Abstract
Cisplatin (CISP) is one of the most commonly used drug for treating various cancers and solid tumours, however, its usage has been heavy restricted due to its deleterious side effects including peripheral neuropathy. Therefore, this study describes the anti-hyperalgesia and anti-allodynia effects of Tiliacora triandra (TTE) in CISP induced peripheral neuropathy. Peripheral neuropathy was established in rats by intraperitoneal injection of 2.5 mg/kg of CISP once a week for 4 weeks and the rats were concurrently treated with TTE (250 and 500 mg/kg, po) daily for 5 weeks. After the treatment, thermal hyperalgesia, motor coordination (rotarod test), mechanical allodynia, acetic acid writhing and formalin tests were evaluated. The rats were sacrificed and blood samples were collected for estimation of haematological parameters. TTE significantly restored motor coordination deficits induced by CISP and TTE-treated rats showed marked improvement in thermal/chemical hyperalgesia and mechanical allodynia. Additionally, treatment with TTE also increased haematological parameters including haemoglobin, platelet count, WBC and RBC compared to CISP-treated group. These findings demonstrated that TTE effectively ameliorated CISP induced peripheral neuropathic pain in a cisplatin paradigm.
Introduction
Cisplatin (CISP) also known as cis-diamminedichloroplatinum is one of the most common platinum based cytotoxic drug used extensively in clinical practice for the treatment of various malignant tumours and cancers including bladder, neck and head, ovarian, testicular and lung cancers. CISP effectively mitigates the replication of cancer cells by restricting DNA replication and repair via its ability to cross link with DNA purine bases resulting in the generation of oxidative stress, mitochondrial dysfunction, DNA damage and apoptosis of cancer cells (Shahid et al. Citation2017; Arafa and Atteia Citation2020; Stathopoulos Citation2010). In spite of the efficacy of CISP against these wide range of malignant and cancerous cells, its toxicity on normal cells as well as other non-targeted organs especially the kidney, brain and testes have raised severe concerns and limited its use. CISP have been extensively reported to instigate neurotoxicity, ototoxicity, hepatotoxicity, nephrotoxicity, gastrointestinal toxicity as well as central and peripheral neuropathy (Barabas et al. Citation2008; Hartmann and Lipp Citation2003; Ullah et al. Citation2018).
Peripheral neuropathy is one of the most common side effects that accompanies the administration of CISP and it is often characterised by gait impairment, sensory disturbances including numbness, pain and parasthesia (Hay Citation2002; Mary Collins et al. Citation2007). The mechanism involved in CISP induced damage is far from been understood, however, some reports have suggested that CISP have the ability to induce dorsal root ganglia damage leading to symmetrical and bilateral sensory symptoms which eventually progresses to proximal stocking-glove distribution (Shahid et al. Citation2017; Akbar et al. Citation2020; Schröder et al. Citation2013). The intricacy of these sequency of adverse events leads to gross deterioration in the quality of life cancer patients using CISP (Ocean and Vahdat Citation2004). Unfortunately, there is no effective therapy in clinical practice that mitigates CISP induced toxicity including neuropathy.
Recently, increasing evidences have suggested the role of natural products especially medicinal plants and their bioactive constituents in the abrogation of chemically induced multiple organ toxicities (Boroja et al. Citation2018; Wang et al. Citation2020). Tiliacora triandra, commonly known as Yanang in Thailand belongs to the Menispermaceae family and it possesses several medicinal properties. In folk medicine T. triandra is used in the treatment of diabetes, malaria, pain, bacterial infection and as a detoxicant (Makinde et al. Citation2020a; Thong-Asa and Bullangpoti Citation2020). Pharmacological studies have also attributed several biological activities to plant or its bioactive compounds including neuroprotection, nephro and testicular-protection and antimycobacterial properties (Makinde et al. Citation2020b; Phunchago et al. Citation2015; Sureram et al. Citation2012; Song et al. Citation2021). However, there is no evidence on the protective effects of T. triandra on CISP induced toxicities including peripheral neuropathy. As such the aim of this study is to investigate the protective effect of T. triandra against CISP-induced peripheral neuropathic pain.
Materials and methods
Plant specimen collection and preparation of T. triandra extract
Naturally grown aerial parts of T. triandra were collected from the southern province of Phatthalung, Thailand in July 2020, and was identified at the Faculty of Traditional Thai Medicine, Prince of Songkla University, Thailand, a voucher specimen with reference number TTM/TT/001/20 was deposited at the herbarium of the faculty. The leaves and twigs of the plant were properly washed under running tap water to remove all debris and dirt. Thereafter, the specimens were dried in a hot air oven at 55 °C for 3 days. After drying, the samples were blended using a mechanical grinder and the powdered sample (500 g) was soaked in 70% ethanol for 24 h, filtered using a Whatman filter paper No.1. The filtrate obtained was evaporated to one third of the original volume. In order to rid of the deep green colour (chlorophyl) from the ethanol extract obtained, the sedimentation process was employed using previously reported protocol (Olatunde et al. Citation2021). Briefly, the extract solution was kept in 4 °C for 24 h and centrifuged at 5000 g at 4 °C for 30 min. The supernatant collected after centrifugation was further filtered, lyophilised and a light brown coloured extract powder obtained was named ‘TTE’. TTE powder was refrigerated until further use.
Animals
Twenty four male Wistar rats (150–180 g) used in this study were housed as six rats per cage and fed with normal rat chow and tap water ad libitum at a constantly maintained environmental conditions of temperature at 22 °C, relative humidity of 60–70% and a 12 h light/dark cycle to stimulate natural rhythm. The animals were allowed one week of acclimatisation before the commencement of the experiment. The protocol for this study was performed as per the guidelines of the care and use of laboratory animals of the National Institute of Health and approval from the Institutional Animal Ethics Committee of Anhui College of Traditional Chinese Medicine was obtained (2020/001).
Experimental design
The rats were randomly divided into four groups, each group containing six rats as follows:
Group I (NC): Normal control group of rats orally administered with normal saline
Group II (CISP): CISP control rats orally administered with normal saline and received intraperitoneal injection of CISP (2.5 mg/kg/week) for 4 weeks.
Group III (TTE250-CISP): Rats orally administered with TTE (250 mg/kg) for 5 weeks, followed by weekly intraperitoneal injection of CISP (2.5 mg/kg/week) for 4 weeks starting from the beginning of the second week of TTE administration.
Group IV (TTE500-CISP): Rats orally administered with TTE (500 mg/kg) for 5 weeks, followed by weekly intraperitoneal injection of CISP (2.5 mg/kg/week) for 4 weeks starting from the beginning of the second week of TTE administration. The choice of TTE and CISP dose in this study was based on previous reports (Arafa and Atteia Citation2020; Makinde et al., Citation2020b; Kandeil et al., Citation2020). The body weights of all the rats were monitored on a weekly basis.
Behavioural studies
Assessment of thermal hyperalgesia
Thermal hyperalgesia was assessed after treatment using the hot plate test. The hotplate was maintained at 52.0 ± 0.5 °C and the thermal withdrawal latency of each on the rats were recorded in seconds at the first sight of hind paw licking or lifting or jumping. A cut off time of 40 s was set so as to avoid tissue damage due to excessive heat.
Tail immersion/warm-water tail flick test
The tail immersion test was performed to access thermal hyperalgesia. The tails of all the animals were immersed in a water bath set at 50.0 ± 0.5 °C until the rats displays the first response of tail flicking, withdrawal or struggle. A cut off time of 12 s was set. Reduced tail withdrawal time illustrates hyperalgesia. All the rats were held in a restrainer so as to maintain uniform stress and avoid bias for all the groups.
Mechanical allodynia
Mechanical allodynia was evaluated after treatment in the hind paws using von Frey filaments. The animals were individually placed on a stainless steel floor and series of von Frey filaments were perpendicularly applied on the hind paw plantar surface until the filament bends for a cut off time of 6 s or until a positive response of paw flinching or lifting occurred and the next von Frey filament with a lessened force was applied, while in the absence of a response the subsequent von Frey filament of greater force was applied. The experiment was repeated five times at 5 mins intervals and the mean value was calculated.
Acetic acid-induced abdominal writhing test
For the assessment of neuropathic pain using acetic acid writhing test, after TTE treatment the animals were administered with an intraperitoneal injection of 1% acetic acid (10 mL/kg). The number of abdominal writhing/stretching movements including body elongation, stretching of back limb, extension of limbs were recorded for 30 min.
Formalin induced nociception test
The formalin test was performed in the rats by injecting 50 µL of 2.5% of formalin solution subcutaneously into the dorsal surface of the right hand paw of each rat. The animals were immediately placed in an open box for observation for 30 min. The early phase (neurogenic phase; 0–5 min after formalin injection) consist of severe licking, biting, lifting or no pain in the injected paw for the first 5 min, while the late phase (inflammatory phase; 15–30 min after formalin injection) featured by biting and licking of the injected paw. The period spent in seconds by the animals in licking or biting of the injected paw was measured with a stopwatch.
Rotarod performance test
A rotarod apparatus was utilised for the experiment. The animals were allowed three sessions of training for three consecutive days. During the training session, the animals were placed on a rotating rod set at 15 rpm and the ability of the rats to remain on the rotarod was recorded in seconds. During the test session, latency of the rats to dismount from the rotarod was recorded. The animals were allowed three trials on the test day and the mean value was recorded.
Animal sacrifice
After the completion of the behavioural studies, the animals were anesthetised using 100 mg/kg of sodium thiopental solution and blood samples were obtained through direct cardiac puncture. The blood collected were kept in EDTA bottles for analysis of haematological parameters
Statistical analysis
Statistical analysis were performed using Graph Pad Prism 5 and experimental data were expressed as mean ± SD. One-way ANOVA accompanied with Bonferroni multiple comparison test was used for statistical analysis. Results were considered significant at P < 0.05.
Results
Effect of TTE on CISP induced weight variation
The effect of TTE on weight variations in after CISP administration is presented in Table . The body weights of CISP control group was significantly reduced after the administration of CISP for 4 weeks when compared to the normal control. However, a contrasting trend was noticed in the TTE (250 and 500 mg/kg) treated groups, TTE notably prevented gross weight reduction compared to CISP control group (Table ).
Table 1. Effect of TTE on body weight and haematological indices in CISP-treated rats.
TTE reduces cisplatin-induced reduction in haematological indices
The results shown in Table indicated that CISP significantly altered haematological parameters as indicated by gross reduction in WBC, platelets, RBC and haemoglobin levels compared to the NC group. Whereas, treatment with TTE produced marked increase in WBC, platelets and RBC levels. The haemoglobin level in the animals treated with TTE (250 and 400 mg/kg) was slightly increased, though not significant compared to CISP-treated rats (Table ).
Effect of TTE on CISP induced thermal hyperalgesia
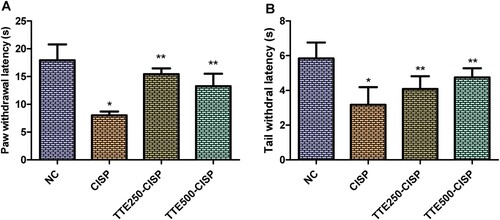
The effect of TTE on thermal hyperalgesia is as shown in Figure . The intraperitoneal injection of CISP once a week for 4 consecutive weeks instigated significant reduction in the paw latency withdrawal of the CISP control group compared to the normal control rats (P < 0.05). Whereas, administration of TTE produced significant increase in withdrawal latency in the hot plate test (Figure (A)). Likewise, in the tail immersion test, TTE extract evoked marked increase in CISP-induced decreased in tail withdrawal latency when compared to the CISP control group (Figure (B)).
Figure 1. Effect of oral administration of TTE on thermal hyperalgesia in cisplatin-treated rats. (A) Hot plate test, (B) Tail immersion test. Values represents mean ± SD (n = 6). Data analysis was performed using one way ANOVA followed by Bonferroni post hoc analysis. *P < 0.05 compared to normal control group; **P < 0.05 compared to cisplatin alone treated group.
Effect of TTE on mechanical allodynia
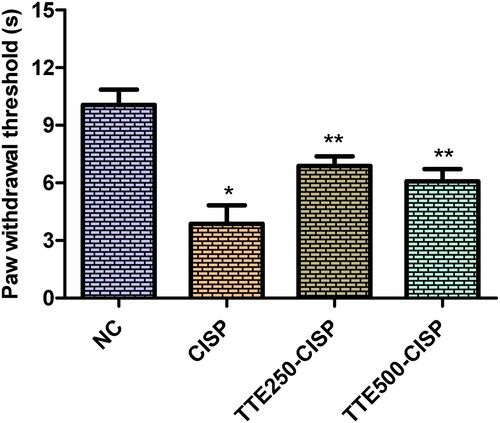
As shown in Figure , the CISP control group showed significant decrease in paw withdrawal threshold compared to the normal control (P < 0.05). Whereas, compared to the CISP control group, the paw withdrawal threshold of rats treated with TTE (250 and 400 mg/kg) significantly increased the paw withdrawal threshold compared to CISP control rats.
Figure 2. Effect of oral administration of TTE on mechanical allodynia in cisplatin-treated rats. Values represents mean ± SD (n = 6). Data analysis was performed using one way ANOVA followed by Bonferroni post hoc analysis. *P < 0.05 compared to normal control group; **P < 0.05 compared to cisplatin alone treated group.
Effect of TTE in the formalin hind paw test
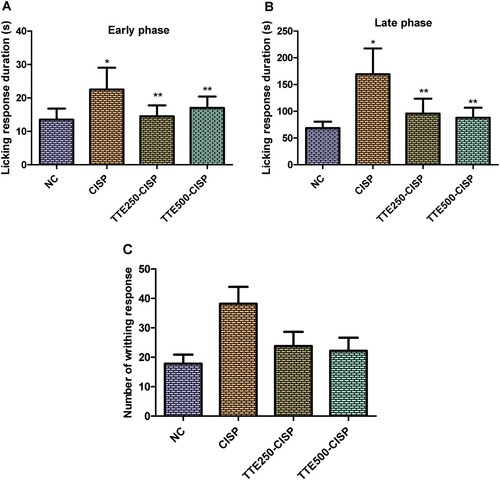
In the neurogenic phase (first phase; 0–5 min), the CISP-TTE treated groups showed weakened and significant nociceptive response compared to CISP control group (Figure (A)). Likewise, the second phase (inflammatory phase; 15–30 min) was marked with significant efficacy of TTE (250 and 500 mg/kg) as indicated by marked reduction in the duration of the pain response (significant reduction in licking time) when compared to the licking time of the rats in CISP control group (Figure (B)).
Figure 3. Effect of oral administration of TTE on nociception in cisplatin-treated rats. (A) First phase of formalin induced paw licking, (B) Second phase of formalin induced paw licking, (C) Acetic acid induced writhing. Values represents mean ± SD (n = 6). Data analysis was performed using one way ANOVA followed by Bonferroni post hoc analysis. *P < 0.05 compared to normal control group; **P < 0.05 compared to cisplatin alone treated group.
Effect of TTE in the acetic acid writhing test
The writhing responses of rats in CISP control group was significantly increased when juxtaposed to the responses evoked by the normal rats. Treatment with TTE (250 and 500 mg/kg) significantly decreased the writhing responses of the rats compared to the CISP control group (Figure C).
Effect of TTE on motor coordination and balance
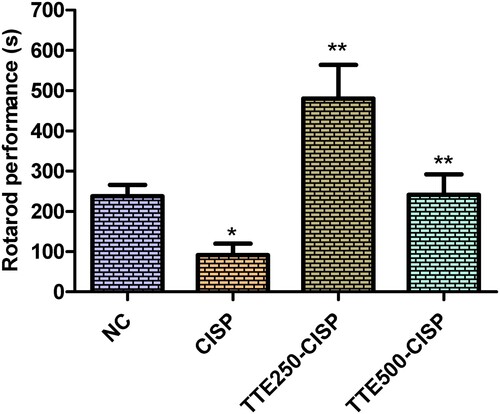
The effect of TTE on motor coordination was evaluated in CISP administered rats using the rotarod experiment. As shown in Figure , a significant decrease was observed in the endurance level of CISP control group rats on the accelerating rotarod when compared with the normal control rats. In contrast, in the TTE-CISP treated groups, the performance of the rats on the rotarod was significantly increased in comparison to the CISP control group (Figure ). Interestingly the dismount latencies in the TTE-treated groups were much lower than the normal control group, while the dismount latency on the rotarod in the TTE200 mg/kg rats was observed to be much better than the TTE400 (Figure ).
Figure 4. Effect of oral administration of TTE on rotarod performance in cisplatin-treated rats. Values represents mean ± SD (n = 6). Data analysis was performed using one way ANOVA followed by Bonferroni post hoc analysis. *P < 0.05 compared to normal control group; **P < 0.05 compared to cisplatin alone treated group.
Discussion
Cancer is one of the leading cause of death worldwide. In 2020, there were an estimated 19.1 million new cases of cancer globally and 10 million cancer deaths (Sung et al. Citation2021). As such combating this dreadful menace has become one of the main priority of global public health. Interestingly, advancement in medical science have produced several anticancer agents including chemotherapy drugs that have increased the life expectancy of many cancer patients (Schirrmacher Citation2019; Zajączkowska et al. Citation2019). Chemotherapeutic drugs plays significant roles in mitigating the progression of cancer, however, the cytotoxic effects of these drugs are not only restricted to the target tissues, but also significantly affects several other organs in the patients resulting in varying degrees of side effects and toxicities (Stankovic et al. Citation2020; Argyriou et al. Citation2012; Trendowski et al. Citation2019; Hakiminia et al. Citation2019; Zajączkowska et al. Citation2019). CISP, a widely used platinum based anticancer and antitumor agent has been widely documented to adversely affect the nervous system leading to peripheral neuropathy characterised by motor, sensory and autonomic dysfunction (Park et al. Citation2013; McWhinney et al. Citation2009). As such there is urgent need for regimens that could effectively taper the adverse reactions related to the use of chemotherapy agents.
This study investigated the anti-hyperalgesia and anti-allodynia properties of Tiliacora triandra (TTE) on CISP induced neuropathic pain. In this study, the rats administered with intraperitoneal injection of CISP (2.5 mg/kg/week) for 4 weeks displayed rapid and significant body weight loss, thermal and chemical hyperalgesia, reduced motor balance and coordination and mechanical allodynia, which are characteristics symptoms observed in peripheral neuropathy. The anti-allodynia and anti-hyperalgesia effects of TTE were assessed using heat hyperalgesia (tail immersion test and hot plate test), acetic acid-induced writhing, formalin and von Frey filaments test. Previous reports have associated the administration of CISP with the evolvement of thermal hyperalgesia and mechanical allodynia in animal models (Shahid et al. Citation2017; Abdelsameea and Kabil Citation2018; Authier et al. Citation2003). CISP can result in acute, transient thermal sensations to irreversible changes in peripheral nerves followed by chronic pain and permanent nerve damage (Seretny et al. Citation2014). The results obtained from this study showed that intraperitoneal injection of CISP ensued in sensory neuropathy culminating into reduced thermal withdrawal latencies suggesting heat hyperalgesia. Our result were coherent with the results from the studies of Kuai et al. and Deng et al. that postulated that CISP induced thermal and mechanical hyperalgesia (Kuai et al. Citation2020; Deng et al. Citation2012). Moreover, Nawaz et al. portrayed that CISP and vincristine decreased the reaction time in models of heat hyperalgesia (Nawaz et al. Citation2018).
The administration of CISP for four consecutive weeks led to a significant increase in mechanical hyperalgesia which was in line with earlier reports (Han et al. Citation2014; Shahid et al. Citation2017). The formation of platinum-DNA products at the spinal ganglion upon administration of CISP can lead to mechanical allodynia (Dzagnidze et al. Citation2007). Additionally, studies have implicated CISP in demyelinating, which is the loss or damage of myelinated fibres, which invariably leads to heat sensitivity and mechanical allodynia (Luo et al. Citation1999; Sisignano et al. Citation2014). Our results indicated that treatment with TTE (250 and 500 mg/kg) markedly attenuated CISP-induced peripheral neuropathy. The animals treated with TTE displayed improved mechanical allodynia suggesting alleviation of neuropathy caused by CISP administration.
Mounting evidences have suggested the negative role CISP plays on locomotion, grip strength and motor coordination. CISP can induce impediment in proprioception resulting in reduced motor coordination as indicated by the reduction in performance and endurance in the rotarod test. This result was coherent with the findings of Abdelsameeaet and Kabil and Bhadri et al. that reported dysfunction in motor coordination after the administration of CISP as evaluated by the time spent on the rotating rod (Abdelsameea and Kabil Citation2018; Bhadri et al. Citation2013). According to the results obtained in this study, the administration of TTE produced continuous and prolonged motor activity as indicated by significant increase of motor performance observed in the rotarod test.
In the formalin test, TTE displayed significant inhibition in both phases including paw licking, biting or shaking in CISP-treated neuropathic rats. The formalin test is one of the most common method used for accessing pain related parameters in animal models. The subcutaneous injection of formalin into the hind paw induces two phases of nociception, the initial phase (neurogenic phase) kick starts immediately after formalin injection for about 5 min, and it arises due to the direct stimulation of primary nociceptive neurons and pain fibres and it is largely dependent on chemical messengers such as serotonin and histamine (López-Cano et al. Citation2017; Martinez et al. Citation2016). The second phase (inflammatory phase) which last between 15 and 30 min after the administration of formalin is associated with the onset of inflammatory reactions which is largely mediated by central nociceptive neurons sensitisation involving the recruitment of cytokines, chemokines and prostaglandins (Martinez et al. Citation2016). The acetic acid writings model is associated with the existence of peritoneum visceral pain involving a complex interplay involving proinflammatory cytokines, prostaglandins, sympathomimetic amines and eicosanoids via the activation of mast cells and peritoneal macrophages (Nawaz et al. Citation2018; Martinez et al. Citation2016). Administration of acetic acid provokes contraction of the abdominal cavity, dorsal abdominal muscles twisting as well as the whole body movement (Le Bars et al. Citation2001; Nawaz et al. Citation2018). The data obtained from the acetic acid writhing test demonstrated that TTE displayed peripheral antinociceptive effects
Our previous studies on the ethanol extract from the aerial parts of Tiliacora triandra (TTE) highlighted the chemical constituents present in the extract including 5,7-dihydroxy-6-oxoheptadecanoic acid, ethyl-5,7-dihydroxy-6-oxooctadecanoate, ethyl linolenate, ethyl linoleate, ethyl pheophorbide A and pheophorbide A (Makinde et al., Citation2020b; Song et al., Citation2021). In addition, other previous report have also indicated several bioactive compounds in the aerial part of T. triandra such as gallic acid, cyanidin, quercetin, condense tannin, triterpene and saponins (Phunchago et al. Citation2015; Wachiryah and Hathaipat Citation2018) Since cisplatin induced pain hypersensitivity and neuropathy has been extensively linked to oxidative stress and inflammation, and several of the aforementioned compounds reported from T. triandra extract have shown considerable antioxidant and anti-inflammatory effects in various oxidative induced disease models (Phunchago et al. Citation2015; Makinde et al. Citation2019, Citation2020a; Song et al., Citation2021), we thus can preliminarily hypothesise that the anti-neuropathic effects of TTE may have been related to its strong antioxidant effects and its ability to reduce inflammation However further mechanistic study is needed in order to validate this hypothesis.
Conclusion
In conclusion, the findings from this study shed a light of the protective effects of TTE on CISP induced peripheral neuropathy. TTE showed anti hyperalgesia effects in formalin and acetic acid test. In addition, TTE ameliorated CISP induced thermal hyperalgesia and mechanical allodynia. Additionally, TTE improved rotarod performance indicating improved motor coordination, while improving CISP induced myelosuppression. These results suggest the potentials of TTE as an effective and safe treatment for CISP induced neuropathy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, as such the supporting data associated with this study is not publicly available.
References
- Abdelsameea AA, Kabil SL. 2018. Mitigation of cisplatin-induced peripheral neuropathy by canagliflozin in rats. Naunyn Schmiedebergs Arch Pharmacol. 391: 945–952.
- Akbar S, Subhan F, Shahid M, Wadood A, Shahbaz N, Farooq U, Ayaz M, Raziq N. 2020. 6-Methoxyflavanone abates cisplatin-induced neuropathic pain apropos anti-inflammatory mechanisms: A behavioral and molecular simulation study. Eur J Pharmacol. 872: 172972.
- Arafa MH, Atteia HH. 2020. Protective role of epigallocatechin gallate in a rat model of cisplatin-induced cerebral inflammation and oxidative damage: impact of modulating NF-κB and Nrf2. Neurotox Res. 37: 380–396.
- Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. 2012. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 82, 51–77.
- Authier N, Gillet J-P, Fialip J, Eschalier A, Coudore F. 2003. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp Neurol. 182: 12–20.
- Barabas K, Milner R, Lurie D, Adin C. 2008. Cisplatin: a review of toxicities and therapeutic applications. Vet Comp Oncol. 6: 1–18.
- Bhadri N, Sanji T, Madakasira Guggilla H, Razdan R. 2013. Amelioration of behavioural, biochemical, and neurophysiological deficits by combination of monosodium glutamate with resveratrol/alpha-lipoic acid/coenzyme Q10 in rat model of cisplatin-induced peripheral neuropathy. Sci World J. 2013: 565813.
- Boroja T, Katanić J, Rosić G, Selaković D, Joksimović J, Mišić D, Stanković V, Jovičić N, Mihailović V. 2018. Summer savory (Satureja hortensis L.) extract: phytochemical profile and modulation of cisplatin-induced liver, renal and testicular toxicity. Food Chem Toxicol. 118: 252–263.
- Deng L, Guindon J, Vemuri VK, Thakur GA, White FA, Makriyannis A, Hohmann AG. 2012. The maintenance of cisplatin- and paclitaxel-induced mechanical and cold allodynia is suppressed by cannabinoid CB2 receptor activation and independent of CXCR4 signaling in models of chemotherapy-induced peripheral neuropathy. Mol Pain. 8: 71.
- Dzagnidze A, Katsarava Z, Makhalova J, Liedert B, Yoon MS, Kaube H, Limmroth V, Thomale J. 2007. Repair capacity for platinum-DNA adducts determines the severity of cisplatin-induced peripheral neuropathy. J Neurosci. 27: 9451–9457.
- Hakiminia B, Goudarzi A, Moghaddas A. 2019. Has vitamin E any shreds of evidence in cisplatin-induced toxicity. J Biochem Mol Toxicol. 33: e22349.
- Han FY, Wyse BD, Smith MT. 2014. Optimization and pharmacological characterization of a refined cisplatin-induced rat model of peripheral neuropathic pain. Behav Pharmacol. 25: 732–740.
- Hartmann JT, Lipp HP. 2003. Toxicity of platinum compounds. Expert Opin Pharmacother. 4: 889–901.
- Hay J. 2002. Quality of life effects of chemotherapy-induced neuropathy in ovarian cancer. Proc Am Soc Clin Oncol. 21: 222.
- Kandeil MA, Gomaa SB, Mahmoud MO. 2020. The effect of some natural antioxidants against cisplatin-induced neurotoxicity in rats: behavioural testing. Heliyon. 6: e04708.
- Kuai CP, Ju LJ, Hu PP, Huang F. 2020. Corydalis saxicola alkaloids attenuate cisplatin-induced neuropathic pain by reducing loss of IENF and blocking TRPV1 activation. Am J Chin Med. 48: 407–428.
- Le Bars D, Gozariu M, Cadden SW. 2001. Animal models of nociception. Pharmacol Rev. 53: 597–652.
- López-Cano M, Fernández-Dueñas V, Llebaria A, Ciruela F. 2017. Formalin murine model of pain. Bio Protoc. 7: e2628.
- Luo FR, Wyrick SD, Chaney SG. 1999. Comparative neurotoxicity of oxaliplatin, ormaplatin, and their biotransformation products utilizing a rat dorsal root ganglia in vitro explant culture model. Cancer Chemother Pharmacol. 44: 29–38.
- Makinde EA, Ovatlarnporn C, Adekoya AE, Nwabor OF, Olatunji OJ. 2019. Antidiabetic, antioxidant and antimicrobial activity of the aerial part of Tiliacora triandra. S Afr J Bot. 125: 337–343
- Makinde EA, Ovatlarnporn C, Sontimuang C, Herbette G, Olatunji OJ. 2020a. Chemical constituents from the aerial part of Tiliacora triandra (Colebr.) diels and their α-glucosidase and α-amylase inhibitory activity. Nat Prod Comm. 15: 1–4.
- Makinde EA, Radenahmad N, Adekoya AE, Olatunji OJ. 2020b. Tiliacora triandra extract possesses antidiabetic effects in high fat diet/streptozotocin-induced diabetes in rats. J Food Biochem. 44: e13239.
- Martinez RM, Zarpelon AC, Domiciano TP, Georgetti SR, Baracat MM, Moreira IC, Andrei CC, Verri WA Jr, Casagrande R. 2016. Antinociceptive effect of Tephrosia sinapou extract in the acetic acid, phenyl-p-benzoquinone, formalin, and complete freund's adjuvant models of overt pain-like behaviour in mice. Scientifica (Cairo). 2016: 8656397.
- Mary Collins M, Linda Abbott R, Julie Aschenbrenner R, Connie Hart B. 2007. Putting evidence into practice®: evidence-based interventions for chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs. 11: 901.
- McWhinney SR, Goldberg RM, McLeod HL. 2009. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 8: 10–16.
- Nawaz NUA, Saeed M, Rauf K, Usman M, Arif M, Ullah Z, Raziq N. 2018. Antinociceptive effectiveness of Tithonia tubaeformis in a vincristine model of chemotherapy-induced painful neuropathy in mice. Biomed Pharmacother. 103: 1043–1051.
- Ocean AJ, Vahdat LT. 2004. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer. 12: 619–625.
- Olatunde OO, Tan SLD, Shiekh KA, Benjakul S, Nirmal NP. 2021. Ethanolic guava leaf extracts with different chlorophyll removal processes: anti-melanosis, antibacterial properties and the impact on qualities of pacific white shrimp during refrigerated storage. Food Chem. 341: 128251.
- Park HJ, Stokes JA, Pirie E, Skahen J, Shtaerman Y, Yaksh TL. 2013. Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesth Analg. 116: 224–231.
- Phunchago N, Wattanathorn J, Chaisiwamongkol K. 2015. Tiliacora triandra, an anti-intoxication plant, improves memory impairment, neurodegeneration, cholinergic function, and oxidative stress in hippocampus of ethanol dependence rats. Oxid Med Cell Longev. 2015: 918426.
- Schirrmacher V. 2019. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (review). Int J Oncol. 54: 407–419.
- Schröder S, Beckmann K, Franconi G, Meyer-Hamme G, Friedemann T, Greten HJ, Rostock M, Efferth T. 2013. Can medical herbs stimulate regeneration or neuroprotection and treat neuropathic pain in chemotherapy-induced peripheral neuropathy? Evid Based Complement Alternat Med. 2013: 423713.
- Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. 2014. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 155: 2461–2470.
- Shahid M, Subhan F, Ahmad N, Sewell RDE. 2017. The flavonoid 6-methoxyflavone allays cisplatin-induced neuropathic allodynia and hypoalgesia. Biomed Pharmacother. 95: 1725–1733.
- Sisignano M, Baron R, Scholich K, Geisslinger G. 2014. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol. 10: 694–707.
- Song P, Sun C, Li J, Long T, Yan Y, Qin H, Makinde EA, Famurewa AC, Jaisi A, Nie Y, Olatunji OJ. 2021. Tiliacora triandra extract and its major constituent attenuates diabetic kidney and testicular impairment by modulating redox imbalance and pro-inflammatory responses in rats. J Sci Food Agric. 101: 1598–1608.
- Stankovic JSK, Selakovic D, Mihailovic V, Rosic G. 2020. Antioxidant supplementation in the treatment of neurotoxicity induced by platinum-based chemotherapeutics-A review. Int J Mol Sci. 21:7753.
- Stathopoulos GP. 2010. Liposomal cisplatin: a new cisplatin formulation. Anti-Cancer Drugs. 21: 732–736.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. 2021. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. https://doi.org/10.3322/caac.21660.
- Sureram S, Senadeera SP, Hongmanee P, Mahidol C, Ruchirawat S, Kittakoop P. 2012. Antimycobacterial activity of bisbenzylisoquinoline alkaloids from Tiliacora triandra against multidrug-resistant isolates of Mycobacterium tuberculosis. Bioorg Med Chem Lett. 22: 2902–2905.
- Thong-Asa W, Bullangpoti V. 2020. Neuroprotective effects of Tiliacora triandra leaf extract in a mice model of cerebral ischemia reperfusion. Avicenna J Phytomed. 10: 202–212.
- Trendowski MR, El Charif O, Dinh PC Jr, Travis LB, Dolan ME. 2019. Genetic and modifiable risk factors contributing to cisplatin-induced toxicities. Clin Cancer Res. 25: 1147–1155.
- Ullah I, Subhan F, Alam J, Shahid M, Ayaz M. 2018. Suppression of cisplatin induced vomiting by Cannabis sativa in pigeons: neurochemical evidences. Front Pharmacol. 9: 231.
- Wachiryah TA, Hathaipat L.2018. Enhancing effect of Tiliacora triandra leaves extract on spatial learning, memory and learning flexibility as well as hippocampal choline acetyltransferase activity in mice. Avicenna J Phytomed. 8: 380–388.
- Wang L, He Y, Li Y, Pei C, Olatunji OJ, Tang J, Famurewa AC, Wang H, Yan B. 2020. Protective effects of nucleosides-rich extract from Cordyceps cicadae against cisplatin induced testicular damage. Chem Biodivers. 17: e2000671.
- Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. 2019. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci. 20: 1451.
