?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Microalgae are increasingly being preferred as sustainable biomass feedstock for biofuels production due to their high growth rate and lipid yield. However, optimal production requires the use of economical native microalgal species which are well adapted to local climatic and ecological conditions. Therefore, this study’s main objective was to isolate and select local microalgal species for prospective biofuels production. Serial dilution and streak plate techniques were used to isolate four species: Chlorella, Scenedesmus, Oscillatoria and Microcystis from Manyame River, Zimbabwe. The isolates were evaluated for their biomass and lipid productivity under artificial lighting (8:16 h light/dark cycle) and a temperature of 25 ± 3°C. Lipid quantification was done gravimetrically, using hexane as an extraction solvent. Each isolate’s performance was compared against that of an imported Chlorella vulgaris strain. Amongst the isolates, Chlorella sp. exhibited a highly competitive growth rate of 0.26 d−1 and lipid yield of 37.16% dry cell weight. In comparison, the imported C. vulgaris had a growth rate of 0.226 d−1 and lipid yield of 35.74% dry cell weight. Although findings suggest that the native Chlorella sp. is the ideal candidate, growth enhancement and genetic engineering techniques may be used to further improve its biomass and lipid yield.
Introduction
Human population growth, urbanization and industrialization are all major factors contributing to the depletion of non-renewable fossil fuels, leading to a global energy crisis (Li et al. Citation2013). Consequently, this crisis has resulted in increased fuel demand and steep pricing (Rawat et al. Citation2011; Rafiee and Khalilpour Citation2019). Third world countries, with no oil reserves, have been the most affected. Zimbabwe has to spend about US$100 million monthly on fuel importation (Sibanda Citation2020). Despite, this fiscal commitment, the imported fuel still falls far below the national demand.
Current scientific researchers have focused on finding sustainable forms of energy. In that regard, esters of vegetable triacylglycerides (TAGs) have been preferred as sustainable alternatives to petroleum diesel. They are commonly produced through the transesterification of TAGs in the presence of a homogeneous (Yang et al. Citation2019), heterogenous (Yang et al. Citation2019) or biological (Tan et al. Citation2016) catalyst. These methyl esters are conventionally referred to as biodiesel. Apart from being sustainable, biodiesel is highly biodegradable (Zivković and Veljković Citation2018), non-toxic (Yusuff et al. Citation2021), environmental friendly with low carbon foot print (Li et al. Citation2020) and possesses an energy density higher than that of petroleum diesel (Tan et al. Citation2016).
Scientists have, for the past decades, been searching for ideal biomass sources to use as feedstock for the biodiesel production process. Plants, such as Glycine max (Tan et al. Citation2016), Zea mays (Veljković et al. Citation2018), Brassica napus (Solis et al. Citation2017), Gossypium hirsutum (Mourshed et al. Citation2020), Jatropha curcas (Kamel et al. Citation2018), Calophyllum inophyllum (Bawane et al. Citation2020) and Elaeis guineensis (Tan et al. Citation2016), have been widely used as biomass feedstock for biodiesel production. However, most of these plants are essential food crops hence their use in biofuels production has adverse effects on food security. In Zimbabwe, biodiesel production from J. curcas gathered momentum in 2007. The project was however affected by shortage of feedstock (Jingura et al. Citation2013). Further, production of J. curcas for biodiesel also demands vast amounts of arable land and thus tends to compete with crop and livestock production. The use of plants as biomass feedstock also has other major setbacks such as their relatively poor growth rate, biomass productivity and lipid yield (Duong et al. Citation2012; Ryu et al. Citation2014). Microalgae, of late, are being preferred as biomass feedstock for biodiesel production due to their robustness, rapid growth, high biomass productivity, substantial lipid yield, efficient use of non-arable land and ability to proliferate in wastewater (Cheah et al. Citation2016).
Microalgae are an extremely diverse group of photosynthetic organisms. This provides a large genomic pool from which potential strains for biodiesel production can be isolated. The applicability of microalgae in biodiesel production has been widely studied (Duong Citation2016; Tan et al. Citation2016; Cobos et al. Citation2017; Kalsum et al. Citation2018; Li et al. Citation2020; Khalaji et al. Citation2021). Nevertheless, selection of a suitable microalgal strain is one of the main challenges affecting commercial microalgal biodiesel production (Mallick et al. Citation2016). Use of native strains for local biofuels production processes is preferable since this ensures ecological security. Further, native algal species are expected to be well adapted to local climatic and ecological conditions. Isolation of pure cultures from their native environment is therefore the first fundamental step towards screening and selection of algal species for biodiesel production. However, phycology is still a relatively understudied discipline in Africa including in countries such as Zimbabwe. The potential of native Zimbabwean microalgal species in biodiesel production is yet to be known since no report in this regard has been recorded thus far. Bioprospecting in such a genetically untapped country may lead to the discovery of novel and/or robust algal strains with highly competitive biomass productivities and extensive oil yields. Hence, the main objective of this study was to isolate and screen microalgal species native to Zimbabwe, with potential use in biodiesel production. Different types of microalgal species were therefore isolated from Manyame River, Zimbabwe. This river drains poorly treated urban wastewater from the City of Harare (Dlamini et al. Citation2016), Norton (Dlamini et al. Citation2016) and Chinhoyi (Norah et al. Citation2015). Thus, this river was considered to be the ideal source of robust and inherently tolerant microalgal species with biological traits of proliferating under extreme urban wastewater environments. The isolated species were assessed for their biomass and lipid yields. Their potential use in biodiesel production was also evaluated against an imported C. vulgaris species. The potential of this imported species in biodiesel production has been determined in many studies (Blinová et al. Citation2015; Lam et al. Citation2017; Kalsum et al. Citation2018; Vishnupriya et al. Citation2019; Ru et al. Citation2020;Khalaji et al. Citation2021). This makes it an ideal candidate for comparative purposes.
Methodology
Field sampling
Water samples were collected, for the isolation of algae, in clean 1 L plastic bottles at four different sites in the upper and middle part of Manyame River, i.e. Lake Chivero, Norton, Zvimba/Kutama and Chinhoyi/Hunyani. Manyame River is known for its high level of nutrients because of draining poorly treated urban wastewater and extensive proliferation of algal blooms (Norah et al. Citation2015; Dlamini et al. Citation2016). These four stations were selected to improve the diversity of isolates as the river is expected to self-purify downstream. Samples were collected at midday, a period under which photosynthetic activity was regarded to be at its peak.
Sample enrichment and isolation of algal strains
Upon arrival at the laboratory, 150 mL of each freshwater sample was enriched with 150 mL of sterile Bold Basal Medium (BBM), in separate transparent glass bottles. The sample enrichment process was also repeated, separately, using F/2 Guillard’s medium and Blue–Green Medium (BG-11). The three different media were used so as to increase the number of species to be isolated since different microalgal species have different media preferences (Idenyi et al. Citation2016; Stemmler et al. Citation2016). During sample enrichment, the cultures were maintained in a controlled culture room for 14 days at 25 ± 3°C under white fluorescent lighting (14 W fluorescent light/TL5). An 8:16 h light–dark cycle was also maintained throughout the culture enrichment period. After initial enrichment, the samples were then subjected to isolation through serial dilutions and streak plating. Serial dilutions are the simplest, most cost-effective and fastest method of isolating algae (Duong Citation2016). In this study, serial dilutions were used to isolate some filamentous microalgae which did not grow well on solidified media.
To isolate non-filamentous microalgae, multiple serial dilutions followed by microscopic examinations (Amscope IN300 TC-FL phase contrast microscope at 20×, 40× and 60×) were carried out to ensure unialgal cultures. After multiple serial dilutions, the samples were then subjected to streak plating on corresponding enrichment media solidified using bacteriological agar (Hi media, India). Repeated streaking was carried out until distinct unialgal colonies were observed. The distinct colonies were then picked and inoculated into separate 250 mL Schott bottles containing corresponding liquid media. Isolated unialgal species were then identified under a phase contrast microscope (Amscope IN300 TC-FL phase contrast microscope at 20×, 40× and 60×) using morphometric based taxonomic keys by Van Vuuren et al. (Citation2006). These unialgal cultures were now considered master cultures for subsequent growth and lipid assays. The master cultures were stored in a controlled culture room at 25 ± 3°C under white fluorescent lighting (14 W fluorescent light/TL5) and 8:16 h light–dark cycle.
Microalgal growth assays
Algal growth assays of isolated strains plus the imported C. vulgaris purchased from Carolina Biological Supply (Burlington, North Carolina, USA, Catalogue No. 15–2075) were conducted in 1L Schott bottles with an initial BBM volume of 800 mL. During the enrichment stage of the samples, high colour (growth) intensities were observed in master cultures with BBM hence growth assays were carried out using BBM as the growth medium. From the master cultures, 10% (v/v) was used as the initial inoculum concentration for each species. The Schott bottles were then exposed to white fluorescent lighting (14 W fluorescent light/TL5) and 8:16 h light/dark cycle. Room temperature was maintained at 25 ± 3°C during the culture period. Cultivation was carried out in triplicates. The cultures were incubated under these conditions for 12 days and gently shaken by hand twice a day to facilitate the distribution of cells and nutrients. Sub-samples of 5 mL were withdrawn at 3 d intervals for the analysis of microalgal growth patterns.
Growth patterns of the microalgal species were determined by measuring optical density (OD) using a Cecil (CE 1021) UV/Visible Spectrophotometer (Cecil Instruments, England) at a wavelength of 680 nm and a light path of 1 cm (Wang et al. Citation2010). The growth rate (GR, per day) was used as an estimate of microalgal biomass production (Wang et al. Citation2010; Osundeko et al. Citation2013). GR was calculated, as suggested by Wang et al. (Citation2010), by fitting the OD observed during the log phase of growth to an exponential function:
where N1 and N0 are the OD680 values at early and late exponential phase, respectively, whilst t1 and t0 are the corresponding days. Algaculture was carried out until day 12 when stationary phases were conclusively observed for all except the native Chlorella sp. Exponential growth rate comparisons could, however, be made since all strains had exhibited their exponential phase of growth. After the 12 d algaculture period, microalgal biomass was harvested through centrifugation (BHG ROTO UNI II, Germany) at 4500 rpm for 20 min. The harvested biomass was dried in an oven at 60°C for 48 h as recommended by Storms et al. (Citation2014). The dry biomass was then used for lipid quantification.
Lipid quantification
Lipid quantification for the studied algal species was carried out using the modified version of Bligh and Dyer’s gravimetric technique as described by Storms et al. (Citation2014). Approximately 40 mg of dry algal biomass was measured and transferred into a mortar pre-washed with hexane. The biomass was ground for 5 min using a pestle. Hexane was then added to the mortar and the resulting slurry homogenized. The resulting mixture was centrifuged (Eppendorf 5804R, Brinkmann Instruments, Westbury, NY) for 20 min at 10 000 rpm. The supernatant was then pipetted into a pre-weighed metal weighing dish and placed in the fume hood to evaporate the hexane. The mass of the oil extracted was determined gravimetrically after hexane had completely evaporated. The mass of the oil extracted was then expressed as a percentage of the dry cell weight (dcw) of algal biomass.
Statistical analysis
Genstat Discovery Edition v4 was used to carry out statistical analyses in this study. The data generated from the microalgal growth and lipid assays met the assumptions of the one-way analysis of variance (one-way ANOVA). Hence, the one-way ANOVA was used to compare mean differences in algal growth and lipid yield. Variations were considered statistically significant at p < 0.05. When mean differences were considered statistically significant, Tukey’s HSD test was carried out for post hoc analysis.
Results
Diversity of isolates
A total of four microalgal species were isolated, namely; Chlorella, Microcystis, Scenedesmus and Oscillatoria. The species belong to two groups of algal taxa which are Chlorophyta (green algae) and Cyanobacteria (blue–green algae). The Cyanobacteria isolates were Microcystis sp. and Oscillatoria sp. whereas Chlorophytes consisted of Chlorella sp. and Scenedesmus sp.
Algal growth
The isolated Chlorella sp. had the highest growth rate amongst the isolates, achieving an exponential growth rate of 0.260 d−1. The achieved growth rate was even higher than that of the imported Chlorella strain (0.226 d−1). Pairwise comparisons did, however, show that the mean growth rate difference between the two Chlorella strains was not statically significant (p > 0.05). Microcystis had the third best growth rate of 0.157 d−1. The slowest growing species were Scenedesmus sp. and Oscillatoria sp., achieving growth rates of 0.122 and 0.082 d−1, respectively (Table ). Statistical analyses showed significant differences (p < 0.05) amongst the means of the isolates’ growth rates.
Table 1. Growth rate performance of isolates against imported Chlorella vulgaris.
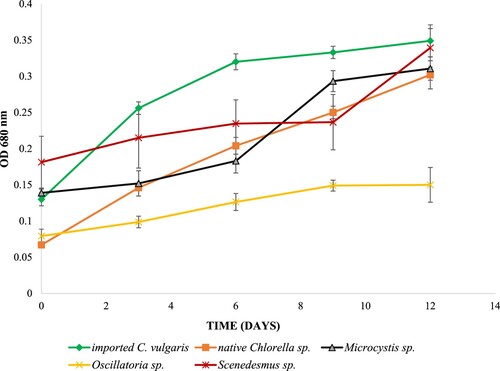
Algal growth patterns, as relative measures of OD680, were also plotted (Figure ). Algaculture was carried out over a period of 12 days. The native Chlorella sp., Oscillatoria sp. and the imported Chlorella sp. did not exhibit lag phases whereas Microcystis sp. and Scenedesmus sp. did. All species did, however, exhibit a 3-day exponential growth period. Comparisons between the two strains with highest growth rates (native and imported Chlorella sp.) indicate that by day 6, the imported Chlorella sp. had already reached its stationary phase of growth whereas the native Chlorella sp. was still exhibiting signs of growth (Figure ).
Lipid yield
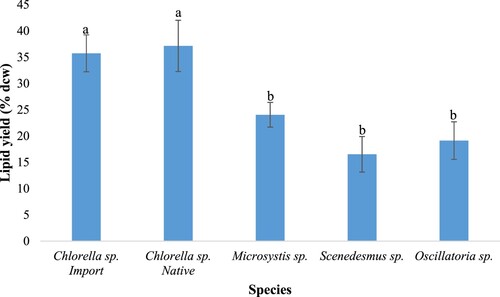
Lipid yield as a percentage of the algal biomass dry weight showed statistically significant mean differences (p<0.05). Both the native and imported Chlorella sp. had high lipid productivity, generating yields of 37.16% and 35.74% dry cell weight (dcw), respectively. However, pairwise comparisons between the two strains proved that their mean differences were not statistically significant (p>0.05). Microcystis sp. also generated a lipid yield (24.05% dcw) higher than that of Oscillatoria sp. (19.13% dcw) and Scenedesmus sp. (16.52% dcw) (Figure ).
Discussion
A total of four microalgal species were isolated, namely; Chlorella, Microcystis, Scenedesmus andOscillatoria. Although Manyame River contains a variety of microalgal species (Tendaupenyu Citation2012; Mhlanga and Mhlanga Citation2013; Mangadze et al. Citation2015), these four were robust, inherently tolerant and quickly adapted to laboratory conditions thus facilitating their isolation. These properties are essential in biodiesel production since they exhibit the extremophilic nature of the algae thus allowing algaculture in various forms of cheap and abundant wastewater. The isolates belonged to two groups of algal taxa which are Chlorophyta (Chlorella and Scenedesmus) and Cyanobacteria (Microcystis and Oscillatoria). Cyanobacteria have for long been identified as major sources of toxins in Lake Chivero and its associated rivers (Mhlanga et al. Citation2006). This group of algae has also been noted to have limited capabilities in biodiesel production due to its preference of glycogen and/or polyhydroxyalkanoates as carbon storage molecules instead of lipids (Lynch et al. Citation2015). On the contrary, Chlorophytes have been extensively studied and their potential use in biodiesel production determined (Lynch et al. Citation2015; Kalsum et al. Citation2018; Li et al. Citation2020; Khalaji et al. Citation2021). They use TAGs as their main carbon storage molecules (Lynch et al. Citation2015). Not only are algae from the Chlorophyta group good lipid producers, they are also relatively easy to culture (Massimi and Kirkwood Citation2016). This boosts their utility as feedstock for biodiesel production.
Growth rates are an important aspect in biodiesel production as they determine the amount of lipid yield per given time. Algal species with high growth rates allow lipid accumulation within the shortest period of time (Massimi and Kirkwood Citation2016). High growth rates also allow microalgae to outgrow most potential contaminants (Luangpipat Citation2013). Thus, comparative growth assays were carried out under laboratory conditions in this study. The native Chlorella sp. had the highest growth rate amongst all the isolates. It managed an exponential growth rate of 0.26d−1. A similar result was observed by Kim et al. (Citation2013) when they subjected the Chlorella sp. to autotrophic cultivation. However, other investigations using the same species reported extensively higher growth rates than the one observed in this study (Duong et al. Citation2015; Juntila et al. Citation2015). Discrepancies can be attributed to variations in culture conditions and genotypic differences. The native Chlorella sp. achieved a growth rate high enough to surpass its foreign counterpart (imported Chlorella sp.). However, pairwise comparisons using Tukey’s HSD test proved that the difference between the growth rates of the two strains is not statistically significant (p>0.05). Laboratory conditions under which the microalgae were exposed were foreign to both strains hence no strain had technical superiority over the other in terms of biomass yield. Nevertheless, the native Chlorella isolate exhibited a desirable trait, in terms of growth, for biodiesel production. The result supports the hypothesis set forth by this study which claims that bioprospecting in a genetically untapped country may actually lead to the discovery of isolates with competitive growth and lipid yields.
The native Chlorella sp., Oscillatoria sp. and imported Chlorella sp. did not have lag phases (Figure ) suggesting their good adaptability to new environmental conditions (Shen et al. Citation2017). Quick adaptability is a desired biological trait and one of the main selection criteria in the production of algal biomass for biofuels since it determines the potential applicability of culturing that microalgal species in cheap but extreme environments such as wastewater (Shen et al. Citation2017). Microcystis sp. and Scenedesmus sp. had lag phases, suggesting poor adaptability to new environmental conditions.
It is also important to note that by day 6, the imported Chlorella sp. had already reached its stationary phase of growth whereas the native Chlorella sp. was still exhibiting signs of growth (Figure ). Under local geographical, climatic and ecological conditions, imported strains are less likely to show dominance and high adaptability as compared to their native counterparts (Duong Citation2016). Thus, the native Chlorella sp. is a more ideal candidate for the biofuels production process. Its extended phase of growth, as compared to its foreign counterpart, allows more accumulation of the essential biomass.
Even though chloropytes are generally known to be robust and capable of high biomass yields, this was not the case with the isolated Scenedesmus sp. Not only did the Scenedesmus sp. have an extended lag phase (Figure ), its exponential growth rate (Table ) and lipid yield were (Figure ) lower than that of the native and imported Chlorella strains. The growth rate observed for Scenedesmus sp. in this study is comparable to the one observed by Hariz and Takriff (Citation2017) when they cultured Scenedesmus in 10% Palm Oil Mill Effluent (POME). Microcystis, an algal genus generally considered not ideal for biodiesel production, had a competitive growth rate better than that of a chlorophyte Scenedesmus. This observation is supported by a previous scientific report which claims that Microcystis sp. possesses a superior growth rate advantage over Scenedesmus sp. when cultured at 20–35 °C temperatures (Yang et al. Citation2018). Microcystis’ growth rate also fell within the range observed by Harke and Gobler (Citation2013) when this microbe was cultured under low phosphorus conditions. Oscillatoria sp. had the lowest growth rate and lipid yield suggesting that it has the least potential application, amongst the isolates, in biodiesel production.
Both the native and imported Chlorella sp. had the highest lipid productivities, generating yields of 37.16% and 35.74% dcw, respectively (Figure ). The results are comparable to the ones reported by other researchers for the Chlorella sp (Lam et al. Citation2017; Gao et al. Citation2019). However, pairwise comparisons between the two strains proved their difference in means is not statistically significant (p > 0.05) implying that under laboratory conditions none of the two strains had significant superiority over the other in terms of GR and lipid yield. Nevertheless, these findings highlight the potential applicability of the native Chlorella in biodiesel production as use of local strains is preferred over imported since these preserve the local genetic pool.
Advances in technology may be further applied to improve the productive performances of the isolated strains. Available technologies such as genetic engineering can be employed to enhance the biomass and lipid yield capacities of the isolates (Duong Citation2016). Technological manipulation and optimization of the culture microenvironment can also improve the isolates’ biomass and lipid yield capacity. The culture environment also influences the type of fatty acids produced and ultimately, the biodiesel quality (Li et al. Citation2020). It has been reported that the lipid yield in cultured algae may increase as nitrogen in the culture media gets depleted (Tan et al. Citation2016). Nitrogen starvation causes the rechanneling of photosynthetic ATP towards lipid biosynthesis (Cobos et al. Citation2017). Induced nitrogen starvation may therefore lead to optimum lipid yield. Spectral intensity also plays a role in improved algae lipid synthesis. Under extreme spectral intensities, algae tend to generate more lipids, a technique believed to be a protective measure against the extreme conditions (Junying et al. Citation2013). Spectral quality can also induce lipid synthesis in microalgae. Blue light enhances the activity of the enzymes carbonic anhydrase and ribulose bisphosphate carboxylase/oxygenase (Rubisco). These enzymes play a crucial role in microalgal carbon cycle and ultimately lipid yield (Vadiveloo et al. Citation2015; Sero et al. Citation2020). Lipid production in algal cells can, therefore, be optimized by inducing nitrogen starvation whilst employing the ideal spectral quality and intensity once the much needed biomass yield has been amassed.
Conclusions
Four microalgal species; Chlorella, Scenedesmus, Oscillatoria and Microcystis were isolated from the nutrient rich Manyame River, Zimbabwe. The potential use of the isolates in biodiesel production was evaluated against an imported Chlorella sp. through the measurement of the species’ growth rate and lipid yield capacity. The native Chlorella sp. exhibited the highest growth rate of 0.260 d−1 and lipid yield of 37.16% dcw amongst the isolates. It also showed favourable signs of adaption to controlled culture conditions since it did not exhibit a lag phase. In comparison, the imported Chlorella sp. managed a growth rate of 0.226 d−1 and lipid yield of 35.74% dcw. It also did not exhibit a lag growth phase. Pairwise comparisons suggested that the differences in biomass and lipid yield capacity of the two Chlorella strains were not statistically significant. However, the native strain is a more ideal candidate for indigenous biodiesel production processes since its preserves the local genetic pool. Further, other isolated microalgae including the imported strain reached stationery phase by day 6 of the experiment whereas the native Chlorella exhibited signs of growth during that same culture period. Findings of this study suggest that bioprospecting for native microalgal strains can actually yield competitive isolates which produce significant amounts of lipids and biomass for the biodiesel production process. Nevertheless, growth enhancement techniques, such as genetic engineering and the technological manipulation of the culture environment, can be used to further improve the native strains’ biomass and lipid yield capacity.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in [figshare] at [https://doi.org/10.6084/m9.figshare.14213495.v1].
Additional information
Funding
References
- Bawane RK, Muthuraja A, Shelke GN, Gangele A. 2020. Impact analysis of Calophyllum inophyllum oil biodiesel on perfomance and emmission characteristic of diesel engine under variation in compression ratio, engine load, and blend proportion. Int J Ambient Energy. 1–12.
- Blinová L, Bartošová A, Gerulová K. 2015. Cultivation of microalgae (Chlorella vulgaris) for biodiesel production. Research Papers Faculty of Materials Science and Technology Slovak University of Technology. 23(36):87–95. doi:10.1515/rput-2015-0010.
- Cheah WY, Ling TC, Show PL, Juan JC, Chang JS, Lee DJ. 2016. Cultivation in wastewaters for energy: A microalgae platform. Appl Energy. 179:609–625. doi:10.1016/j.apenergy.2016.07.015.
- Cobos M, Paredes JD, Maddox JD, Vargas-Arana G, Flores L, Aguilar CP, Marapara JL, Castro JC. 2017. Isolation and characterization of Native Microalgae from the Peruvian Amazon with potential for biodiesel production. Energies. 10(2):224. doi:10.3390/en10020224.
- Dlamini S, Nhapi I, Gumindoga W, Nhiwatiwa T, Dube T. 2016. Assessing the feasibility of integrating remote sensing and in-situ measurements in monitoring water quality status of Lake Chivero, Zimbabwe. Physics and Chemistry of the Earth, Parts A/B/C. 93:2–11. doi:10.1016/j.pce.2016.04.004.
- Duong VT. 2016. Isolation and evaluation of microalgae strains from The Northern Territory and Queensland - Australia that have adapted to accumulate triacylglycerides and protein as storage [Doctoral disseertation, The University of Queensland].
- Duong VT, Li Y, Nowak E, Schenk PM. 2012. Microalgae isolation and selection for prospective biodiesel production. Energies. 5(6):1835–1849. doi:10.3390/en5061835.
- Duong VT, Thomas-Hall SR, Schenk PM. 2015. Growth and lipid accumulation of microalgae from fluctuating brackish and sea water locations in South east queensland—Australia. Front Plant Sci. 6:359. doi:10.3389/fpls.2015.00359.
- Gao F, Yang HL, Li C, Peng YY, Lu MM, Jin WH, Bao JJ, Guo YM. 2019. Effect of organic carbon to nitrogen ratio in wastewater on growth, nutrient uptake and lipid accumulation of a mixotrophic culture Chlorella sp. Bioresour Technol. 282:118–124.
- Hariz HB, Takriff MS. 2017. Growth and biomass production of native microalgae Chlorella sp., chlamydomonas sp. and Scenedesmus sp. cultivated in Palm Oil Mill Effluent (POME) at different Cultivation conditions. Transactions on Science and Technology. 4(3):298–311.
- Harke M. J, Gobler C. J. 2013. Global tanscriptional responses of the toxic cyanobacterium, Microcystis aeruginosa, to nitrogen stress, phosphorus stress, and growth on organic matter. PLos One. 8(7):e69834.
- Idenyi JN, Ebenyi LN, Ogah O, Nwali BU, Ogbanshi ME. 2016. Effect of different growth media on the cell densities of freshwater microalgae isolates. IOSR Journal of Pharmacy and Biological Sciences Ver. 11(3):24–28. doi:10.9790/3008-1103042428.
- Jingura RM, Musademba D, Kamusoko R. 2013. A review of the state of biomass energy technologies in Zimbabwe. Renewable Sustainable Energy Rev. 26:652–659. doi:10.1016/j.rser.2013.05.036.
- Juntila DJ, Bautista MA, Monotilla W. 2015. Biomass and lipid production of a local isolate Chlorella sorokiniana under mixotrophic growth conditions. Bioresour Technol. 191:395–398. doi:10.1016/j.biortech.2015.03.098.
- Junying ZHU, Junfeng RONG, Baoning ZONG. 2013. Factors in mass cultivation of microalgae for biodiesel. Chin J Catal. 34(1):80–100. doi:10.1016/S1872-2067(11)60497-X.
- Kalsum, U., Mahmuddin, Mahfud, M., & Roesyadi, A. (2018). Biodiesel production from Chlorella vulgaris via homogenous acid catalyzed in situ transesterification with microwave irradiation. IOP Conference Series: Earth and Environmental Science, 175(1). doi:10.1088/1755-1315/175/1/012018
- Kamel DA, Farag HA, Amin NK, Zatout AA, Ali RM. 2018. Smart utilization of Jatropha (Jatropha curcas linnaeus) seeds for biodiesel production: optimization and mechanism. Ind Crops Prod. 111:407–413.
- Khalaji M, Hosseini SA, Ghorbani R, Agh N, Rezaei H, Kornaros M, Koutra E. 2021. Treatment of dairy wastewater by microalgae Chlorella vulgaris for biofuels production. Biomass Conversion and Biorefinery. 1–7.
- Kim S, Park JE, Cho YB, Hwang SJ. 2013. Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions. Bioresour Technol. 144:8–13. doi:10.1016/j.biortech.2013.06.068.
- Lam MK, Yusoff MI, Uemura Y, Lim JW, Khoo CG, Lee KT, Ong HC. 2017. Cultivation of Chlorella vulgaris using nutrients source from domestic wastewater for biodiesel production: growth condition and kinetic studies. Renewable Energy. 103:197–207.
- Li WW, Yu HQ, He Z. 2013. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 7(3):911–924. doi:10.1039/C3EE43106A.
- Li G, Zhang J, Li H, Hu R, Yao X, Liu Y, Zhou Y, Lyu T. 2020. Towards high-quality biodiesel production from microalgae using original and anaerobically-digested livestock wastewater. Chemosphere. doi:10.1016/j.chemosphere.2020.128578.
- Luangpipat T. 2013. Photobioreactor production of microalgae for potential fuel oils [Doctoral dissertation, Massey University]. http://hdl.handle.net/10179/4937.
- Lynch F, Santana-sánchez A, Jämsä M, Sivonen K, Aro E, Allahverdiyeva Y. 2015. Screening native isolates of cyanobacteria and a green alga for integrated wastewater treatment, biomass accumulation and neutral lipid production. Algal Res. 11:411–420. doi:10.1016/j.algal.2015.05.015.
- Mallick N, Bagchi SK, Koley S, Singh AK. 2016. Progress and challenges in microalgal biodiesel production. Front. Microbiol. 7:1019.
- Mangadze T, Bere T, Mwedzi T. 2015. Epilithic diatom flora in contrasting land-use settings in tropical streams, Manyame catchment, Zimbabwe. Hydrobiologia. 753(1):163–173. doi:10.1007/s10750-015-2203-7.
- Massimi R, Kirkwood AE. 2016. Screening microalgae isolated from urban storm- and wastewater systems as feedstock for biofuel. PeerJ. 4:e2396. doi:10.7717/peerj.2396.
- Mhlanga L, Day J, Cronberg G, Chimbari M, Siziba N, Annadotter H, Mhlanga L, Day J, Cronberg G, Chimbari M. 2006. Cyanobacteria and cyanotoxins in the source water from Lake Chivero, Harare, Zimbabwe, and the presence of cyanotoxins in drinking water. Afr J Aquat Sci. 31(2):165–173. doi:10.2989/16085910609503888.
- Mhlanga L, Mhlanga W. 2013. Dynamics of a cyanobacterial bloom in a hypereutrophic reservoir, Lake Chivero, Zimbabwe. Afr J Aquat Sci. 38(3):313–321. doi:10.2989/16085914.2013.827565.
- Mourshed M, Ghosh SK, Islam MW. 2020. Experimental investigation of cotton (Gossypium hirsutum) seed oil and neem (azadirachta indica) seed oil methyl esters as biodiesel on DI (direct injection) engine. Int J Ambient Energy. 1–11.
- Norah M, Shumirai Z, Zelm ML, Upenyu M. 2015. Impacts of untreated sewage discharge on water quality of middle Manyame river: A case of Chinhoyi town Zimbabwe. Int J Environ Monitoring Analysis. 3(3):133–138. doi:10.11648/j.ijema.20150303.14.
- Osundeko O, Davies H, Pittman JK. 2013. Oxidative stress-tolerant microalgae strains are highly efficient for biofuel feedstock production on wastewater. Biomass Bioenergy. 56:284–294. doi:10.1016/j.biombioe.2013.05.027.
- Rafiee A, Khalilpour RK. 2019. Renewable hybridization of oil and gas supply chains. In: Polygeneration with polystorage for chemical and energy hubs. Academic Press; p. 331–372.
- Rawat I, Kumar RR, Mutanda T, Bux F. 2011. Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energy. 88(10):3411–3424. doi:10.1016/j.apenergy.2010.11.025.
- Ru ITK, Sung YY, Jusoh M, Wahid MEA, Nagappan T. 2020. Chlorella vulgaris: A perspective on its potential for combining high biomass with high value bioproducts. Applied Phycology. 1(1):2–11.
- Ryu BG, Kim EJ, Kim HS, Kim J, Choi YE, Yang JW. 2014. Simultaneous treatment of municipal wastewater and biodiesel production by cultivation of Chlorella vulgaris with indigenous wastewater bacteria. Biotechnol Bioprocess Eng. 19(2):201–210. doi:10.1007/s12257-013-0250-3.
- Sero ET, Siziba N, Bunhu T, Shoko R, Jonathan E. 2020. Biophotonics for improving algal photobioreactor performance: A review. Int J Energy Res. 44(7):5071–5092. doi:10.1002/er.5059.
- Shen L, Ndayambaje JD, Murwanashyaka T, Cui W, Manirafasha E, Chen C, Wang Y, Lu Y. 2017. Assessment upon heterotrophic microalgae screened from wastewater microbiota for concurrent pollutants removal and biofuel production. Bioresour Technol. 245:386–393. doi:10.1016/j.biortech.2017.07.177.
- Sibanda G. 2020. ‘Dealers still diverting fuel.’ The Herald. https://www.herald.co.zw/dealers-still-diverting-fuel/.
- Solis JL, Berkemar AL, Alejo L, Kiros Y. 2017. Biodiesel from rapeseed oil (Brassica napus) by supported Li2O and MgO. Int J Energy and Environ Eng. 8(1):9–23.
- Stemmler K, Massimi R, Kirkwood AE. 2016. Growth and fatty acid characterization of microalgae isolated from municipal waste-treatment systems and the potential role of algal-associated bacteria in feedstock production. PeerJ. 4:e1780. doi:10.7717/peerj.1780.
- Storms, Z.J., Cameron, E., de la Hoz Siegler, H., & Mccaffrey, W.C. 2014. A simple and rapid protocol for measuring neutral lipids in algal cells using fluorescence. J Visualized Exp: JoVE, 87. doi:10.3791/51441
- Tan CH, Chen CY, Show PL, Ling TC, Lam HL, Lee DJ, Chang JS. 2016. Strategies for enhancing lipid production from indigenous microalgae isolates. J Taiwan Institute Chemical Eng. 63:189–194.
- Tendaupenyu P. 2012. Nutrient limitation of phytoplankton in five impoundments on the Manyame river, Zimbabwe. Water SA. 38(1):97–104. doi:10.4314/wsa.v38i1.12.
- Vadiveloo A, Moheimani NR, Cosgrove JJ, Bahri PA, Parlevliet D. 2015. Effect of different light spectra on the growth and productivity of acclimated nannochloropsis sp. (eustigmatophyceae). Algal Res. 8:121–127. doi:10.1016/j.algal.2015.02.001.
- Van Vuuren JS, Taylor J, Gerber A. 2006. A guide for the identification of microscopic algae in South African freshwaters. Department of water affairs and forestry. Potchefstroom, South Africa: North-West University.
- Veljković VB, Biberdžić MO, Banković-Ilić IB, Djalović IG, Tasić MB, Nježić ZB, Stamenković OS. 2018. Biodiesel production from corn oil: A review. Renewable Sustainable Energy Rev. 91:531–548. doi:10.1016/j.rser.2018.04.024.
- Vishnupriya M, Ramesh K, Sivakumar P, Balasubramanian R, Sircar A. 2019. Kinetic and thermodynamic studies on the extraction of bio oil from Chlorella vulgaris and the subsequent biodiesel production. Chem Eng. 206(3):409. doi:10.1080/00986445.2018.1494582.
- Wang L, Min M, Li Y, Chen P, Chen Y, Liu Y, Wang Y, Ruan R. 2010. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol. 162(4):1174–1186.
- Yang C, He L, Guan Q, Chen J, Miao R, Tao L, Hu N, Li B. 2019. Synthesis of NiMo/La-Al2O3 powders for efficient catalytic transesterification of triglyceride with the high yield of 92.5%. Environ Technol. 1–8.
- Yang J, Tang H, Zhang X, Zhu X, Huang Y, Yang Z. 2018. High temperature and pH favor Microcystis aeruginosa to outcompete Scenedesmus obliquus. Environ Sci Pollution Res. 25(5):4794–4802. doi:10.1007/s11356-017-0887-0.
- Yusuff AS, Gbadamosi AO, Popoola LT. 2021. Biodiesel production from transesterified waste cooking oil by zinc-modified anthill catalyst: parametric optimization and biodiesel properties improvement. J Environ Chem Eng. doi:10.1016/j.jece.2020.104955.
- Zivković S, Veljković M. 2018. The environmental impacts the of production and use of biodiesel. Environ Sci Pollution Res. 25(1):191–199.