Abstract
Currently, interstitial lung disease (ILD) has no unified treatment plan. Home-based pulmonary rehabilitation (PR), as a non-pharmacological treatment, is a non-invasive and low-cost option for improving exercise capacity and quality of life. This study explores the impact of home-based PR on lung function, and the quality of life of patients with ILD. In this randomized prospective study, 60 patients with ILD were randomly assigned to the home-based PR intervention and traditional drug treatment groups, and they were followed for 12 months. The outcome measures were the 6-min walk distance (6MWD), exercise-related dyspnea, and St George’s Respiratory Questionnaire score (SGRQ). It found that the 6MWD results were significantly improved from 348.5 ± 106.1 m at baseline to 403.6 ± 100.3 m on the completion of PR and 443.8 ± 96.5 at 1-year follow-up (P = 0.001). Compared with the control group, the home-based PR group had slightly improved pulmonary function. Furthermore, the severity of dyspnea evaluated with mMRC in the home-based PR group improved from 2.9 ± 1.1 at baseline to 2.2 ± 0.9 after the home-based PR program, and this improvement persisted after 12 months (P = 0.001). Additionally, home-based PR for patients with ILD improved the functional pulmonary performance and the SGRQ score results.
Introduction
Interstitial lung disease (ILD) is a group of acute and chronic lung disorders characterized by varying degrees of inflammation and fibrosis (Wallis and Spinks Citation2015; Dowman et al. Citation2017; Raj et al. Citation2017). Patients with ILD typically suffer from progressive breathlessness, reduced lung function, decreased exercise tolerance, and decreased quality of life. As a group of heterogeneous diseases, ILD currently has no unified treatment plan, and its treatment may involve drug and non-drug modalities (Meyer Citation2014). However, pharmacologic treatments have little effect on improving patients’ quality of life, and reductions in the health-related quality of life (HRQoL) occur (Chang et al. Citation1999).
Pulmonary rehabilitation (PR), as a non-pharmacological treatment, has been established as a feasible intervention for patients with chronic obstructive pulmonary disease to strengthen respiratory muscles, improve exercise tolerance, prevent pulmonary complications, and, ultimately, improve HRQoL (Camillo et al. Citation2016; Coquart et al. Citation2017; Augustin et al. Citation2018; Coquart et al. Citation2019). Training strategies include respiratory muscle training, health education, and psychological support. However, only a few studies have applied PR to ILD patients, and this makes it challenging to recommend PR as regular therapy (Sciriha et al. Citation2019).
Therefore, in the present study, we aimed to explore the effect of home-based PR on lung function, quality of life, and the mental health of patients with ILD to provide evidence on the benefits of PR.
Materials and methods
Participants
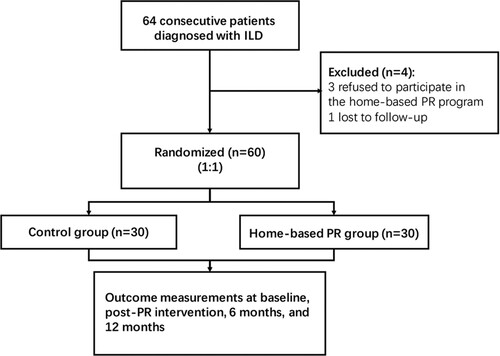
Patients with ILD who were admitted to the Department of Respiratory Medicine in Heilongjiang Provincial Hospital between March 2017 and June 2018 were enrolled in this research. The diagnosis of ILD was based on the criteria of the official American Thoracic Society (ATS)/European Respiratory Society (ERS)/Japanese Respiratory Society (JRS)/Latin American Thoracic Association (ALAT) statement (Raghu et al. Citation2011). Among the 64 ILD patients included in our study, 58 (90.6%) were idiopathic pulmonary fibrosis patients, 3 (4.7%) were non-specific interstitial pneumonia, 1 (1.6%) were secondary to asbestosis, 2 (3.1%) were due to rheumatoid arthritis. In this study, ILD patients were enrolled into the program who were medically stable and free from exacerbations evaluated by the respiratory physicians in the 3 months before the initiation of the study. As the condition of these patients could not further improve after drug treatment. This was aimed at minimizing the possible effects of drugs and also providing new ideas for the treatment of the patients during the late stage. Subjects with severe orthopedic or neurologic disorders, unstable cardiovascular disease, cancer that were unable to PR training were excluded. Patients who were recently changed in medications or lost follow-up were also excluded. This study was approved by Heilongjiang Provincial Hospital and all patients voluntarily participated in this study, and they signed informed consent forms. The ILD patients were numbered according to the order in which they participated in the lung rehabilitation, and they were divided into the control and the home-based PR groups using a random number table method with a 1:1 allocation ratio.
Home-based PR
Patients in the control group received conventional drug treatment (corticosteroids and/or immunomodulators), and those in the PR group received 12 weeks of home-based PR and conventional drug treatment. The home-based PR program was conducted as follows: (1) Sports training included whole-body endurance and local muscle training. The patients participated in exercise activities, including walking, brisk walking, jogging, climbing stairs, and cycling, for 3–5 times per week. Patients were required not to show apparent shortness of breath or severe cough after the exercise. (2) Respiratory muscle training included abdominal muscle training, balloon blowing, candle blowing, lip-diaphragm breathing, and systemic breathing exercises. Before the training, clinicians distributed picture materials, CD-ROMs, and finger clip oximeters to the patients, and they provided them with initial guidance. After the patients had mastered the operating rules, they were instructed to conduct home training. Rehabilitation and respiratory physicians chose different sports training and respiratory muscle exercise methods, strengths, and frequencies for patients depending on their conditions. (3) For the long-term oxygen therapy (LTOT), patients with indications were asked to inhale oxygen through the nasal catheter for more than 15 h a day; the required oxygen flow was 1–3 L/min (Bell et al. Citation2017). (4) Health education involved knowledge lectures, including guidance on medication, correct and safe oxygen inhalation, prevention of upper respiratory tract infections, tobacco control, and precautions during training, conducted to empower patients by creating awareness of the disease and improving treatment compliance and self-confidence. (5) For psychological and behavioral interventions, respiratory physicians and family members of patients cooperated with counselors to provide psychological counseling to patients and relieve their anxiety and depression.
Outcome measures
Baseline characteristics, including age, sex, body mass index, and pulmonary function, were recorded. Outcome measures were completed at baseline, post-PR intervention (after 6 weeks in control group), and 12 months after treatment to assess the durability of the program. The primary outcomes were the quality of life in ILD patients, which was evaluated using the St. George’s Respiratory Questionnaire (SGRQ), with higher scores indicating lower quality of life (Jones et al. Citation1992), and the 6-min walk distance (6MWD) conducted in a corridor following the ATS and ERS recommendations(Mylius et al. Citation2016). Spirometry was used to evaluate patients’ forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and carbon monoxide diffusing capacity (DLCO) (Culver et al. Citation2017). The severity of dyspnea was evaluated using the modified Medical Research Committee (mMRC) dyspnea scale ranging from 0 (no breathlessness) to 4 (too breathless to leave the house) (Rajala et al. Citation2017).
Statistical analysis
The SPSS 22.0 software (IBM Corporation, Armonk, NY, USA) was used for all data analysis. Data are expressed as mean ± SD. The unpaired Student’s t-test and the Mann–Whitney U test were used to compare the two groups (control vs home-based group) at baseline, post-PR and at 12 months of follow-up. In each group, changes in parameters (SGRQ, 6MWD, FVC, FEV1, DLCO, and mMRC) over time were compared with repeated measures analysis of variance, and Tukey post hoc tests were conducted when a significant difference was detected. The chi-squared test was used to compare categorical data. P < 0.05 was considered statistically significant.
Results
Sixty-four ILD patients were enrolled in this study. Of these, 4 participants did not complete the follow-up; 3 refused to continue and 1 lost contact (Figure ). Thirty patients (14 males and 16 females) were randomized into the home-based PR group, and the remaining 30 (15 males and 15 females) formed the control group. All the participants allocated to the home-based PR group completed the 12-week program. There was no significant difference between the baseline characteristics and the clinical variables of the home-based PR and control groups (Table ). Both groups had mild restrictive dysfunction with decreased FEV1 and FVC (nearly 10% of predicted), and DLCO as revealed by the respiratory function test.
Table 1. Baseline characteristics in ILD patients.
The results of the evaluation of functional capacity (FVC, FEV1, FEV1/FVC, DLCO, and 6MWT), dyspnea (mMRC), and quality of life (SGRQ) during the study are presented in Table . No adverse events or deaths occurred during the home-based PR treatment. After the home-based PR intervention, the pulmonary function, dyspnea, quality of life and exercise capacity significantly improved from the baseline, also in comparison to the control group who did not show any significant change in the outcome measures (Table ). Compared with the control group, the home-based PR group had slightly improved pulmonary function. Meanwhile, the severity of dyspnea during daily life activities in the home-based PR group improved from 2.9 ± 1.1 to 1.9 ± 0.8 on mMRC, which was significantly lower compared with the findings of the control group at 12 months (P = 0.019) (Figure a). Moreover, a significant increase in 6MWD from 348.5 ± 106.1 m at baseline to 403.6 ± 100.3 m (P = 0.015) on the completion of PR and 443.8 ± 96.5 (P = 0.001) at 1-year follow up was observed (Figure b). As the SGRQ score results showed (Figure c), the HRQoL of the ILD patients also improved (P = 0.001 at 12 weeks, and P = 0.002 at 12 months).
Figure 2. Changes in the outcome measures in the control and home-based PR groups over the duration of study (a) mMRC, (b) 6MWD, and (c) SGRQ.
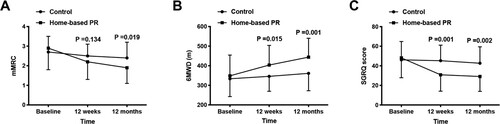
Table 2. Changes of pulmonary function and other measures at baseline, 12 weeks, and 12 months.
Discussion
Recent studies have shown that PR can improve lung function, exercise endurance and quality of life, and blood gas status and reduce the degree of shortness in breathing in chronic obstructive pulmonary disease patients (do Nascimento et al. Citation2015; Maekura et al. Citation2015; Camillo et al. Citation2016; Houchen-Wolloff et al. Citation2018; Grosbois et al. Citation2019). It is hypothesized that PR can also be used as a treatment method in ILD patients and delay the progression of the disease; however, there are currently no detailed guidelines on the use of PR in ILD patients (Igarashi et al. Citation2018). Several studies have shown that the PR program has several benefits tailored to improving the prognosis of ILD patients (Sciriha et al. Citation2019; Wallaert et al. Citation2019; Yuen et al. Citation2019). Few studies focus on the long-term effects of home-based PR programs on patients. In this study, we determined that home-based PR was an effective treatment in improving pulmonary function in patients with ILD. Dyspnea, exercise capacity, and general HRQoL were significantly improved after 12 weeks of home-based PR, and these effects were still observable at the 12-month follow-up.
Pulmonary function is significantly reduced in ILD patients due to lung inflammation, as reflected by the reductions in FEV1 and FVC (% of predicted) (Raghu et al. Citation2011). PR mainly includes respiratory training and exercise therapy, and these practical physical exercises can strengthen the endurance of respiratory muscles, especially the diaphragm, and prevent respiratory muscle fatigue (Nici et al. Citation2006; Kenn et al. Citation2013). Our study found that home-based PR resulted in significant increments in FVC and FEV1. However, in the study of Sciriha et al., there was no significant change in pulmonary function (Sciriha et al. Citation2019), although the effects of exercise on pulmonary function in ILD patients have been essentially established (Kenn et al. Citation2013; Vainshelboim et al. Citation2014). This may be due to the different frequencies and intensities of the PR programs. Several ILD patients do not continue with rehabilitation after benefiting from short-term treatment, and PR for ILD patients should be individualized and comprehensive. However, strategies for improving patient compliance, standardizing sports training methods and exercise intensity, formulating monitoring indicators and judgment standards, and scientifically evaluating the effect of PR need to be investigated (Morisset et al. Citation2016).
The quality of life of a majority of ILD patients progressively deteriorates due to dyspnea during the exercises. Therefore, any treatment to alleviate dyspnea should be tailored to improve functional capacity and quality of life in ILD patients. As the SGRQ score is closely associated with dyspnea, we also evaluated the changes in SGRQ score. As expected, the home-based PR intervention significantly decreased the SGRQ score, and this was consistent with previous findings (Kaymaz et al. Citation2013; da Fontoura et al. Citation2018; Sciriha et al. Citation2019). Meanwhile, a significant increase in 6MWD after the 12-week home-based PR was observed, and home-based PR can be recommended for patients with ILD based on this improvement.
This study has limitations. The primary limitation is that the patients were few. Therefore, further studies with a larger sample are required to corroborate our findings. Additionally, our study was conducted in a single-center, and we recommend multi-center studies with longer follow-ups to generalize our findings.
In summary, home-based PR may improve the functional exercise capacity of ILD patients by improving dyspnea, increasing exercise capacity, and improving the general HRQoL. Home-based PR may be convenient and effective for improving the quality of life of patients with ILD.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Augustin I, Wouters E, Houben-Wilke S, Gaffron S, Janssen D, Franssen F, Spruit M. 2018. Comprehensive lung function assessment does not allow to infer response to pulmonary rehabilitation in patients with COPD. J Clin Med. 8(1):27–38. doi:10.3390/jcm8010027.
- Bell EC, Cox NS, Goh N, Glaspole I, Westall GP, Watson A, Holland AE. 2017. Oxygen therapy for interstitial lung disease: a systematic review. Eur Respir Rev. 26(143):160080–160086. doi:10.1183/16000617.0080-2016.
- Camillo CA, Langer D, Osadnik CR, Pancini L, Demeyer H, Burtin C, Gosselink R, Decramer M, Janssens W, Troosters T. 2016. Survival after pulmonary rehabilitation in patients with COPD: impact of functional exercise capacity and its changes. Int J Chron Obstruct Pulmon Dis. 11:2671–2679. doi:10.2147/COPD.S113450.
- Chang JA, Curtis JR, Patrick DL, Raghu G. 1999. Assessment of health-related quality of life in patients With interstitial lung disease. Chest. 116(5):1175–1182. doi:10.1378/chest.116.5.1175.
- Coquart JB, Heutte N, Terce G, Grosbois J-M. 2019. Convergent validity and minimal clinically important difference of the maugeri foundation respiratory failure questionnaire (MRF-28) and the chronic obstructive pulmonary disease-specific health-related quality of life questionnaire (VQ11). Int J Chron Obstruct Pulmon Dis. 14:2895–2903. doi:10.2147/COPD.S222165.
- Coquart JB, Le Rouzic O, Racil G, Wallaert B, Grosbois JM. 2017. Real-life feasibility and effectiveness of home-based pulmonary rehabilitation in chronic obstructive pulmonary disease requiring medical equipment. Int J Chron Obstruct Pulmon Dis. 12:3549–3556. doi:10.2147/COPD.S150827.
- Culver BH, Graham BL, Coates AL, Wanger J, Berry CE, Clarke PK, Hallstrand TS, Hankinson JL, Kaminsky DA, MacIntyre NR, et al. 2017. Recommendations for a standardized pulmonary function report. An Official American Thoracic Society technical statement. Am J Respir Crit Care Med. 196(11):1463–1472. doi:10.1164/rccm.201710-1981ST.
- da Fontoura FF, Berton DC, Watte G, Florian J, Schio SM, Camargo JJP, Teixeira PJZ, Moreira JDS. 2018. Pulmonary rehabilitation in patients with advanced idiopathic pulmonary fibrosis referred for lung transplantation. J Cardiopulm Rehabil Prev. 38(2):131–134. doi:10.1097/HCR.0000000000000315.
- do Nascimento ES, Sampaio LM, Peixoto-Souza FS, Dias FD, Gomes EL, Greiffo FR, Ligeiro de Oliveira AP, Stirbulov R, Vieira RP, Costa D. 2015. Home-based pulmonary rehabilitation improves clinical features and systemic inflammation in chronic obstructive pulmonary disease patients. Int J Chron Obstruct Pulmon Dis. 10:645–653.
- Dowman LM, McDonald CF, Hill CJ, Lee AL, Barker K, Boote C, Glaspole I, Goh NSL, Southcott AM, Burge AT, et al. 2017. The evidence of benefits of exercise training in interstitial lung disease: a randomised controlled trial. Thorax. 72(7):610–619. doi:10.1136/thoraxjnl-2016-208638.
- Grosbois J-M, Coquart J, Fry S, Le Rouzic O, Grosbois T, Wallaert B, Chenivesse C. 2019. Long-term effect of home-based pulmonary rehabilitation in severe asthma. Respir Med. 157:36–41. doi:10.1016/j.rmed.2019.08.015.
- Houchen-Wolloff L, Williams JE, Green RH, Woltmann G, Steiner MC, Sewell L, Morgan MD, Singh SJ. 2018. Survival following pulmonary rehabilitation in patients with COPD: the effect of program completion and change in incremental shuttle walking test distance. Int J Chron Obstruct Pulmon Dis. 13:37–44. doi:10.2147/COPD.S143101.
- Igarashi A, Iwanami Y, Sugino K, Gocho K, Homma S, Ebihara S. 2018. Using 6-min walk distance expressed as a percentage of reference to evaluate the effect of pulmonary rehabilitation in elderly patients With interstitial lung disease. J Cardiopulm Rehabil Prev. 38(5):342–347. doi:10.1097/HCR.0000000000000305.
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. 1992. A self-complete measure of health status for chronic airflow limitation: The St. George’s respiratory questionnaire. Am Rev Respir Dis. 145(6):1321–1327. doi:10.1164/ajrccm/145.6.1321.
- Kaymaz D, Ergun P, Candemir I, Utku E, Demir N, Sengul F, Egesel N, Demir P. 2013. Pulmonary rehabilitation in interstitial lung diseases. Tuberk Toraks. 61(4):295–302. doi:10.5578/tt.6291.
- Kenn K, Gloeckl R, Behr J. 2013. Pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis–a review. Respiration. 86(2):89–99. doi:10.1159/000354112.
- Maekura R, Hiraga T, Miki K, Kitada S, Miki M, Yoshimura K, Yamamoto H, Kawabe T, Mori M. 2015. Personalized pulmonary rehabilitation and occupational therapy based on cardiopulmonary exercise testing for patients with advanced chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 10:1787–1800. doi:10.2147/COPD.S86455.
- Meyer KC. 2014. Diagnosis and management of interstitial lung disease. Transl Respir Med. 2(1):1–13. doi:10.1186/2213-0802-2-4.
- Morisset J, Dube BP, Garvey C, Bourbeau J, Collard HR, Swigris JJ, Lee JS. 2016. The unmet educational needs of patients with interstitial lung disease. setting the stage for tailored pulmonary rehabilitation. Ann Am Thorac Soc. 13(7):1026–1033. doi:10.1513/AnnalsATS.201512-836OC.
- Mylius CF, Paap D, Takken T. 2016. Reference value for the 6-minute walk test in children and adolescents: a systematic review. Expert Rev Respir Med. 10(12):1335–1352. doi:10.1080/17476348.2016.1258305.
- Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, Carone M, Celli B, Engelen M, Fahy B, et al. 2006. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 173(12):1390–1413. doi:10.1164/rccm.200508-1211ST.
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier J-F, Flaherty KR, Lasky JA, et al. 2011. An Official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 183(6):788–824. doi:10.1164/rccm.2009-040GL.
- Raj R, Raparia K, Lynch DA, Brown KK. 2017. Surgical lung biopsy for interstitial lung diseases. Chest. 151(5):1131–1140. doi:10.1016/j.chest.2016.06.019.
- Rajala K, Lehto JT, Sutinen E, Kautiainen H, Myllärniemi M, Saarto T. 2017. mMRC dyspnoea scale indicates impaired quality of life and increased pain in patients with idiopathic pulmonary fibrosis. ERJ Open Res. 3(4):1–8. doi:10.1183/23120541.00084-2017.
- Sciriha A, Lungaro-Mifsud S, Fsadni P, Scerri J, Montefort S. 2019. Pulmonary rehabilitation in patients with interstitial lung disease: the effects of a 12-week programme. Respir Med. 146:49–56. doi:10.1016/j.rmed.2018.11.007.
- Vainshelboim B, Oliveira J, Yehoshua L, Weiss I, Fox BD, Fruchter O, Kramer MR. 2014. Exercise training-based pulmonary rehabilitation program is clinically beneficial for idiopathic pulmonary fibrosis. Respiration. 88(5):378–388. doi:10.1159/000367899.
- Wallaert B, Duthoit L, Drumez E, Behal H, Wemeau L, Chenivesse C, Grosbois JM. 2019. Long-term evaluation of home-based pulmonary rehabilitation in patients with fibrotic idiopathic interstitial pneumonias. ERJ Open Res. 5(2):1–9. doi:10.1183/23120541.00045-2019.
- Wallis A, Spinks K. 2015. The diagnosis and management of interstitial lung diseases. BMJ. 350(350):1–12. doi:10.1136/bmj.h2072.
- Yuen HK, Lowman JD, Oster RA, de Andrade JA. 2019. Home-based pulmonary rehabilitation for patients with idiopathic pulmonary fibrosis: a pilot study. J Cardiopulm Rehabil Prev. 39(4):281–284. doi:10.1097/HCR.0000000000000418.