Abstract
The main objective of the current study was to examine the anticancer effects of naturally occurring Hirsutenone in human thyroid cancer cells along with investigating its effects on cell migration and invasion, apoptosis and Wnt/Beta-catenin signalling pathway. MTT assay and clonogenic assay were used to study effects on cell viability and cancer colony formation. Fluorescence microscopy along with flow cytometry was used to study apoptotic effects induced by Hirsutenone. Effects on Wnt/Beta-catenin signalling pathway were examined by western blot assay. Results indicated that Hirsutenone led to potent cell proliferation inhibition along with reducing cancer colony formation in a dose-dependent manner. Hirsutenone induced apoptotic effects as indicated by acridine orange/ethidium bromide staining assay as well as annexin-V FITC assay. The percentage of both early and late apoptotic cells increased significantly with increasing doses of Hirsutenone. Hirsutenone also caused activation of CASPASE-3, CASPASE-9, and BAX while as it led to downregulation of BCL-2. Hirsutenone also caused the inhibition of cell migration and cell invasion along with causing suppression of Wnt/beta-catenin signalling pathway. In conclusion, Hirsutenone exhibits strong anticancer properties in human thyroid cancer cells mediated via apoptosis, cell migration and invasion inhibition and suppression of Wnt/beta-catenin signalling pathway.
Introduction
Uncontrolled proliferation rate and acquired high metastatic potential of thyroid cells result in the development of thyroid cancer. The global thyroid cancer occurrence till 2017 reached to 3.2 million (Kim et al. Citation2020). Thyroid cancer incidences are three times high in women than in men and it is ranked as 5th among the top cancers prevailing in women (Siegel et al. Citation2019; Seib and Sosa Citation2019). The development of thyroid cancer is associated with a number of predisposing genetic factors. Multiple endocrine neoplasia type 2 and exposure to radiation are the major risk factors for the development of thyroid cancer (Genetics of endocrine and neuroendocrine neoplasias (pdq(r)): Health professional version Citation2002; Sanabria et al. Citation2018). The higher frequency of thyroid cancer incidence is believed to be of external radiotherapy during childhood (Drozd et al. Citation2018). Thyroid cancer shows different histopathologic types like undifferentiated and differentiated thyroid cancers. Among these differentiated thyroid cancer shows higher lethality as well as mortality (Nikiforov and Nikiforova Citation2011). Different therapies have been developed for the management of thyroid cancer including radioiodine therapy, heat therapy, chemotherapy and surgery. Surgically whole thyroid organ is removed, leaves the patient with deficiency of thyroid secretory hormones for which daily tablets are prescribed. Due to acquired drug resistance after long time exposure to chemopreventive drugs, novel chemotherapy candidates are required to overcome drug resistance. Diarylheptanoids are naturally occurring class of chemical entities bearing a 1,7-diphenylheptane skeleton. From past few decades, diarylheptanoids have attained research interest due to their significant biological behaviour. Diverse pharmacological activities of diarylheptanoids have been reported in recent studies including estrogenic, anti-emetic, anti-influenza, pro-apoptotic and anti-inflammatory (Yang et al. Citation2002; Tian et al. Citation2009; Sawamura et al. Citation2010; Lai et al. Citation2012; Thongon et al. Citation2017). Researches have also revealed cytotoxicity of diarylheptanoids against different human cancer cell lines including B16 murine melanoma model (Lee et al. Citation2013; Xi et al. Citation2015). Hirsutenone belongs to diarylheptanoids and it has been isolated from A. hirsuta (Tung et al. Citation2010). It has been reported to exhibit an array of biological activities like anti-atopic dermatitis, anti-tumour, anti-inflammatory (Kim et al. Citation2006; Jeong et al. Citation2010; Lee et al. Citation2012). As the previous studies were indicative of anticancer potential of hirsutenone diarylheptanoid, current investigation was designed to further explore its anticancer effects against thyroid cancer. The effects of hirsutenone on cell migration and invasion, apoptosis initiation and targeting Wnt/Beta-catenin signalling pathway were also examined.
Materials and method
Chemicals, reagents, cell culture and conditions
Hirsutenone (Q4961, purity > 97%) was purchased from Sigma-Aldrich (USA). Stock solution of Hirsutenone (500 mM) was prepared in dimethyl sulfoxide (DMSO) and stored at −20 °C. It was then diluted with Dulbecco's Modified Eagle Medium before being used in different assays and the final concentration of DMSO was kept below 0.2%. All the chemicals unless otherwise mentioned were bought from Sigma-Aldrich. The thyroid cancer cells (MDA-T32) and normal thyroid cells (follicular epithelial cell line-Nthy-ori 3-1) were obtained from Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, China. These cell lines were maintained in a 5% CO2 incubator set at 37°C under moist conditions till use.
Proliferation assay
The cellular proliferation of thyroid MDA-T32 cancer cells and normal thyroid cells (follicular epithelial cell line-Nthy-ori 3-1) was monitored by the execution of MTT cell viability assay. Thyroid MDA-T32 cancer cells and normal follicular epithelial cells were placed in 96-well plates with a density of 4 × 104 cells/well and precultured for 24 h. Afterwards, cells were treated with varying hirsutenone drug doses viz. 0, 10, 20, 40, 80 and 160 µM, in a 5% CO2 incubator at 37°C for 24, 48 and 72 h under humidified conditions. Thereafter, cells were supplemented with MTT stock solution 20 µl (5 mg/ml concentration) and subjected to further incubation for 5 h. The MTT exposure resulted in the formation of blue formazan crystals, which were then dissolved in dimethyl sulfoxide (DMSO). Finally, 96-well microplate reader (Spectra MAX 340, Molecular Devices Co., CA, USA) was used to calculate absorbance at 630 nm and 540 nm for optical density measurements.
Clonogenic assay
In 6-well plates, human thyroid MDA-T32 cancer cells (600 cells/well) were seeded and then subjected to hirsutenone drug treatment for 48 h at altering doses of control, 20, 80 and 160 µM. Controls only received 0.1% DMSO. After hirsutenone drug exposure, media was completely removed and replaced with fresh media. Cells were then incubated for 10 days followed by fixation in ethanol (70%) and crystal violet staining. Experiments were executed in triplicates and cells were counted using light microscope (Tokyo, Japan).
AO/EB staining assay
To detect apoptotic cell death, cellular morphology was detected via AO/EB staining assay. Thyroid MDA-T32 cancer cells were harvested at full confluence followed by trypsinisation with 0.25% trypsin. Trypsinized cells were placed in 96-well plate with a density of 2 × 104 cells/ml bearing FCS (fetal calf serum). Each well plate was subjected to varying hirsutenone doses 0, 20, 80, and 160 µM, with incubation at 37 °C. Cells were again supplemented with trypsin (20 µl) and then placed onto glass slides. AO/EB staining solution (100 µg/ml each) was placed over slides and covered with coverslips. Finally, the morphology of hirsutenone treated thyroid MDA-T32 cancer cells was observed under fluorescent microscope (OLYMPUS, Japan).
Annexin V/PI assay
For quantification of thyroid MDA-T32 apoptotic cancer cells by annexin V-FITC/PI assay, Annexin V Apoptosis Detection Kit (BD, USA) was used. Cells were harvested and subjected to varying hirsutenone drug doses viz. 0, 20, 80, and 160 µM. Treated cells were then exposed to Annexin V Apoptosis Detection Kit for about 15 min firmly following manufacturer’s guideline at room temperature. Afterwards, flow cytometric analysis was performed to check the percentage of apoptotic cells.
Wound healing assay for cell migration analysis
Human thyroid MDA-T32 cancer cells were harvested from complete medium bearing 6-well plates at 75% of growth confluence. Afterwards, a 1-mm plastic tip was used to scratch a wound in each well followed by 3 times washing PBA washing carefully. All the debris was removed and cells were treated with changing hirsutenone doses viz. 0, 20, 80, and 160 µM. The distance between migrated cells and scratched wound was examined through an inverted microscope.
Cell invasion analysis
To detect the cell invasion potency of thyroid MDA-T32 cancer cells transwell chamber assay was performed. Polycarbonate membrane of 8 lm pore size and 5 mm radius was used for cell invasion analysis and transwell chambers were coated with Matrigel (Corning Costar, Cambridge, MA). Human thyroid MDA-T32 cancer cells were first trypsinized and then suspended with a final density of 4 × 105 cells/ml in serum free medium (L15 medium). Transwell chambers were added with changing hirsutenone doses, viz. 0, 20, 80, and 160 µM. Cell suspensions were then added to upper chambers of the transwell and lower chambers were placed with 5% FBS and medium only. Transwell chambers were then incubated for 24 h in a 5% CO2 incubator at 37oC. Cotton swab was then used to clean off the non-invaded cells followed by fixation with methanol. Eosin and hematoxylin (Nanjing Sunshine Biotechnology Ltd, China) were then used to stain the invaded cells and microscopically 10 fields were counted for each experiment.
Western blotting analysis
For western blotting assay, MDA-T32 cells were cultured in 6-well plates and subjected to varying hirsutenone drug doses viz. 0, 20, 80, and 160 µM for 24 h. Afterwards, lysing was performed and lysates were subjected to 10 min of boiling for denaturation. The quantification of protein content within each lysate was performed by BCA assay and total protein content was separated by SDS-PAGE. Thereafter, proteins were electrophoretically transferred to PVDF (polyvinylidene difluoride) membranes which were then blocked by using 10% skimmed milk. Membranes were then probed with antibodies of target proteins followed by overnight secondary antibody exposure at 4°C. For immunoblotting the following antibodies were used: anti-β-catenin (1:2000) (#9562) (Santa Cruz Biotechnology), anti- GSK3B (1:2000) (#9369), anti-phosphorylated-p- GSK3B (1:2000) (#9322), anti-Wnt3a (1:1000) (#2391) (Cell Signalling Technology), anti-GAPDH (1:2000) (#5174) (Cell Signalling Technology). Finally, SuperSignal West Pico Chemiluminescent substrate was used to develop images of target bands.
Statistical analysis
Data from performed triplicate experiments are represented as mean ± SD values unless otherwise stated. Statistical analysis was carried by using one-way ANOVA and comparison for each experimental group by Student’s t test. P < 0.05 was taken as statistically significant.
Results
Hirsutenone inhibited the proliferation of MDA-T32 cells
To estimate cellular proliferation of thyroid MDA-T32 cancer cells, MTT assay was performed. MDA-T32 cells were subjected to hirsutenone treatment (0, 10, 20, 40, 80 and 160 µM) for different time intervals of 24 h, 48 h, and 72 h. The results revealed that hirsutenone (Figure ) drug induced antiproliferative effects on thyroid MDA-T32 cancer cells both in a time and dose-reliant fashion (P < 0.05). The number of viable cells declined from nearly 100% to about 25% (0–160 µM) after 24 h of exposure. It was observed to decline from 90% to near about 15% and from 85% to near about 10% after 48 h and 72 h drug exposure, respectively (Figure A). Further, the anticancer effects of hirsutenone were also tested in normal thyroid cell line namely follicular epithelial cell line-Nthy-ori 3-1. The results are shown in Figure (B) and indicate that hirsutenone selectively targeted MDA-T32 thyroid cancer cells at almost every dose used in the current experiment (P < 0.05). Hirsutenone showed more cytotoxic effects in MDA-T32 thyroid cancer cells than the normal follicular epithelial cells (Nthy-ori 3-1) indicating selective anticancer potential of this molecule.
Figure 2. (A) Results of MTT assay. Thyroid MDA-T32 cancer cells were treated with hirsutenone at varying indicated doses for 24, 48 and 72 h. It was observed that the viability of target cells reduced in a dose as well as time-dependent manner. (B) MTT assay results of the effect of hirsutenone on the viability of normal thyroid cells namely follicular epithelial cell line-Nthy-ori 3-1. Results indicated that normal cells showed lesser cytotoxicity as compared to the cancer cells. Data is shown as means ± standard deviation with repetitions n = 3. *P < 0.05.
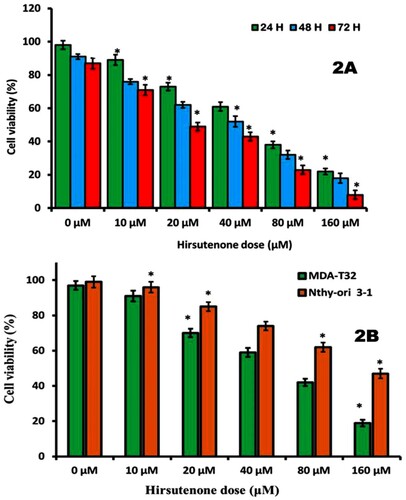
Proliferation inhibition of MDA-T32 cell colonies by hirsutenone
The effect of hirsutenone on thyroid cancer cell colony proliferation was evaluated by clonogenic assay. Cells were treated with varying hirsutenone drug doses for 48 h and then left untouched for 10 days. Results revealed that the number of cell colonies decreased significantly with increase in hirsutenone drug dose (Figure A). The number of colonies at control was shown to be nearly 350, on exposure to hirsutenone drug it reduced to near about 270 at 20 µM (P < 0.05). On exposure to higher hirsutenone concentrations (160 µM), the number of MDA-T32 cell colonies was restricted to about 20 (Figure B) (P < 0.05). Therefore, hirsutenone drug was seen to have anti-clonogenic potential against thyroid MDA-T32 cancer cells.
Figure 3. (A) Pictures representing thyroid MDA-T32 cancer cell colonies after 48 h of hirsutenone treatment and 10 days of incubation. Experiments were performed three times. (B) Graphical representation of number of cell colonies after hirsutenone exposure. It was observed that number of cell colonies appeared to be decreasing with increasing drug doses. Data is revealed as means ± standard deviation with replications n = 3. *P < 0.05.
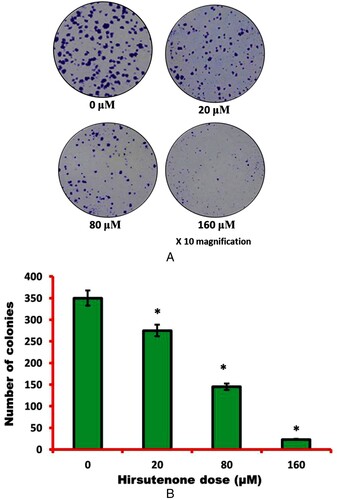
Induction of apoptosis by hirsutenone drug in MDA-T32 cells
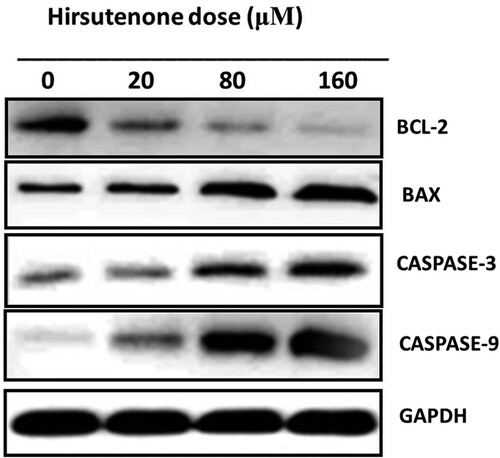
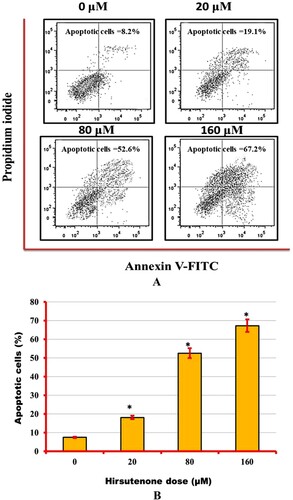
After observation of anti-proliferative and anti-clonogenic potency of hirsutenone drug, an effort was made to find its underlying mechanism. As apoptosis is termed as type-1 PCD, it was tested in hirsutenone treated MDA-T32 cells. For apoptosis analysis AO/EB staining assay was performed to monitor cell morphology and annexing V/PI staining assay was executed for apoptosis quantification. Results from morphological assessment showed specific morphological features that supported apoptotic cell death. Cells were observed with fragmented DNA, condensed nuclei and membrane blebbing (Figure ). Thus indicated apoptotic cell death in cancerous thyroid cells. The annexin V/PI staining assay also showed increased percentage of apoptotic cells (Figure A). It was observed that the number of apoptotic cells increased significantly (P < 0.05) from near about 5% at control to about 70% at higher drug doses (0–160 µM) (Figure B). Western blotting assay was performed to check molecular mechanism of apoptosis. Results indicated that the expression of pro-apoptotic proteins increased and those of anti-apoptotic proteins decreased with enhanced hirsutenone doses. The expression of BAX and CASPASE (−3, −8 and −9) enhanced while as BCL-2 was restricted in hirsutenone treated MDA-T32 cells (Figure ). This indicated that the apoptosis inducing potential of hirsutenone drug is mediated through Caspase activation.
Figure 4. To examine cell morphology of hirsutenone treated thyroid MDA-T32 cancer cells, AO/EB staining assay was performed. Results revealed apoptotic cell morphology as represented by arrows like nuclear condensation, DNA fragmentation and membrane blebbing.
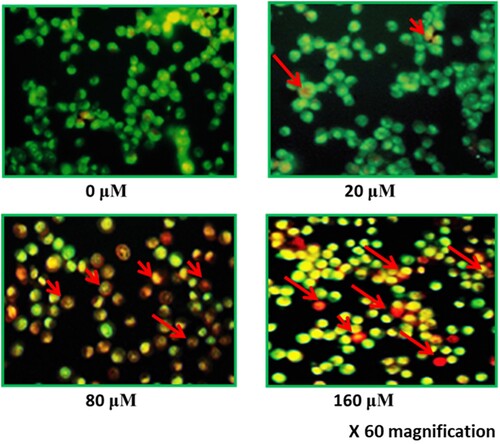
Figure 5. (A) Flow cytometric analysis of hirsutenone treated MDA-T32 cells after performing annexin V/PI staining assay. Results representing increasing number of apoptotic cell percentage with increasing drug doses. Data is shown as means ± standard deviation with replications n = 3. P < 0.05. (B)0 Graphical representation of increased number of apoptotic cells after execution of annexin V/PI staining assay at indicated doses. Data is shown as means ± standard deviation with replications n = 3. *P < 0.05.
Inhibition of cell migration and invasion by hirsutenone drug in MDA-T32 cells
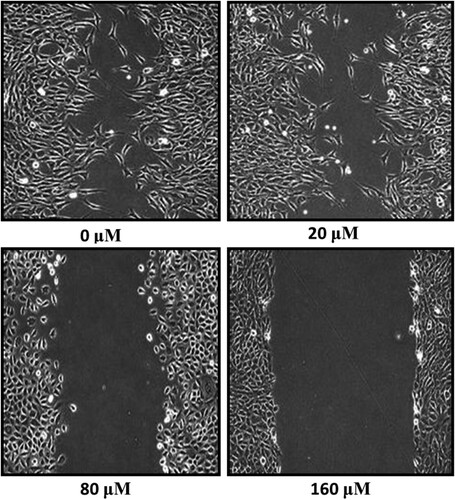
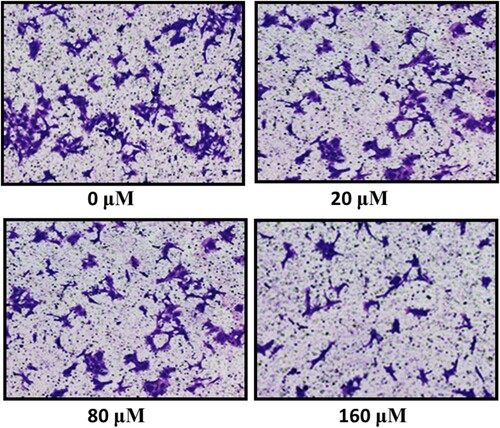
Subsequently, the detection of invasive potential and cell migratory capacity of thyroid MDA-T32 cancer cells in response to the hirsutenone drug treatment was performed. Cell migration capability was tested by wound healing assay and it was observed that MDA-T32 cells showed significant reduction in migration rates as compared to the control. After 24 h of scratching a wound in hirsutenone treated cells and controls, it was observed that wound was about to close in case of controls and in treated cells wound length remained almost unchanged (Figure ). The invasion rate was assessed via transwell chambers assay. The rate of invasion at controls in comparison to positive controls remained unchanged and in case of positive controls invasion rate decreased at lower drug doses. While at higher hirsutenone drug doses invasion was suppressed to minimum (Figure )
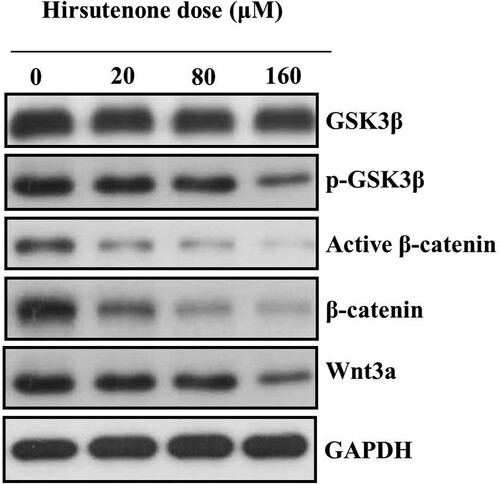
Hirsutenone inhibited the Wnt/beta-catenin signalling pathway in MDA-T32 cells
Western blotting analysis was executed to monitor the activity of Wnt/beta-catenin signalling pathway allied genes. The Wnt/beta-catenin signalling pathway plays important roles in maintaining regular growth and differentiation in cancer cells. However, apoptotic cells show inactive Wnt/beta-catenin signalling pathway while normal-cancer cells show enhanced activity of Wnt/beta-catenin signalling pathway. Therefore, we investigated the activity of Wnt/beta-catenin signalling pathway in hirsutenone treated MDA-T32 cells to further validate apoptotic cell death. Results revealed that the expressions of Wnt-3a, beta-catenin, active beta-catenin and phosphorylated-GSK3β reduced significantly and activity of GSK3β remained almost unchanged (Figure ). Thus it was evidenced that antiproliferative effects of hirsutenone were due its Wnt/beta-catenin signalling pathway inhibition potency.
Discussion
Programmed cell death (PCD) is one of the vital processes by which multicellular organisms maintain health of tissues and organs. When the equilibrium between cell death and survival rate gets disturbed by some internal or external factors, PCD plays an essential role in deciding the fate of carcinogenesis (Laubenbacher et al. Citation2009), (Hanahan and Weinberg Citation2011). Necrosis, apoptosis and autophagy are three main types of PCD and are easily differentiated on the basis of cellular morphology (Bialik et al. Citation2010; Tan et al. Citation2009). Apoptosis (Type I PCD) is characterized by specific biochemical and cellular morphology changes like nuclear condensation, cell shrinkage, membrane blebbing and nuclear fragmentation, and adhesion loss to extracellular matrix and neighbours (Kerr et al. Citation1972; Nishida et al. Citation2008). Biochemical changes that are evident in an apoptotic cell include phosphatidylserine externalization, inter-nucleosomal fragments, chromosomal DNA cleavage, and specific proteolysis (Cohen et al. Citation1994), (Martin and Green Citation1995).
Herein, our results showed that hirsutenone induced selective antiproliferative effects in thyroid MDA-T32 cancer cells without causing too much damage to the normal cells used in this experiment (normal follicular epithelial cells, Nthy-ori 3-1). Hirsutenone inhibited migration and invasion of MDA-T32 cells and also its anticancer effects were found to be apoptosis mediated.
Comparing our results with the published literature, it seems various authors have reported the anticancer properties of this molecule although in other cancer forms. Hirsutenone has been reported to suppress human prostate cancer by targeting Akt1 and Akt2 and anchorage-dependent and independent cell growth of PC3 and LNCaP human prostate cancer cells (Kang et al. Citation2015). In yet another interesting study, authors reported that hirsutenone enhanced CDDP-dependent apoptosis in chemoresistant ovarian cancer cells by down-regulating XIAP through degradation via the proteasome-ubiquitin pathway and by inducing AIF translocation to the nucleus (Farrand et al. Citation2014). Further, the induction of apoptosis was associated with the ability of hirsutenone to inhibit PI3K/AKT function (Farrand et al. Citation2014). Therefore, hirsutenone showed apoptosis mediated antiproliferative effects in MDA-T32 cells as indicated by the results both at morphologically and at molecular levels.
Wnt/β-catenin signalling pathway plays an imperative role for proliferation, differentiation and apoptosis. The β-catenin is continuously degraded by a destruction complex containing the scaffold protein Axin, the enzyme glycogen synthase kinase-3β (GSK3β) and the adenomatosis polyposis coli (APC) protein but this degradation occurs in the absence of Wnt proteins. There is huge convincing evidence which shows that unusual activation of Wnt/β-catenin signalling pathway leads the triggering, disease progression, and distant metastasis in various human malignancies including thyroid cancer. There seems to be a pressing need to design and develop drugs which will mediate various stages of the Wnt/β-catenin signalling pathway (Klaus and Birchmeier Citation2008; JPt et al. Citation2010; de Sousa et al. Citation2011; King et al. Citation2012). Studies have reported that the activity of caspases results in the disruption of Wnt/beta-catenin signalling pathway which results in the inactivation of this pathway. Caspases being proapoptotic proteins therefore show the inactivation of Wnt/beta-catenin signalling pathway in apoptotic cells. Therefore, we investigated hirsutenone treated MDA-T32 cells for the activity of Wnt/beta-catenin signalling pathway to verify apoptotic cell death. Our findings revealed that the expressions of Wnt-3a, beta-catenin, active beta-catenin and phosphorylated-GSK3β reduced significantly and activity of GSK3β remained almost unchanged. Hence, results indicated that hirsutenone caused the inactivation of Wnt/beta-catenin signalling pathway in MDA-T32 cells which caused the promotion of apoptotic cell death.
The motivation for classical cancer therapy has mainly focused on targeting cell proliferation at the primary malignant site without any discrimination towards the normal cells leading to unwanted serious side effects. Later on this approach was improved by the incorporation of new targeted therapies and immunotherapies which led to reduced normal cell cytotoxicity leading to lesser side effects. These targeted therapies specifically target cancer cells without harming normal cells. Nevertheless, to treat cancer more efficiently, we should further emphasis on preventing the development and growth of metastatic carcinoma cells. In this regard inhibition of cancer cell migration and invasion become highly significant as they have a role in cancer metastasis. Those molecules which could target both cancer cell migration and invasion may play critical roles in anticancer therapies by preventing migration of cancer cells to new tissues (Chabner and Roberts Citation2005; Pardoll Citation2012). In the current study, it was shown that hirsutenone significantly inhibited both cancer cell migration as well as cell invasion and as such could prove to be a promising anticancer agent.
Conclusion
In conclusion, this study indicates that hirsutenone molecule shows anticancer and anti-metastatic potential against thyroid cancer cells and can be a promising lead molecule.
Data availability statement
The data which was generated and analysed during current study are always available from the corresponding author and on request will be made available. The data is freely available from the figshare repository (https://figshare.com/) and can be easily accessed at: https://figshare.com/articles/dataset/Antiproliferative_effects_of_Hirsutenone_in_thyroid_cancer_cells/12780818.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bialik S, Zalckvar E, Ber Y, Rubinstein AD, Kimchi A. 2010. Systems biology analysis of programmed cell death. Trends Biochem Sci. 35:556–564.
- Chabner BA, Roberts TG Jr. 2005. Timeline: chemotherapy and the war on cancer. Nat Rev Cancer. 5(1):65–72.
- Cohen GM, Sun XM, Fearnhead H, MacFarlane M, Brown DG, Snowden RT, et al. 1994. Formation of large molecular weight fragments of DNA is a key committed step of apoptosis in thymocytes. J Immunol. 153:507–516.
- de Sousa EM, Vermeulen L, Richel D, Medema JP. 2011. Targeting Wnt signaling in colon cancer stem cells. Clin Cancer Res. 17(4):647–653.
- Drozd VM, Branovan I, Shiglik N, Biko J, Reiners C. 2018. Thyroid cancer induction: nitrates as independent risk factors or risk modulators after radiation exposure, with a focus on the chernobyl accident. Eur Thyroid J. 7:67–74.
- Farrand L, Kim JY, Byun S, et al. 2014. The diarylheptanoid hirsutenone sensitizes chemoresistant ovarian cancer cells to cisplatin via modulation of apoptosis-inducing factor and X-linked inhibitor of apoptosis. J Biol Chem. 289(3):1723–1731.
- Genetics of endocrine and neuroendocrine neoplasias (pdq(r)): Health professional version. 2002. In Pdq cancer information summaries; National Cancer Institute (NCI): Bethesda, MD, USA.
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell. 144:646–674.
- Jeong MS, Choi SE, Kim JY, Kim JS, Kim EJ, Park KH, Lee do I, Joo SS, Lee CS, Bang H, et al. 2010. Atopic dermatitis-like skin lesions reduced by topical application and intraperitoneal injection of hirsutenone in NC/Nga mice. Clin Dev Immunol. 2010:article 618517.
- JPt M, Wang SC, Hebrok M, Morris John P. 2010. KRAS, hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 10(10):683–695.
- Kang S, Kim JE, Li Y, Jung SK, Song NR, Thimmegowda NR, Kim BY, Lee HJ, Bode AM, Dong Z, Lee KW. 2015. Hirsutenone in Alnus extract inhibits akt activity and suppresses prostate cancer cell proliferation. Mol Carcinog. 54(11):1354–1362.
- Kerr JFR, Wyllie AH, Currie AR. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 26:239–257.
- Kim J, Gosnell JE, Roman SA. 2020. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 16:17–29.
- Kim JH, Lee KW, Lee MW, Lee HJ, Kim SH, Surh YJ. 2006. Hirsutenone inhibits phorbol ester-induced upregulation of COX-2 and MMP-9 in cultured human mammary epithelial cells: NF-kappaB as a potential molecular target. FEBS Lett. 580:385–392.
- King TD, Suto MJ, Li Y. 2012. The Wnt/beta-catenin signaling pathway: a potential therapeutic target in the treatment of triple negative breast cancer. J Cell Biochem. 113(1):13–18.
- Klaus A, Birchmeier W. 2008. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 8(5):387–398.
- Lai Y-C, Chen C-K, Lin W-W, Lee S-S. 2012. A comprehensive investigation of anti-inflammatory diarylheptanoids from the leaves of Alnus formosana. Phytochemistry. 73:84–94.
- Laubenbacher R, Hower V, Jarrah A, Torti SV, Shulaev V, Mendes P, et al. 2009. A systems biology view of cancer. Biochim Biophys Acta. 1796:129–139.
- Lee CS, Jang ER, Kim YJ, Myung SC, Kim W, Lee MW. 2012. Diarylheptanoid hirsutenone enhances apoptotic effect of TRAIL on epithelial ovarian carcinoma cell lines via activation of death receptor and mitochondrial pathway. Invest New Drugs. 30:548–557.
- Lee O, Kim J, Choi YW, Lee M, Park G, Oh C. 2013. Efficacy of oregonin investigated by non-invasive evaluation in a B16 mouse melanoma model. Exp Dermatol. 22:832–849.
- Martin SJ, Green DR. 1995. Protease activation during apoptosis: death by a thousand cuts? Cell. 82:349–352.
- Nikiforov YE, Nikiforova MN. 2011. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 7:569–580.
- Nishida K, Yamaguchi O, Otsu K. 2008. Crosstalk between autophagy and apoptosis in heart disease. Circ Res. 103:343–351.
- Pardoll DM. 2012. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 12(4):252–264.
- Sanabria A, Kowalski LP, Shah JP, Nixon IJ, Angelos P, Williams MD, Rinaldo A, Ferlito A. 2018. Growing incidence of thyroid carcinoma in recent years: factors underlying overdiagnosis. Head Neck. 40(4):855–866.
- Sawamura R, Shimizu T, Sun Y, Yasukawa K, Miura M, Toriyama M, Motohashi S, Watanabe W, Konno K, Kurokawa M. 2010. In vitro and in vivo anti-influenza virus activity of diarylheptanoids isolated from alpiniaofficinarum. Antivir Chem Chemoth. 21:33–41.
- Seib CD, Sosa JA. 2019. Evolving understanding of the epidemiology of thyroid cancer. Endocrinol Metab Clin North Am. 48(1):23–35.
- Siegel RL, Miller KD, Jemal A. 2019 Jan. Cancer statistics, 2019. CA Cancer J Clin. 69(1):7–34.
- Tan ML, Ooi JP, Ismail N, Moad AI, Muhammad TS. 2009. Programmed cell death pathways and current antitumor targets. Pharmacol Res. 26:1547–1560.
- Thongon N, Boonmuen N, Suksen K, Wichit P, Chairoungdua A, Tuchinda P, Suksamrarn A, Winuthayanon W, Piyachaturawat P. 2017. Selective estrogen receptor modulator (SERM)-like activities of diarylheptanoid a phytoestrogen from curcuma comosa, in breast cancer cells, pre-osteoblastcells, and rat uterine tissues. J Agric Food Chem. 65:3490–3496.
- Tian Z, An N, Zhou B, Xiao P, Kohane IS, Wu E. 2009. Cytotoxic diarylheptanoidinduces cell cycle arrest and apoptosis via increasing ATF3 and stabilizingp53 in SH-SY5Y cells. Cancer Chemoth Pharm. 63:1131–1139.
- Tung NH, Kim SK, Ra JC, Zhao YZ, Sohn DH, Kim YH. 2010. Antioxidative and hepatoprotective diarylheptanoids from the bark of Alnus japonica. Planta Med. 76:626–670.
- Xi Y, Gao H, Callaghan MU, Fribley AM, Garshott DM, Xu Z-X, Zeng Q, Li Y-L. 2015. Induction of BCL2-interacting killer BIK, is mediated for anti-cancer activity of curcumin in human head and neck squamous cell carcinoma cells. J Cancer. 6:327–332.
- Yang Y, Kinoshita K, Koyama K, Takahashi K, Kondo S, Watanabe K. 2002. Structure-antiemetic-activity of some diarylheptanoids and their analogues. Phytomedicine. 9:146–152.