Abstract
Researches on drug delivery system (DDS) have made incredible progress, but there are still problems such as insufficient targeting and undesirable treatment efficiency. To improve the above problems, a stem cell-based DDS was developed. Stem cell and its derivatives (cell membranes, extracellular vesicles/exosomes) have been used as important drug delivery vehicles in the treatment of a number of human key diseases, such as genetic diseases, cancer and so on. For stem cells highly express chemokine receptors such as CCR2 and CXCR4, which can specifically bind to chemokines or cytokines produced in tissue injury sites, so they exhibit centripetal and homing properties, which is the reason why they are employed as DDS vehicles. Stem cell-based DDS effectively enhance the targeting and efficiency of drugs with low immunogenicity. We review the extremely prominent superiority of stem cells and its derivatives as drug delivery vehicles. Especially summarize the different targeting strategies and the major application in anti-tumor, anti-inflammatory, tissue repair and regenerative medicine fields. The challenges of stem cell-based targeted DDS are still how to enhance their targeting, selectivity, effectiveness, reducing immunogenicity, and fully exerting its immunomodulatory effects. We think stem cell and its derivatives-based DDS will demonstrate their significant prospects in the future.
Introduction
The drug delivery system (DDS) can transport the drug from outside of the body to a specific location in the body through the delivery vehicle (Zhou et al. Citation2018; Saeedi et al. Citation2019). It is a specialized system that comprehensively regulates the space, time, and dose distribution of the drug in the body to improve pharmacological activity and reduce side effects (Mu and Holm Citation2018; Li et al. Citation2019). Cellular and molecular accuracy of the DDS has provided new possibilities for diagnosis and treatment of disease (Poon et al. Citation2020). All DDS is constructed to increase the therapeutic concentration of the drug at the target site and maintain a certain duration of action (Gozde and Ufuk Citation2018; Zhou et al. Citation2018). Usually, nanoparticles, bacteria, and viruses are used as the delivery vehicles to protect the drug against degradation, and targeted to the target location (Severino et al. Citation2019; Mitchell et al. Citation2021).
In recent years, the innovation of nano-carriers has promoted the development of DDS. Although conventional nano-medicine transport carriers can overcome the shortcomings of traditional drugs such as poor selectivity and large toxic and side effects, they still have certain limitations. Nanoparticles (NPs) will accumulate outside the target tissue, resulting in low enrichment efficiency in the target tissue and causing side effects to the human body. In addition, the size, shape, surface chemistry, hardness, and chemical composition of the delivery vehicle will also affect DDS and reduce delivery efficiency (Danhier Citation2016; Nakamura et al. Citation2016). Therefore, how to effectively arrive at the specific biological target is still a difficult problem. In addition, nano DDS lacks international standards and evaluation methods in toxicology, biocompatibility, targeting, etc., and the complexity of the manufacturing process also limits its industrial production (Wang et al. Citation2019). NPs administered to the tumor site mainly rely on the enhanced permeability and retention (EPR) effect of tumor to increase the accumulation of the drug in cancer tissue (Fang et al. Citation2011). But in fact, the effect of EPR is mediocre. The total accumulative drugs in target site transferred by NPs increased only less than 2 times compared with other key normal organs. So, nanomedicine is almost unanimous in clinically failing to improve the efficacy. All of the above limits the application of artificial nano-drug transport carriers in the field of biomedicine.
With the maturity of cell engineering technology, cell-based targeted drug delivery strategy is a new direction in the research of DDS targeting strategy, among which red blood cells, macrophages, neutrophils and stem cells are often used as carriers for targeted drug delivery (Gao et al. Citation2017; An et al. Citation2019; Wu et al. Citation2019). Stem cells are a type of multipotent cells with self-renewal and self-replication capabilities (Preynat-Seauve and Krause Citation2011; Teng et al. Citation2018). Under certain conditions, it can differentiate into a variety of functional cells, such as muscle, bone, fat and other cells, then form tissues and organs under appropriate conditions. With the rapid development of molecular biology and transplantation technology, stem cells have been broadly utilized within the areas of regenerative medicine and tissue engineering due to their multi-directional differentiation and immunomodulatory properties (Preynat-Seauve and Krause Citation2011; Mills et al. Citation2017; Han et al. Citation2019). As of July 2021, there are as many as 8,560 stem cell products for clinical research with registration applications worldwide, of which 838 are in phase III clinical trials. However, only a few mesenchymal stem cell (MSC) products have been commercialized (Table ). But there is interesting thing, some highly toxic chemotherapy drugs have little effect on the viability, migration, cell cycle, or differentiation potential of mesenchymal stem cells (MSCs) (Wang et al. Citation2018). At the same time, stem cells highly express chemokine receptors such as CCR2 and CXCR4, which can specifically bind to chemokines or cytokines produced in tissue injury sites. So, stem cells exhibit centripetal and homing properties, which is the reason why they are employed as DDS vehicles. Recently, MSCs have been used as ‘Trojan horses’ to deliver nanospheres for photothermal treatment of tumors (Luo et al. Citation2020). It can be seen that drug delivery is an important direction for the future development of stem cell technology.
Table 1. Researches and developments of stem cell products.
In this paper, we review the extremely prominent superiority of stem cells and its derivatives as drug delivery vehicles. Especially summarize the different targeting strategies and the major application in anti-tumor, anti-inflammatory, tissue repair and regenerative medicine fields. The challenges of stem cell-based targeted DDS are still how to enhance their targeting, selectivity, effectiveness, reducing immunogenicity, and fully exerting its immunomodulatory effects. We think stem cell and its derivatives-based DDS will demonstrate their significant prospects in the future.
Homing properties of stem cells
Stem cells are present in almost all multicellular organisms. According to the source of stem cells, they are classified into embryonic stem cells, adult stem cells, tissue-resident stem cells, and induced pluripotent stem cells. Meanwhile, according to the differentiation ability of stem cells, they can be divided into Totipotent Cells, Pluripotent Cells, Multipotent Cells, Oligopotent Cells, Unipotent Cells (Kolios and Moodley Citation2013). The stem cells currently used as drug delivery vehicles are mainly MSCs, such as hBMSC (Muslimov et al. Citation2020), human umbilical cord blood MSC (Cao et al. Citation2018), human adipose MSC (Herea et al. Citation2019), and human gingival MSC (Coccè et al. Citation2017). Among the various characteristics of stem cells (Table ), the homing properties and low immunogenicity are two key factors for stem cells to be used as drug delivery vehicles.
Table 2. Characteristics of stem cell.
The homing of stem cell to tissue injury and tumor sites is the most important reason for their targeted delivery of drugs. ‘Homing’ usually refers to the life process of specific cell groups in the circulatory system, such as HSC, that migrate to the microenvironment of biological tissues or organs to maintain or reshape their cell fate. Stem cell homing was defined as the arrest of stem cell within the vasculature of a tissue, and then across the process of migration of vascular endothelial cells to the target tissue (Karp and Leng Teo Citation2009). The niche of stem cells is a special microenvironment that is the basis for the existence of stem cells. It not only provides nutrients to stem cells, but also guides the actions of stem cells and determines the direction of stem cell differentiation. Changes in the microenvironment are the initiating factors for the homing of stem cells. Different microenvironments secrete different signal molecules and regulate the behavior of stem cells through different signal pathways, so that stem cells can grow and attract more stem cells to the tissue. Receptors such as chemokines and growth factors are widely expressed on the surface of stem cells. The homing process also involves the participation of chemokines, adhesion molecules, growth factors, enzymes and other ligands and their receptors. The expression of the corresponding receptor binding has the effect of driving the homing of stem cells. The homing process of stem cells to the lesion site includes the following four stages (Figure ): (1) produce a chemical stimulus response to show the tendency to the tissue injury site; (2) rolling, stagnation, adhesion and crawling on endothelial cells; (3) Trans endothelial migration (TEM) to the target tissue; (4) Migration in the interstitial of the target tissue and specific interaction with the target cell (Thanuja et al. Citation2018).
Figure 1. Homing/migration of MSCs towards target tissue/cells. Stem cells are injected into the blood vessel, and then participate in the surface adhesion factor-mediated stagnation on the vascular endothelial cells and rolling, adhesion and crawling. Subsequently, stem cells are induced to migrate across endothelial cells through receptor-ligand interaction. Later, chemoattractant-mediated migration in the interstitial of the target tissue and specific interaction with the target cell.
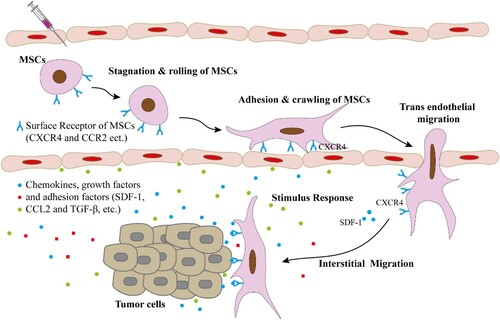
Chemokines (SDF-1), growth factors (TGF-β) and adhesion factors (CAM-1) are produced in the disease environment and participate in the homing of MSCs. CC and CXC receptors (such as CCR2 and CXCR4, etc.) highly expressed on the surface of stem cell, combining MSC with chemokines forcefully drive the homing of MSCs (Karp and Leng Teo, Citation2009; Thanuja et al. Citation2018). Crosstalk between SDF-1 and the natural CXCR4 receptor of stem cell significantly controls homing, and receptor ligand response is the key to mediate stem cell tropism (Timin et al. Citation2019). A research letter published in Nature (2018) reported that using advanced live imaging and a cell-labelling system, Pan et al. (Li et al. Citation2018a) performed high-resolution analyses of the HSPC homing in caudal hematopoietic tissue of zebrafish (equivalent to the fetal liver in mammals), and revealed the role of the vascular architecture in the regulation of HSPC retention. HSPCs entering hematopoietic tissues through its vascular cell adhesion molecule (VCAM-1) and then combined with ITGA4 receptor on the surface of HSPC, then HSPCs are brought into specific venous capillaries, where they stay for a long time for proliferation and differentiation, so as to achieve the homing of HSPCs.
On the other hand, stem cell homing mechanism has a certain memory function, it can remember the damage that has occurred, and store the memory of the corresponding trauma or inflammation. Stem cells will regularly cooperate with the immune system to use the memory of injury to improve the tissue’s future response to injury or pathogenic attacks, thereby accelerating the healing of new wounds. MSCs can escape the surveillance of the immune system and will not be cleared immediately after the drug is loaded into the body. HBMSCs derived from allogeneic cartilage does not affect the maturation of immature dendritic cells (DCs), cannot stimulate the immunogenic response of DCs in vitro or activate T cells indirectly through DCs (Kiernan et al. Citation2018). Stem cells can exist in allograft without functional change after allotransplantation. Without the occurrence of allogeneic rejection, stem cells gradually clear and release drugs in the body to achieve the purpose of treatment (Chiu Citation2005). The homing and low immunogenicity properties of stem cells make them irreplaceable advantages in the treatment of diseases, especially refractory diseases.
Stem cell and its derivatives as drug vehicle to deliver therapeutic drugs
Approaches of stem cells deliver therapeutic drugs
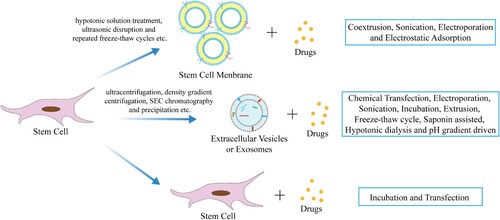
Studies have found that stem cells can deliver a variety of therapeutic drugs, such as apoptosis inducers, cytotoxic chemotherapeutic drugs, anti-angiogenic factors, immunomodulators, oncolytic viruses, drug-loaded NPs/microparticles, tissues or tumor-specific prodrugs (Krueger et al. Citation2018). Except stem cell itself, the cell membranes, extracellular vesicles or exosomes in stem cell also can be used as and drug vehicle (Figure ), and we call them ‘stem cell derivatives’.
Figure 2. Stem cell and its derivatives as drug vehicle to deliver therapeutic drugs. Cell membranes, extracellular vesicles or exosomes, derived from stem cells, and stem cells themselves are all good drug delivery vehicles which loaded with therapeutic drugs in various ways, thereby delivering the drugs to the target site for the treatment of diseases.
Stem cell membrane as drug vehicles
As the research on biomimetic DDS becomes more and more mature, the research on stem cells as drug carriers have achieved exciting research results. In the initial stage of research, the reason why cell membranes are widely studied is that they are easy to separate and contain a large number of recognition sites including cell–cell receptors (ICAM, VAM), chemokine receptor (CXCR), and cell-matrix receptor (integrins) (Majumdar et al. Citation2003; Wang et al. Citation2021). These recognition sites play a key role in the interaction between MSCs and tumor cells (Gao et al. Citation2016a). Almeida et al. (Citation2016) found that the CXCR2 receptor on MSC can recognize the NAP-2 cytokine of NK cells, so that MSC can be recruited to the injured site to regulate tissue repair or regeneration. As we described earlier, CXCR4 expressed on the MSC membrane shows strong inflammation targeting. The use of human adipose-derived MSC membrane-coated NPs (BSMNC) overexpressing CXCR4 showed a good ability to penetrate the endothelial cell barrier in the treatment of hindlimb ischemia (Bose et al. Citation2018). Due to the existence of recognition sites on the surface of the cell membrane, stem cell membranes are often used to coat drug-loaded NPs to improve the targeting ability of drug-loaded NPs. Yang et al. (Citation2018) developed a poly (lactic-co-glycolic acid) (PLGA) NPs with a layer of plasma membrane from UCMSC coating on the surface for tumor-targeted delivery of chemotherapy drugs. The results showed that the functionalization of the MSCs cell membrane significantly increased the tumor-targeting and accumulation of the NPs, significantly inhibited the growth of tumors, and induced apoptosis in tumor cells.
Stem cell derived extracellular vesicles or exosomes as drug vehicles
The concept of exosome was first proposed by Trams et al. (Citation1981). The cargo of EVs comprises small and long, coding and non-coding RNAs (e.g. mRNA, miRNA, lncRNA), lipids, proteins, as well as active chemotherapy drugs. EVs seem to have many features of an ideal carrier system, such as being naturally protected from degradation in the circulation, possess intrinsic cell targeting properties, and being able to overcome natural barriers such as the blood–brain barrier. Furthermore, EVs likely utilize endogenous mechanisms for uptake, intracellular trafficking, and subsequent delivery of their content in recipient cells. Importantly, nearly non-immunogenic when used autologously ensures the safety of EV administration in humans (Vader et al. Citation2016). The study by Ma et al. (Citation2017) found that exosomes extracted from BM-MSCs transfected with miR-221 oligonucleotides can be used as efficient nanocarriers. Lv et al. (Citation2020) loaded miR-21-5p mimics into human adipose stem cell-derived exosomes (HASC-exos) through electroporation, and it was found that engineering exosomes can promote diabetic wound healing. The work of Pascucci et al. (Citation2014) demonstrated for the first time that MSCs can package and deliver active drugs through their microvesicles (MVS) for the outstanding targeting ability to home in on tumor tissue. Subsequently, Kalimuthu et al. (Citation2018a). Co-incubated hBMSCs with paclitaxel (PTX) to obtain PTX-loaded EMs (PTX-MSC-EMs), found that PTX-MSC-EMs had a significant inhibitory effect on tumor growth in vivo.
Stem cell itself as drug vehicles
In addition to stem cell-derived cell membranes and extracellular vesicles or exosomes, stem cells are being studied more extensively as drug vehicles. The co-incubation of adipose-derived MSCs and paclitaxel in a closed bioreactor can expand a large number of drug-loaded stem cells in a short period of time, providing ideas for future industrial production (Lisini et al. Citation2020). Adipose-derived stem cells are now postulated as a potential ‘Trojan horse’ to vehicle and deliver free chemotherapy drug PTX and PTX-loaded DBCO-functionalized NPs to tumor sites and inhibit the growth of tumor cells (Borghese et al. Citation2020; Layek et al. Citation2020). Due to tumor tropism, integration in the tumor stroma, and excellent immune properties, MSC delivers therapeutic/cytotoxic drugs to the tumor site and unloads. This will greatly increase the concentration of the agent, enhance its therapeutic effect, and lowering systemic toxicity (Saulite et al. Citation2018; Almeida-Porada et al. Citation2020).
The utilization of NPs for loading chemotherapeutic drugs effectively protects MSCs from direct interaction with toxic drugs, and formation efflux transporters on MSCs. They selectively return to the tumor site and retained, thereby creating a cellular drug bank that releases the drug over a longer time period (Pessina et al. Citation2011; Sadhukha et al. Citation2014; Layek et al. Citation2018; Wang et al. Citation2018). For example, In the lung metastasis model, the systemic delivery of loaded MSCs can mediate the accumulation of BPCD in the lung to 93% after 24 h, and stay in the lung for at least 2 weeks (Yao et al. Citation2017). The study of Layek et al. (Citation2020). showed that paclitaxel-NPs loaded-MSCs can be released within 10 days under simulated conditions in vitro, with a cumulative release rate of about 92%. In addition, the acidic environment of tumor is more conducive to the release of drugs. Timin et al. confirmed that the cumulative release rate of VCR microcapsules at pH 4.0 for 5 days is 4.2 times that of pH 7.4 (Citation2019). The release of drugs in stem cell-based drug delivery systems depends on the P-gp-mediated efflux of stem cells and the disintegration of intracellular hybrid nanostructures. The ability of NPs enter into MSCs carrier is enhanced, thereby promoting the accumulation of NPs in tumor tissues and exerting their therapeutic effects. (Xu et al. Citation2018; Layek et al. Citation2020).
In vivo pharmacokinetics of drug-loaded stem cells
The dynamic process of stem cells in vivo is the guarantee of successfully delivery drug, which is loaded by stem cell and its derivatives as drug delivery vehicles. In animal experiments, there are two main methods for MSC transplantation, including (1) local infusion into the liver through hepatic portal vein injection, and (2) systemic infusion into the body via peripheral intravenous injection, arterial injection and intraperitoneal injection (Sun et al. Citation2014). The clinical treatment of MSC includes in situ injection, intravenous injection, arterial injection and so on. However, due to the simplicity of intravenous injection, systemic diseases such as graft-versus-host disease, Crohn’s disease, and systemic lupus erythematosus are still dominated by intravenous injection.
Intravenous injection of MSCs has a nonspecific biodistribution in lung, liver, spleen and other visceral tissues, and there is some retention, which reduces the concentration to the target site (Sadhukha et al. Citation2014; Kalimuthu et al. Citation2017; Kalimuthu et al. Citation2018b). Post-injection of MSCs in normal animals, most of the cells are retained initially in the lungs. Subsequently, the cells in the lungs gradually decrease and redistribute to the liver, spleen and kidney tissues (Layek et al. Citation2020). There is a positive effect of the adhesion molecules VLA-4/VCAM-1 on MSCs/endothelial cells during the interaction of MSCs with lung tissue (Fischer et al. Citation2009; Lee et al. Citation2009; Kerkelä et al. Citation2013). The redistribution of MSC in the body may be due to the phagocytosis of monocytes mediating the clearance of MSCs in the body, and subsequently, the relocation of monocytes from the lungs to the blood and liver (de Witte et al. Citation2018). The distribution of stem cells in the body under diseased conditions is different from that of normal animals. The same as previously described, stem cells tend to migrate to tissue damage sites. For example, a mouse study showed that both whole-body irradiation and local irradiation (such as selective irradiation of the abdomen or legs) affect the distribution of intravenous injections. Compared with untreated animals, NOD/SCID mice infused with hMSCs increased the number of hMSCs in the brain, heart, bone marrow and muscle after whole-body irradiation. In addition, selective irradiation of extremities or abdomen will also increase the implantation of hMSCs in bare skin or muscle (Leibacher and Henschler Citation2016). The reason for this phenomenon may be that the tissue damage caused by irradiation induces the migration of stem cells. DIR-labeled MSCs were injected intravenously into 4T1 lung metastasis model mice, and most of the fluorescent signals were concentrated in the lungs (93%) and lasted for two weeks (Yao et al. Citation2017). Strangely, a study showed that the fluorescence signal of fluorescent magnetic nanoparticle-labeled MSCs (FMNP-MSCs) is not detected in mouse heart, liver, spleen, lung, kidney and brain, but the fluorescence signal in tumor tissue is increasing (Ruan et al. Citation2012). Of course, this may also be caused by the limitations of detection methods. That’s all, the targeted migration of MSCs in vivo is the key to the high efficiency and safety of clinical treatment.
There is, in addition, one further point to make. For the treatment of brain cancer, innate tumor resistance and presence of the blood–brain barrier (BBB) require the development of multi-modal therapeutic regimens. Diffuse intrinsic pontine glioma (DIPG) is among the deadliest of pediatric brain tumors. Radiotherapy is the standard-of-care treatment for DIPG. Balyasnikova1 et al. (Chastkofsky et al. Citation2021) explored the use of MSC for oncolytic virus (OV) delivery. They found MSCs loaded with OV disseminate within a tumor and release OV throughout the DIPG brainstem xenografts in mice. The result showed that administration of OV-loaded MSCs with radiotherapy to mice bearing brainstem DIPG xenografts results in more prolonged survival relative to that conferred by either therapy alone (P < 0.01).
Specifically, MSCs preferentially migrate to the tumor area, and then releases a large number of chemokines into the tumor microenvironment through paracrine action, enhance the EPR of tumor, and thus integrates into the tumor tissue (Ruan et al. Citation2012; Li et al. Citation2015; Xu et al. Citation2018).
Methods of loading cargos with stem cell and its derivatives-based DDS
The drug loading methods for stem cell and its derivatives-based DDS are still a limitation. There is only co-incubation, ultrasonic and transfection. These methods are based on the different characteristics of vehicle materials, drugs and end-organ therapy. Review of previous research helps to investigate available opportunities in the field of recent drug delivery studies, and we show them in Figure .
The most important drug loading method for stem cells DDS is co-incubation, mainly to load exogenous small molecule compounds and drug-loaded nanoparticles, such as when Dental pulp stem cells (DPSC) incubated with PTX, PTX endocytosed into DPSC (Salehi et al. Citation2018). Nucleic acid and protein drugs are mainly transfected to stem cells. Anti-inflammatory factor IL-4 was transfected into MSCs, then formed MSCs (IL-4+) spheres treated OA rats and showed cartilage protection and anti-inflammatory effects (Song et al. Citation2020). Huang et al. (Citation2019b) constructed a stable FoxO1 overexpressing periodontal ligament stem cell line by lentivirus transfection, which promoted the osteogenic differentiation of periodontal ligament stem cells while exerting the antioxidant effect of FoxO1.
Stem cell membranes are separated and coated on the surface of drug-loaded NPs to deliver drugs. The functionalization of drug-loaded NPs with natural cell membranes typically comprises three main steps, (1) the isolation of stem cell membrane, (2) the synthesis of the drug-loaded NPs, and (3) final fusion of stem membrane with the NPs (Zhai et al. Citation2017). The isolation and breakage of the stem cell membrane can be done by various means, such as hypotonic solution treatment, ultrasonic disruption and repeated freeze–thaw cycles, etc. (Xu et al. Citation2020). Gao et al. (Citation2016b) physically mixed the stem cell membrane with SUCNPs@mSiO2 NPs and then co-extruded 11 passes through a 200 nm pore polycarbonate membrane to obtain the stem cell membrane-coated nanoparticles. Yang et al. (Citation2018) used the method of ultrasonicated to coat the PLGA NPs mixed in the stem cell membrane solution with the membrane. Cell membrane-coated drug-loaded NPs can also be formed through electroporation and electrostatic attractions (Xu et al. Citation2020).
The stem cell-derived extracellular vesicles or exosomes are isolated and participate in the delivery of their own active substances and foreign cargos. According to the shape, size, density or surface composition of exosomes, they can be separated from stem cells by various methods, such as centrifugation ultracentrifugation, density gradient centrifugation, SEC chromatography and precipitation, etc. (Gutierrez-Millan et al. Citation2021). Exosomes load nucleic acids and small molecular therapeutics using various methods including chemical transfection, electroporation, sonication, co-incubation, extrusion, freeze–thaw cycle, saponin assisted, hypotonic dialysis and pH gradient driven. (Chinnappan et al. Citation2020; Mehryab et al. Citation2020) Furthermore, the surface modification to the stem cell-derived extracellular vesicles or exosomes can dramatically influence their application values. Zhu et al. (Citation2019) designed a cRGD-modified embryonic stem cell exosomes, and loaded PTX by co-incubation. Bagheri et al. (Citation2020) prepared DOX-loaded exosomes by electroporation, and modified the exosomes with MUC1 aptamers. Zhou et al. (Citation2020) loaded PTX and gemcitabine monophosphate (GEMP) into BMSCs exosomes by electroporation.
Location of drugs in stem cell and its derivatives-based DDS
The form of drugs existing in stem cell and its derivatives-based DDS is very important for their fate. The ways in which stem cells deliver therapeutic drugs have been extensively studied (Figure ). Either drugs enter the cytoplasm via passive transport (Banerji and Hayes Citation2007) and active endocytosis (Rejman et al. Citation2004) of the cells, or through nano or micro, the non-covalent and covalent coupling of the carriers and the cell membrane anchors the drug carriers on the surface of the stem cell membrane (Timin et al. Citation2019).
Figure 3. Location of drugs in stem cell and its derivatives-based DDS. a, Surface adsorption caused by electrostatic force, van der Waals force and hydrogen bond. b, Endocytosis of stem cells. c, Chemical bond modification. d, Antigen antibody reaction. e, Biotin reacts with avidin. f, Effects of cell penetrating peptide.
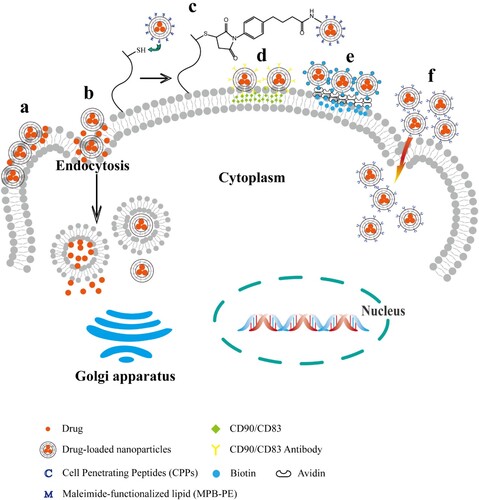
Drugs in stem cells cytoplasm
Stem cells can effectively internalize small molecule drugs or drug-loaded particles through endocytosis, and can achieve drug loading through simple co-cultivation. Lisini et al. (Citation2020) incubated ADMS with paclitaxel to obtain a large number of drug-loaded stem cells for tumor treatment. Timin et al. (Citation2019) incubated 0.6 μm, 2.0 μm and 5.0 μm silica-coated capsules with MSCs and found that the size of the particles would affect the endocytosis of MSC, and 0.6 μm capsules could be better internalized. The receptor protein on the surface of the stem cell membrane could bind the surface-modified ligand–protein of the NPs, subsequently, the NPs are wrapped by the curved and recessed cell membrane (Doherty and McMahon Citation2009). Huang et al. developed a magnetic ternary nanohybrid (MTN) system. HA-SPIO prepared by ligand exchange reaction was anticipated to facilitate stem cell uptake of cationic nano complexes through dual CD44 and magneto-targeting effects (Huang et al. Citation2019a).
Drugs anchored on the cell membrane surface
Drug-loaded NPs can adhere to the surface of stem cells through non-covalent interactions between polymer materials and stem cells, such as van der Waals forces, electrostatic forces, hydrogen bonding, and hydrophobic forces (Villa et al. Citation2015). Yao et al. (Citation2017) reported NPs can be anchored on the cell membranes of MSCs through three types of membrane-engineered methods: insertion or fusion by lipid, biomarker-mediated and chemical modification. They took advantage of the high non-covalent affinity of biotin and avidin, selected biotin to modify MSCs membrane and doxorubicin (DOX) conjugates, and used avidin as a bridge between DOX conjugates and MSCs. Xu et al. (Citation2019) also connected the drug-loaded polymer NPs to BMSCs through biotin–avidin to construct a cell-NPs hybrid carrier. Li et al. (Citation2011) demonstrated that silica nano-doxorubicin particles can be anchored on MSCs through specific antibody–antigen recognition on the cell membrane interface. The loaded MSCs can effectively track glioma cells and significantly promote tumor cell apoptosis. Many cell surfaces have high-level functional groups, such as sulfhydryl, amine, sialic acid residues, etc. NPs can be chemically modified to promote MSCs covalent binding with cell carriers (Pang et al. Citation2017). For example, Stephan et al. (Citation2010) detected high levels of reduced thiol groups on the surface of T cells, B cells, and hematopoietic stem cells. They covalently attached reactive maleimide group-modified NPs to the surface of thiol-rich T cells through thiol–maleimide conjugation.
Applications of stem cell-based DDS
Stem cells deliver cargos for tumor treatment
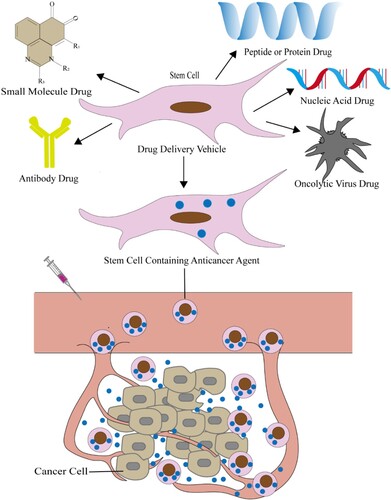
Stem cells have been certified to be used as drug delivery vehicles for the treatment of various cancers (Figure ), such as melanoma, ovarian, hepatocarcinoma, glioblastoma, lymphoma, lung cancer, breast cancer, colon, prostate cancer, and so on (Li et al. Citation2015; Levy et al. Citation2016). Addition of paclitaxel, doxorubicin, or gemcitabine to the conditioned media of MSCs from gum papilla can inhibit the growth of squamous cell carcinoma (Coccè et al. Citation2017). In order to optimize the pharmacokinetics of antitumor drugs, Timin et al. (Citation2019) prepared new silica-coated capsules that loaded the highly toxic antitumor drug vincristine (VCR), and further combined with the hMSCs. hMSCs can metastasize to the tumor site and improve VCR efficiency. The infer molecular mechanisms is that hMSCs loaded with VCR capsules may through the CXCL12 (SDF-1)/CXCR4 pathway actively migrating to the tumor. Muslimov et al. (Citation2020) developed a photosensitive bionic DDS based on MSCs. Firstly, they prepared multilayer submicron capsules modified with Au-NRs and coated with silicon oxide. Then MSCs delivered vincristine-loaded microcapsules into tumor spheres, triggered drug release under NIR laser irradiation can lead to the effective death of tumor spheres. Yao et al. (Citation2017) used the dual drug loading method of endocytosis and membrane binding for the first time to maximize the number of DOX conjugates in BMSCs, and compared with free DOX and its conjugates, DOX conjugates-loaded BMSCs could significantly inhibit tumor growth and prolong the lifespan of tumor-bearing mice. Moku et al. (Citation2019) used a maleimide–thiol covalent modification to couple the transcriptional of transactivator (TAT) peptide to the surface of PLGA NPs to improve the accumulation and retention of NPs in MSCs. In this study, by increasing the drug loading of MSC, the tumor growth of lung cancer mice was significantly inhibited and the survival rate was improved.
Figure 4. Stem cell as drug vehicle deliver anticancer agents to treat cancer. Stem cells as a vehicle to contain drugs for the treatment of cancer, including small molecule chemotherapeutics, antibody drugs, nucleic acid drugs, polypeptide or protein drugs and oncolytic virus drugs. The drug-loaded stem cells get into the cancer tissue through the blood and release anti-cancer agents. Thereby exerting a therapeutic effect.
Stem cells deliver cargos for inflammation-related diseases
Extensive research has proved that stem cells as a carrier play a role in many inflammatory diseases, such as neuroinflammation, osteoarthritis, periodontitis inflammation of myocardial damage and so on (Regmi et al. Citation2019). In the treatment of inflammation-related diseases, stem cells are mostly used as gene or protein carriers to exert their immunomodulatory and anti-inflammatory properties. For example, in the treatment of traumatic brain injury (TBI) in rats, MSCs overexpressing IL-10 reduced neuroinflammation markers and transformed the expression of classic inflammation (CD86) into an alternative inflammatory state (CD163) (Maiti et al. Citation2019; Peruzzaro et al. Citation2019). The results suggest that transplantation of MSCs-IL-10 may be an effective strategy to protect neurons from damage caused by TBI. In addition, BMSCs overexpressing platelet-derived growth factor (PDGF) or heme oxygenase-1 (HO-1) can improve the symptoms of surgery-induced canine OA (Oh et al. Citation2021). Wang et al. (Citation2015) treated DSS-induced experimental colitis with IL-37b gene-modified mouse bone marrow MSCs (MSC-IL-37b) which could express and secret IL-37b had significant therapeutic efficacy against colitis.
Stem cells deliver cargos for tissue repair and regenerative medicine
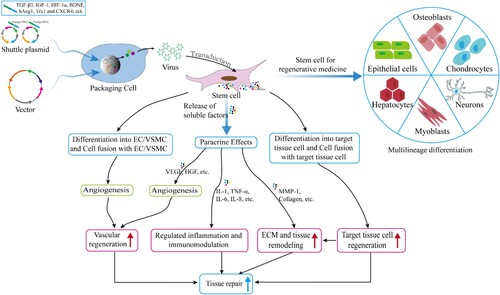
Stem cells have broad application prospects in tissue repair and regenerative medicine, and have made exciting achievements in the research of tissue repair such as skin (Li et al. Citation2018b), myocardial and spinal cord (Sheyn et al. Citation2016) injury repair. Stem cells have strong paracrine activity, including the secretion of growth factors, cytokines and hormones, which are the primary mechanism for promoting tissue repair (Figure ). The paracrine action of stem cells can activate the SDF-1α/CXCR4 signaling pathway, which promotes the migration of stem cells to the tissue injury site (Haider et al. Citation2008). The migration ability of MSCs with high expression of CXCR4 is enhanced, which promotes the regeneration of intervertebral discs (Wei et al. Citation2016). Stem cells are used as drug carriers for tissue repair, often through genetic engineering to transform stem cells. Transplantation of genetically recombinant stem cells is an attractive tissue-repair strategy, which have roles as both therapeutics and carriers for gene delivery to wound sites (Peng et al. Citation2011). Angiopoietin-1 (Ang1), insulin-like growth factor (IGF-1) and hypoxia-inducible factor-1α (HIF-1α)-modified MSCs can promote heart repair after myocardial infarction (Sun et al. Citation2007; Haider et al. Citation2008; Huang et al. Citation2014). Suresh et al. (Citation2015) used the same method to modify MSCs with Thioredoxin-1 (Trx1) to reduce myocardial fibrosis and improve heart function. Stem cells play a beneficial role in the healing of damaged tissue by directly differentiating to many different resident cell types and/or secreting several trophic factors to aid tissue repair (Hong et al. Citation2012).
Figure 5. Stem cells deliver cargos for tissue repair and regenerative medicine. Stem cell are used in regenerative medicine through transplantation due to their strong self-renewal and multilineage differentiation characteristics, including bone regeneration, cartilage regeneration, myocardial regeneration, nerve regeneration and so on. In addition, stem cell promotes vascular regeneration through powerful paracrine effects, enhance immune regulation and reduce inflammation, promote extracellular matrix and tissue remodeling, thereby promoting tissue damage repair. Stem cell promotes the overexpression of genes such as TGF-β3 and IGF-1 through genetic engineering, and promote tissue repair.
Challenges and prospects
In the application as a drug delivery vehicle, it is necessary to select the source of the MSC for different treatment target sites. For example, ADMSCs have the ability to homing to injured liver. Zhao et al. (Citation2014) used ADMSCs as drug delivery vehicles for the study of liver injury and liver cancer. In the research related to stem cell therapy, the source of stem cells involves ethical issues, such as embryonic stem cells, somatic cell nuclear transfer cells, and induced pluripotent stem cells. However, not all stem cells involve ethical issues. For example, adult stem cells or umbilical cord blood stem cells are not involved ethical issues (de Miguel-Beriain Citation2015). In view of the ethical issues of stem cells and strong paracrine capabilities, cell-free therapy based on stem cells will become a future research trend. Stem cell membranes, stem cell-derived extracellular vesicles, stem cell secretion groups, stem cell extracellular matrix, etc., will likely have the potential to replace or even exceed stem cell applications. Israel has developed an innovative compound EXO-CD24 based on CD-24 exosomes. The exogenous CD-24 protein is packaged in exosomes and is directly delivered to the patient’s lungs by nasal inhalation to eliminate COVID-19 caused the fatal ‘cytokine storm’. The safety of stem cell therapy is also a matter of close attention. Studies have shown that MSC has the effect of inhibiting tumor activity, and at the same time, there is also research evidence that it has the effect of promoting tumor development (Rhee et al. Citation2015). However, no matter whether it is the anti-tumor effect of MSC or the role of promoting tumor development, the specific mechanism of its effect has not been unified.
From the surface modification of NPs to the construction of DDS based on stem cells in vivo, the research of targeting in DDS has been seeking a breakthrough, and at the same time, amazing research results have been achieved. Studies have shown that MSC can directionally migrate to tissue damage sites through SDF1/CXCR4 signal axis (Marquez-Curtis and Janowska-Wieczorek Citation2013). For the study of MSC targeting mechanism and the monitoring of its migration process in vivo, further development is needed. The low immunogenicity of stem cells makes them better for drug delivery. In addition, stem cells secrete inflammatory factors and immunosuppressive factors to cooperate with the innate immune system and the acquired immune system, thereby playing the role of immune regulation (Qi et al. Citation2018). For example, stem cells affect the maturation, migration, polarization and function of macrophages through paracrine release factors. They can also affect the maturation of dendritic cells and effectively regulate the activation and functional differentiation of T cells (Lin and Du Citation2018). Therefore, the combination of stem cell immunotherapy and drug delivery has bright application prospects in future disease treatment.
Author contributions
The manuscript was written through contributions of all authors. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data created or analyzed in this study.
Additional information
Funding
References
- Almeida CR, Caires HR, Vasconcelos DP, Barbosa MA. 2016. NAP-2 Secreted by human NK cells can stimulate mesenchymal stem/stromal cell recruitment. Stem Cell Rep. 6(4):466–473. doi:10.1016/j.stemcr.2016.02.012.
- Almeida-Porada G, Atala AJ, Porada CD. 2020. Therapeutic mesenchymal stromal cells for immunotherapy and for gene and drug delivery. Mol Ther Methods Clin Dev. 16:204–224. doi:10.1016/j.omtm.2020.01.005.
- An L, Wang Y, Lin J, Tian Q, Xie Y, Hu J, et al. 2019. Macrophages-mediated delivery of small gold nanorods for tumor hypoxia photoacoustic imaging and enhanced photothermal therapy. ACS Appl Mater Interfaces. 11(17):15251–15261. doi:10.1021/acsami.9b00495.
- Bagheri E, Abnous K, Farzad SA, Taghdisi SM, Ramezani M, Alibolandi M. 2020. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 261:118369. doi:10.1016/j.lfs.2020.118369.
- Banerji SK, Hayes MA. 2007. Examination of nonendocytotic bulk transport of nanoparticles across phospholipid membranes. Langmuir: ACS J Surf Colloids. 23(6):3305–3313.
- Borghese C, Casagrande N, Corona G, Aldinucci D. 2020. Adipose-derived stem cells primed with paclitaxel inhibit ovarian cancer spheroid growth and overcome paclitaxel resistance. Pharmaceutics. 12(5). doi:10.3390/pharmaceutics12050401
- Bose RJ, Kim BJ, Arai Y, Han IB, Moon JJ, Paulmurugan R, et al. 2018. Bioengineered stem cell membrane functionalized nanocarriers for therapeutic targeting of severe hindlimb ischemia. Biomaterials. 185:360–370. doi:10.1016/j.biomaterials.2018.08.018.
- Cao S, Guo J, He Y, Alahdal M, Tang S, Zhao Y, et al. 2018. Nano-loaded human umbilical cord mesenchymal stem cells as targeted carriers of doxorubicin for breast cancer therapy. Artif Cells Nanomed Biotechnol. 46(sup1):642–652. doi:10.1080/21691401.2018.1434185.
- Chastkofsky MI, Pituch KC, Katagi H, Zannikou M, Ilut L, Xiao T, et al. 2021. Mesenchymal stem cells successfully deliver oncolytic virotherapy to diffuse intrinsic pontine glioma. Clin Cancer Res. 27(6):1766–1777. doi:10.1158/1078-0432.CCR-20-1499.
- Chinnappan M, Srivastava A, Amreddy N, Razaq M, Pareek V, Ahmed R, et al. 2020. Exosomes as drug delivery vehicle and contributor of resistance to anticancer drugs. Cancer Lett. 486:18–28. doi:10.1016/j.canlet.2020.05.004.
- Chiu RC. 2005. ‘Stealth immune tolerance’ in stem cell transplantation: potential for ‘universal donors’ in myocardial regenerative therapy. J Heart Lung Transplant. 24(5):511–516. doi:10.1016/j.healun.2004.11.010.
- Coccè V, Farronato D, Brini AT, Masia C, Giannì AB, Piovani G, et al. 2017. Drug loaded gingival mesenchymal stromal cells (GinPa-MSCs) inhibit in vitro proliferation of oral squamous cell carcinoma. Sci Rep. 7(1):9376. doi:10.1038/s41598-017-09175-4.
- Danhier F. 2016. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. 244(Pt A):108–121. doi:10.1016/j.jconrel.2016.11.015.
- de Miguel-Beriain I. 2015. The ethics of stem cells revisited. Adv Drug Deliv Rev. 82–83:176–180. doi:10.1016/j.addr.2014.11.011.
- de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A, Korevaar SS, et al. 2018. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells. 36(4):602–615. doi:10.1002/stem.2779.
- Doherty GJ, McMahon HT. 2009. Mechanisms of endocytosis. Annu Rev Biochem. 78:857–902. doi:10.1146/annurev.biochem.78.081307.110540.
- Fang J, Nakamura H, Maeda H. 2011. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 63(3):136–151. doi:10.1016/j.addr.2010.04.009.
- Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. 2009. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 18(5):683–692. doi:10.1089/scd.2008.0253.
- Gao C, Lin Z, Jurado-Sanchez B, Lin X, Wu Z, He Q. 2016a. Stem cell membrane-coated nanogels for highly efficient in vivo tumor targeted drug delivery. Small. 12(30):4056–4062. doi:10.1002/smll.201600624.
- Gao C, Lin Z, Wu Z, Lin X, He Q. 2016b. Stem-cell-membrane camouflaging on near-infrared photoactivated upconversion nanoarchitectures for in vivo remote-controlled photodynamic therapy. ACS Appl Mater Interfaces. 8(50):34252–34260. doi:10.1021/acsami.6b12865.
- Gao M, Liang C, Song X, Chen Q, Jin Q, Wang C, et al. 2017. Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy. Adv Mater. 29(35). doi:10.1002/adma.201701429.
- Gozde U, Ufuk G. 2018. Smart drug delivery systems in cancer therapy. Curr Drug Targets. 19(3):202–212. doi:10.2174/1389450117666160401124624.
- Gutierrez-Millan C, Calvo Díaz C, Lanao JM, Colino CI. 2021. Advances in exosomes-based drug delivery systems. Macromol Biosci. 21(1):e2000269. doi:10.1002/mabi.202000269.
- Hagenhoff A, Bruns CJ, Zhao Y, von Luttichau I, Niess H, Spitzweg C, et al. 2016. Harnessing mesenchymal stem cell homing as an anticancer therapy. Expert Opin Biol Ther. 16(9):1079–1092. doi:10.1080/14712598.2016.1196179.
- Haider H, Jiang S, Idris NM, Ashraf M. 2008. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 103(11):1300–1308. doi:10.1161/CIRCRESAHA.108.186742.
- Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. 2019. Mesenchymal stem cells for regenerative medicine. Cells. 8(8). doi:10.3390/cells8080886.
- Herea D-D, Labusca L, Radu E, Chiriac H, Grigoras M, Panzaru OD, et al. 2019. Human adipose-derived stem cells loaded with drug-coated magnetic nanoparticles for in-vitro tumor cells targeting. Mater Sci Eng C-Mater Biol Appl. 94:666–676. doi:10.1016/j.msec.2018.10.019.
- Hong HS, Kim YH, Son Y. 2012. Perspectives on mesenchymal stem cells: tissue repair, immune modulation, and tumor homing. Arch Pharmacal Res. 35(2):201–211. doi:10.1007/s12272-012-0201-0.
- Huang B, Qian J, Ma J, Huang Z, Shen Y, Chen X, et al. 2014. Myocardial transfection of hypoxia-inducible factor-1α and co-transplantation of mesenchymal stem cells enhance cardiac repair in rats with experimental myocardial infarction. Stem Cell Res Ther. 5(1):22. doi:10.1186/scrt410.
- Huang RY, Lin YH, Lin SY, Li YN, Chiang CS, Chang CW. 2019a. Magnetic ternary nanohybrids for nonviral gene delivery of stem cells and applications on cancer therapy. Theranostics. 9(8):2411–2423. doi:10.7150/thno.29326.
- Huang X, Chen H, Xie Y, Cao Z, Lin X, Wang Y. 2019b. Foxo1 Overexpression ameliorates TNF-alpha-induced oxidative damage and promotes osteogenesis of human periodontal ligament stem cells via antioxidant defense activation. Stem Cells Int. 2019:2120453. doi:10.1155/2019/2120453.
- Kalimuthu S, Gangadaran P, Rajendran RL, Zhu L, Oh JM, Lee HW, et al. 2018a. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front Pharmacol. 9:1116. doi:10.3389/fphar.2018.01116.
- Kalimuthu S, Oh JM, Gangadaran P, Zhu L, Lee HW, Rajendran RL, et al. 2017. In vivo tracking of chemokine receptor CXCR4-engineered mesenchymal stem cell migration by optical molecular imaging. Stem Cells Int. 2017:8085637. doi:10.1155/2017/8085637.
- Kalimuthu S, Zhu L, Oh JM, Gangadaran P, Lee HW, Baek SH, et al. 2018b. Migration of mesenchymal stem cells to tumor xenograft models and in vitro drug delivery by doxorubicin. Int J Med Sci. 15(10):1051–1061. doi:10.7150/ijms.25760.
- Karp JM, Leng Teo GS. 2009. Mesenchymal stem cell homing: the devil Is in the details. Cell Stem Cell. 4(3):206–216. doi:10.1016/j.stem.2009.02.001.
- Karp JM, Leng Teo GS. 2009. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 4(3):206–216. doi:10.1016/j.stem.2009.02.001.
- Kerkelä E, Hakkarainen T, Mäkelä T, Raki M, Kambur O, Kilpinen L, et al. 2013. Transient proteolytic modification of mesenchymal stromal cells increases lung clearance rate and targeting to injured tissue. Stem Cells Transl Med. 2(7):510–520. doi:10.5966/sctm.2012-0187.
- Kiernan CH, KleinJan A, Peeters M, Wolvius EB, Farrell E, Brama PAJ. 2018. Allogeneic chondrogenically differentiated human bone marrow stromal cells do not induce dendritic cell maturation. J Tissue Eng Regen Med. 12(6):1530–1540. doi:10.1002/term.2682.
- Kolios G, Moodley Y. 2013. Introduction to stem cells and regenerative medicine. Respiration. 85(1):3–10. doi:10.1159/000345615.
- Krueger TEG, Thorek DLJ, Denmeade SR, Isaacs JT, Brennen WN. 2018. Concise review: mesenchymal stem cell-based drug delivery: the good, the bad, the ugly, and the promise. Stem Cells Transl Med. 7(9):651–663. doi:10.1002/sctm.18-0024.
- Layek B, Sadhukha T, Panyam J, Prabha S. 2018. Nano-engineered mesenchymal stem cells increase therapeutic efficacy of anticancer drug through true active tumor targeting. Mol Cancer Ther. 17(6):1196–1206. doi:10.1158/1535-7163.Mct-17-0682.
- Layek B, Shetty M, Nethi SK, Sehgal D, Starr TK, Prabha S. 2020. Mesenchymal stem cells as guideposts for nanoparticle-mediated targeted drug delivery in ovarian cancer. Cancers (Basel). 12(4). doi:10.3390/cancers12040965.
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. 2009. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 5(1):54–63. doi:10.1016/j.stem.2009.05.003.
- Leibacher J, Henschler R. 2016. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 7:7. doi:10.1186/s13287-015-0271-2.
- Levy O, Brennen WN, Han E, Rosen DM, Musabeyezu J, Safaee H, et al. 2016. A prodrug-doped cellular Trojan horse for the potential treatment of prostate cancer. Biomaterials. 91:140–150. doi:10.1016/j.biomaterials.2016.03.023.
- Li C, Wang J, Wang Y, Gao H, Wei G, Huang Y, et al. 2019. Recent progress in drug delivery. Acta Pharmaceutica Sinica B. 9(6):1145–1162. doi:10.1016/j.apsb.2019.08.003.
- Li D, Xue W, Li M, Dong M, Wang J, Wang X, et al. 2018a. VCAM-1(+) macrophages guide the homing of HSPCs to a vascular niche. Nature. 564(7734):119–124. doi:10.1038/s41586-018-0709-7.
- Li L, Guan Y, Liu H, Hao N, Liu T, Meng X, et al. 2011. Silica nanorattle-doxorubicin-anchored mesenchymal stem cells for tumor-tropic therapy. ACS Nano. 5(9):7462–7470. doi:10.1021/nn202399w.
- Li M, Qiu L, Hu W, Deng X, Xu H, Cao Y, et al. 2018b. Genetically-modified bone mesenchymal stem cells with TGF-beta3 improve wound healing and reduce scar tissue formation in a rabbit model. Exp Cell Res. 367(1):24–29. doi:10.1016/j.yexcr.2018.02.006.
- Li Z, Fan D, Xiong D. 2015. Mesenchymal stem cells as delivery vectors for anti-tumor therapy. Stem Cell Investig. 2:6. doi:10.3978/j.issn.2306-9759.2015.03.01.
- Lin L, Du L. 2018. The role of secreted factors in stem cells-mediated immune regulation. Cell Immunol. 326:24–32. doi:10.1016/j.cellimm.2017.07.010.
- Lisini D, Nava S, Frigerio S, Pogliani S, Maronati G, Marcianti A, et al. 2020. Automated large-scale production of paclitaxel loaded mesenchymal stromal cells for cell therapy applications. Pharmaceutics. 12:5. doi:10.3390/pharmaceutics12050411.
- Luo M, Zhou Y, Gao N, Cheng W, Wang X, Cao J, et al. 2020. Mesenchymal stem cells transporting black phosphorus-based biocompatible nanospheres: active Trojan horse for enhanced photothermal cancer therapy. Chem Eng J. 385. doi:10.1016/j.cej.2019.123942.
- Lv Q, Deng J, Chen Y, Wang Y, Liu B, Liu J. 2020. Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Mol Pharm. 17(5):1723–1733. doi:10.1021/acs.molpharmaceut.0c00177.
- Ma M, Chen S, Liu Z, Xie H, Deng H, Shang S, et al. 2017. miRNA-221 of exosomes originating from bone marrow mesenchymal stem cells promotes oncogenic activity in gastric cancer. Onco Targets Ther. 10:4161–4171. doi:10.2147/OTT.S143315.
- Maiti P, Peruzzaro S, Kolli N, Andrews M, Al-Gharaibeh A, Rossignol J, et al. 2019. Transplantation of mesenchymal stem cells overexpressing interleukin-10 induces autophagy response and promotes neuroprotection in a rat model of TBI. J Cell Mol Med. 23(8):5211–5224. doi:10.1111/jcmm.14396.
- Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, et al. 2003. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 10(2):228–241. doi:10.1007/bf02256058.
- Marquez-Curtis LA, Janowska-Wieczorek A. 2013. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. Biomed Res Int. 2013:561098. doi:10.1155/2013/561098.
- Mehryab F, Rabbani S, Shahhosseini S, Shekari F, Fatahi Y, Baharvand H, et al. 2020. Exosomes as a next-generation drug delivery system: An update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 113:42–62. doi:10.1016/j.actbio.2020.06.036.
- Mills KM, Szczerkowski JLA, Habib SJ. 2017. WNT ligand presentation and reception: from the stem cell niche to tissue engineering. Open Biol. 7:8. doi:10.1098/rsob.170140.
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. 2021. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 20(2):101–124. doi:10.1038/s41573-020-0090-8.
- Moku G, Layek B, Trautman L, Putnam S, Panyam J, Prabha S. 2019. Improving payload capacity and anti-tumor efficacy of mesenchymal stem cells using TAT peptide functionalized polymeric nanoparticles. Cancers (Basel). 11(4). doi:10.3390/cancers11040491.
- Mu H, Holm R. 2018. Solid lipid nanocarriers in drug delivery: characterization and design. Expert Opin Drug Deliv. 15(8):771–785. doi:10.1080/17425247.2018.1504018.
- Muslimov AR, Timin AS, Bichaykina VR, Peltek OO, Karpov TE, Dubavik A, et al. 2020. Biomimetic drug delivery platforms based on mesenchymal stem cells impregnated with light-responsive submicron sized carriers. Biomater Sci. 8(4):1137–1147. doi:10.1039/c9bm00926d.
- Nakamura Y, Mochida A, Choyke PL, Kobayashi H. 2016. Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug Chem. 27(10):2225–2238. doi:10.1021/acs.bioconjchem.6b00437.
- Oh J, Son YS, Kim WH, Kwon OK, Kang BJ. 2021. Mesenchymal stem cells genetically engineered to express platelet-derived growth factor and heme oxygenase-1 ameliorate osteoarthritis in a canine model. J Orthop Surg Res. 16(1):43. doi:10.1186/s13018-020-02178-4.
- Pang L, Zhang C, Qin J, Han L, Li R, Hong C, et al. 2017. A novel strategy to achieve effective drug delivery: exploit cells as carrier combined with nanoparticles. Drug Deliv. 24(1):83–91. doi:10.1080/10717544.2016.1230903.
- Pascucci L, Cocce V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. 2014. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 192:262–270. doi:10.1016/j.jconrel.2014.07.042.
- Peng LH, Fung KP, Leung PC, Gao JQ. 2011. Genetically manipulated adult stem cells for wound healing. Drug Discov Today. 16(21–22):957–966. doi:10.1016/j.drudis.2011.07.009.
- Peruzzaro ST, Andrews MMM, Al-Gharaibeh A, Pupiec O, Resk M, Story D, et al. 2019. Transplantation of mesenchymal stem cells genetically engineered to overexpress interleukin-10 promotes alternative inflammatory response in rat model of traumatic brain injury. J Neuroinflammation. 16(1):2. doi:10.1186/s12974-018-1383-2.
- Pessina A, Bonomi A, Coccè V, Invernici G, Navone S, Cavicchini L, et al. 2011. Mesenchymal stromal cells primed with paclitaxel provide a new approach for cancer therapy. PLoS One. 6(12):e28321. doi:10.1371/journal.pone.0028321.
- Poon W, Kingston BR, Ouyang B, Ngo W, Chan WCW. 2020. A framework for designing delivery systems. Nat Nanotechnol. 15(10):819–829. doi:10.1038/s41565-020-0759-5.
- Preynat-Seauve O, Krause K-H. 2011. Stem cell sources for regenerative medicine: the immunological point of view. Semin Immunopathol. 33(6):519–524. doi:10.1007/s00281-011-0271-y.
- Qi K, Li N, Zhang Z, Melino G. 2018. Tissue regeneration: The crosstalk between mesenchymal stem cells and immune response. Cell Immunol. 326:86–93. doi:10.1016/j.cellimm.2017.11.010.
- Regmi S, Pathak S, Kim JO, Yong CS, Jeong JH. 2019. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur J Cell Biol. 98(5–8):151041. doi:10.1016/j.ejcb.2019.04.002.
- Rejman J, Oberle V, Zuhorn IS, Hoekstra D. 2004. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 377(Pt 1):159–169.
- Rhee KJ, Lee JI, Eom YW. 2015. Mesenchymal stem cell-mediated effects of tumor support or suppression. Int J Mol Sci. 16(12):30015–30033. doi:10.3390/ijms161226215.
- Ruan J, Ji J, Song H, Qian Q, Wang K, Wang C, et al. 2012. Fluorescent magnetic nanoparticle-labeled mesenchymal stem cells for targeted imaging and hyperthermia therapy of in vivo gastric cancer. Nanoscale Res Lett. 7(1):309. doi:10.1186/1556-276x-7-309.
- Sadhukha T, O’Brien TD, Prabha S. 2014. Nano-engineered mesenchymal stem cells as targeted therapeutic carriers. J Control Release. 196:243–251. doi:10.1016/j.jconrel.2014.10.015.
- Saeedi M, Eslamifar M, Khezri K, Dizaj SM. 2019. Applications of nanotechnology in drug delivery to the central nervous system. Biomed Pharmacother. 111:666–675. doi:10.1016/j.biopha.2018.12.133.
- Salehi H, Al-Arag S, Middendorp E, Gergely C, Cuisinier F, Orti V. 2018. Dental pulp stem cells used to deliver the anticancer drug paclitaxel. Stem Cell Res Ther. 9(1):103. doi:10.1186/s13287-018-0831-3.
- Saulite L, Pleiko K, Popena I, Dapkute D, Rotomskis R, Riekstina U. 2018. Nanoparticle delivery to metastatic breast cancer cells by nanoengineered mesenchymal stem cells. Beilstein J Nanotechnol. 9:321–332. doi:10.3762/bjnano.9.32.
- Severino P, da Silva CF, Andrade LN, de Lima Oliveira D, Campos J, Souto EB. 2019. Alginate nanoparticles for drug delivery and targeting. Curr Pharm Des. 25(11):1312–1334. doi:10.2174/1381612825666190425163424.
- Sheyn D, Shapiro G, Tawackoli W, Jun DS, Koh Y, Kang KB, et al. 2016. PTH induces systemically administered mesenchymal stem cells to migrate to and regenerate spine injuries. Mol Ther. 24(2):318–330. doi:10.1038/mt.2015.211.
- Song SY, Hong J, Go S, Lim S, Sohn HS, Kang M, et al. 2020. Interleukin-4 gene transfection and spheroid formation potentiate therapeutic efficacy of mesenchymal stem cells for osteoarthritis. Adv Healthc Mater. 9(5):e1901612. doi:10.1002/adhm.201901612.
- Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. 2010. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 16(9):1035–1041. doi:10.1038/nm.2198.
- Sun L, Cui M, Wang Z, Feng X, Mao J, Chen P, et al. 2007. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem Biophys Res Commun. 357(3):779–784. doi:10.1016/j.bbrc.2007.04.010.
- Sun L, Fan X, Zhang L, Shi G, Aili M, Lu X, et al. 2014. Bone mesenchymal stem cell transplantation via four routes for the treatment of acute liver failure in rats. Int J Mol Med. 34(4):987–996. doi:10.3892/ijmm.2014.1890.
- Suresh SC, Selvaraju V, Thirunavukkarasu M, Goldman JW, Husain A, Alexander Palesty J, et al. 2015. Thioredoxin-1 (Trx1) engineered mesenchymal stem cell therapy increased pro-angiogenic factors, reduced fibrosis and improved heart function in the infarcted rat myocardium. Int J Cardiol. 201:517–528. doi:10.1016/j.ijcard.2015.08.117.
- Teng CF, Jeng LB, Shyu WC. 2018. Role of insulin-like growth factor 1 receptor signaling in stem cell stemness and therapeutic efficacy. Cell Transplant. 27(9):1313–1319. doi:10.1177/0963689718779777.
- Thanuja MY, Anupama C, Ranganath SH. 2018. Bioengineered cellular and cell membrane-derived vehicles for actively targeted drug delivery: So near and yet so far. Adv Drug Deliv Rev. 132:57–80. doi:10.1016/j.addr.2018.06.012.
- Thanuja MY, Anupama C, Ranganath SH. 2018. Bioengineered cellular and cell membrane-derived vehicles for actively targeted drug delivery: so near and yet so far. Adv Drug Deliv Rev. 132:57–80. doi:10.1016/j.addr.2018.06.012.
- Timin AS, Peltek OO, Zyuzin MV, Muslimov AR, Karpov TE, Epifanovskaya OS, et al. 2019. Safe and effective delivery of antitumor drug using mesenchymal stem cells impregnated with submicron carriers. ACS Appl Mater Interfaces. 11(14):13091–13104. doi:10.1021/acsami.8b22685.
- Timin AS, Peltek OO, Zyuzin MV, Muslimov AR, Karpov TE, Epifanovskaya OS, et al. 2019. Safe and effective delivery of antitumor drug using mesenchymal stem cells impregnated with submicron carriers. ACS Appl Mater Interfaces. 11(14):13091–13104. doi:10.1021/acsami.8b22685.
- Trams EG, Lauter CJ, Salem N, Jr., Heine U. 1981. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 645(1):63–70. doi:10.1016/0005-2736(81)90512-5.
- Vader P, Mol EA, Pasterkamp G, Schiffelers RM. 2016. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 106(Pt A):148–156. doi:10.1016/j.addr.2016.02.006.
- Villa CH, Pan DC, Zaitsev S, Cines DB, Siegel DL, Muzykantov VR. 2015. Delivery of drugs bound to erythrocytes: new avenues for an old intravascular carrier. Ther Delivery. 6(7):795–826. doi:10.4155/tde.15.34.
- Wang M, Xin Y, Cao H, Li W, Hua Y, Webster TJ, et al. 2021. Recent advances in mesenchymal stem cell membrane-coated nanoparticles for enhanced drug delivery. Biomater Sci. 9(4):1088–1103. doi:10.1039/d0bm01164a.
- Wang W, Lu K-J, Yu C-H, Huang Q-L, Du Y-Z. 2019. Nano-drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnol. 17(1). doi:10.1186/s12951-019-0514-y.
- Wang WQ, Dong K, Zhou L, Jiao GH, Zhu CZ, Li WW, et al. 2015. IL-37b gene transfer enhances the therapeutic efficacy of mesenchumal stromal cells in DSS-induced colitis mice. Acta Pharmacol Sin. 36(11):1377–1387. doi:10.1038/aps.2015.51.
- Wang X, Gao J, Ouyang X, Wang J, Sun X, Lv Y. 2018. Mesenchymal stem cells loaded with paclitaxel-poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy. Int J Nanomedicine. 13:5231–5248. doi:10.2147/ijn.S167142.
- Wei JN, Cai F, Wang F, Wu XT, Liu L, Hong X, et al. 2016. Transplantation of CXCR4 overexpressed mesenchymal stem cells augments regeneration in degenerated intervertebral discs. DNA Cell Biol. 35(5):241–248. doi:10.1089/dna.2015.3118.
- Wu HH, Zhou Y, Tabata Y, Gao JQ. 2019. Mesenchymal stem cell-based drug delivery strategy: from cells to biomimetic. J Control Release. 294:102–113. doi:10.1016/j.jconrel.2018.12.019.
- Xu C, Feng Q, Yang H, Wang G, Huang L, Bai Q, et al. 2018. A light-triggered mesenchymal stem cell delivery system for photoacoustic imaging and chemo-photothermal therapy of triple negative breast cancer. Adv Sci (Weinh). 5(10):1800382. doi:10.1002/advs.201800382.
- Xu CH, Ye PJ, Zhou YC, He DX, Wei H, Yu CY. 2020. Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater. 105:1–14. doi:10.1016/j.actbio.2020.01.036.
- Xu M, Asghar S, Dai S, Wang Y, Feng S, Jin L, et al. 2019. Mesenchymal stem cells-curcumin loaded chitosan nanoparticles hybrid vectors for tumor-tropic therapy. Int J Biol Macromol. 134:1002–1012. doi:10.1016/j.ijbiomac.2019.04.201.
- Yang N, Ding Y, Zhang Y, Wang B, Zhao X, Cheng K, et al. 2018. Surface functionalization of polymeric nanoparticles with umbilical cord-derived mesenchymal stem cell membrane for tumor-targeted therapy. ACS Appl Mater Interfaces. 10(27):22963–22973. doi:10.1021/acsami.8b05363.
- Yao S, Li X, Liu J, Sun Y, Wang Z, Jiang Y. 2017. Maximized nanodrug-loaded mesenchymal stem cells by a dual drug-loaded mode for the systemic treatment of metastatic lung cancer. Drug Deliv. 24(1):1372–1383. doi:10.1080/10717544.2017.1375580.
- Zhai Y, Su J, Ran W, Zhang P, Yin Q, Zhang Z, et al. 2017. Preparation and application of cell membrane-camouflaged nanoparticles for cancer therapy. Theranostics. 7(10):2575–2592. doi:10.7150/thno.20118.
- Zhao J, Vykoukal J, Abdelsalam M, Recio-Boiles A, Huang Q, Qiao Y, et al. 2014. Stem cell-mediated delivery of SPIO-loaded gold nanoparticles for the theranosis of liver injury and hepatocellular carcinoma. Nanotechnology. 25(40):405101. doi:10.1088/0957-4484/25/40/405101.
- Zheng G, Ge M, Qiu G, Shu Q, Xu J. 2015. Mesenchymal stromal cells affect disease outcomes via macrophage polarization. Stem Cells Int. 2015:989473. doi:10.1155/2015/989473.
- Zhou F, Teng F, Deng P, Meng N, Song Z, Feng R. 2018. Recent progress of nano-drug delivery system for liver cancer treatment. Anticancer Agents Med Chem. 17(14):1884–1897. doi:10.2174/1871520617666170713151149.
- Zhou Y, Zhou W, Chen X, Wang Q, Li C, Chen Q, et al. 2020. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm Sin B. 10(8):1563–1575. doi:10.1016/j.apsb.2019.11.013.
- Zhu Q, Ling X, Yang Y, Zhang J, Li Q, Niu X, et al. 2019. Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Adv Sci (Weinh). 6(6):1801899. doi:10.1002/advs.201801899.
