Abstract
We explored the therapeutic effects of liraglutide combined with metformin and Diamicron in elderly patients with type 2 diabetes mellitus (T2DM) and the effects of this regimen on glucose – lipid metabolism and islet β-cell function. Ninety-two elderly patients with T2DM admitted to our hospital were selected as research participants. Of these, 42 patients treated with metformin and Diamicron were included in the control group (CG) and the remaining 50 patients treated with liraglutide combined with metformin and Diamicron were enrolled in the research group (RG). The total rate of efficacy in RG was significantly higher than that in CG (P < 0.05); however, there was no significant difference in the incidence of adverse reactions (P > 0.05). Moreover, no marked differences were observed in glucose–lipid metabolism indexes and islet β-cell function between the two groups before treatment (P > 0.05). After treatment, fasting plasma glucose, 2-h postprandial blood glucose, and glycosylated hemoglobin levels remarkably decreased in both groups, and these levels were significantly lower in RG than in CG (P < 0.001). Liraglutide combined with metformin and Diamicron can effectively improve glucose – lipid metabolism in elderly patients with T2DM and enhance islet β-cell function with improved clinical efficacy.
Introduction
In recent years, the number of patients with diabetes has increased, leading to a serious burden on families and society (Hagon-Traub et al. Citation2020). As per statistics (Ma Citation2018), diabetes has now affected approximately 440 million people worldwide. The majority of patients with diabetes suffer from type 2 diabetes mellitus (T2DM), and approximately 1 in 11 adults worldwide has diabetes, 90% of which have T2DM (Zheng et al. Citation2018). T2DM, a common chronic disease, generally manifests as disorders of glucose – lipid metabolism as well as increased fasting plasma glucose (FPG) and postprandial blood glucose levels. Moreover, it is characterized by insulin resistance, progressive islet β-cell function decline, and hyperglycemia due to insufficient insulin secretion and is mostly found in elderly patients (DeFronzo Citation2004). The onset and development of T2DM is relatively slow; T2DM is often clinically asymptomatic, which makes it difficult for patients to notice it in the early stages. As the disease progresses, complications, such as diabetic foot and diabetic nephropathy, can be induced, leading to a significant increase in mortality and disability rates. This seriously affects the quality of life as well as safety of patients (Larsson et al. Citation2018). At present, there is no radical cure for T2DM. The main goal of T2DM treatment in clinical settings is to eliminate clinical symptoms, correct the metabolic disorder of the body, and stabilize blood glucose of the patient for as long as possible (Strain et al. Citation2018). Metformin, one of the most commonly used oral antidiabetic drugs in clinical practice, primarily inhibits the mitochondrial respiratory chain in the liver, activates 5′-adenosine monophosphate-activated protein kinase (AMPK), and improves insulin sensitivity via its influence on fat metabolism, thereby reducing the expression of glucose-producing enzymes and controlling blood glucose (Rena et al. Citation2017). However, the use of metformin alone to control the levels of blood glucose makes it difficult to achieve the desired effect of blood glucose control and can cause adverse reactions in patients, resulting in unfavorable hypoglycemic effects. Diamicron, also known as gliclazide, can reduce glycogenolysis in the liver, prevent platelet aggregation, and restore the function of vascular endothelial cells, thereby alleviating the clinical symptoms of patients (Colagiuri et al. Citation2018). The combination of these two commonly used drugs can complement their shortcomings; however, the symptoms of insulin resistance in patients are more obvious because the blood lipid indexes are affected. Liraglutide, a new hypoglycemic drug (Mann et al. Citation2017), can promote insulin secretion, improve pancreatic islet β-cell function, and enhance the tissue insulin sensitivity. At present, there are few studies on the combination of metformin, Diamicron, and liraglutide for treating elderly patients with T2DM at home and abroad. Therefore, this study analyzed and studied the application value of liraglutide combined with metformin and Diamicron for treating elderly patients with T2DM to provide a valuable reference and guidance for future clinical diagnosis and treatment of T2DM.
Materials and methods
Clinical data
A total of 92 patients with T2DM who were admitted to our hospital from December 2017 to October 2019 were included in this study. Of these, 42 patients treated with metformin and Diamicron were included in the control group (CG) and the remaining 50 patients treated with liraglutide combined with metformin and Diamicron were included in the research group (RG). The present study was approved by the medical ethics committee of Taizhou Peoples Hospital (approval number ChiCTR1800016834), and all the enrolled participants provided informed consent.
Inclusion and exclusion criteria
The inclusion criteria were as follows: those who met the diagnostic criteria for T2DM as per the ‘Guidelines for the Prevention and Treatment of Type 2 Diabetes in China (2013 Edition)’; were aged >60 years; had no heart, liver, kidney, and other important organ dysfunctions; and who provided informed consent after being informed of this study. The exclusion criteria included those who had T2DM combined with lung infection or infectious diseases; had chronic complications of diabetes; or had mental disorders or communication disorders
Methods
Except routine treatment, CG received metformin (Changchun Changqing Pharmaceutical Group Co., Ltd., State Drug Approval Document Number: H22020483) at a dose of <2 g twice daily and Diamicron (Servier Pharmaceuticals, France, State Drug Approval Document Number: H20110194) at a dose of 30–120 mg once daily at breakfast. RG received CG regimen in addition to liraglutide (Denmark Novo Nordisk, State Drug Approval Document Number: S20110020) that was subcutaneously injected at a dose of 0.6 mg/day, increased to 1.2 mg/day a week later, and then gradually increased to 1.8 mg/day. The treatment lasted for 3 months.
Outcome measures
Treatment efficacy was defined as follows: markedly effective (clinical features and indicators were normal), effective (clinical features and indicators were significantly improved), and ineffective (clinical features and indicators did not change). Detection of glucose – lipid metabolism indexes was performed as follows: 5 ml of peripheral venous blood was collected from both groups before and after treatment. After centrifugation, an automatic biochemical analyzer was used to measure the glucose metabolism indexes of FPG, 2-h postprandial blood glucose (2hPG), and glycosylated hemoglobin (HbA1c) as well as the blood lipid indexes of total cholesterol (TC), triglycerides (TGs), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C). Islet β-cell function was analyzed as per the insulin-related indexes, including the homeostatic model assessment of insulin resistance (HOMA-IR) and homeostatic model assessment of β-cell function (HOMA-β). These indexes were measured using an automatic immunochemiluminescence instrument and compared between the two groups. In addition, adverse reactions were compared between the two groups.
Statistical methods
Data were analyzed using SPSS 24.0 (Shanghai Yuchuang Network Technology Co., Ltd.), and the required graphs were plotted with GraphPad Prism 5. Categorical data were expressed as percentages, and the chi-square test was used for intergroup comparisons of these variables. Quantitative data were expressed as means ± standard deviation, and the t-test was used for intergroup comparisons of these variables. P < 0.05 indicated statistical significance.
Results
General information
There was no significant difference in age, sex, body mass index, course of disease, family history, education, place of residence, and smoking between the two groups (P > 0.05), and these characteristics were comparable (Table ).
Table 1. Comparison of demographic characteristics of the patients [n (%)].
Comparison of clinical efficacy
RG showed an increase in the total rate of efficacy compared with CG (96.00% vs. 83.33%; P < 0.05; Table ).
Table 2. Comparison of clinical efficacy between the two groups [n (%)].
Comparison of glucose metabolism indexes
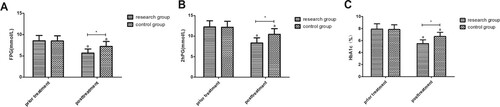
The glucose metabolism indexes decreased in both groups after treatment (P < 0.001), and the levels in RG were lower than those in CG (P < 0.001; Figure ).
Comparison of lipid metabolism indexes
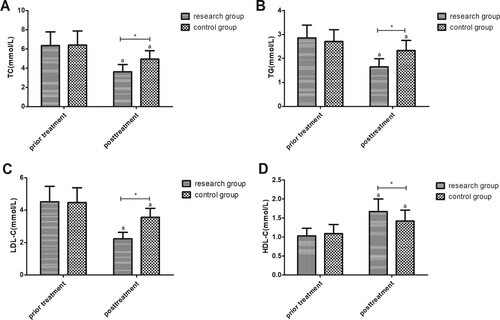
HDL-C levels increased but the other lipid metabolism indexes (TC, TG, and LDL-C) decreased in the two groups after treatment (P < 0.001); the differences in RG were greater than those in CG (Figure ).
Comparison of insulin-related indexes
After treatment, two groups showed increased HOMA-β and decreased HOMA-IR (P < 0.05); the differences in RG were more significant than those in CG (P < 0.001; Table ).
Table 3. Comparison of insulin-related indexes between the two groups before and after treatment.
Comparison of the incidence of adverse reactions
There were no significant differences in the incidence of adverse reactions, such as hypoglycemia, hypotension, nausea and vomiting, and constipation and diarrhea, between the two groups (P > 0.05; Table ).
Table 4. Comparison of adverse reactions between the two groups [n (%)].
Discussion
Diabetes mellitus, a disease that poses a serious threat to human health and safety, is characterized by a high incidence, high disability rate, and high mortality rate due to complications (Duncan et al. Citation2017). As per statistics (Rowley et al. Citation2017), a 50% higher risk of death from any cause is observed in adults with diabetes than in those without diabetes. T2DM is the main type of diabetes (Eraky et al. Citation2018), and as a lifelong, complex metabolic and endocrine disorder, T2DM pathogenesis is complex and may be related to heredity, obesity, age, and lifestyle (Sohail et al. Citation2017). Studies (Xu et al. Citation2017; Yu et al. Citation2018) have shown that an increase in insulin resistance and the incidence of hyperlipidemia has led to the global epidemic of T2DM. At present, the clinical treatment of T2DM centers more on good quality of life and extension of the lifespan of patients (Collier et al. Citation2020). Drug therapy, diet, and exercise interventions are presently the mainstream treatments for T2DM. However, there are many adverse drug reactions, particularly in elderly patients who often suffer from a variety of diseases and require different drugs. Moreover, the interactions between drugs are difficult for patients to tolerate; hence, the use of drugs is limited to a certain extent. As the disease progresses, the impairment of islet β-cell function and insulin resistance are gradually aggravated. Furthermore, the sensitivity of patients to drug treatment gradually decreases, which prevents the traditional hypoglycemic regimen commonly used in clinical settings from achieving the ideal clinical effects. Metformin is a clinically preferred oral hypoglycemic agent and is widely used to treat diabetes; however, it is prone to inducing adverse events, such as hypoglycemia, after a single administration (Sanchez-Rangel and Inzucchi Citation2017). Therefore, determining a more stable and safer combination of drugs has gradually become a research hotspot of researchers at home and abroad (Overbeek et al. Citation2017). As a second-generation sulfonylurea drug, Diamicron can repair islet β-cell function and has better blood glucose control effects (Al-Omary Citation2017). Liraglutide is a novel hypoglycemic agent that can correct T2DM pathogenesis (Pokala et al. Citation2017). At present, there are few studies on the combination of these three drugs for treating elderly patients with T2DM, and the value of applying this regimen has not been completely determined. Therefore, in this study, the efficacy of liraglutide combined with metformin and Diamicron for treating elderly patients with T2DM was compared, and the changes in glucose – lipid metabolism and islet β-cell function during treatment were observed to demonstrate the value of combination therapy with these three drugs for treating T2DM.
A superior treatment effect was observed in RG than in CG. FPG, 2hPG, and HbA1c levels were significantly lower after the different treatments than before treatment, and the improvement was more obvious in RG than in CG. In addition, the blood lipid indexes of patients in the two groups significantly decreased after treatment, except HDL-C that increased. These changes were more significant in RG than in CG. Moreover, HOMA-β in RG was remarkably higher than that in CG after treatment, whereas HOMA-IR demonstrated opposite results. Kalra et al. study (Kalra and Das Citation2017) showed that metformin combined with Diamicron can effectively control the blood glucose levels of most patients with T2DM with a low risk of hypoglycemia. Another study by Ayers et al. (Ayers et al. Citation2017) demonstrated that liraglutide significantly decreased HbA1c levels at 12 and 24 weeks and was more effective in treating T2DM compared with placebo. The reason for the difference observed in the results of this study may be that during hyperglycemia, liraglutide promotes islet β-cell autophagy by regulating AMPK while protecting islet β-cells from hyperglycemia and inhibiting islet β-cell apoptosis by enhancing autophagy (Miao et al. Citation2018). Besides, some studies have suggested that (Peradze et al. Citation2019) treating obese patients without obvious T2DM with high-dose liraglutide in a short period of time would cause changes in lipid – lipoprotein and the levels of hormones, which can reduce the risk of atherosclerosis and cardiovascular disease. Its mechanism may be that the improved blood glucose control decreases the expression of apoCIII, a key regulator of hypertriglyceridemia, in patients with hyperglycemia (Matikainen et al. Citation2019). Moreover, the results of Huang et al. study (Huang et al. Citation2019) revealed that in rats treated with liraglutide and insulin, the level of HDL-C level was significantly increased, whereas the levels of TC, TG, and LDL-C were decreased, which was consistent with the results of our study and could be mutually corroborated. A further comparison of the adverse reactions of drug use identified that there was no significant difference in the incidence of adverse reactions between the two groups, indicating that the safety of drug use was not affected by the addition of liraglutide. In recent years, it has also been reported that (Ko et al. Citation2017) combination drug therapy shows better hypoglycemic effects and fewer adverse effects than monotherapy.
This study has some limitations. For example, the sample size base was small, and the population was relatively single. In addition, the specific mechanism of the combination of the three drugs in elderly patients with T2DM needs further discussion and exploration. We will continue to follow-up the research participants for a longer time and update our study results in the future.
In conclusion, liraglutide combined with metformin and Diamicron can effectively improve glucose – lipid metabolism and islet β-cell function in elderly patients with T2DM with better clinical efficacy.
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Al-Omary FAM. 2017. Gliclazide. Profiles Drug Subst Excip Relat Methodol. 42:125–192. eng.
- Ayers D, Kanters S, Goldgrub R, Hughes M, Kato R, Kragh N. 2017. Network meta-analysis of liraglutide versus dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes in Japanese patients. Curr Med Res Opin. 33(9):1653–1661. eng.
- Colagiuri S, Matthews D, Leiter LA, Chan SP, Sesti G, Marre M. 2018. The place of gliclazide MR in the evolving type 2 diabetes landscape: A comparison with other sulfonylureas and newer oral antihyperglycemic agents. Diabetes Res Clin Pract. 143:1–14. eng.
- Collier A, Meney C, Hair M, Cameron L, Boyle JG. 2020. Cancer has overtaken cardiovascular disease as the commonest cause of death in Scottish type 2 diabetes patients: A population-based study (the ayrshire diabetes follow-up cohort study). J Diabetes Investig. 11(1):55–61. eng.
- DeFronzo RA. 2004. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 88(4):787–835, ix. eng.
- Duncan BB, Schmidt MI, Ewerton C, Moradi-Lakeh M, Passos VMA, França EB, Marinho F, Mokdad AH. 2017. The burden of diabetes and hyperglycemia in Brazil-past and present: findings from the global burden of disease study 2015. Diabetol Metab Syndr. 9:18. eng.
- Eraky SM, Abdel-Rahman N, Eissa LA. 2018. Modulating effects of omega-3 fatty acids and pioglitazone combination on insulin resistance through toll-like receptor 4 in type 2 diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids. 136:123–129. eng.
- Hagon-Traub I, Chinet L, Georges A, Schlüter V. 2020. [Cantonal diabetes program - a response at local level to address public health issues related to non-communicable diseases]. Rev Med Suisse. 16(682):366–369.. fre.
- Huang Q, Liu C, Li JR, Zhang L, Huang FC, Wang D, Luo YJ. 2019. Incremental effect of liraglutide on traditional insulin injections in rats with type 2 diabetes mellitus by maintaining glycolipid metabolism and cardiovascular function. Exp Ther Med. 17(3):1863–1869. eng.
- Kalra S, Das AK. 2017. Epidemiologic surveillance of glycemic response to a scored, breakable, extended release, fixed dose combination of gliclazide and metformin in persons with type 2 diabetes. J Assoc Physicians India. 65(6):38–41. eng.
- Ko SH, Hur KY, Rhee SY, Kim NH, Moon MK, Park SO, Lee BW, Kim HJ, Choi KM, Kim JH. 2017. Antihyperglycemic agent therapy for adult patients with type 2 diabetes mellitus 2017: a position statement of the Korean diabetes association. Diabetes Metab J. 41(5):337–348. eng.
- Larsson SC, Wallin A, Håkansson N, Stackelberg O, Bäck M, Wolk A. 2018. Type 1 and type 2 diabetes mellitus and incidence of seven cardiovascular diseases. Int J Cardiol. 262:66–70. eng.
- Ma RCW. 2018. Epidemiology of diabetes and diabetic complications in China. Diabetologia. 61(6):1249–1260. eng.
- Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B, Buse JB. 2017. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 377(9):839–848. eng.
- Matikainen N, Söderlund S, Björnson E, Pietiläinen K, Hakkarainen A, Lundbom N, Taskinen MR, Borén J. 2019. Liraglutide treatment improves postprandial lipid metabolism and cardiometabolic risk factors in humans with adequately controlled type 2 diabetes: A single-centre randomized controlled study. Diabetes Obes Metab. 21(1):84–94. eng.
- Miao X, Gu Z, Liu Y, Jin M, Lu Y, Gong Y, Li L, Li C. 2018. The glucagon-like peptide-1 analogue liraglutide promotes autophagy through the modulation of 5'-AMP-activated protein kinase in INS-1 β-cells under high glucose conditions. Peptides. 100:127–139. eng.
- Overbeek JA, Heintjes EM, Prieto-Alhambra D, Blin P, Lassalle R, Hall GC, Lapi F, Bianchini E, Hammar N, Bezemer ID, et al. 2017. Type 2 diabetes mellitus treatment patterns across Europe: A population-based multi-database study. Clin Ther. 39(4):759–770. eng.
- Peradze N, Farr OM, Perakakis N, Lázaro I, Sala-Vila A, Mantzoros CS. 2019. Short-term treatment with high dose liraglutide improves lipid and lipoprotein profile and changes hormonal mediators of lipid metabolism in obese patients with no overt type 2 diabetes mellitus: a randomized, placebo-controlled, cross-over, double-blind clinical trial. Cardiovasc Diabetol. 18(1):141. eng.
- Pokala N, Adams-Huet B, Li X, Harrison LB, Vanderheiden A, Lingvay I. 2017. The effect of baseline characteristics on clinical efficacy of liraglutide in patients treated with high-dose insulin. Diabetes Obes Metab. 19(10):1454–1457. eng.
- Rena G, Hardie DG, Pearson ER. 2017. The mechanisms of action of metformin. Diabetologia. 60(9):1577–1585. eng.
- Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. 2017. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 20(1):6–12. eng.
- Sanchez-Rangel E, Inzucchi SE. 2017. Metformin: clinical use in type 2 diabetes. Diabetologia. 60(9):1586–1593. eng.
- Sohail MU, Althani A, Anwar H, Rizzi R, Marei HE. 2017. Role of the gastrointestinal tract microbiome in the pathophysiology of diabetes mellitus. J Diabetes Res. 2017:9631435. eng.
- Strain WD, Hope SV, Green A, Kar P, Valabhji J, Sinclair AJ. 2018. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty. A national collaborative stakeholder initiative. Diabet Med. 35(7):838–845. eng.
- Xu Y, Niu Y, Gao Y, Wang F, Qin W, Lu Y, Hu J, Peng L, Liu J, Xiong W. 2017. Borapetoside E, a clerodane diterpenoid extracted from tinospora crispa, improves hyperglycemia and hyperlipidemia in high-Fat-diet-induced type 2 diabetes mice. J Nat Prod. 80(8):2319–2327. eng.
- Yu X, Xu L, Zhou Q, Wu S, Tian J, Piao C, Guo H, Zhang J, Li L, Wu S, et al. 2018. The efficacy and safety of the Chinese herbal formula, JTTZ, for the treatment of type 2 diabetes with obesity and hyperlipidemia: A multicenter randomized, positive-controlled, open-label clinical trial. Int J Endocrinol. 2018:9519231. eng.
- Zheng Y, Ley SH, Hu FB. 2018. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 14(2):88–98. eng.