a-3p expression is downregulated in patients with pharyngeal cancer-induced dysphagia (PCD). (A) miR-33a-3p expression is downregulated in tumor tissues and adjacent healthy tissues in patients with PCD. (B) miR-33a-3p expression is downregulated in tumor cells and muscle satellite cells collected from patients with PCD, n = 10 for each group. **P < 0.01, n.s., not significant. One-way analysis of variance followed by Tukey’s multiple comparisons test. Error bars indicate SEM.
miR-33a-3p targets myf5 and sox11
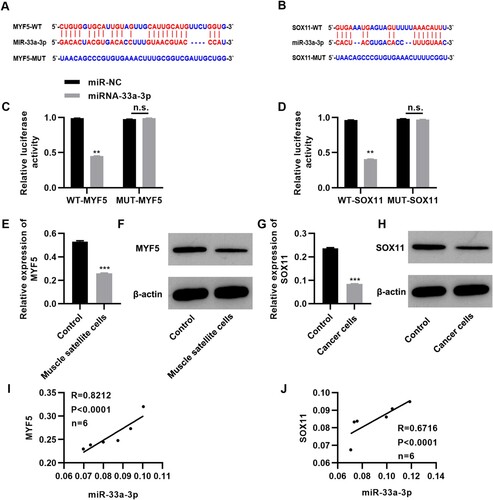
To determine the molecular mechanism of miR-33a-5p in PCD, we analyzed the targets of miR-33a-3p in TargetScan (http://www.targetscan.org/vert_72/) and miRDB (http://mirdb.org/miRDB/) databases, and as a result, myf5, which encodes a myogenic regulatory factor, and sox11, a tumor suppressor gene, were screened as the potential targets of miR-33a-3p (Cai et al. Citation2020; Yu et al. Citation2020). The results were validated using the dual-luciferase reporter gene assay (Figure A-B). RT–PCR was performed to determine the expression patterns of patients with PCD. myf5 expression was downregulated in the MSCs isolated from patients’ tissues. Similarly, sox11 expression was downregulated in tumor cells (Figure E-H, P < 0.001). Correlation analysis indicated that miR-33a-3p expression was positively correlated with myf5 and sox11 expression (Figure I-J). These results indicate that miR-33a-3p may have a potential positive regulatory effect on the expression of myf5 and sox11.
Figure 2. miR-33a-3p targets myf5 and sox11. (A-B) Bioinformatics prediction showed that miR-33a-3p may target myf5 and sox11; (C-D) The relative luciferase activity of the region in miR-33a-3p binding to the wild-type or mutant 3'-UTR of myf5 and sox11, n = 3 for each group; (E-H) myf5 expression is downregulated in muscle satellite cells (E-F) and sox11 expression is downregulated in tumor cells (G-H), n = 6 in each group; (I-J) Correlation between the expression level of miR-33a-3p and those of myf5 and sox11. **P < 0.01, ***P < 0.001, n.s., not significant. Mann–Whitney U test (C-G).
Tongguan Liyan Decoction promotes the proliferation and enhances the viability of MSCs
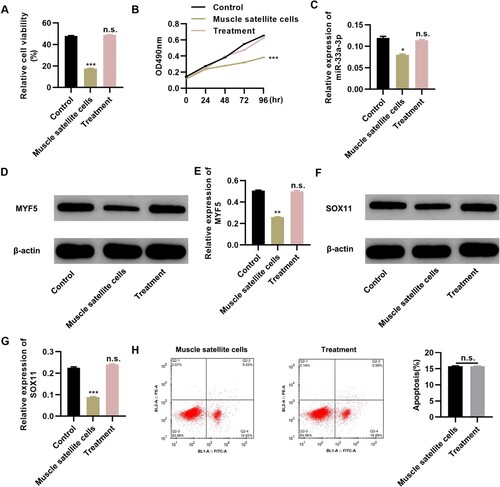
To determine the potential therapeutic efficacy of Tongguan Liyan Decoction in elderly patients with PCD, we performed in vitro experiments using MSCs collected from these patients. Tongguan Liyan Decoction increased the viability of MSCs to a level similar to that of healthy MSCs (Figure A, P < 0.001) as well as enhanced the proliferative ability of MSCs, as demonstrated by the MTT assay (Figure B, P < 0.001). Tongguan Liyan Decoction upregulated miR-33a-3p expression (Figure C, P < 0.05) as well as myf5 and sox11 expression (Figure D-G; P < 0.01 or 0.001) but had no effect on apoptosis (Figure H). These results indicate that Tongguan Liyan Decoction could improve the viability of MSCs in patients with PCD, which had a positive effect on restoring the pharyngeal swallowing ability of the patients, and the effect of Tongguan Liyan Decoction on MSCs might be achieved via the regulation of miR-33a-3p expression.
Figure 3. Tongguan Liyan Decoction promotes the proliferation and viability of muscle satellite cells (MSCs). (A-B) Tongguan Liyan Decoction enhances the proliferation and viability of MSCs. (C-G) RT-PCR and western blot analysis results show the expression profiles of miR-33a-3p (C), myf5 (D-E), and sox11 (F-G). (H) Tongguan Liyan Decoction did not alter the apoptosis of MSCs. n = 6 in each group. *P < 0.05, **P < 0.01, ***P < 0.001. n.s., not significant. One-way analysis of variance followed by Tukey’s multiple comparisons test (A-C, E, and G), and Mann–Whitney U test (H).
Tongguan Liyan Decoction inhibits pharyngeal tumor cell proliferation
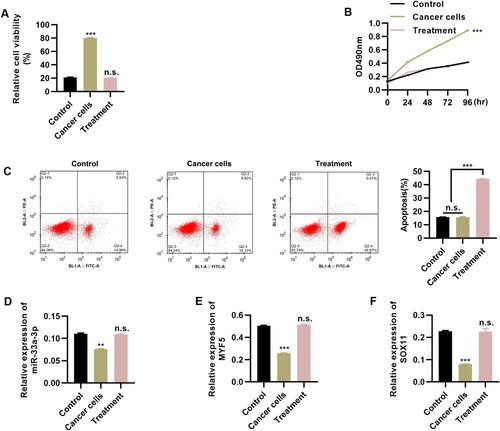
To investigate whether Tongguan Liyan Decoction could ameliorate the symptoms of pharyngeal tumors, we performed in vitro experiments using tumor cells. We cultured tumor cells in DMEM medium containing Tongguan Liyan Decoction (50 μL/mL). The viability and proliferation rate of tumor cells were clearly repressed following the addition of Tongguan Liyan Decoction (Figure A-B, P < 0.001). Flow cytometric analysis revealed that Tongguan Liyan Decoction also accelerated the apoptosis of tumor cells (Figure C, P < 0.001). In addition, RT–PCR analysis revealed that the addition of Tongguan Liyan Decoction upregulated the expression levels of miR-33a-3p (Figure D, P < 0.01), myf5 (Figure E, P < 0.001), and sox11 (Figure F, P < 0.001). The results of the in vitro experiments proved that Tongguan Liyan Decoction could not only restore the viability of MSCs in vitro but also inhibit the proliferation of pharyngeal cancer cells in vitro. This indicated that Tongguan Liyan Decoction had a certain effect on PCDtreatment.
Figure 4. Tongguan Liyan Decoction inhibits the proliferation of tumor cells. (A-C) Tongguan Liyan Decoction represses the viability (A) and proliferation (B) of pharyngeal cancer cells, with increased apoptosis (C); (D-F) Tongguan Liyan Decoction upregulates the expression levels of miR-33a-3p (D), myf5 (E), and sox11 (F). n = 6 in each group. **P < 0.01, ***P < 0.001. n.s., not significant. One-way analysis of variance followed by Tukey’s multiple comparisons test.
Different roles of pcDNA3-FLAG3-myf5 and pcDNA3.1-sox11 plasmids in in vitro cultured cells
To further elucidate the roles of myf5 and sox11, we used MSCs and tumor cells collected from the patients to perform transfection using pcDNA3-FLAG3-myf5 and pcDNA3.1-sox11 plasmids. We found that transfection of pcDNA3-FLAG3-myf5 led to upregulation of myf5 expression (Figure A, P < 0.001) and MSC viability and proliferation (Figure B-C, P < 0.001). However, these changes were reversed by the transfection of pcDNA3.1-sox11 (Figure D-F, P < 0.001). The experimental results of plasmids once again verified the important role of myf5 and sox11 in regulating the occurrence and development of PCD.
Figure 5. Regulation of myf5 and sox11 overexpression in in vitro cultured cells. (A-C) pcDNA3-FLAG3-myf5 plasmid upregulates myf5 expression, and myf5 overexpression promotes MSC viability (B) and proliferation (C). (D-E) sox11 overexpression decreases tumor cell proliferation (D) and viability (E). (F) pcDNA3.1-sox11 plasmid upregulates the expression level of sox11. n = 6 in each group. ***P < 0.001. n.s., not significant. Mann–Whitney U test.
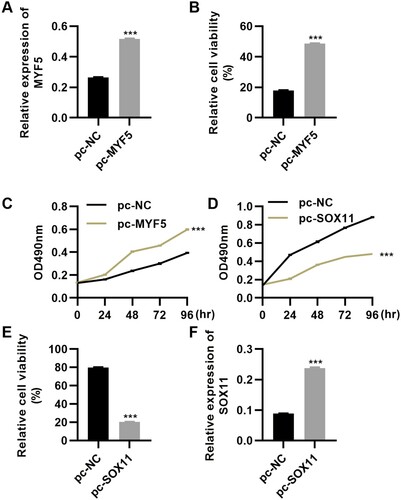
Transfection of miR-33a-3p inhibitor reduces HSkMSC viability and promotes NP69 cell proliferation by downregulating the expression levels of myf5 and sox11
To verify the role of miR-33a-3p in promoting the recovery of PCD, we used miR-33a-3p inhibitor to transfect HSkMSCs and NP69 cells. The results showed that the transfection of miR-33a-3p inhibitor inhibited the expression of miR-33a-3p in HSkMSCs and NP69 cells (Figure A and B, P < 0.001) and further downregulated the expression levels of myf5 and sox11 (Figure C-F, P < 0.001). Cell proliferation and cell viability assays showed that transfection of miR-33a-3p inhibitor inhibited the proliferation and viability of HSkMSCs (Figure G and H, P < 0.001) but promoted the proliferation and viability of NP69 cells (Figure I and J, P < 0.001). These results suggest that inhibition of miR-33a-3p expression in normal HSkMSCs leads to pathological changes in HSkMSC cells, which may be the principal cause of dysphagia. The enhancement of the proliferation and viability of NP69 cells may inhibit the expression of miR-33a-3p and lead to the possibility of tumorigenicity of NP69 cells.
Figure 6. miR-33a-3p inhibitor reduced the viability of HSkMSCs and promoted the proliferation of NP69 cells. (A and B) miR-33a-3p inhibitor downregulated miR-33a-3p expression in HSkMSCs (A) and NP69 cells (B); (C-F) miR-33a-3p inhibitor downregulated the expression of myf5 and sox11 in HSkMSCs (C and E) and NP69 cells (D and F); (G-J) miR-33a-3p inhibitor inhibited the proliferative ability (G) and viability (I) of HSkMSCs and promoted the proliferative ability (H) and viability (J) of NP69 cells. Mann–Whitney U test.
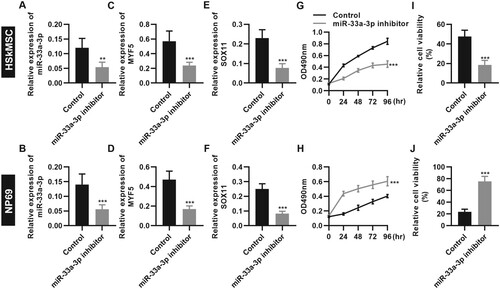
Tongguan Liyan Decoction exerts a potential therapeutic effect on PCD rats
To further clarify the efficacy of Tongguan Liyan Decoction on PCD, we established a rat PCD model and performed in vivo experiments. We found that following continuous administration of Tongguan Liyan Decoction, the pathological condition of the pharyngeal tumors in PCD rats was significantly improved, with a tremendous increase in diet (Figure A and B, P < 0.001). RT–PCR results also confirmed that Tongguan Liyan Decoction upregulated the expression level of miR-33a-3p and its downstream targets myf5 and sox11 in tumors (Figure C-E, P < 0.001). The in vivo experiments verified that Tongguan Liyan Decoction had a good effect on PCD and may regulate the expression of miR-33a-3p to upregulate the expression levels of its downstream target genes myf5 and sox11, thereby achieving the main purpose of improving PCD symptoms in rats.
Figure 7. Tongguan Liyan Decoction ameliorated the pathological conditions of rat pharyngeal cancer-induced dysphagia (PCD) models. (A) HE staining results indicated that Tongguan Liyan Decoction improves the pathological conditions of pharyngeal tissues in PCD rats; (B) Tongguan Liyan Decoction increases the diet intake of PCD rats; (C-E) Tongguan Liyan Decoction upregulates the expression of miR-33a-3p (C), myf5 (D), and sox11 (E) in PCD rats. n = 6 in each group. **P < 0.01, ***P < 0.001. n.s., not significant. One-way analysis of variance followed by Tukey’s multiple comparison test (B-E).
Discussion
Pharyngeal cancer is one of the most common diseases worldwide (Bosetti et al. Citation2020), and continuous improvements in medical treatments have decreased the mortality rate of patients with pharyngeal cancer (Bhide et al. Citation2009; Huo et al. Citation2019 Bosetti et al. Citation2020;). However, pharyngeal cancer still results in severe symptoms such as mucositis, dysphagia, dysgeusia, and thirst (Christmas and Rogus-Pulia Citation2019; Espinosa-Val et al. Citation2020). Dysphagia is the most frequent and challenging adverse event caused by the surgical treatment of pharyngeal cancer (Christmas and Rogus-Pulia Citation2019; Savas and Yilmaz Citation2019) and often has long-term or even permanent effects on the quality of life and social functioning of patients (Christmas and Rogus-Pulia Citation2019; Marmor et al. Citation2020).
In the present study, the correlation between miR-33a-3p expression and the development of PCD was examined in patients with PCD. The results showed that miR-33a-3p expression was downregulated in patients with PCD and was identified to have two downstream targets: myf5 and sox11; further, the expression level of miR-33a-3p was positively correlated with those of myf5 and sox11.
As per traditional knowledge, dysphagia is associated with insufficient viability of MSCs, particularly in elderly patients (Christmas and Rogus-Pulia Citation2019; Everton et al. Citation2020; Van den Steen et al. Citation2020). Thus, the viability and proliferation of MSCs are essential components for the prophylaxis and treatment of dysphagia (Qu-Petersen et al. Citation2002; Tiburcy et al. Citation2019; Lin et al. Citation2020). Interestingly, results of the in vitro experiments indicated that Tongguan Liyan Decoction enhances the viability and proliferation of MSCs, without any effect on the apoptosis of MSCs, demonstrating the potential efficacy of this decoction on dysphagia. Subsequent qRT-PCR analysis also revealed that Tongguan Liyan Decoction upregulated the expression levels of miR-33a-3p and its downstream targets myf5 and sox11. Nevertheless, tumor cells manifested different outcomes in addition to having a positive effect on the expression levels of miR-33a-5p, myf5, and sox11. Different genes manifest different functions in various cells (Curjuric et al. Citation2012; Bao et al. Citation2018; Tsujimura et al. Citation2019). Accordingly, we infer that the upregulation of myf5 and sox11 expression triggers different outcomes. myf5 enhances MSC viability and proliferation; these effects are abolished when it is expressed in tumor cells. This may be attributable to differences in the receptors; however, this could not been proved in this study. We confirmed that Tongguan Liyan Decoction ameliorated the pathological conditions of PCD rats in addition to the aforementioned positive role of Tongguan Liyan Decoction in in vitro cultured cells. These findings may be of great significance for patients with dysphagia caused by radiotherapy for the treatment of pharyngeal cancer.
In conclusion, the findings of this study suggest a path for elucidating the pathogenesis of dysphagia caused by radiotherapy or surgical treatment for pharyngeal cancer and that Tongguan Liyan Decoction can prevent the development of dysphagia and inhibit tumorigenesis, highlighting the importance of using traditional Chinese medicine preparations for the clinical treatment of cancer and providing potential therapeutic targets for the treatment of dysphagia caused by radiotherapy. Our findings are conducive to widening the application of traditional Chinese medicine preparations for the treatment of dysphagia and provide therapeutic strategies for the treatment of dysphagia caused by radiotherapy for pharyngeal cancer.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research, the participants of this study did not agree to their data being shared publicly, so supporting data is not available.
Additional information
Funding
References
- Bao M, Song Y, Xia J, Li P, Liu Q, Wan Z. 2018. miR-1269 promotes cell survival and proliferation by targeting tp53 and caspase-9 in lung cancer. Onco Targets Ther. 11:1721–1732.
- Bhide SA, Gulliford S, Kazi R, El-Hariry I, Newbold K, Harrington KJ, Nutting CM. 2009. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 93(3):539–544.
- Bosetti C, Carioli G, Santucci C, Bertuccio P, Gallus S, Garavello W, Negri E, La Vecchia C. 2020. Global trends in oral and pharyngeal cancer incidence and mortality. Int J Cancer. 147(4):1040–1049.
- Cai S, Zhu Q, Guo C, Yuan R, Zhang X, Nie Y, Chen L, Fang Y, Chen K, Zhang J, et al. 2020. MLL1 promotes myogenesis by epigenetically regulating Myf5. Cell Prolif. 53(2):e12744.
- Chen Q, Wang M, Wu S. 2020. The lncRNA MCF2L-AS1 controls osteogenic differentiation by regulating miR-33a. Cell Cycle. 19(9):1059–1065.
- Christmas C, Rogus-Pulia N. 2019. Swallowing disorders in the older population. J Am Geriatr Soc. 67(12):2643–2649.
- Curjuric I, Imboden M, Nadif R, Kumar A, Schindler C, Haun M, Kronenberg F, Kunzli N, Phuleria H, Postma DS, et al. 2012. Different genes interact with particulate matter and tobacco smoke exposure in affecting lung function decline in the general population. PLoS One. 7(7):e40175.
- Deantonio L, Masini L, Brambilla M, Pia F, Krengli M. 2013. Dysphagia after definitive radiotherapy for head and neck cancer. Correlation of dose-volume parameters of the pharyngeal constrictor muscles. Strahlenther Onkol. 189(3):230–236.
- Espinosa-Val MC, Martin-Martinez A, Graupera M, Arias O, Elvira A, Cabre M, Palomera E, Bolivar-Prados M, Clave P, Ortega O. 2020. Prevalence, risk factors, and complications of oropharyngeal dysphagia in older patients with dementia. Nutrients. 12(3):863.
- Everton LF, Benfield JK, Hedstrom A, Wilkinson G, Michou E, England TJ, Dziewas R, Bath PM, Hamdy S. 2020. Psychometric assessment and validation of the dysphagia severity rating scale in stroke patients. Sci Rep. 10(1):7268.
- Huo D, Jiang S, Qin Z, Feng Y, Yang R, Lv L, Li Y. 2019. Omethoate induces pharyngeal cancer cell proliferation and G1/S cell cycle progression by activation of Akt/GSK-3beta/cyclin D1 signaling pathway. Toxicology. 427:152298.
- Ku PK, Holsinger FC, Chan JY, Yeung ZC, Chan BY, Tong MC, Starmer HM. 2020. Management of dysphagia in the head and neck cancer patient during COVID-19 pandemic: a practical strategy. Head Neck. 42(7):1491–1496.
- Lambros MP, Kondapalli L, Parsa C, Mulamalla HC, Orlando R, Pon D, Huang Y, Chow MS. 2015. Molecular signatures in the prevention of radiation damage by the synergistic effect of N-acetyl cysteine and qingre liyan decoction, a traditional Chinese medicine, using a 3-dimensional cell culture model of oral mucositis. Evid Based Complement Alternat Med. 2015:425760.
- Li L, Zhang X, Bu F, Chen N, Zhang H, Gu J. 2019. Simultaneous determination of eight constituents in rat plasma by HPLC-MS/MS and its application to a pharmacokinetic study after oral administration of Shejin-liyan Granule. Biomed Chromatogr. 33(11):e4648.
- Lin C, Han G, Ning H, Song J, Ran N, Yi X, Seow Y, Yin H. 2020. Glycine enhances satellite cell proliferation, cell transplantation, and oligonucleotide efficacy in dystrophic muscle. Mol Ther. 28(5):1339–1358.
- Marmor S, Cohen S, Fujioka N, Cho LC, Bhargava A, Misono S. 2020. Dysphagia prevalence and associated survival differences in older patients with lung cancer: a SEER-medicare population-based study. J Geriatr Oncol. 11(7):1115–1117.
- Onoda S, Kinoshita M, Ariyoshi Y. 2020. Relationship between the incidence of postoperative fistula or dysphagia and resection style, gastric tube formation, and irradiation following free jejunal flap transfer. Plast Reconstr Surg Glob Open. 8(2):e2663.
- Ott MO, Bober E, Lyons G, Arnold H, Buckingham M. 1991. Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development. 111(4):1097–1107.
- Panda AC, Abdelmohsen K, Martindale JL, Di Germanio C, Yang X, Grammatikakis I, Noh JH, Zhang Y, Lehrmann E, Dudekula DB, et al. 2016. Novel RNA-binding activity of MYF5 enhances Ccnd1/cyclin D1 mRNA translation during myogenesis. Nucleic Acids Res. 44(5):2393–2408.
- Picelli A, Modenese A, Poletto E, Businaro V, Varalta V, Gandolfi M, Bonetti B, Smania N. 2020. May ultrasonography be considered a useful tool for bedside screening of dysphagia in patients with acute stroke? A cohort study. Minerva Med. https://doi.org/10.23736/S0026-4806.20.06571-4.
- Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, et al. 2002. Identification of a Novel population of muscle Stem cells in mice: potential for muscle regeneration. J Cell Biol. 157(5):851–864.
- Savas S, Yilmaz M. 2019. Self reported dysphagia is not associated with sarcopenia defined by the revised EWGSOP2 criteria and regional thresholds at the hospital among ambulatory Older patients. Mater Sociomed. 31(4):253–257.
- Tiburcy M, Markov A, Kraemer LK, Christalla P, Rave-Fraenk M, Fischer HJ, Reichardt HM, Zimmermann WH. 2019. Regeneration competent satellite cell niches in rat engineered skeletal muscle. FASEB Bioadv. 1(12):731–746.
- Tomifuji M, Araki K, Yamashita T, Mizokami D, Kamide D, Suzuki H, Miyagawa Y, Tanaka S, Taniai S, Shiotani A. 2016. Risk factors for dysphagia after transoral videolaryngoscopic surgery for laryngeal and pharyngeal cancer. Head Neck. 38(2):196–201.
- Tsujimura M, Kusamori K, Nishikawa M. 2019. Rapid regulation of human mesenchymal stem cell proliferation using inducible caspase-9 suicide gene for safe cell-based therapy. Int J Mol Sci. 20(22):5759.
- Van den Steen L, Baudelet M, Tomassen P, Bonte K, De Bodt M, Van Nuffelen G. 2020. The effect of tongue-strengthening exercises on tongue strength and swallowing-related parameters in chronic radiation-associated dysphagia. Head Neck. 42(9):2298–2307.
- Wang L, Shen YF, Shi ZM, Shang XJ, Jin DL, Xi F. 2018. Overexpression miR-211-5p hinders the proliferation, migration, and invasion of thyroid tumor cells by downregulating SOX11. J Clin Lab Anal. 32(3):e22293.
- Wu MH, Yuan B, Liu QF, Wang Q. 2007. Study of qingre liyan decoction in treating and preventing acute radioactive oral mucositis. Chin J Integr Med. 13(4):280–284.
- Xu Z, Zhang L, Yu Q, Zhang Y, Yan L, Chen ZJ. 2019. The estrogen-regulated lncRNA H19/miR-216a-5p axis alters stromal cell invasion and migration via ACTA2 in endometriosis. Mol Hum Reprod. 25(9):550–561.
- Yu B, Li W, Al F, Chen Z. 2017. MicroRNA-33a deficiency inhibits proliferation and fibrosis through inactivation of TGF-beta/smad pathway in human cardiac fibroblasts. Pharmazie. 72(8):456–460.
- Yu F, Wu F, Li F, Liao X, Wang Y, Li X, Wang C, Shi Y, Ye L. 2020. Wnt7b-Induced Sox11 functions enhance self-renewal and osteogenic commitment of bone marrow mesenchymal stem cells. Stem Cells. 38(8):1020–1033.
- Yuen MTY, Tsang RK, Wong IYH, Chan DKK, Chan FSY, Law SYK. 2019. Long-term pharyngeal dysphagia after esophagectomy for esophageal cancer-an investigation using videofluoroscopic swallow studies. Dis Esophagus. 32(1). https://doi.org/10.1093/dote/doy068.
- Zhang C, Chen K, Wei R, Fan G, Cai X, Xu L, Cen B, Wang J, Xie H, Zheng S, et al. 2020. The circFASN/miR-33a pathway participates in tacrolimus-induced dysregulation of hepatic triglyceride homeostasis. Signal Transduct Target Ther. 5:23.
- Zhang X, Sun CY, Zhang YB, Guo HZ, Feng XX, Peng SZ, Yuan J, Zheng RB, Chen WP, Su ZR, et al. 2016. Kegan Liyan oral liquid ameliorates lipopolysaccharide-induced acute lung injury through inhibition of TLR4-mediated NF-kappaB signaling pathway and MMP-9 expression. J Ethnopharmacol. 186:91–102.
